Hydrogels as a Replacement Material for Damaged Articular Hyaline Cartilage
Abstract
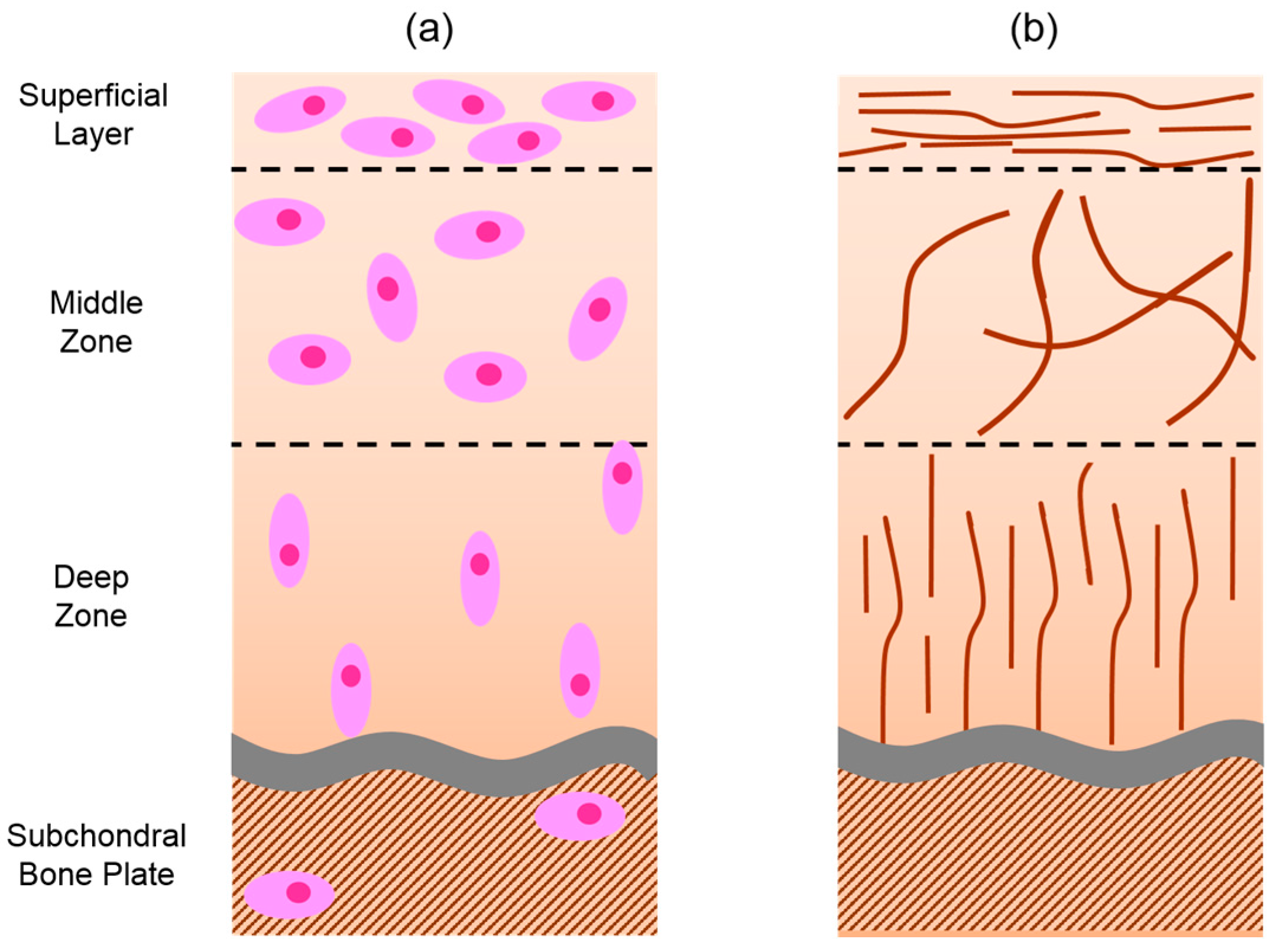
:1. Hyaline Cartilage Structure
2. Causes of Cartilage Damage
3. Current Strategies for Cartilage Defect Repair
4. Synthetic Materials Used in Joint Replacement and Cartilage Repair
5. Alternative Materials—Hydrogels
6. Hydrogel Integration
7. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DN | double network |
| ACI | autologous chondrocyte implantation |
| CoCr | cobalt chrome |
| MoM | metal on metal |
| UHMW-PE | ultra-high molecular weight polyethylene |
| PE | poly(ethylene) |
| PMPC | poly(2-methacryloyloxyethyl phophorylcholine) |
| MPDSAH | 3-dimethyl (3-(N-methacrylamido)propyl) ammonium propane sulfonate |
| PAA | poly(acrylic acid) |
| PVA | poly(vinyl acid) |
| PAAm | poly(acrylamide) |
| PEG | poly(ethylene glycol) |
| PAMPS | poly(2-acrylamido-2-methylpropanesulfonic acid) |
| PCDME | poly(N-(carboxymethyl)-N,N-dimethyl-2-(methacryloyloxy) ethanaminium) |
| PMMA | poly(methyl methacrylate) |
| EDC | 1-ethyl-(3-3-dimethylaminopropyl) carbodamide |
| MBAA | N-N’-methylenebis(acrylamide) |
| CS | chondroitin sulphate |
| HEMA | hydroxyethyl methacrylate |
References
- Poole, C.A.; Flint, M.H.; Beaumont, B.W. Chondrons extracted from canine tibial cartilage: Preliminary report on their isolation and structure. J. Orthop. Res. 1988, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.A. Articular cartilage chondrons: Form, function and failure. J. Anat. 1997, 191, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.E.; Seedhom, B.B. Thickness of human articular cartilage in joints of the lower limb. Ann. Rheum. Dis. 1999, 58, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Guo, X.E. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Zehbe, R.; Haibel, A.; Riesemeier, H.; Gross, U.; Kirkpatrick, C.J.; Schubert, H.; Brochhausen, C. Going beyond histology. Synchrotron micro-computed tomography as a methodology for biological tissue characterization: From tissue morphology to individual cells. J. R. Soc. Interface 2010, 7, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lian, Q.; He, J.; Zhao, J.; Jin, Z.; Li, D. Study on the microstructure of human articular cartilage/bone interface. J. Bionic Eng. 2011, 8, 251–262. [Google Scholar] [CrossRef]
- Mollenhauer, J.A.; Burkardt, C.; Nisch, W.; Bossert, J.; Hempel, H.J.; Jandt, K.D.; Muehleman, C. Definition of the joint cartilage-bone interface by topological scanning technologies: Considerations for optimized material interfaces in implant technology. Adv. Eng. Mater. 2007, 9, 1097–1103. [Google Scholar] [CrossRef]
- Stockwell, R.A. Biology of Cartilage Cells; Biology Structure and Function Books, 1st ed.; Cambridge University Press: Cambridge, UK, 1979. [Google Scholar]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, M.V.; Pleshko, N. Wavelength-dependent penetration depth of near infrared radiation into cartilage. Analyst 2015, 140, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.M.; Borthakur, A.; Kaufman, J.H.; Leigh, J.S.; Reddy, R. Water distribution patterns inside bovine articular cartilage as visualized by 1H magnetic resonance imaging. Osteoarthr. Cartil. 2001, 9, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Spencer, N.D. Achieving Ultralow Friction by Aqueous Brush-Assisted Lubrication; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Mow, V.C.; Huiskes, R. Basic Orthopaedic Biomechanics & Mechano-Biology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Gannon, A.R.; Nagel, T.; Kelly, D.J. The role of the superficial region in determining the dynamic properties of articular cartilage. Osteoarthr. Cartil. 2012, 20, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Ateshian, G.A. The role of interstitial fluid pressurization in articular cartilage lubrication. J. Biomech. 2009, 42, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Soltz, M.A.; Ateshian, G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J. Biomech. 1998, 31, 927–934. [Google Scholar] [CrossRef]
- Park, S.; Krishnan, R.; Nicoll, S.B.; Ateshian, G.A. Cartilage interstitial fluid load support in unconfined compression. J. Biomech. 2003, 36, 1785–1796. [Google Scholar] [CrossRef]
- Mow, V.C.; Gu, W.Y.; Chen, F.H. Chapter 5 Structure and Function of Articular Cartilage and Meniscus, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Kovach, I.S. A molecular theory of cartilage viscoelasticity. Biophys. Chem. 1996, 59, 61–73. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Boileau, C.; Brunet, J.; Boily, M.; Lajeunesse, D.; Reboul, P.; Laufer, S.; Martel-Pelletier, J. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone 2004, 34, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ying, Z.; Duan, X.; Tan, H.; Yang, B.; Guo, L.; Chen, G.; Dai, G.; Ma, Z.; Yang, L. Histomorphometric analysis of adult articular calcified cartilage zone. J. Struct. Biol. 2009, 168, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Oegema, T.R., Jr.; Carpenter, R.J.; Hofmeister, F.; Thompson, R.C., Jr. The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc. Res. Techniq. 1997, 37, 324–332. [Google Scholar] [CrossRef]
- Imhof, H.; Breitenseher, M.; Kainberger, F.; Rand, T.; Trattnig, S. Importance of subchondral bone to articular cartilage in health and disease. Top. Magn. Reson. Imaging 1999, 10, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M.; Kettunen, J.; Paananen, H.; Aalto, T.; Battié, M.C.; Impivaara, O.; Videman, T.; Sarna, S. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995, 38, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Repo, R.; Finlay, J. Survival of articular cartilage after controlled impact. J. Bone Jt. Surg. Am. 1977, 59, 1068–1076. [Google Scholar]
- Torzilli, P.A.; Grigiene, R.; Borrelli, J.J.; Helfet, D.L. Effect of impact load on articular cartilage: Cell metabolism and viability, and matrix water content. J. Biomech. Eng. 1999, 121, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Hadler, N.M.; Gillings, D.B.; Imbus, H.R.; Levitin, P.M.; Makuc, D.; Utsinger, P.D.; Yount, W.J.; Slusser, D.; Moskovitz, N. Hand structure and function in an industrial setting. Arthritis Rheum. 1978, 21, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Duthon, V.B.; Charbonnier, C.; Kolo, F.C.; Magnenat-Thalmann, N.; Becker, C.D.; Bouvet, C.; Coppens, E.; Hoffmeyer, P.; Menetrey, J. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy 2013, 29, 411–419. [Google Scholar] [CrossRef] [PubMed]
- McMillan, G.; Nichols, L. Osteoarthritis and meniscus disorders of the knee as occupational diseases of miners. Occup. Environ. Med. 2005, 62, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Gemne, G.; Saraste, H.; Christ, E.; Dupuis, H.G. Bone and joint pathology in workers using hand-held vibrating tools: An overview. Scand. J. Work Environ. Health 1987, 13, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Grogan, S.; Schäfer, D.; Heberer, M.; Mainil-Varlet, P.; Martin, I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr. Cartil. 2004, 12, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, R.L.; Negendank, W.G.; Teitge, R.A.; Reed, A.H.; Miller, P.R.; Fernandez-Madrid, F. Factors affecting articular cartilage thickness in osteoarthritis and aging. J. Rheumatol. 1994, 21, 1310–1318. [Google Scholar] [PubMed]
- Lotz, M.; Loeser, R.F. Effects of aging on articular cartilage homeostasis. Bone 2012, 51, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Roughley, P.J.; Rosenberg, L.C. Age-Related changes in cartilage proteoglycans: Quantitative electron microscopic studies. Microsc. Res. Techniq. 1994, 28, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.L.; Feik, S.A.; Clement, J.G. Increase in pore area, and not pore density, is the main determinant in the development of porosity in human cortical bone. J. Anat. 2006, 209, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.L. Septic arthritis. Lancet 1998, 351, 197–202. [Google Scholar] [CrossRef]
- Angly, B.; Steiger, R.; Zimmerli, W. Septic arthritis of finger joints. Handchir. Mikrochir. Plast. Chir. 2007, 39, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Louati, K.; Miquel, A.; Behin, A.; Benveniste, O.; Sellam, J. Quickly progressive amyotrophy of the thigh: An unusual cause of rapid chondrolysis of the knee. Jt. Bone Spine 2015, 82, 203–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duff-Barclay, I.; Spillman, D.T. Total human hip joint prostheses—A laboratory study of friction and wear. Proc. Inst. Mech. Eng. 1966, 181, 90–103. [Google Scholar] [CrossRef]
- Chamberlain, M.A.; Care, G.; Harfield, B. Physiotherapy in osteoarthrosis of the knees. A controlled trial of hospital versus home exercises. Int. Rehabil. Med. 1982, 4, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.C.; Lam, J.K.; Siu, E.; Fung, C.S.; Li, K.T.; Lam, M.W. Physiotherapist-designed aquatic exercise programme for community-dwelling elders with osteoarthritis of the knee: A Hong Kong pilot study. Hong Kong Med. J. 2014, 20, 16–23. [Google Scholar] [PubMed]
- Hurley, M.V.; Walsh, N.; Bhavnani, V.; Britten, N.; Stevenson, F. Health beliefs before and after participation on an exercised-based rehabilitation programme for chronic knee pain: Doing is believing. BMC Musculoskelet. Disord. 2010, 11, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.W.; Dieterichs, C. The results of arthroscopic lavage and debridement of osteoarthritic knees based on the severity of degeneration. Arthroscopy 2003, 19, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Cipriano, C.A.; Moric, M.; Sporer, S.M.; Della Valle, C.J. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J. Arthroplast. 2012, 27, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Moseley, J.B.; O’Malley, K.; Petersen, N.J.; Menke, T.J.; Brody, B.A.; Kuykendall, D.H.; Hollingsworth, J.C.; Ashton, C.M.; Wray, N.P. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N. Eng. J. Med. 2002, 347, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Rodkey, W.G.; Singleton, S.B.; Briggs, K.K. Microfracture technique forfull-thickness chondral defects: Technique and clinical results. Oper. Tech. Orthop. 1997, 7, 300–304. [Google Scholar] [CrossRef]
- Mithoefer, K.; Williams, R.J.; Warren, R.F.; Potter, H.G.; Spock, C.R.; Jones, E.C.; Wickiewicz, T.L.; Marx, R.G. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. J. Bone Jt. Surg. Am. 2006, 88, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; Williams, R.J.; Warren, R.F.; Potter, H.G.; Spock, C.R.; Jones, E.C.; Wickiewicz, T.L.; Marx, R.G. The microfracture technique for the treatment of articular cartilage lesions in the knee. J. Bone Jt. Surg. Am. 2005, 87, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Eyre, D.R.; Koide, S.; Glimcher, M.J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J. Bone Jt. Surg. Am. 1980, 62, 79–89. [Google Scholar]
- Peterson, L.; Brittberg, M.; Kiviranta, I.; Åkerlund, E.L.; Lindahl, A. Autologous chondrocyte transplantation: Biomechanics and long-term durability. Am. J. Sports Med. 2002, 30, 2–12. [Google Scholar] [PubMed]
- Kreuz, P.C.; Steinwachs, M.R.; Erggelet, C.; Krause, S.J.; Konrad, G.; Uhl, M.; Südkamp, N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 2006, 14, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Jt. Am. 1993, 75, 532–553. [Google Scholar]
- Bentley, G.; Biant, L.C.; Vijayan, S.; Macmull, S.; Skinner, J.A.; Carrington, R.W.J. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. Bone Jt. J. 2012, 94, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Perdisa, F.; Tetta, C.; Di Martino, A.; Marcacci, M. Arthroscopic mosaicplasty: Long-term outcome and joint degeneration progression. Knee 2015, 22, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Vásárhelyi, G.; Hangody, L.R.; Sükösd, Z.; Tibay, G.; Bartha, L.; Bodó, G. Autologous osteochondral grafting—Technique and long-term results. Injury 2008, 39, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Erggelet, C.; Sittinger, M.; Lahm, A. The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects of the knee joint. Arthroscopy 2003, 19, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An educational review of cartilage repair: Precepts & practice—Myths & misconceptions—Progress & prospects. Osteoarthr. Cartil. 2015, 23, 334–350. [Google Scholar] [PubMed]
- Foran, J.R.H.; Brown, N.M.; Della Valle, C.J.; Berger, R.A.; Galante, J.O. Long-term survivorship and failure modes of unicompartmental knee srthroplasty. Clin. Orthop. Relat. Res. 2012, 471, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Steele, R.G.; Hutabarat, S.; Evans, R.L.; Ackroyd, C.E.; Newman, J.H. Survivorship of the St Georg Sled medial unicompartmental knee replacement beyond ten years. Bone Jt. J. 2006, 88B, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Bhumiratana, S.; Eton, R.E.; Oungoulian, S.R.; Wan, L.Q.; Ateshian, G.A.; Vunjak-Novakovic, G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc. Natl. Acad. Sci. USA 2014, 111, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, N. Development of cartilage tissue engineering techniques based on biomedical research. J. Orthop. Sci. 2014, 19, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Kock, L.; van Donkelaar, C.; Ito, K. Tissue engineering of functional articular cartilage: The current status. Cell Tissue Res. 2012, 347, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, J. Articular cartilage tissue engineering: Development and future: A review. J. Musculoskelet. Pain 2014, 22, 68–77. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A. Problem of stress shielding and improvement to the hip implant designs: A review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar]
- Black, J.; Hastings, G. Handbook of Biomaterial Properties; Chapman and Hall: London, UK, 1998; Volume 65. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total hip arthroplasty-over 100 years of operative history. Orthop. Rev. 2011, 3, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W. The Chemistry of Medical and Dental Materials: Chapter 4 Metals; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Schaffer, A.W.; Schaffer, A.; Pilger, A.; Engelhardt, C.; Zweymueller, K.; Ruediger, H.W. Increased blood cobalt and chromium after total hip replacement. Clin. Toxicol. 1999, 37, 839–844. [Google Scholar] [CrossRef]
- Dunstan, E.; Sanghrajka, A.P.; Tilley, S.; Unwin, P.; Blunn, G.; Cannon, S.R.; Briggs, T.W.R. Metal ion levels after metal-on-metal proximal femoral replacements, a 30-year follow up. J. Bone Jt. Surg. Am. 2005, 87B, 628–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jantzen, C.; Jørgensen, H.L.; Duus, B.R.; Sporring, S.L.; Lauritzen, J.B. Chromium and cobalt ion concentrations in blood and serum following various types of metal-on-metal hip arthroplasties. Acta Orthop. 2013, 84, 229–236. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, M.; Kirsch-Volders, M.; Lison, D. Cobalt and antimony: Genotoxicity and carcinogenicity. Mutat. Res. Fund. Mol. Mech. Mutagen. 2003, 533, 135–152. [Google Scholar] [CrossRef]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhabra, G.; Sood, A.; Fisher, B.; Cartwright, L.; Saunders, M.; Evans, W.H.; Surprenant, A.; Lopez-Castejon, G.; Mann, S.; Davis, S.A.; et al. Nanoparticles can cause DNA damage across a cellular barrier. Nat. Nano 2009, 4, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Dieppe, P.; Porter, M.; Blom, A.W. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: Linkage study between the National Joint Registry of England and Wales and hospital episode statistics. BMJ 2012, 344, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lalmohamed, A.; MacGregor, A.J.; de Vries, F.; Leufkens, H.G.; van Staa, T.P. Patterns of risk of cancer in patients with metal-on-metal hip replacements versus other bearing surface types: A record linkage study between a prospective joint registry and general practice electronic health records in England. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, K.T.; Visuri, T.; Pulkkinen, P.; Eskelinen, A.; Remes, V.; Virolainen, P.; Junnila, M.; Pukkala, E. Cancer incidence and cause-specific mortality in patients with metal-on-metal hip replacements in Finland. Acta Orthop. 2014, 85, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Brewster, D.H.; Stockton, D.L.; Reekie, A.; Ashcroft, G.P.; Howie, C.R.; Porter, D.E.; Black, R.J. Risk of cancer following primary total hip replacement or primary resurfacing arthroplasty of the hip: A retrospective cohort study in Scotland. Br. J. Cancer 2013, 108, 1883–1890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kendal, A.R.; Prieto-Alhambra, D.; Arden, N.K.; Carr, A.; Judge, A. Mortality rates at 10 years after metal-on-metal hip resurfacing compared with total hip replacement in England: Retrospective cohort analysis of hospital episode statistics. BMJ 2013, 347, 1–12. [Google Scholar] [CrossRef] [PubMed]
- UK Government. Medical Safety Alert, Metal-on-Metal (MoM) Hip Replacements-Guidance on Implantation and Patient Management. Available online: https://www.gov.uk/drug-device-alerts/metal-on-metal-mom-hip-replacements-guidance-on-implantation-and-patient-management (accessed on 6 March 2016).
- Cohen, D. Out of joint: The story of the ASR. BMJ 2011, 342, 1–7. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Stryker Initiates Voluntary Product Recall of Modular-Neck Stems. Available online: http://www.fda.gov/Safety/Recalls/ucm311043.htm (accessed on 6 March 2016).
- U.S. Food and Drug Administration. Recalls Specific to Metal-on-Metal Hip Implants. Available online: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241770.htm#1 (accessed on 6 March 2016).
- NJR Steering Committee. National Joint Registry for England, Wales and Northern Ireland, 12th Annual Report; National Joint Registry: Hemel Hempstead, UK, 2015; pp. 1–179. [Google Scholar]
- MacDonald, S.J.; McCalden, R.W.; Chess, D.G.; Bourne, R.B.; Rorabeck, C.H.; Cleland, D.; Leung, F. Metal-on-metal versus polyethylene in hip arthroplasty: A randomized clinical trial. Clin. Orthop. Relat. Res. 2003, 406, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, X.; Wang, S.; Chen, A.; Wu, W.; Long, R. A novel cartilage tissue construction based on artificial cells and matrix-shaping. Mater. Lett. 2015, 159, 24–27. [Google Scholar] [CrossRef]
- Mollon, B.; Kandel, R.; Chahal, J.; Theodoropoulos, J. The clinical status of cartilage tissue regeneration in humans. Osteoarthr. Cartil. 2013, 21, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Kyomoto, M.; Moro, T.; Takatori, Y.; Kawaguchi, H.; Ishihara, K. Cartilage-mimicking, high-density brush structure improves wear resistance of crosslinked polyethylene: A pilot study. Clin. Orthop. Relat. Res. 2011, 469, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Kyomoto, M.; Ishihara, K.; Saiga, K.; Hashimoto, M.; Tanaka, S.; Ito, H.; Tanaka, T.; Oshima, H.; Kawaguchi, H.; et al. Grafting of poly(2-methacryloyloxyethyl phosphorylcholine) on polyethylene liner in artificial hip joints reduces production of wear particles. J. Mech. Behav. Biomed. Mater. 2014, 31, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kyomoto, M.; Moro, T.; Saiga, K.; Hashimoto, M.; Ito, H.; Kawaguchi, H.; Takatori, Y.; Ishihara, K. Biomimetic hydration lubrication with various polyelectrolyte layers on cross-linked polyethylene orthopedic bearing materials. Biomaterials 2012, 33, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Takatori, Y.; Kyomoto, M.; Ishihara, K.; Hashimoto, M.; Ito, H.; Tanaka, T.; Oshima, H.; Tanaka, S.; Kawaguchi, H. Long-term hip simulator testing of the artificial hip joint bearing surface grafted with biocompatible phospholipid polymer. J. Orthop. Res. 2014, 32, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Iwasaki, Y.; Ebihara, S.; Shindo, Y.; Nakabayashi, N. Photoinduced graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on polyethylene membrane surface for obtaining blood cell adhesion resistance. Colloids Surface B 2000, 18, 325–335. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Liu, J.; Zhang, Y. Preparation and antifouling property of polyethersulfone ultrafiltration hybrid membrane containing halloysite nanotubes grafted with MPC via RATRP method. Desalination 2014, 344, 313–320. [Google Scholar] [CrossRef]
- Ishihara, K. Bioinspired phospholipid polymer biomaterials for making high performance artificial organs. Sci. Tech. Adv. Mater. 2000, 1, 131. [Google Scholar] [CrossRef]
- Palmer, R.R.; Lewis, A.L.; Kirkwood, L.C.; Rose, S.F.; Lloyd, A.W.; Vick, T.A.; Stratford, P.W. Biological evaluation and drug delivery application of cationically modified phospholipid polymers. Biomaterials 2004, 25, 4785–4796. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-D.; Yao, K.; Zhang, H.; Huang, X.-J.; Xu, Z.-K. Surface modification of silicone intraocular lens by 2-methacryloyloxyethyl phosphoryl-choline binding to reduce Staphylococcus epidermidis adherence. Clin. Exp. Ophthalmol. 2007, 35, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xiong, D.; Wang, K. The mechanical properties of the ultra high molecular weight polyethylene grafted with 3-dimethy (3-(N-methacryamido) propyl) ammonium propane sulfonate. J. Mech. Behav. Biomed. Mater. 2014, 35, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xiong, D.; Wang, K. Biotribological properties of UHMWPE grafted with AA under lubrication as artificial joint. J. Mater. Sci. Mater. Med. 2013, 24, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Briscoe, W.H.; Armes, S.P.; Klein, J. Lubrication at physiological pressures by polyzwitterionic brushes. Science 2009, 323, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Deng, Y.; Wang, N.; Yang, Y. Influence of surface PMPC brushes on tribological and biocompatibility properties of UHMWPE. Appl. Surf. Sci. 2014, 298, 56–61. [Google Scholar] [CrossRef]
- Greene, G.W.; Olszewska, A.; Osterberg, M.; Zhu, H.; Horn, R. A cartilage-inspired lubrication system. Soft Matter 2014, 10, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Yarimitsu, S.; Nakashima, K.; Sakai, N.; Yamaguchi, T.; Sawae, Y.; Suzuki, A. Biphasic and boundary lubrication mechanisms in artificial hydrogel cartilage: A review. Proc. Inst. Mech. Eng. H 2015, 229, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.A.; Correia, C.; Oliveira, J.M.; Sousa, R.A.; Espregueira-Mendes, J.; Reis, R.L. Cartilage repair using hydrogels: A critical review of in vivo experimental designs. ACS Biomater. Sci. Eng. 2015, 1, 726–739. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, R.; Cheng, Y.; Fu, J. Super-tough double-network hydrogels reinforced by covalently compositing with silica-nanoparticles. Soft Matter 2012, 8, 6048–6056. [Google Scholar] [CrossRef]
- Gao, G.; Du, G.; Cheng, Y.; Fu, J. Tough nanocomposite double network hydrogels reinforced with clay nanorods through covalent bonding and reversible chain adsorption. J. Mater. Chem. B 2014, 2, 1539–1548. [Google Scholar] [CrossRef]
- Dong, W.; Huang, C.; Wang, Y.; Sun, Y.; Ma, P.; Chen, M. Superior mechanical properties of double-network hydrogels reinforced by carbon nanotubes without organic modification. Int. J. Mol. Sci. 2013, 14, 22380–22394. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Du, G.; Sun, Y.; Fu, J. Self-healable, tough, and ultrastretchable nanocomposite hydrogels based on reversible polyacrylamide/montmorillonite adsorption. ACS Appl. Mater. Interfaces 2015, 7, 5029–5037. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of sodium alginate/poly(N-isopropylacrylamide)/clay semi-IPN magnetic hydrogels. Polym. Bull. 2012, 68, 1153–1169. [Google Scholar] [CrossRef]
- Skelton, S.; Bostwick, M.; O’Connor, K.; Konst, S.; Casey, S.; Lee, B.P. Biomimetic adhesive containing nanocomposite hydrogel with enhanced materials properties. Soft Matter 2013, 9, 3825–3833. [Google Scholar] [CrossRef]
- Fei, X.; Xu, S.; Feng, S.; Lin, J.; Lin, J.; Shi, X.; Wang, J. Mechanically strengthened double network composite hydrogels with high water content: A preliminary study. J. Polym. Res. 2011, 18, 1131–1136. [Google Scholar] [CrossRef]
- Li, P.; Siddaramaiah; Kim, N.H.; Heo, S.-B.; Lee, J.-H. Novel PAAm/laponite clay nanocomposite hydrogels with improved cationic dye adsorption behavior. Compos. Part B Eng. 2008, 39, 756–763. [Google Scholar] [CrossRef]
- Moutos, F.T.; Freed, L.E.; Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Kurokawa, T.; Gong, J.P. Effect of void structure on the toughness of double network hydrogels. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1246–1254. [Google Scholar] [CrossRef]
- Bai, H.; Polini, A.; Delattre, B.; Tomsia, A.P. Thermoresponsive composite hydrogels with aligned macroporous structure by ice-templated assembly. Chem. Mater. 2013, 25, 4551–4556. [Google Scholar] [CrossRef] [PubMed]
- Tsukeshiba, H.; Huang, M.; Na, Y.-H.; Kurokawa, T.; Kuwabara, R.; Tanaka, Y.; Furukawa, H.; Osada, Y.; Gong, J.P. Effect of polymer entanglement on the toughening of double network hydrogels. J. Phys. Chem. B 2005, 109, 16304–16309. [Google Scholar] [CrossRef] [PubMed]
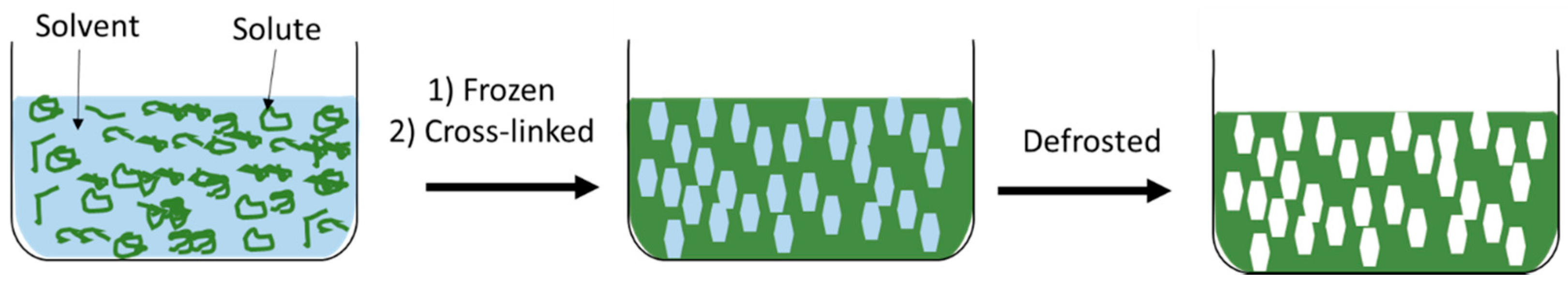
- Kumar, A.; Mishra, R.; Reinwald, Y.; Bhat, S. Cryogels: Freezing unveiled by thawing. Mater. Today 2010, 13, 42–44. [Google Scholar] [CrossRef]
- Kathuria, N.; Tripathi, A.; Kar, K.K.; Kumar, A. Synthesis and characterization of elastic and macroporous chitosan–gelatin cryogels for tissue engineering. Acta Biomater. 2009, 5, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Seven, F. The use of superporous p(AAc (acrylic acid)) cryogels as support for Co and Ni nanoparticle preparation and as reactor in H2 production from sodium borohydride hydrolysis. Energy 2014, 71, 170–179. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Zubov, A.L.; Titova, E.F. Swelling behavior of poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. Enzyme Microb. Tech. 1996, 18, 561–569. [Google Scholar] [CrossRef]
- Hwang, Y.; Zhang, C.; Varghese, S. Poly(ethylene glycol) cryogels as potential cell scaffolds: Effect of polymerization conditions on cryogel microstructure and properties. J. Mater. Chem. 2010, 20, 345–351. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, A. Multi-featured macroporous agarose–alginate cryogel: Synthesis and characterization for bioengineering applications. Macromol. Biosci. 2011, 11, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Cartiva Cartiva SCI. Chronic Toe Pain? Available online: http://cartiva.net/index (accessed on 9 March 2016).
- Cartiva Cartiva SCI. Synthetic Cartilage Implant Designed to Restore Natural Joint Mechanics in Osteoarthritis Patients. Available online: http://cartiva.net/synthetic-cartilage-implant-designed-to-restore-natural-joint-mechanics-in-osteoarthritis-patients-2.html (accessed on 9 March 2016).
- Sciarretta, F.V. 5 to 8 years follow-up of knee chondral defects treated by PVA-H hydrogel implants. Eur. Rev. Med. Pharmacol. 2013, 17, 3031–3038. [Google Scholar]
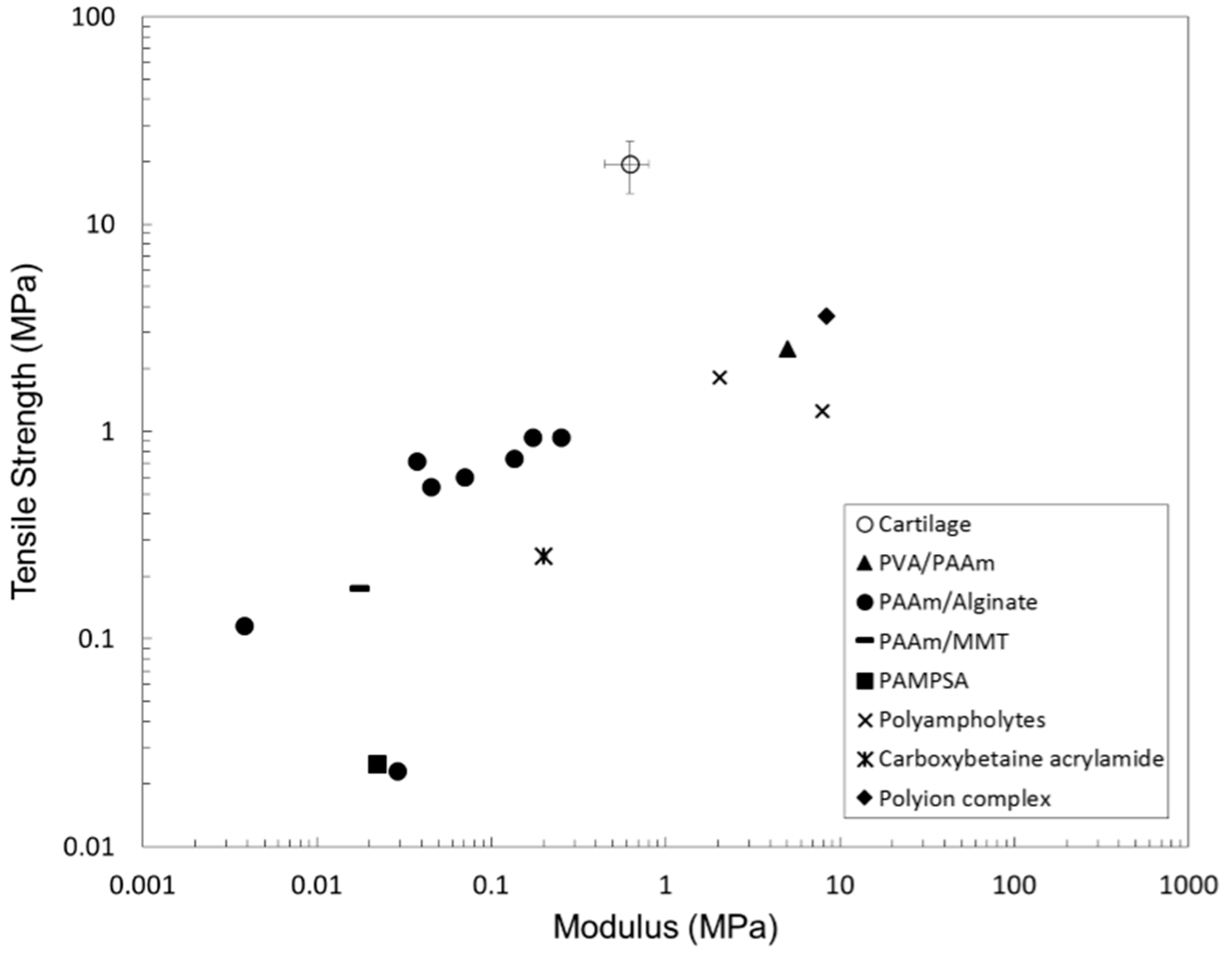
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Ihsan, A.B.; Li, X.; Guo, H.; Gong, J.P. Free reprocessability of tough and self-healing hydrogels based on polyion complex. ACS Macro Lett. 2015, 4, 961–964. [Google Scholar] [CrossRef]
- Gong, J.P. Materials both tough and soft. Science 2014, 344, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Miserez, A.; Weaver, J.C.; Chaudhuri, O. Biological materials and molecular biomimetics—Filling up the empty soft materials space for tissue engineering applications. J. Mater. Chem. B 2015, 3, 13–24. [Google Scholar] [CrossRef]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef]
- Li, J.; Illeperuma, W.R.K.; Suo, Z.; Vlassak, J.J. Hybrid hydrogels with extremely high stiffness and toughness. ACS Macro Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef]
- Lin, S.; Cao, C.; Wang, Q.; Gonzalez, M.; Dolbow, J.E.; Zhao, X. Design of stiff, tough and stretchy hydrogel composites via nanoscale hybrid crosslinking and macroscale fiber reinforcement. Soft Matter 2014, 10, 7519–7527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef] [PubMed]
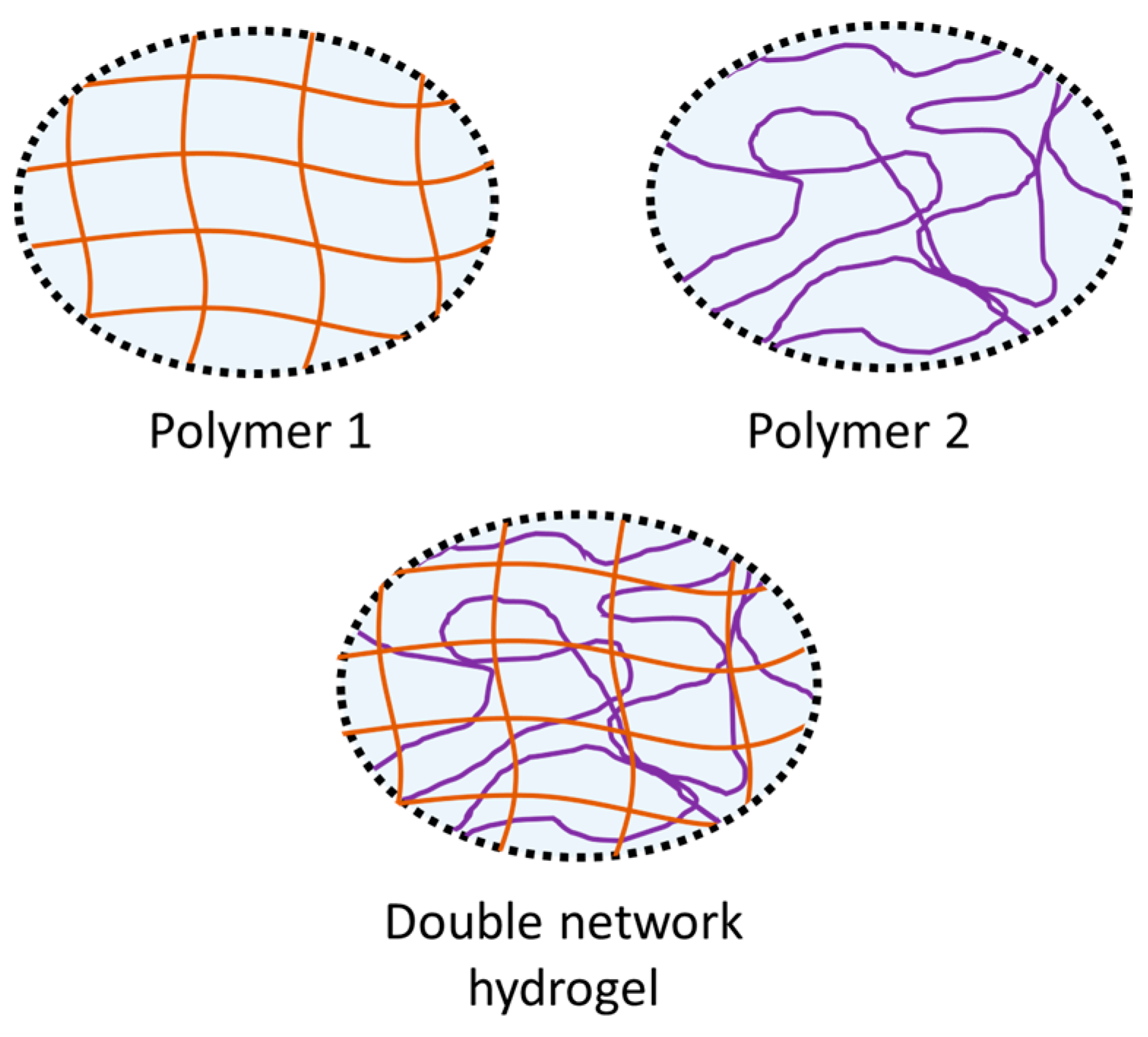
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Peak, C.; Wilker, J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Myung, D.; Waters, D.; Wiseman, M.; Duhamel, P.-E.; Noolandi, J.; Ta, C.N.; Frank, C.W. Progress in the development of interpenetrating polymer network hydrogels. Polym. Adv. Technol. 2008, 19, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Akasaki, T.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Nonoyama, T.; Taira, T.; Saruwatari, Y.; Ping Gong, J. Double network hydrogels from polyzwitterions: High mechanical strength and excellent anti-biofouling properties. J. Mater. Chem. B 2013, 1, 3685–3693. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Rakovsky, A.; Marbach, D.; Lotan, N.; Lanir, Y. Poly(ethylene glycol)-based hydrogels as cartilage substitutes: Synthesis and mechanical characteristics. J. Appl. Polym. Sci. 2009, 112, 390–401. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Li, Z.P.; Zhang, F.; Peng, X.C.; Zhou, Z.H. Influence of process variables on the physical properties of gelatin/SA/HYA composite hydrogels. Polym. Plast. Technol. 2014, 53, 935–940. [Google Scholar] [CrossRef]
- Bai, T.; Liu, S.; Sun, F.; Sinclair, A.; Zhang, L.; Shao, Q.; Jiang, S. Zwitterionic fusion in hydrogels and spontaneous and time-independent self-healing under physiological conditions. Biomaterials 2014, 35, 3926–3933. [Google Scholar] [CrossRef] [PubMed]
- Samanta, H.S.; Ray, S.K. Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohyd. Polym. 2014, 99, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Rosenwasser, M.P.; Buckwalter, J.A.; Malinin, T.I.; Mow, V.C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J. Orthop. Res. 1991, 9, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Mansour, J.M. Biomechanics of cartilage. In Kinesiology: The Mechanics and Pathomechanics of Human Movement; Oatis, C., Ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.-Y.; Chen, Y.M.; Zhou, J.; Suo, Z. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Li, L.; Wang, T.; Ding, Y.; Liu, G.; Zhang, G. A self-healing polymeric material: From gel to plastic. J. Mater. Chem. A 2014, 2, 11049–11053. [Google Scholar] [CrossRef]
- Marya, S.K.S.; Bawari, R.K. Total Hip Replacment Surgery: Principles and Techniques; Jitender Brothers Medical Publishers Ltd.: New Delhi, India, 2010. [Google Scholar]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Haller, C.M.; Buerzle, W.; Kivelio, A.; Perrini, M.; Brubaker, C.E.; Gubeli, R.J.; Mallik, A.S.; Weber, W.; Messersmith, P.B.; Mazza, E.; et al. Mussel-mimetic tissue adhesive for fetal membrane repair: An ex vivo evaluation. Acta Biomater. 2012, 8, 4365–4370. [Google Scholar] [CrossRef] [PubMed]
- Ryou, M.; Thompson, C.C. Tissue adhesives: A review. Tech. Gastrointest. Endosc. 2006, 8, 33–37. [Google Scholar] [CrossRef]
- Sajid, M.S.; Craciunas, L.; Sains, P.; Singh, K.K.; Baig, M.K. Use of antibacterial sutures for skin closure in controlling surgical site infections: A systematic review of published randomized, controlled trials. Gastroenterol. Rep. 2013, 1, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Skilbeck, C.J. Sutures, ligatures and knots. Surgery 2011, 29, 63–66. [Google Scholar] [CrossRef]
- Moy, R.L.; Waldman, B.; Hein, D.W. A review of sutures and suturing techniques. J. Dermatol. Surg. Oncol. 1992, 18, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Dafford, E.E.; Anderson, P.A. Comparison of dural repair techniques. Spine J. 2015, 15, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Félix, S.P.; Pereira Lopes, F.R.; Marques, S.A.; Martinez, A.M.B. Comparison between suture and fibrin glue on repair by direct coaptation or tubulization of injured mouse sciatic nerve. Microsurgery 2013, 33, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.P.; Daniels, A.U.; Ronken, S.; García, H.A.; Friederich, N.F.; Kurokawa, T.; Gong, J.P.; Wirz, D. Acrylamide polymer double-network hydrogels: Candidate cartilage repair materials with cartilage-like dynamic stiffness and attractive surgery-related attachment mechanics. Cartilage 2011, 2, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Stähli, A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthr. Cartil. 2008, 16, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Cassar-Gheiti, A.J.; Byrne, D.P.; Kavanagh, E.; Mulhall, K.J. Comparison of four chondral repair techniques in the hip joint: A biomechanical study using a physiological human cadaveric model. Osteoarthr. Cartil. 2015, 23, 1018–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oka, M. Biomechanics and repair of articular cartilge. J. Orthop. Sci. 2001, 6, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Oka, M.; Kobayashi, M.; Gu, H.-O.; Li, Z.-L.; Nakamura, T.; Ikada, Y. Significance of interstitial bone ingrowth under load-bearing conditions: A comparison between solid and porous implant materials. Biomaterials 1996, 17, 1141–1148. [Google Scholar] [CrossRef]
- Oka, M.; Chang, Y.-S.; Nakamura, T.; Ushio, K.; Toguchida, J.; Gu, H.-O. Synthetic osteochondral replacement of the femoral articular surface. Bone Jt. J. 1997, 79B, 1003–1007. [Google Scholar] [CrossRef]
- Cook, J.L.; Kuroki, K.; Bozynski, C.C.; Stoker, A.M.; Pfeiffer, F.M.; Cook, C.R. Evaluation of synthetic osteochondral implants. J. Knee Surg. 2014, 27, 295–302. [Google Scholar] [PubMed]
- Hodge, W.A.; Fijan, R.S.; Carlson, K.L.; Burgess, R.G.; Harris, W.H.; Mann, R.W. Contact pressures in the human hip joint measured in vivo. Proc. Natl. Acad. Sci. USA 1986, 83, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beddoes, C.M.; Whitehouse, M.R.; Briscoe, W.H.; Su, B. Hydrogels as a Replacement Material for Damaged Articular Hyaline Cartilage. Materials 2016, 9, 443. https://doi.org/10.3390/ma9060443
Beddoes CM, Whitehouse MR, Briscoe WH, Su B. Hydrogels as a Replacement Material for Damaged Articular Hyaline Cartilage. Materials. 2016; 9(6):443. https://doi.org/10.3390/ma9060443
Chicago/Turabian StyleBeddoes, Charlotte M., Michael R. Whitehouse, Wuge H. Briscoe, and Bo Su. 2016. "Hydrogels as a Replacement Material for Damaged Articular Hyaline Cartilage" Materials 9, no. 6: 443. https://doi.org/10.3390/ma9060443
APA StyleBeddoes, C. M., Whitehouse, M. R., Briscoe, W. H., & Su, B. (2016). Hydrogels as a Replacement Material for Damaged Articular Hyaline Cartilage. Materials, 9(6), 443. https://doi.org/10.3390/ma9060443









