Synergetic Effect of Ultrasound, the Heterogeneous Fenton Reaction and Photocatalysis by TiO2 Loaded on Nickel Foam on the Degradation of Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
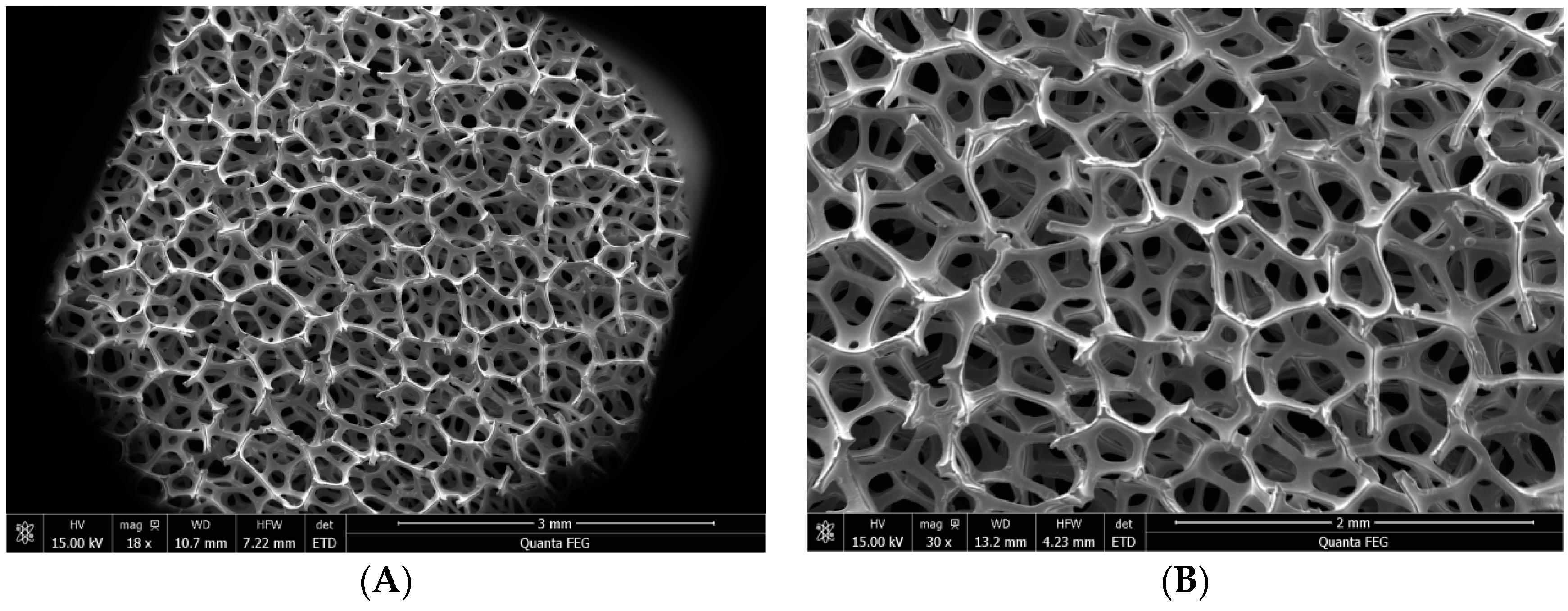
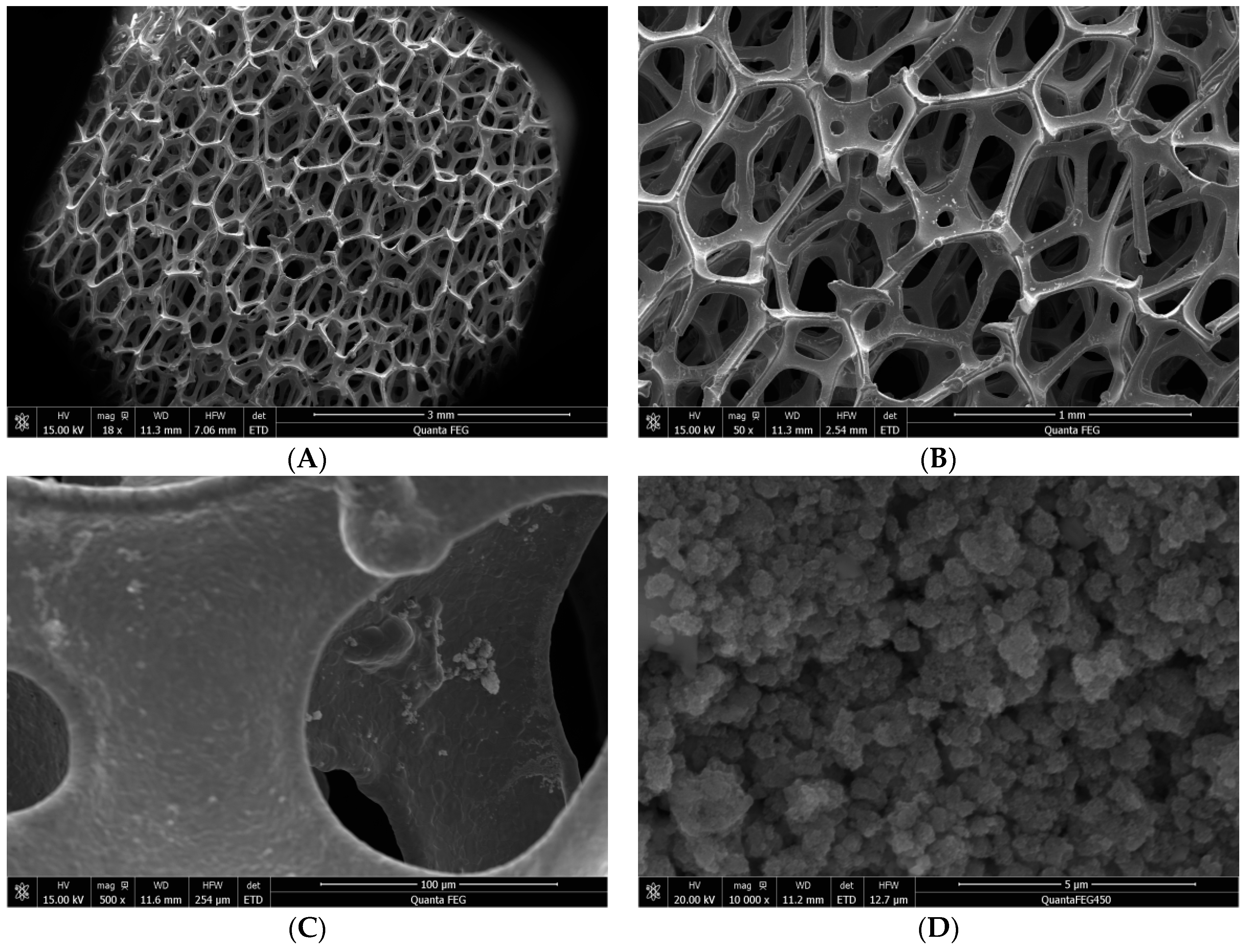
2.2. Preparation of TiO2 Loaded on NF
2.3. Catalytic Activity Testing
2.3.1. Analysis of Ni2+
2.3.2. Quenching of Catalysis
2.3.3. Degradation of Acetochlor
2.3.4. Measurement of Hydroxyl Radical
3. Results and Discussion
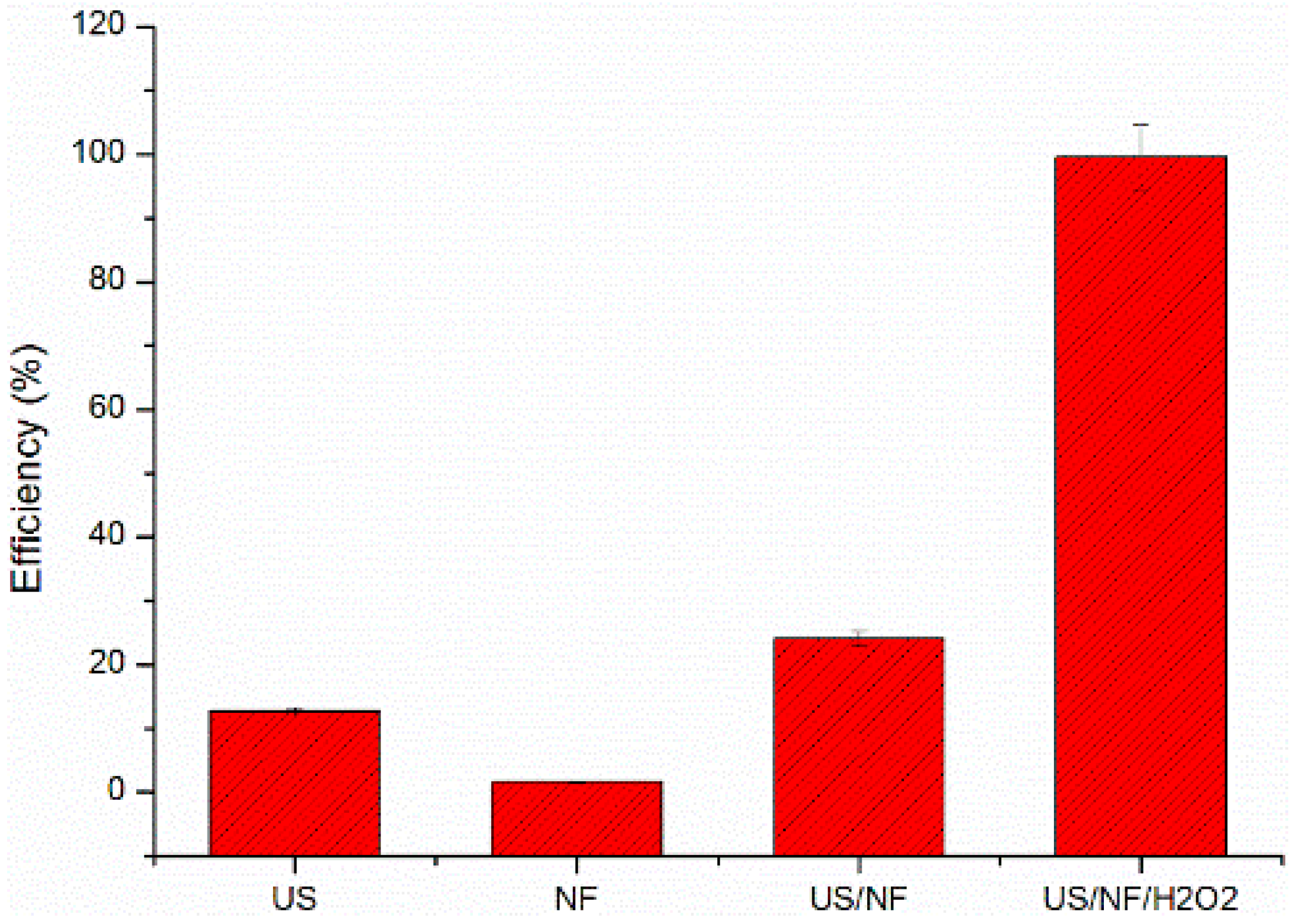
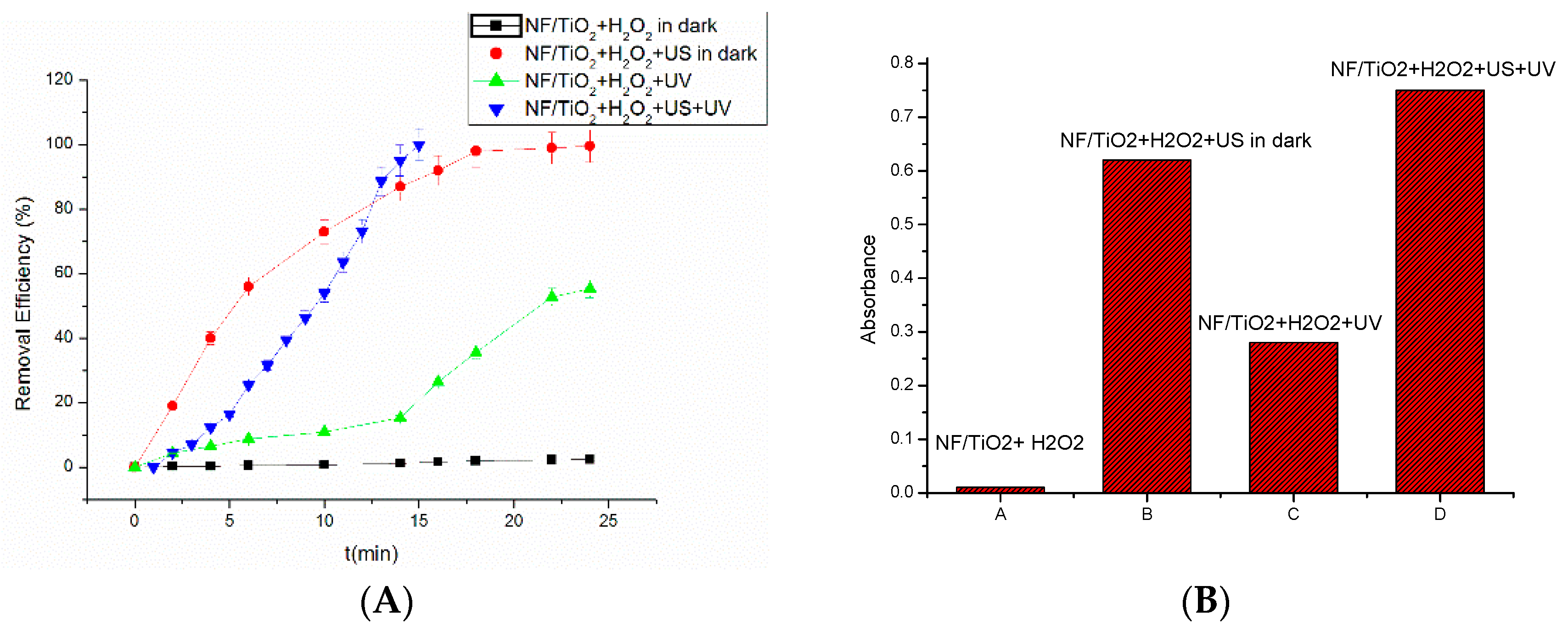
3.1. Removal of RhB under Different Fenton Reaction Systems
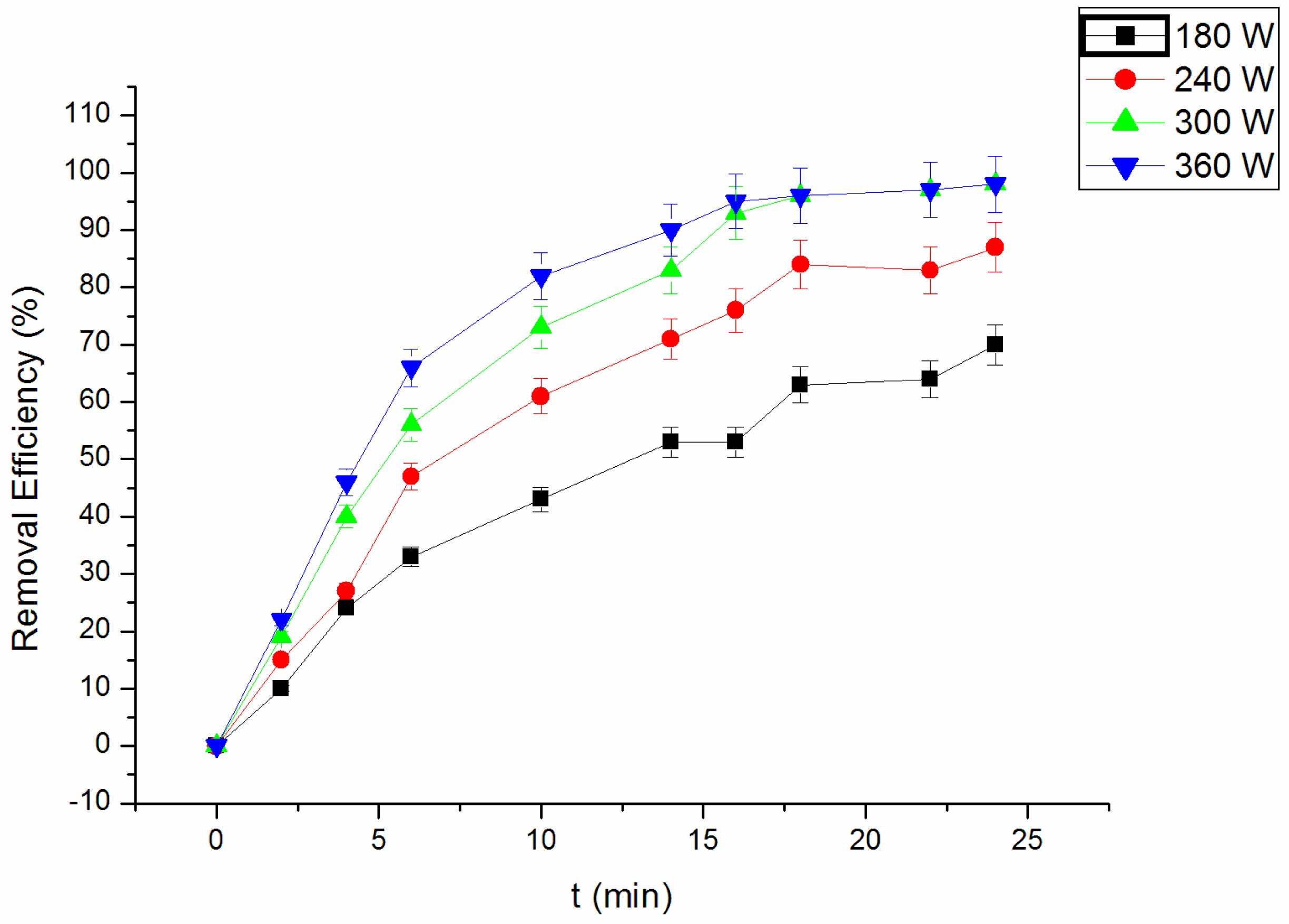
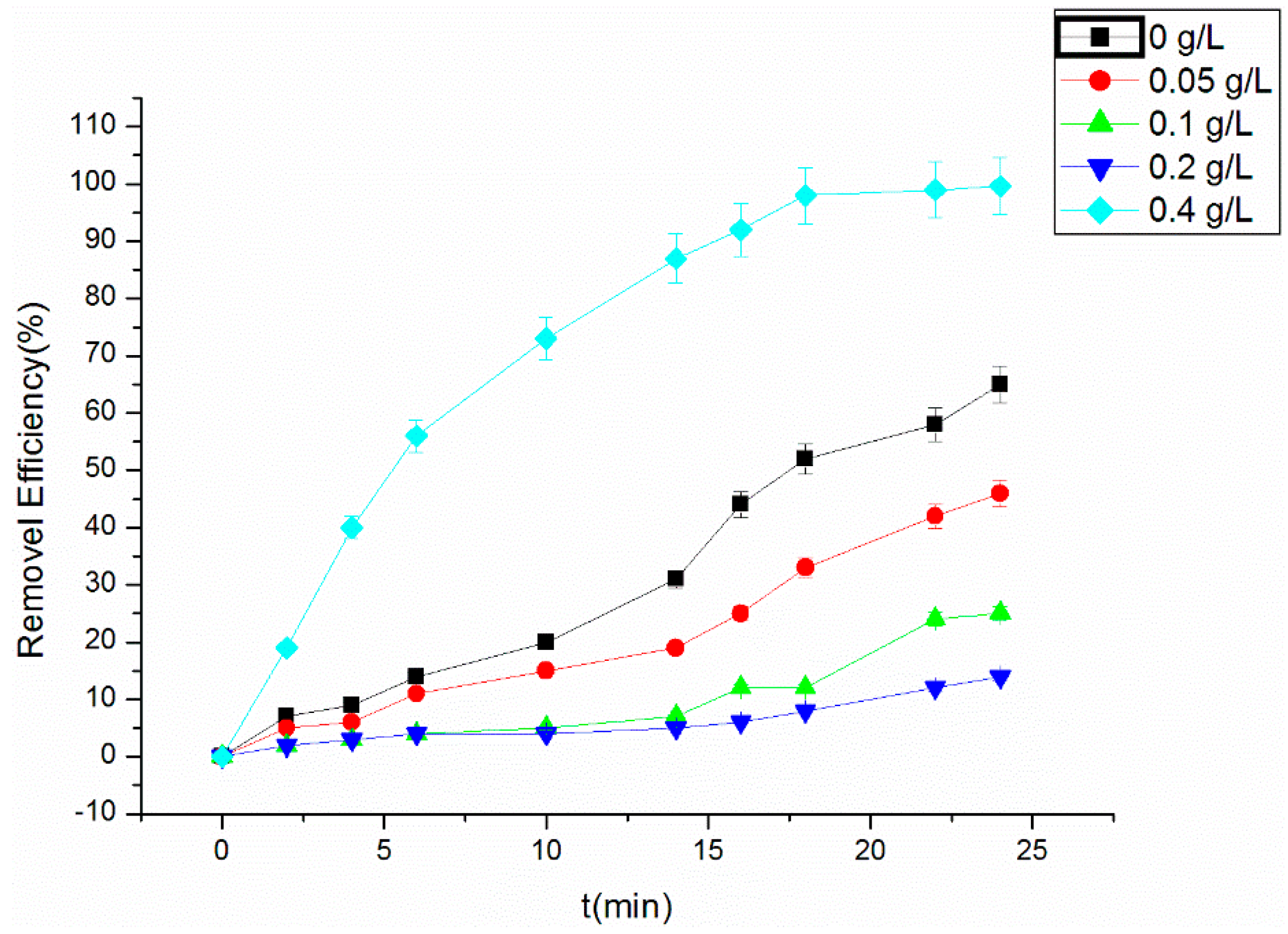
3.2. Effect of Ultrasonic Power on Degradation of RhB
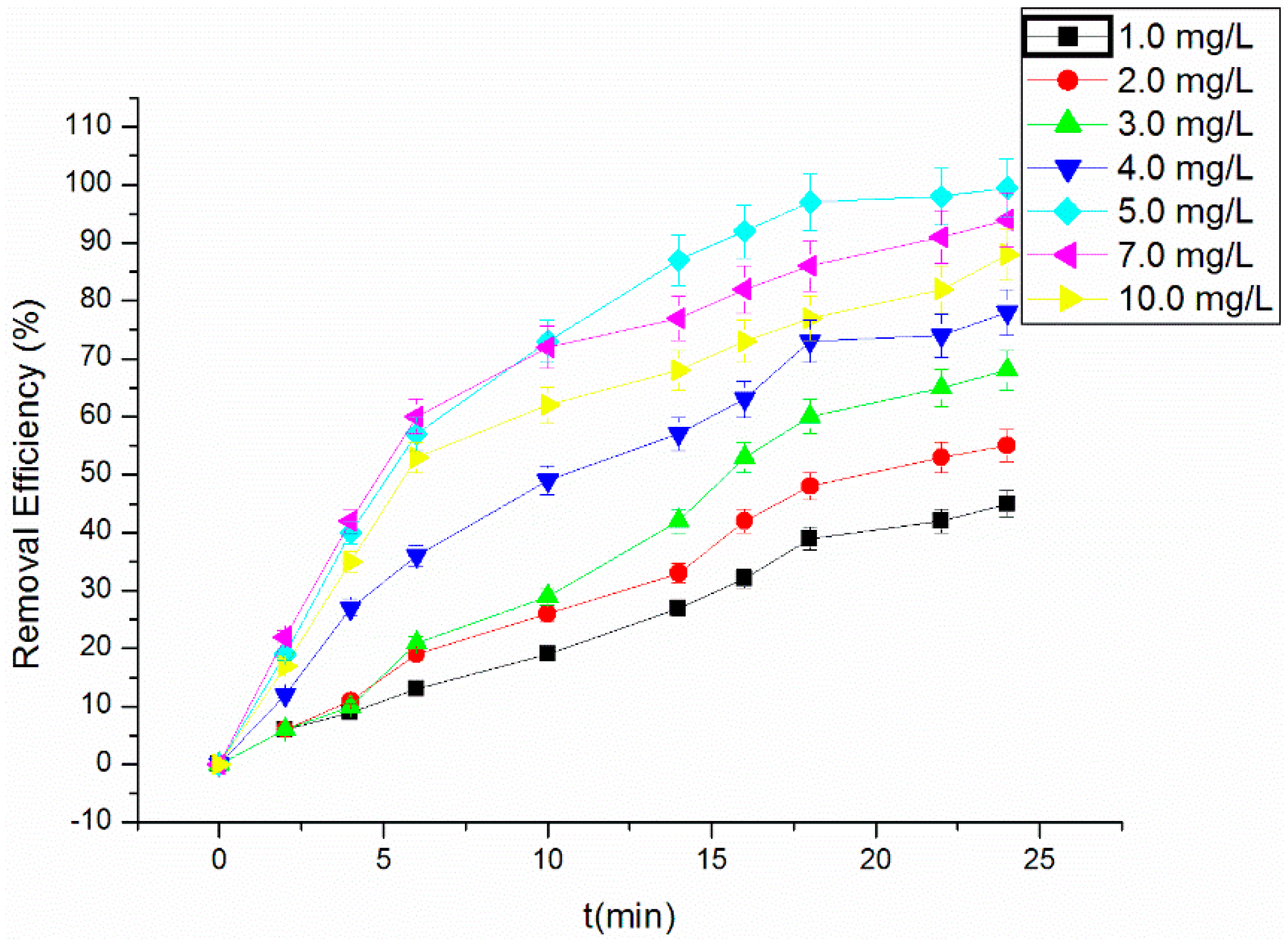
3.3. Effect of H2O2 Concentration on the Degradation of RhB
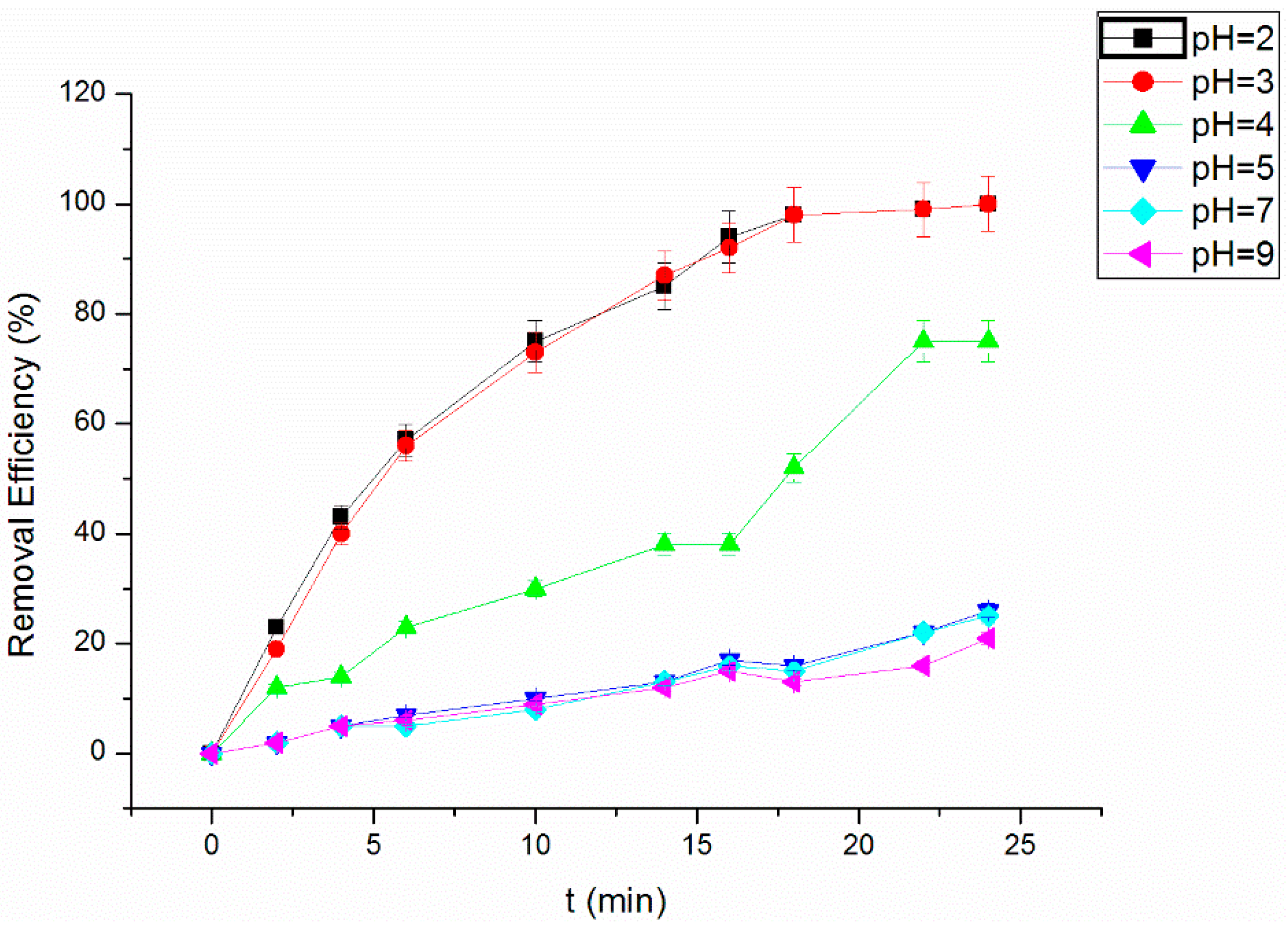
3.4. Effect of Initial pH Value on the RhB Removal Efficiency
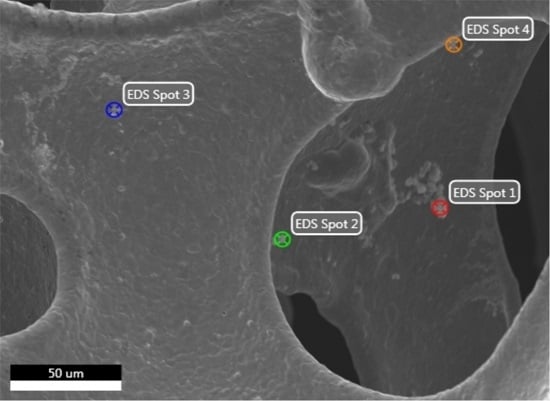
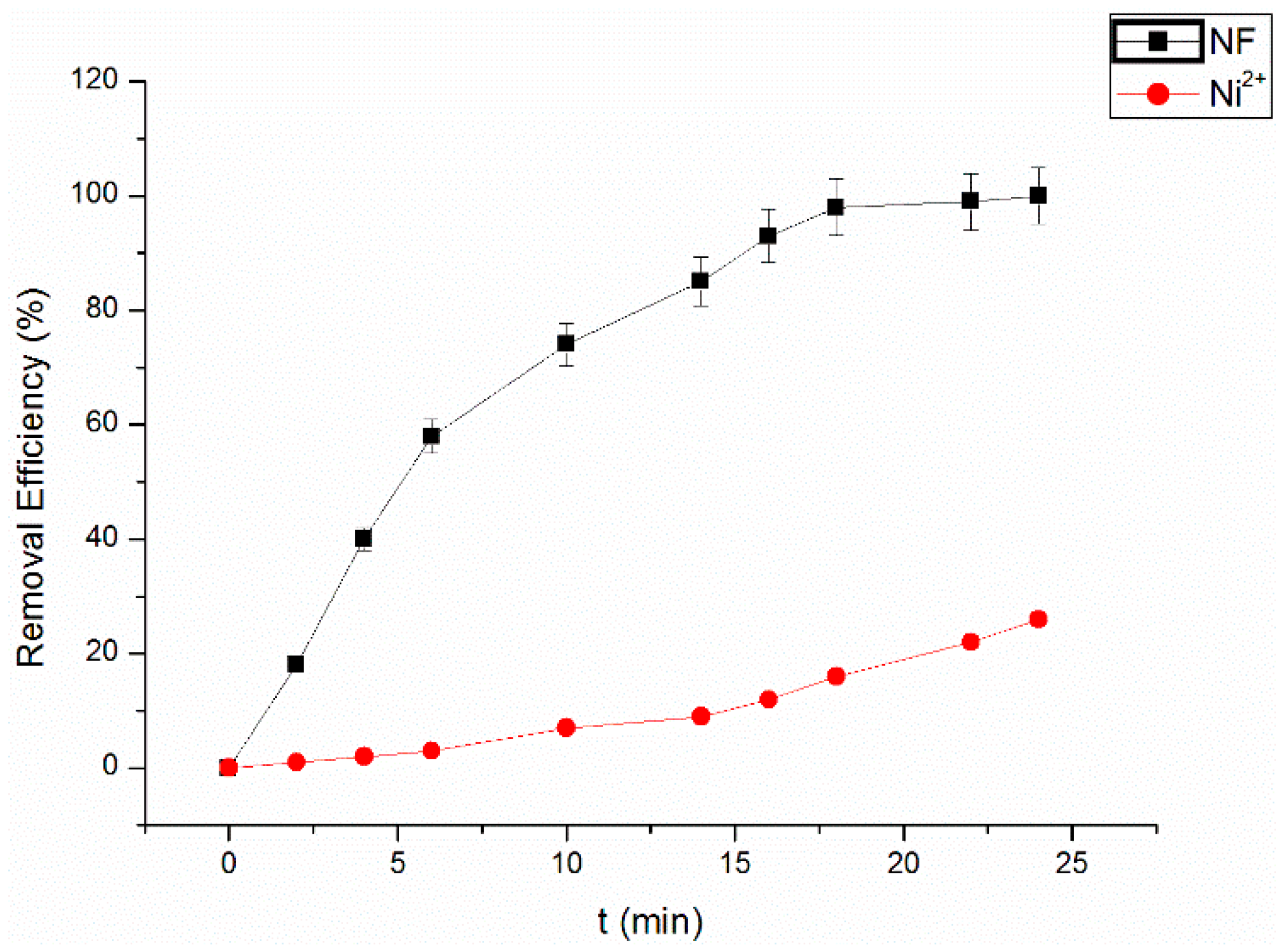
3.5. Dissolution of Nickel from NF
3.6. Quenching of the Fenton Reaction
3.7. The Synergetic Effect of the Fenton Reaction and Photocatalysis
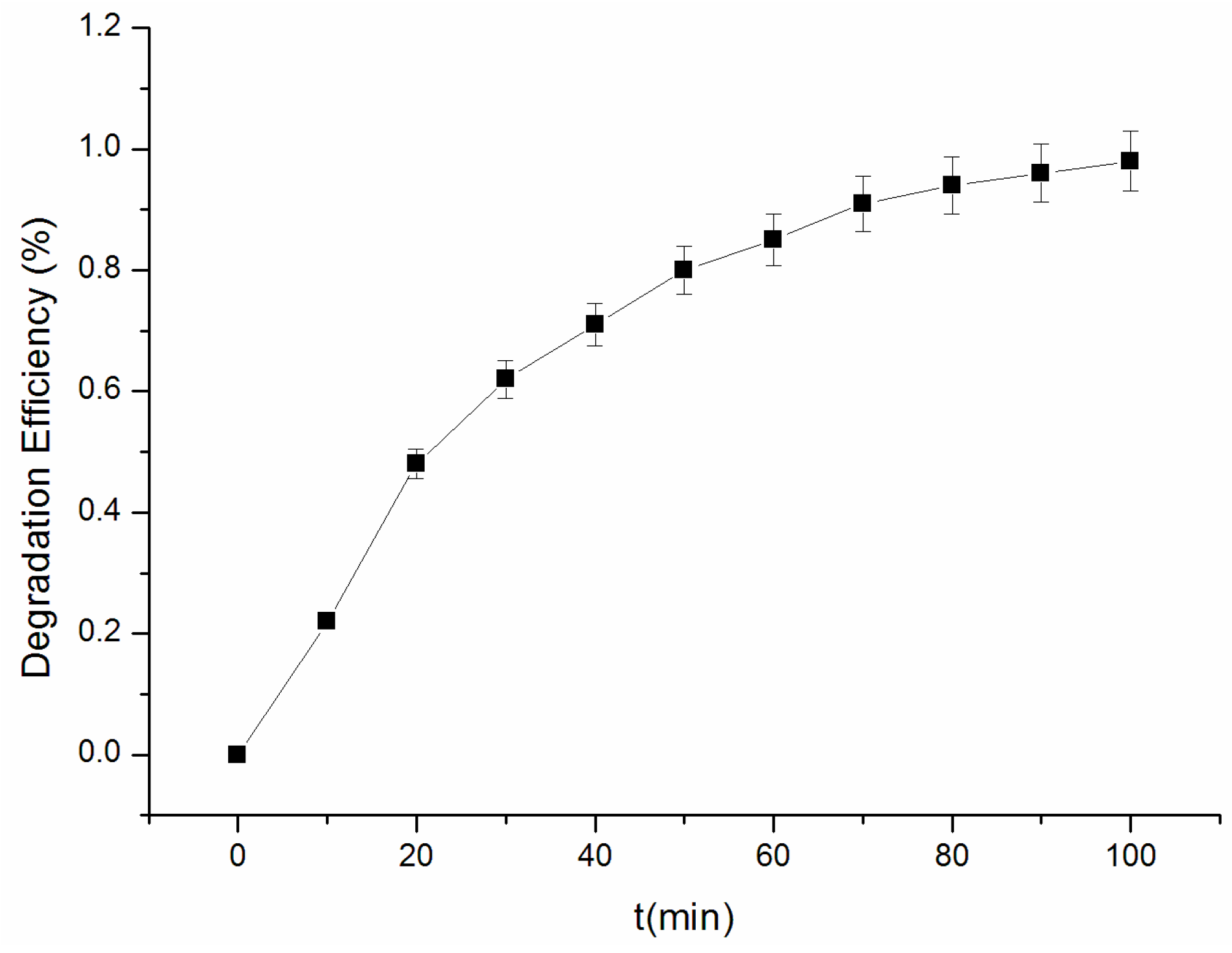
3.8. Degradation of Acetochlor by the Synergetic Effect
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Halim, A.A.; Aziz, H.A.; Megat, M.A.; Shah, K.; Nordin, M. Ammoniacal nitrogen and COD removal from semi-aerobic landfill leachate using a composite adsorbent: Fixed bed column adsorption performance. J. Hazard. Mater. 2010, 175, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Lo, W.; Chan, G.Y.S. Radicals-catalyzed oxidation reactions for degradation of recalcitrant compounds from landfill leachate. Chem. Eng. J. 2006, 125, 35–57. [Google Scholar] [CrossRef]
- Meeroff, D.E.; Bloetscher, F.; Reddy, D.V.; Gasnier, F.; Jain, S.; McBarnette, A.; Hamaguchi, H. Application of photochemical technologies for treatment of landfill leachate. J. Hazard. Mater. 2012, 209, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, R.; Lin, J.; Zhu, Y. Significant photocatalytic enhancement inmethylene blue degradation of TiO2 photocatalysts via graphene-like carbonin situ hybridization. Appl. Catal. B Environ. 2010, 100, 179–183. [Google Scholar] [CrossRef]
- Fu, Y.; Xiong, P.; Chen, H.; Sun, X.; Wang, X. High photocatalytic activity ofmagnetically separable manganese ferrite-graphene heteroarchitectures. Ind. Eng. Chem. Res. 2012, 51, 725–731. [Google Scholar] [CrossRef]
- Chandren, S.; Ohtania, B. Preparation characterization and photocatalyticperformance of titania particles encapsulated in hollow silica shells as an efficient photocatalyst for redox-combined stereoselective synthesis of l-pipecolinic acid from l-lysine. J. Photochem. Photobiol. A 2012, 246, 50–59. [Google Scholar] [CrossRef]
- Cantavenera, M.J.; Catanzaro, I.; Loddo, V.; Palmisano, L.; Sciandrello, G. Photocatalytic degradation of paraquat and genotoxicity of its intermediate products. J. Photochem. Photobiol. A 2007, 185, 277–282. [Google Scholar] [CrossRef]
- Liu, T.; Xia, X.; Liu, S.; Mou, X.; Qiu, Y. Acceleration of denitrification in turbid rivers due to denitrification occurring on suspended sediment in oxic water. Environ. Sci. Technol. 2013, 47, 4053–4061. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Yang, Y.; Li, Y.; Cao, H.; Wang, Y.; Zhang, Y. Heterogeneous Fenton-like degradation of 4-chlorophenol using iron/ordered mesoporous carbon catalyst. J. Environ. Sci. 2014, 26, 1171–1179. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. Highly stable Fe/gamma-Al2O3 catalyst for catalytic wetperoxide oxidation. J. Chem. Technol. Biotechnol. 2011, 86, 497–504. [Google Scholar] [CrossRef]
- Tang, T.; Li, K.; Shen, Z.; Sun, T.; Wang, Y.; Jia, J. Facile synthesis of polypyrrole functionalized nickel foam with catalytic activity comparable to Pt for the poly-generation of hydrogen and electricity. J. Power Sources 2016, 301, 54–61. [Google Scholar] [CrossRef]
- Song, S.; Wu, M.; Liu, Y.; Zhu, Q.; Tsiakaras, P.; Wang, Y. Efficient and Stable Carbon-coated Nickel Foam Cathodes for the Electro-Fenton Process. Electrochim. Acta 2015, 176, 811–818. [Google Scholar] [CrossRef]
- Singh, S.K.; Xu, Q. Bimetallic nickel-iridium nanocatalysts for hydrogen generation by decomposition of hydrous hydrazine. Chem. Commun. 2010, 46, 6545–6547. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.G.W.; Leddy, J.; Minteer, S.D. Enhanced alcohol electrocatalysis with the introduction of magnetic composites into nickel electrocatalysts. Chem. Commun. 2012, 48, 11972–11974. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Chen, Q.; Ji, F.; Guo, Q.; Fan, J.; Xu, X. Combination of heterogeneous Fenton reaction and photocatalysis using Co–TiO2 nanocatalyst for activation of KHSO5 with visible light ultrasound at ambient conditions. J. Environ. Sci. 2014, 26, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, K. Reduced graphene oxide-TiO2 nanocomposite with high photocatalystic activity for the degradation of rhodamine B. J. Mol. Catal. A 2011, 345, 101–107. [Google Scholar] [CrossRef]
- Pham, T.; Brar, S.; Tyagi, R.; Surampalli, R. Influence of ultrasonication and Fenton oxidation pre-treatment on rheological characteristics of wastewater sludge. Ultrason. Sonochem. 2010, 17, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lindley, C.E.; Stewart, J.T.; Sandstrom, M.W. Determination of low concentration of Acetochlor in water by automated solid-phase extraction and gas chromatograph with mass-selective detection. J. AOAC Int. 1996, 74, 962–966. [Google Scholar]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef]
- Ollis, D.; Silva, C.G.; Faria, J. Simultaneous photochemical and photocatalyzed liquid phase reactions: Dye decolorization kinetics. Catal. Today 2015, 240, 80–85. [Google Scholar] [CrossRef]
- Xiong, J.; Dong, X.; Dong, Y.; Hao, X. Stuart Hampshire, Dual-production of nickel foam supported carbon nanotubes and hydrogen by methane catalytic decomposition. Int. J. Hydrog. Energy 2012, 37, 12307–12316. [Google Scholar] [CrossRef]
- Holstvoogd, R.D.; Swaaij, W.P.M.V.; Dierendonck, L.L.V. The absorption of gases in aqueous activated carbon slurries enhanced by adsorbing or catalytic particles. Chem. Eng. Sci. 1988, 43, 2181–2187. [Google Scholar] [CrossRef]
- Yusof, N.S.M.; Babgi, B.; Alghamdi, Y.; Aksu, M.; Madhavan, J.; Ashokkumar, M. Physical and chemical effects of acoustic cavitation in selected ultrasoniccleaning applications. Ultrason. Sonochem. 2016, 29, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tu, J.; Niu, F.; Yang, P. Cavitation dose in an ultrasonic cleaner and its dependence on experimental parameters. Appl. Acoust. 2016, 101, 179–184. [Google Scholar] [CrossRef]
- Romero, V.; González, O.; Bayarri, B.; Marco, P.; Giménez, J.; Esplugas, S. Degradation of Metoprolol by photo-Fenton: Comparison of different photoreactors performance. Chem. Eng. J. 2016, 283, 639–648. [Google Scholar] [CrossRef]
- Xin, Z.; Jacques, B., Jr.; Daniel, D.; Hui, Z.; Sebastien, R. Modulating the copper oxide morphology and accessibility by using micro-/mesoporous SBA-15 structures as host support: Effect on the activity for the CWPO of phenol reaction. Appl. Catal. B Environ. 2012, 121, 123–134. [Google Scholar]
- Huang, R.; Fang, Z.; Fang, X.; Tsang, E.P. Pickling waste liquor. J. Colloid Interface Sci. 2014, 436, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chu, G.; Kong, D.; Luo, Y.; Zhang, J.; Zou, H.; Zhang, L.; Chen, J. Mass transfer intensification in a rotating packed bed with surface-modified nickel foam packing. Chem. Eng. J. 2016, 285, 236–242. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, G.; Tang, J.; Ren, Y.; Yang, J. One-dimensional NiCO2O4 nanowire arrays grown on nickel foam for high-performance lithium-ion batteries. J. Power Sources 2015, 299, 97–103. [Google Scholar] [CrossRef]
- Chu, G.; Sang, L.; Du, X.; Luo, Y.; Zou, H.; Chen, J. Studies of CO2 absorption and effective interfacial area in a two-stage rotating packed bed with nickel foam packing. Chem. Eng. Process. Process Intensif. 2015, 90, 34–40. [Google Scholar] [CrossRef]
- Theron, P.; Pichat, P.; Guillard, C.; Petrier, C.; Chopin, T. Degradation of phenyltrifluoromethylketone in water by separate or simultaneous use of TiO2 photocatalysis and 30 or 515 kHz ultrasound. Phys. Chem. Chem. Phys. 1999, 1, 4663–4668. [Google Scholar] [CrossRef]
- Theron, P.; Pichat, P.; Guillard, C.; Petrier, C. Water treatment by TiO2 photocatalysis and/or ultrasound: Degradation of phenyltrifluoromethylketone, a trifluoroacetic acid-forming pollutant, and octan-1-ol, a very hydrophobic pollutant. Water Sci. Technol. 2001, 44, 263–270. [Google Scholar] [PubMed]
- Liu, S.; Chen, Y.; Yu, H.; Zhang, S. Kinetics and mechanisms of radiation-induced degradation of acetochlor. Chemosphere 2005, 59, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xu, H.; Xu, S.; Ma, F. The effect of tourmaline on cell membrane of Nitrosomonas europaea and biodegradation of micropollutant. Surf. Interface 2014, 46, 564–569. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Xu, S.; Li, G.; Yang, J. Synergetic Effect of Ultrasound, the Heterogeneous Fenton Reaction and Photocatalysis by TiO2 Loaded on Nickel Foam on the Degradation of Pollutants. Materials 2016, 9, 457. https://doi.org/10.3390/ma9060457
Qiu S, Xu S, Li G, Yang J. Synergetic Effect of Ultrasound, the Heterogeneous Fenton Reaction and Photocatalysis by TiO2 Loaded on Nickel Foam on the Degradation of Pollutants. Materials. 2016; 9(6):457. https://doi.org/10.3390/ma9060457
Chicago/Turabian StyleQiu, Shan, Shanwen Xu, Guangming Li, and Jixian Yang. 2016. "Synergetic Effect of Ultrasound, the Heterogeneous Fenton Reaction and Photocatalysis by TiO2 Loaded on Nickel Foam on the Degradation of Pollutants" Materials 9, no. 6: 457. https://doi.org/10.3390/ma9060457
APA StyleQiu, S., Xu, S., Li, G., & Yang, J. (2016). Synergetic Effect of Ultrasound, the Heterogeneous Fenton Reaction and Photocatalysis by TiO2 Loaded on Nickel Foam on the Degradation of Pollutants. Materials, 9(6), 457. https://doi.org/10.3390/ma9060457