Innovative Fly Ash Geopolymer-Epoxy Composites: Preparation, Microstructure and Mechanical Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Specimen Preparation
2.2.1. Preparation of Metakaolin-Based Geopolymer (G-MK)
2.2.2. Preparation of Fly Ash-Based Geopolymer (G-FA)
2.2.3. Preparation of the EPOXY-Geopolymer Composites
2.3. Curing Treatments
3. Results and Discussion
3.1. Characterization
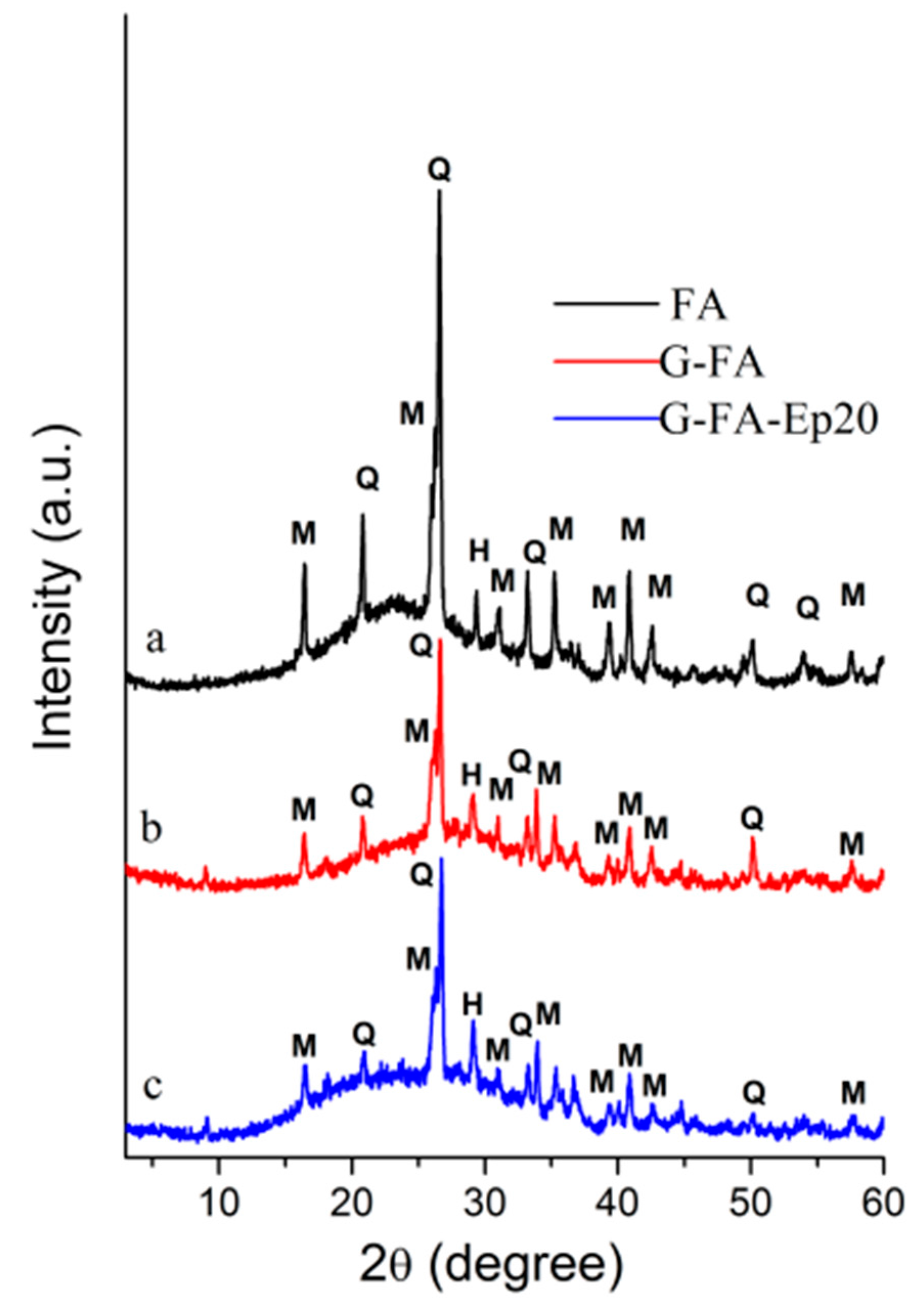
3.1.1. X-ray Diffraction Characterization
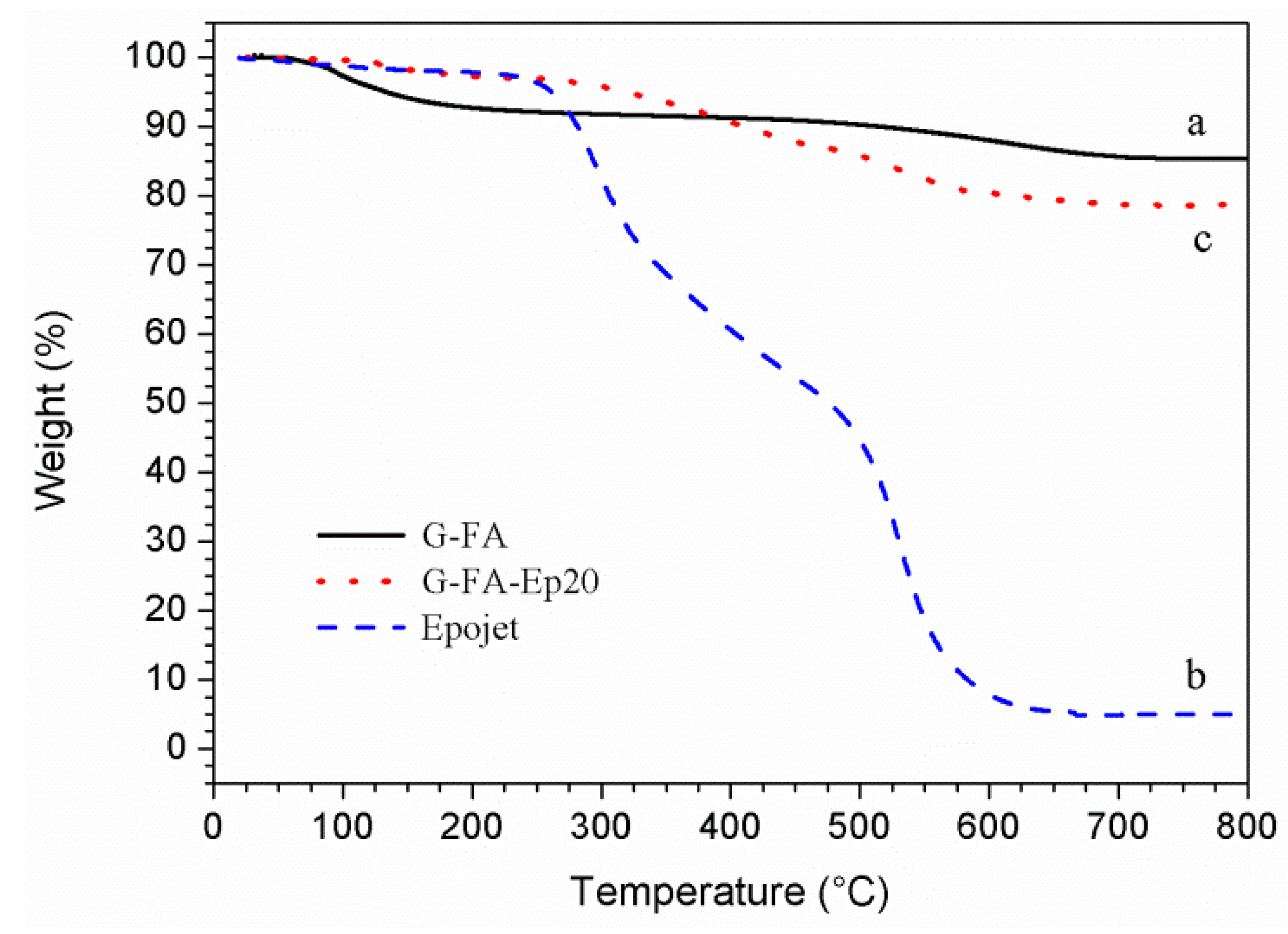
3.1.2. Thermal Analysis
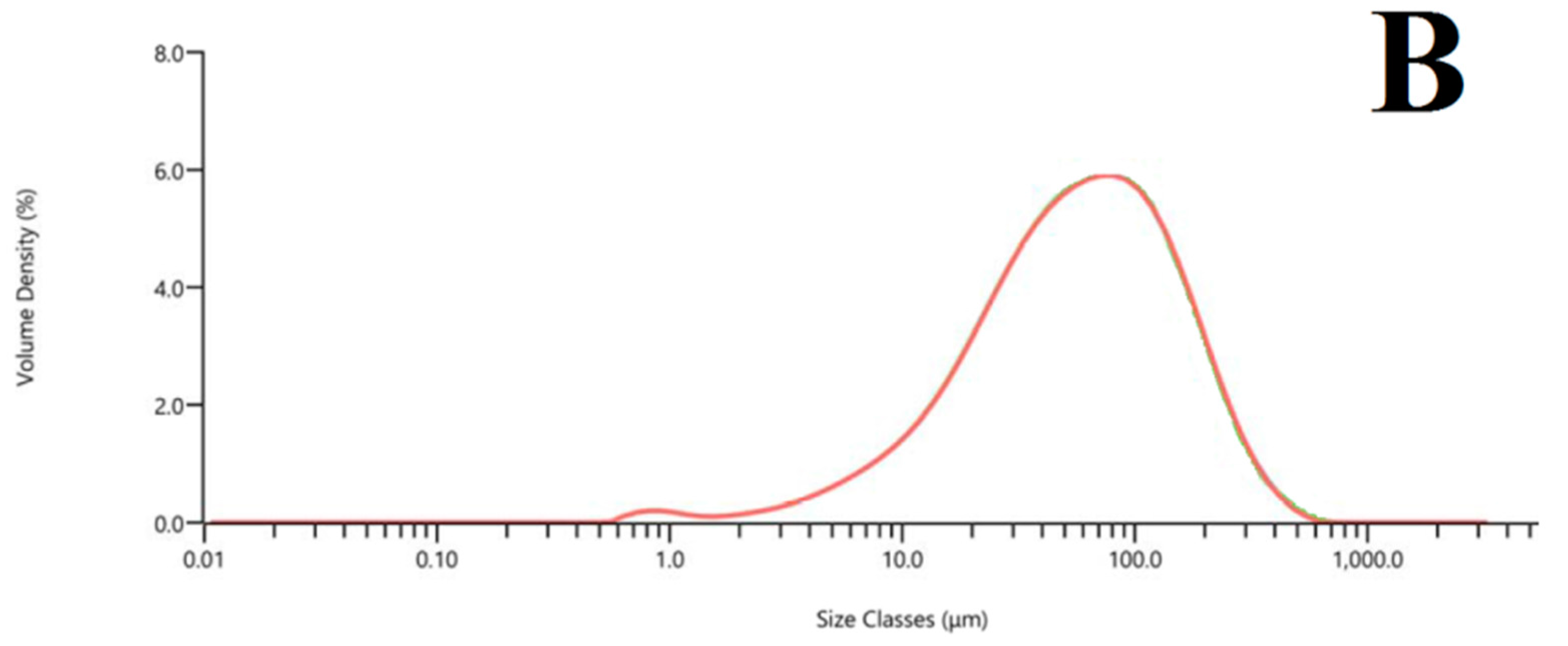
3.1.3. Microstructural Analysis of Fly Ash
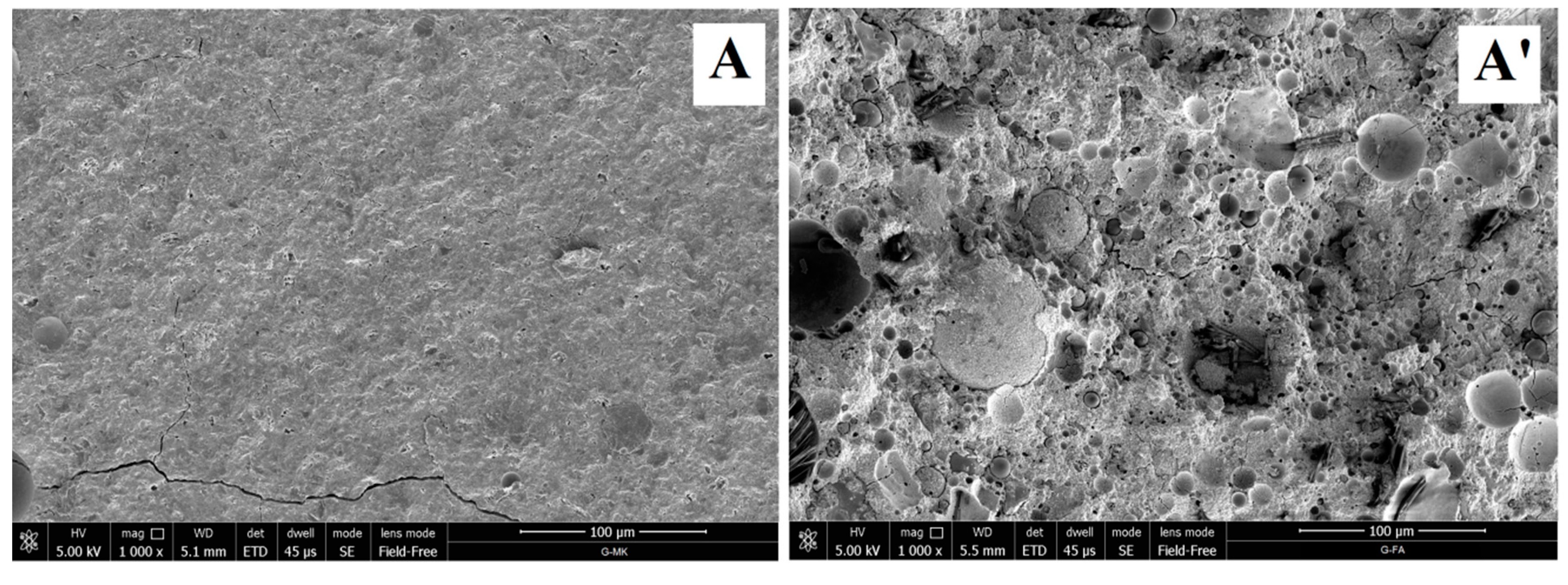
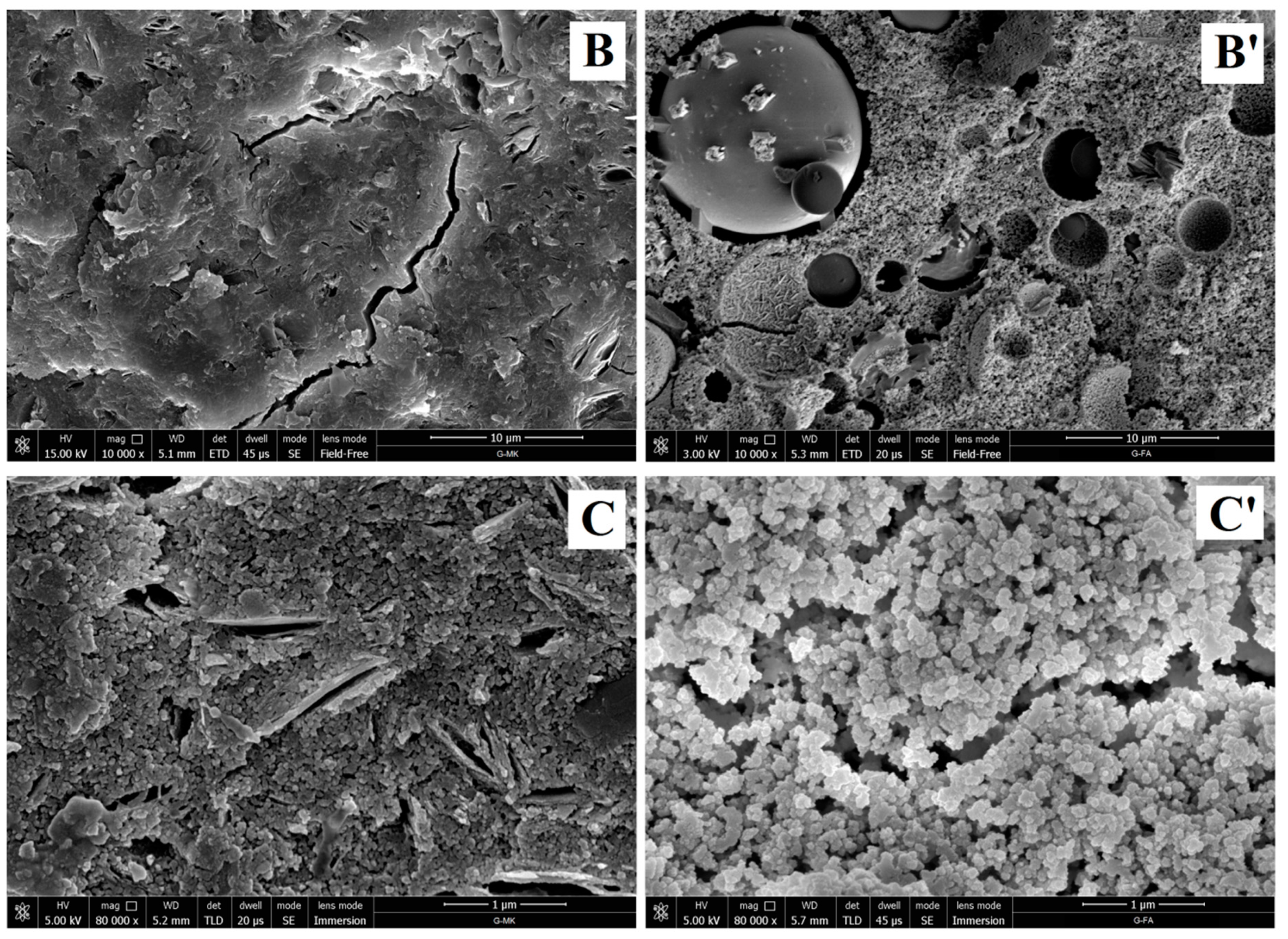
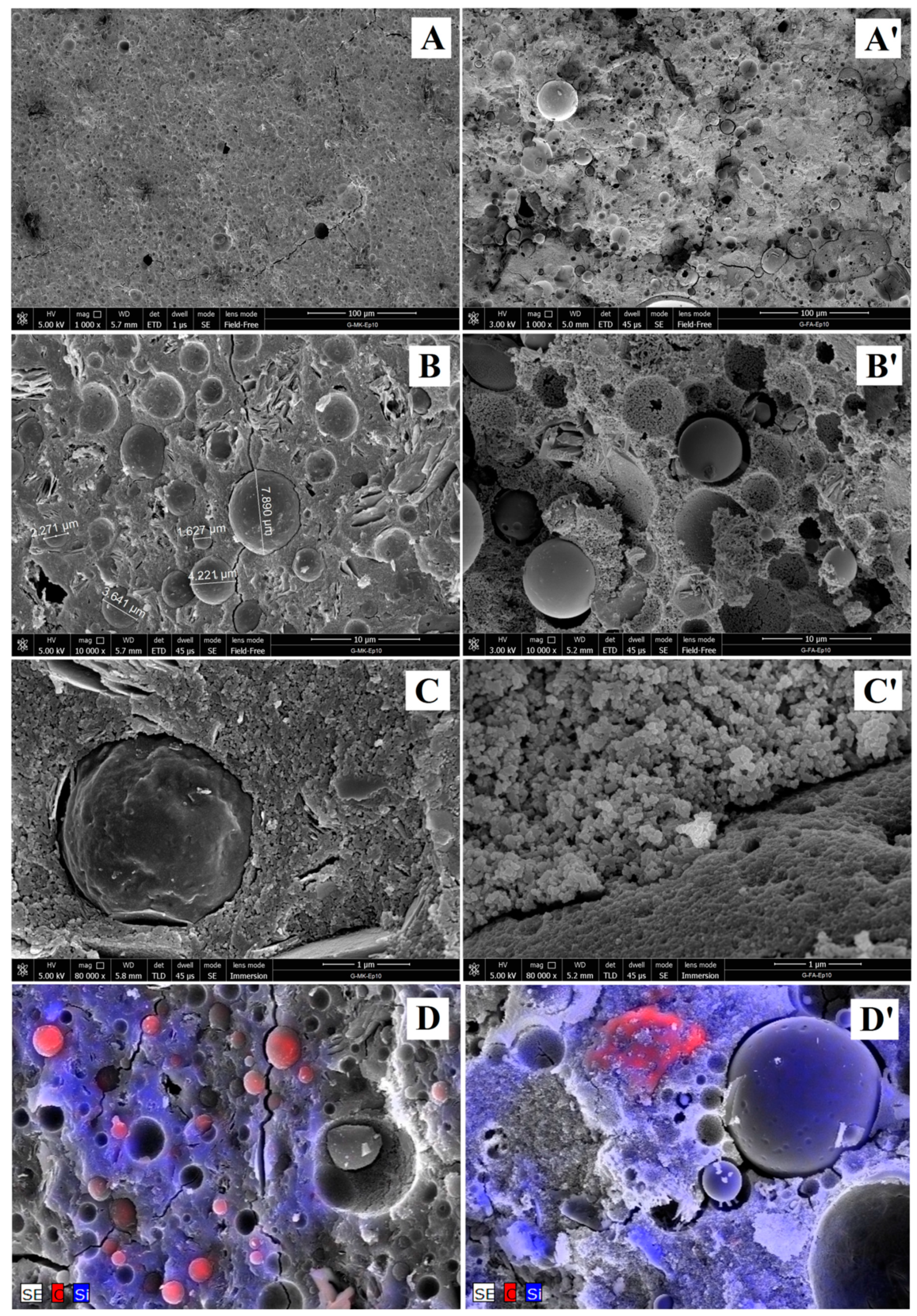
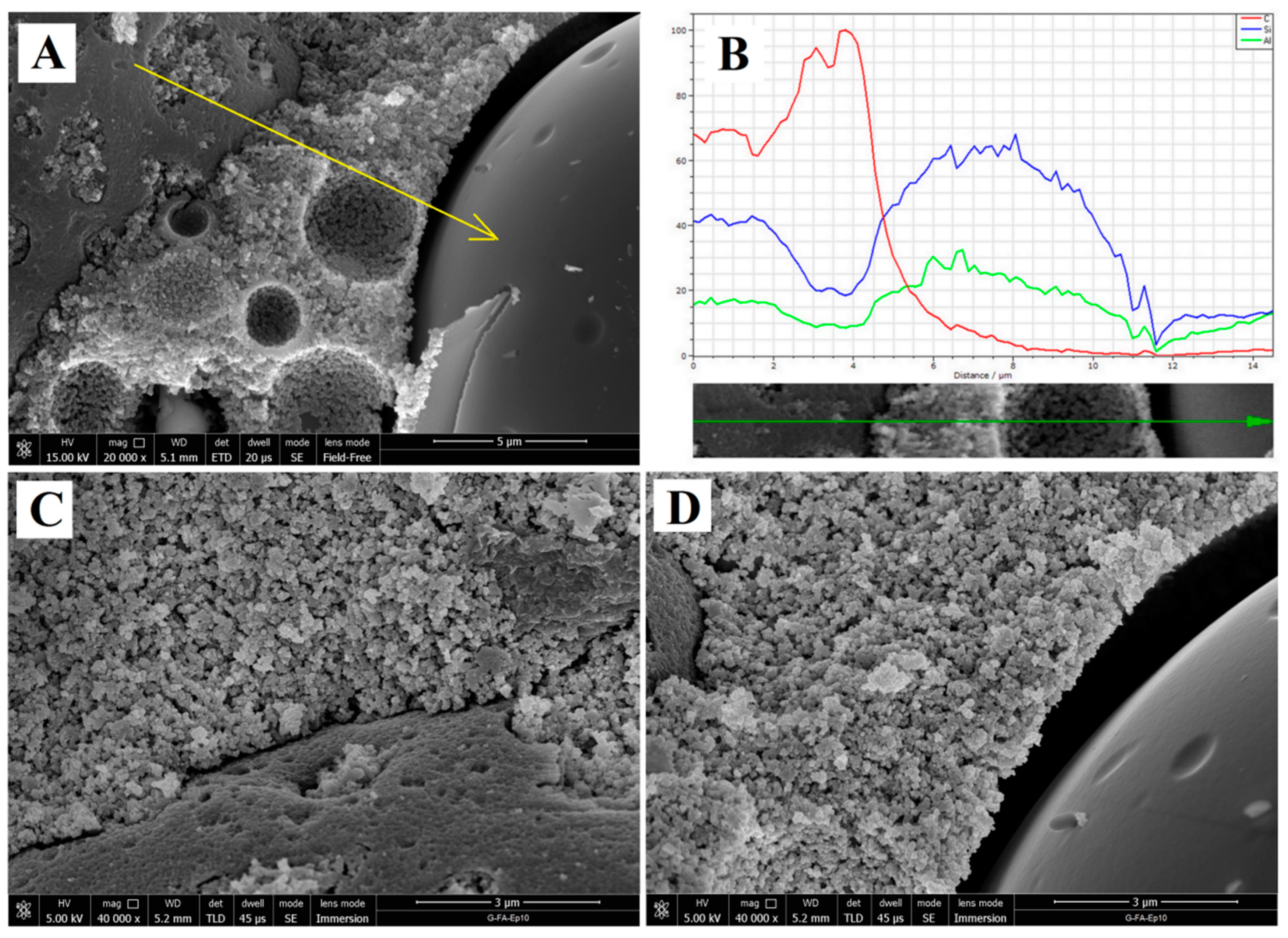
3.1.4. Microstructural Analysis of Geopolymers and Geopolymer Based Composites
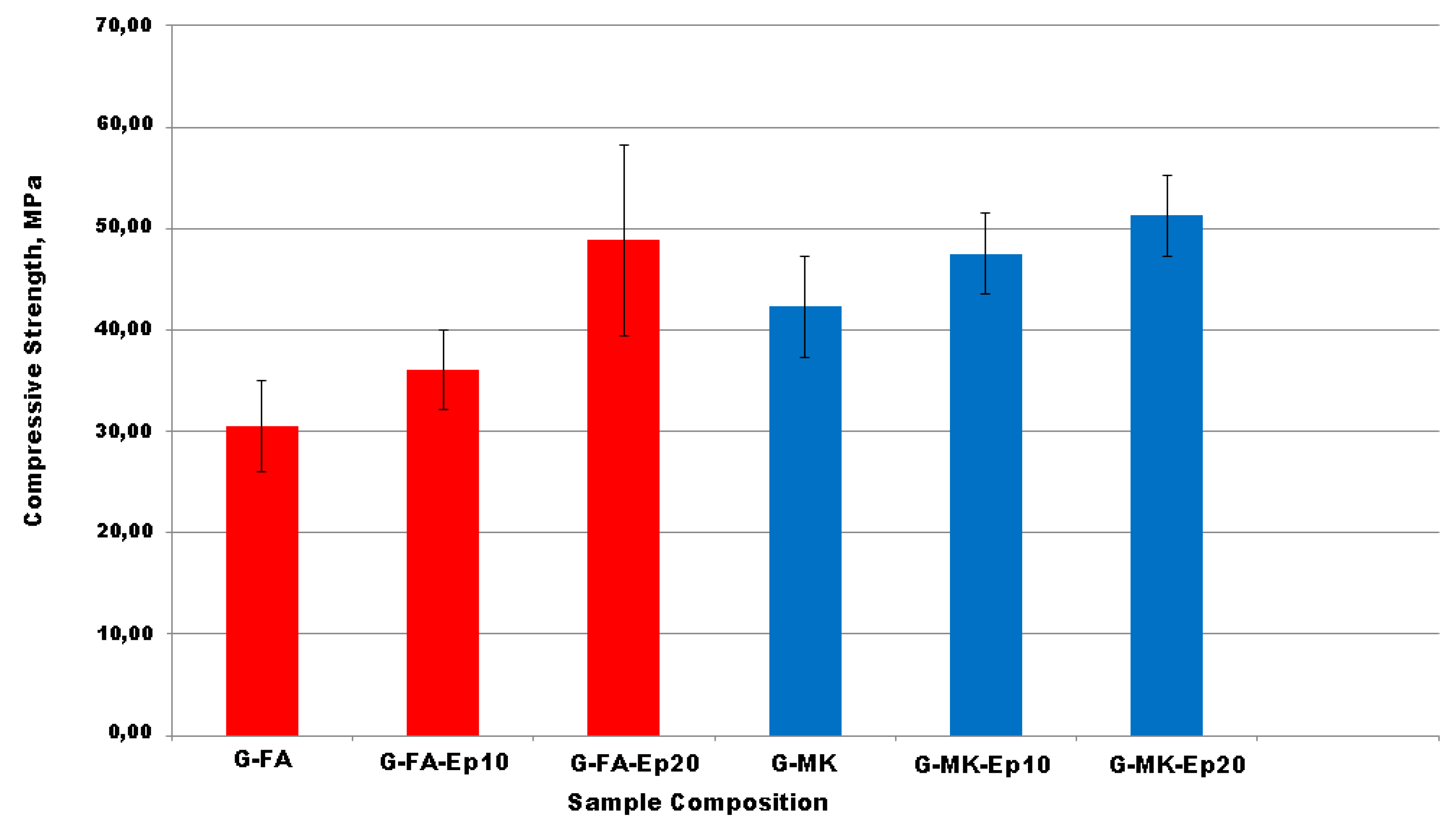
3.1.5. Compressive Strength Test
4. Conclusions
- the compressive strength of the composite materials is significantly better than that of the neat geopolymer;
- fly ash-based composite materials represent a valid alternative in place of more expensive raw materials, such as metakaolin, in particular for those applications for which it is important to save materials and limit the costs;
- the procedure is inexpensive and uses easily available reagents.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 3rd ed.; Institut Gèopolymère: Saint Quentin, France, 2011. [Google Scholar]
- Cioffi, R.; Maffucci, L.; Santoro, L. Optimization of geopolymer synthesis by calcination and polycondensation of a kaolinitic residue. Resour. Conserv. Recycl. 2003, 40, 27–38. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Concrete Technology, Past, Present and Future; Davidovits, J.; Metha, P.K. (Eds.) ACI SP-144; American Concrete Institute: Detroit, MI, USA, 1994; pp. 383–384.
- Arm, M. KTH Land and Water Resources Engineering; KTH Royal Institute of Technology: Stockholm, Sweden, 2003; p. 5. [Google Scholar]
- Concrete Technology for a Sustainable Development in the 21st Century; Malhotra, V.M.; Gjorv, O.E.; Sakai, K. E & FN Spon: London, UK, 2000; pp. 226–235. [Google Scholar]
- McCaffrey, R. Climate change and the cement industry. In Global Cement and Lime Magazine; Environmental Special Issue; Climate Strategies: Cambridge, UK, 2002; pp. 15–19. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Goodwin, R.W. Combustion Ash/Residue Management: An Engineering Perspective; Noyes Publications: London, UK, 1993. [Google Scholar]
- Berry, E.E.; Malhotra, V.M. Fly ash for use in concrete—A critical review. J. Am. Concr. Inst. 1980, 77, 59–73. [Google Scholar]
- Matsunaga, T.; Kim, J.K.; Hardcastle, S.; Rohatgi, P.K. Crystallinity and selected properties of fly ash particles. Mater. Sci. Eng. A 2002, 325, 333–343. [Google Scholar] [CrossRef]
- Puertas, F.; Martìnez-Ramìrezez, S.; Alonso, S.; Vàzquez, T. Alkali-activated fly ash/slag cement strength behavior and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Colangelo, F.; Cioffi, R. Low temperature alkaline activation of weathered fly ash: Influence of mineral admixtures on early age performance. Constr. Build. Mater. 2015, 86, 169–177. [Google Scholar] [CrossRef]
- Safiuddin, M.; Jumaat, M.Z.; Salam, M.A.; Islam, M.S.; Hashim, R. Utilization of solid wastes in construction materials. Int. J. Phys. Sci. 2010, 13, 1952–1963. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and related Alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–232. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Li, Z.; Chen, R.; Zhang, L. Utilization of chitosan biopolymer to enhance fly ash based geopolymer. J. Mater. Sci. 2013, 48, 7986–7993. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Li, Z. Infrared spectroscopy study of structural nature of geopolymeric products. J. Wuhan Univ. Technol. 2008, 23, 522–527. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, X.; Zhu, H.; Hua, S.; Chen, Y. Preparation and mechanical properties of polypropylene fiber reinforced calcined kaolin-fly ash based geopolymer. J. Cent. South Univ. Technol. 2009, 16, 49–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Li, Z. Impact behavior and microstructural characteristics of PVA fiber reinforced fly ash-geopolymer boards prepared by extrusion technique. J. Mater. Sci. 2006, 41, 2787–2794. [Google Scholar]
- Zhang, S.; Gong, K.; Lu, J. Novel modification method for inorganic geopolymer by using water soluble organic polymers. Mater. Lett. 2004, 58, 1292–1296. [Google Scholar] [CrossRef]
- Zhao, Q.; Nair, B.; Rahimian, T.; Balaguru, P. Novel geopolymer based composites with enhanced ductility. J. Mater. Sci. 2007, 42, 3131–3137. [Google Scholar] [CrossRef]
- Ricciotti, L.; Borbone, F.; Carella, A.; Centore, R.; Roviello, A.; Barra, M.; Roviello, G.; Ferone, C.; Minarini, C.; Morvillo, P. Synthesis of highly regioregular poly[3-(4-alkoxyphenyl)-thiophene]s by oxidative catalysis using copper complexes. J. Polym. Sci. A Polym. Chem. 2013, 51, 4351–4360. [Google Scholar] [CrossRef]
- Carella, A.; Borbone, F.; Roviello, G.; Caruso, U.; Ferone, C.; Ricciotti, L.; Pirozzi, B.; Persico, P.; Schieroni, A.; Roviello, A. Rigid chain ribbon-like metallopolymers. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2412–2421. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Xu, D.L.; Wang, B.Q.; Xu, G.M.; Yang, D.F.; Wang, N.; Liu, H.C.; Wang, Y.C. A novel method for preparation of organic resins reinforced geopolymer composites. J. Mater. Sci. 2010, 45, 1189–1192. [Google Scholar] [CrossRef]
- Ferone, C.; Roviello, G.; Colangelo, F.; Cioffi, R.; Tarallo, O. Novel hybrid organic-geopolymer materials. Appl. Clay Sci. 2013, 73, 42–50. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Cioffi, R.; Tarallo, O. Synthesis and characterization of novel epoxy geopolymer hybrid composites. Materials 2013, 6, 3943–3962. [Google Scholar] [CrossRef]
- Colangelo, F.; Roviello, G.; Ricciotti, L.; Ferone, C.; Cioffi, R. Preparation and characterization of new geopolymer-epoxy resin hybrid mortars. Materials 2013, 6, 2989–3006. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Tarallo, O. Fire resistant melamine based organic-geopolymer hybrid composites. Cem. Concr. Compos. 2015, 59, 89–99. [Google Scholar] [CrossRef]
- Ricciotti, L.; Roviello, G.; Tarallo, O.; Borbone, F.; Ferone, C.; Colangelo, F.; Catauro, M.; Cioffi, R. Synthesis and characterizations of melamine-based epoxy resins. Int. J. Mol. Sci. 2013, 14, 18200–18214. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Mechanical performances of weathered coal fly ash based geopolymer bricks. Procedia Eng. 2011, 21, 745–752. [Google Scholar] [CrossRef]
- Epojet. Available online: http://www.mapei.com/public/COM/products/367_epojet_gb.pdf (accessed on 9 June 2016).
- Criado, M.; Fernández-Jiménez, A.; de la Torre, A.G.; Aranda, M.A.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Chen-Tan, N.W.; van Riessen, A.; Chi, V.L.Y.; Southam, D.C. Determining the reactivity of a fly ash for production of geopolymer. J. Am. Ceram. Soc. 2009, 92, 881–887. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Proffen, T.; van Deventer, J.S.J. The effects of temperature on the local structure of metakaolin-based geopolymer binder: A neutron pair distribution function investigation. J. Am. Ceram. Soc. 2010, 93, 3486–3492. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Van Riessen, A.; Chen-Tan, N. Beneficiation of collie fly ash for synthesis of geopolymer Part 2—Geopolymers. Fuel 2013, 111, 829–835. [Google Scholar] [CrossRef]
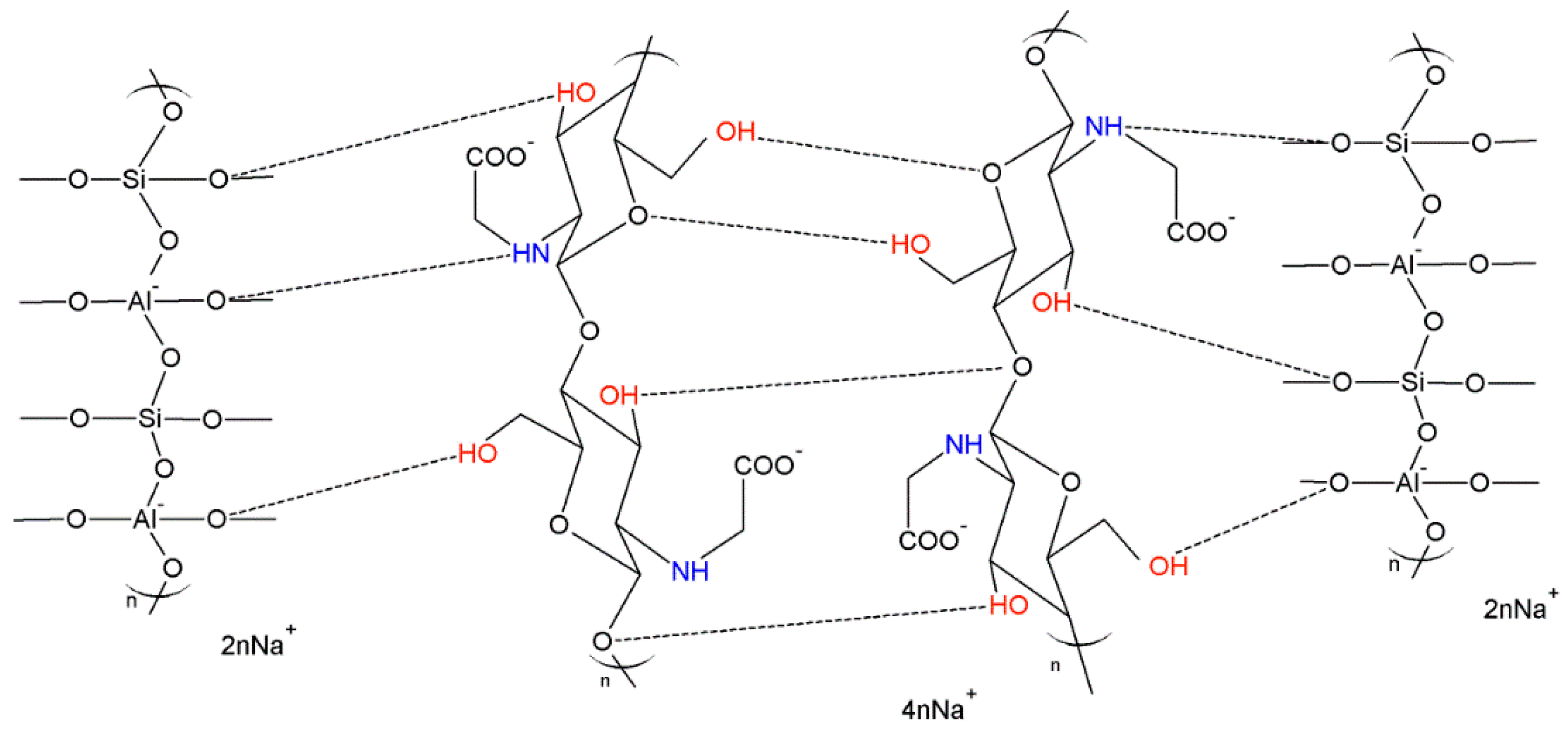
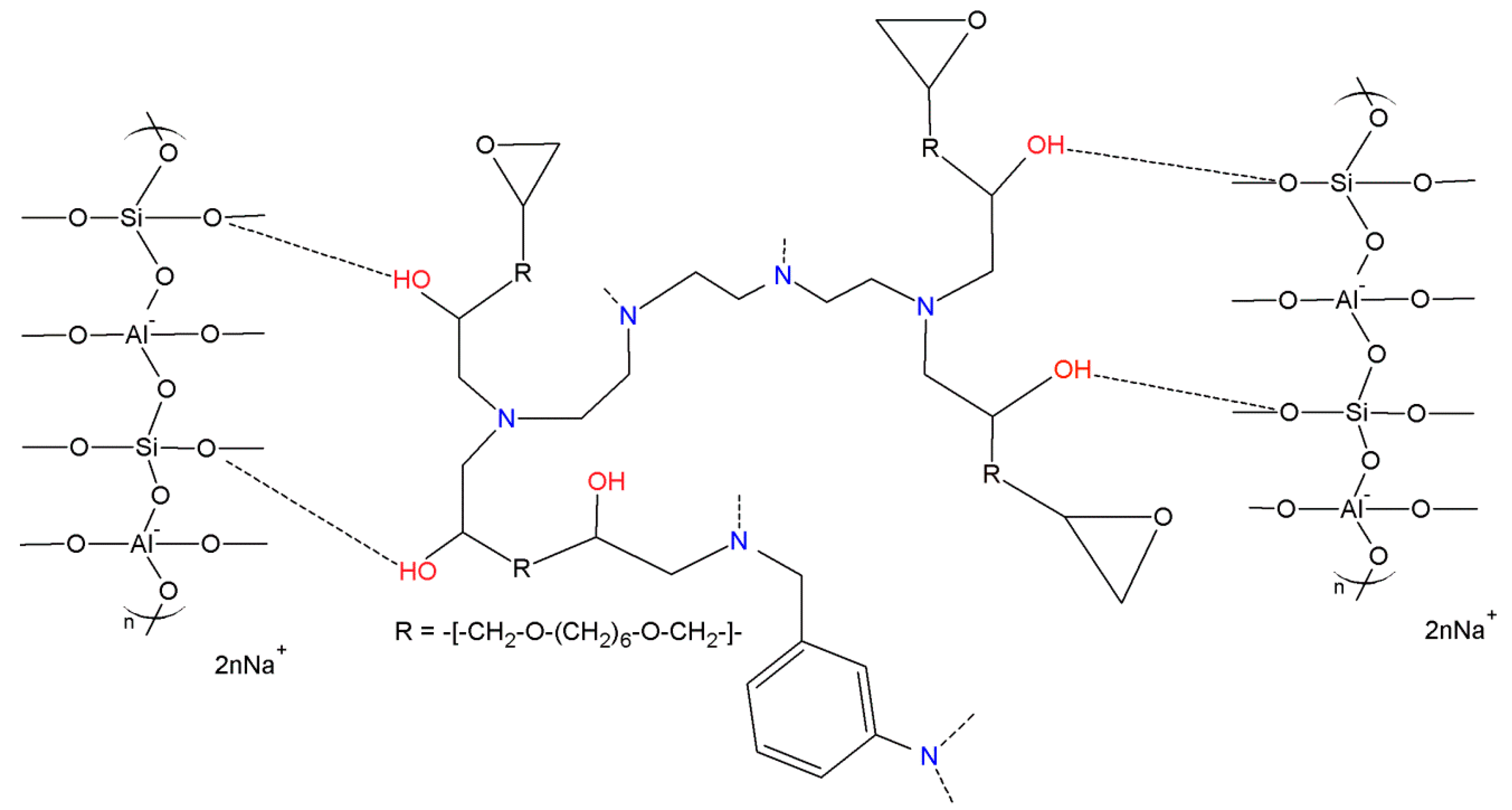

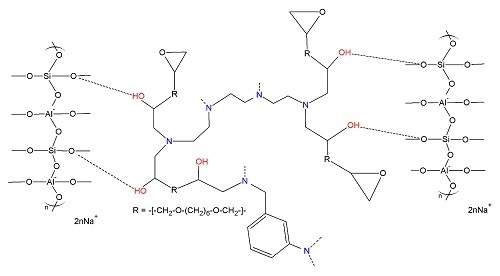
| Fly Ash | |||||||
| Al2O3 | SiO2 | K2O | Fe2O3 | Na2O | MgO | CaO | others |
| 28.12 | 53.75 | 1.89 | 6.99 | 0.87 | 1.59 | 4.32 | 2.47 |
| Metakaolin | |||||||
| Al2O3 | SiO2 | K2O | Fe2O3 | TiO2 | MgO | CaO | others |
| 41.90 | 52.90 | 0.77 | 1.60 | 1.80 | 0.19 | 0.17 | 0.67 |
| Sodium Silicate Solution | |||||||
| SiO2 | Na2O | H2O | |||||
| 27.40 | 8.15 | 64.45 | |||||
| Mix ID | MK | FA | SS | NaOH | NaOH soln | Resin |
|---|---|---|---|---|---|---|
| G-MK | 41.6 | - | 50.0 | 8.4 | - | - |
| G-MK-Ep10 | 37.4 | - | 45.0 | 7.6 | - | 10 |
| G-MK-Ep20 | 33.3 | - | 40.0 | 6.7 | - | 20 |
| G-FA | - | 60.2 | 19.9 | - | 19.9 | - |
| G-FA-Ep10 | 54.2 | 17.9 | - | 17.9 | 10 | |
| G-FA-Ep20 | 48.2 | 15.9 | - | 15.9 | 20 |
| Mix ID | Weight Loss Starting Temperature (°C) | Weight Loss Ending Temperature (°C) | Weight Loss at 200 °C (wt %) | Weight Loss at 400°C (wt %) | Residual at 800°C (wt %) |
|---|---|---|---|---|---|
| G-FA | 30 | 750 | 7.2 | 8.7 | 85 |
| Epojet | 250 | 650 | 2.1 | 39.4 | 5 |
| G-FA-Ep20 | 30 | 700 | 2.6 | 9.2 | 78 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roviello, G.; Ricciotti, L.; Tarallo, O.; Ferone, C.; Colangelo, F.; Roviello, V.; Cioffi, R. Innovative Fly Ash Geopolymer-Epoxy Composites: Preparation, Microstructure and Mechanical Properties. Materials 2016, 9, 461. https://doi.org/10.3390/ma9060461
Roviello G, Ricciotti L, Tarallo O, Ferone C, Colangelo F, Roviello V, Cioffi R. Innovative Fly Ash Geopolymer-Epoxy Composites: Preparation, Microstructure and Mechanical Properties. Materials. 2016; 9(6):461. https://doi.org/10.3390/ma9060461
Chicago/Turabian StyleRoviello, Giuseppina, Laura Ricciotti, Oreste Tarallo, Claudio Ferone, Francesco Colangelo, Valentina Roviello, and Raffaele Cioffi. 2016. "Innovative Fly Ash Geopolymer-Epoxy Composites: Preparation, Microstructure and Mechanical Properties" Materials 9, no. 6: 461. https://doi.org/10.3390/ma9060461
APA StyleRoviello, G., Ricciotti, L., Tarallo, O., Ferone, C., Colangelo, F., Roviello, V., & Cioffi, R. (2016). Innovative Fly Ash Geopolymer-Epoxy Composites: Preparation, Microstructure and Mechanical Properties. Materials, 9(6), 461. https://doi.org/10.3390/ma9060461