A Series of Zr-Based Bulk Metallic Glasses with Room Temperature Plasticity
Abstract
:1. Introduction
2. Experimental
2.1. Compositional Design
2.2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author contributions
Conflicts of interest
References
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T. Fabrication of bulk glassy Zr55Al10Ni5Cu30 alloy of 30 mm in diameter by a suction casting method. Mater. Trans. 1996, 37, 185–187. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, G.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Super plastic bulk metallic glasses at room temperature. Science 2007, 315, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhang, W.; Dong, C.; Qiang, J.B.; Yubuta, K.; Makino, A.; Inoue, A. Unusual compressive plasticity of a centimeter-diameter Zr-based bulk metallic glass with high Zr content. J. Alloys Compd. 2010, 504S, S2–S5. [Google Scholar] [CrossRef]
- Hui, X.; Liu, S.N.; Pang, S.J.; Zhuo, L.C.; Zhang, T.; Chen, G.L.; Liu, Z.K. High-zirconium-based bulk metallic glasses with large plasticity. Scr. Mater. 2010, 63, 239–242. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Fujitab, K.; Yavari, A.R.; Inoue, A. Malleable hypoeutectic Zr-Ni-Cu-Al bulk glassy alloys with tensile plastic enlongation at room temperature. Philos. Mag. Lett. 2009, 89, 322–324. [Google Scholar] [CrossRef]
- Yang, Y.W.; Hua, N.B.; Li, R.; Pang, S.J.; Zhang, T. High-Zirconium bulk metallic glasses with high strength and large ductility. Sci. China G 2013, 56, 540–544. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Zhang, W.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Inoue, A. Stable flowing of localized shear bands in soft bulk metallic glasses. Acta Mater. 2010, 58, 904–909. [Google Scholar] [CrossRef]
- Hua, N.B.; Huang, L.; He, W.; Pang, S.J.; Zhang, T. A Ni-free high-zirconium-based bulk metallic glass with enhanced plasticity and biocompatibility. J. Non-Cryst. Solids 2013, 376, 133–138. [Google Scholar] [CrossRef]
- Gan, S.F.; Chenuo, W.; Liu, Z.; Ch, K.C.; Zhang, H.J.; Wang, J.F.; Yu, P. A plastic Ni-free Zr-based bulk metallic glass with high specific strength and good corrosion properties in simulated body fluid. Mater. Lett. 2012, 84, 81–84. [Google Scholar]
- Li, H.F.; Zheng, Y.; Xu, F.; Jiang, J.Z. In vitro investigation of novel Ni free Zr-based bulk metallic glass as potential biomaterials. Mater. Lett. 2012, 75, 74–76. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Huang, L.; Wu, W.; Luo, X.K.; Shi, M.J.; Liaw, P.K.; He, W.; Zhang, T. Novel low Cu content and Ni-free Zr-based bulk metallic glasses for biomedical applications. J. Non-Cryst. Solids 2013, 363, 1–5. [Google Scholar] [CrossRef]
- Caron, A.; Wunderlich, R.; Louzguine-Luzgin, D.V.; Xie, G.; Inoue, A.; Fecht, H.-J. Influence of minor aluminum concentration changes in zirconium-based bulk metallic glasses on the elastic, anelastic and plastic properties. Acta Mater. 2010, 58, 2004–2013. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, K.S.; Das, J.; Thomas, J.; Kühn, U.; Eckert, J. Improved plasticity of bulk metallic glasses upon cold rolling. Scr. Mater. 2010, 62, 678–681. [Google Scholar] [CrossRef]
- Scudino, S.; Jerliu, B.; Surreddi, K.B.; Kühn, U.; Eckert, J. Effect of cold rolling on compressive and tensile mechanical properties of Zr52.5Ti5Cu18Ni14.5Al10 bulk metallic glass. J. Alloys Compd. 2011, 509, S128–S130. [Google Scholar] [CrossRef]
- Tariq, N.H.; Naeem, M.; Akhter, J.I.; Hasan, B.A. Plasticity enhancement in Zr based bulk metallic glass by sand blasting. Mater. Chem. Phys. 2011, 126, 207–211. [Google Scholar] [CrossRef]
- Wang, W.H. The elastic properties, elastic models and elastic perspectives of metallic glasses. Prog. Mater. Sci. 2012, 57, 487–656. [Google Scholar] [CrossRef]
- Cai, A.H.; Xiong, X.; Liu, Y.; An, W.K.; Tan, J.Y.; Pan, Y. Design of new Zr-Al-Ni-Cu bulk metallic glasses. J. Alloys Compd. 2009, 468, 432–437. [Google Scholar] [CrossRef]
- Cai, A.H.; Chen, H.; An, W.K.; Tan, J.Y.; Zhou, Y. Relationship between melting enthalpy ΔHm and critical cooling rate Rc for bulk metallic glasses. Mater. Sci. Eng. A 2007, 457, 6–12. [Google Scholar] [CrossRef]
- Cai, A.H.; Sun, G.X.; Pan, Y. Evaluation of the parameters related to glass-forming ability of bulk metallic glasses. Mater. Des. 2006, 27, 479–488. [Google Scholar] [CrossRef]
- Cai, A.H.; Ding, D.W.; Xiong, X.; Liu, Y.; An, W.K.; Zhou, G.J.; Luo, Y.; Li, T.L.; Li, X.S. Design of Zr-Al-Ni-Cu bulk metallic glasses with network structures. Mater. Des. 2014, 63, 233–237. [Google Scholar] [CrossRef]
- An, W.K.; Ding, D.W.; Cai, A.H.; Zhou, G.J.; Luo, Y.; Li, J.H.; Peng, Y.Y. Mechanism, condition and characteristics for the formation of the network structure in Zr-Al-Ni-Cu bulk metallic glasses. Sci. China G 2015, 58, 066101. [Google Scholar] [CrossRef]
- Cai, A.H.; Xiong, X.; Liu, Y.; An, W.K.; Tan, J.Y. Artificial neural network modeling of reduced glass transition temperature of glass forming alloys. Appl. Phys. Lett. 2008, 92, 111909. [Google Scholar] [CrossRef]
- Cai, A.H.; Xiong, X.; Liu, Y.; An, W.K.; Tan, J.Y.; Luo, Y. Artificial neural network modeling for undercooled liquid region of glass forming alloys. Comput. Mater. Sci. 2010, 48, 109–114. [Google Scholar] [CrossRef]
- Cai, A.H.; Liu, Y.; An, W.K.; Zhou, G.J.; Luo, Y.; Li, T.L.; Li, X.S.; Tan, X.F. Prediction of critical cooling rate for glass forming alloys by artificial neural network. Mater. Des. 2013, 52, 671–676. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand Sect. A 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Lasocka, T.M. The effect of scanning rate on glass transition temperature of splat-cooled Te85Ge15. Mater. Sci. Eng. 1976, 23, 173–177. [Google Scholar] [CrossRef]
- Cai, A.H.; Xiong, X.; Liu, Y.; Li, J.H.; An, W.K.; Luo, Y. Characteristics of near-eutectic and off-eutectic Zr-Al-Ni-Cu glass forming alloys. Mater. Sci. Eng. A 2009, 516, 100–102. [Google Scholar] [CrossRef]
- Cai, A.H.; Liu, Y.; Wu, H.; Ding, D.W.; An, W.K.; Zhou, G.J.; Luo, Y.; Peng, Y.Y. Phase formation, glass forming ability, mechanical and thermal properties of Cu50Zr50-xAlx (0 ≤ x ≤ 11.0) glass forming alloys. Sci. China Mater. 2015, 58, 584–594. [Google Scholar] [CrossRef]
- Yu, P.; Bai, H.Y.; Wang, W.H. Superior glass-forming ability of CuZr alloys from minor additions. J. Mater. Res. 2006, 21, 1674–1679. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhao, D.Q.; Pan, M.X.; Shek, C.H.; Wang, W.H. Mechanical heterogeneity and mechanism of plasticity in metallic glasses. Appl. Phys. Lett. 2009, 94, 031904. [Google Scholar] [CrossRef]
- Du, X.H.; Huang, J.C.; Hsieh, K.C.; Lai, Y.H.; Chen, H.M.; Jang, J.S.C.; Liaw, P.K. Two-glassy-phase bulk metallic glass with remarkable plasticity. Appl. Phys. Lett. 2007, 91, 131901. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Zhao, K.; Xia, X.X.; Bai, H.Y.; Zhao, D.Q.; Wang, W.H. Room temperature homogeneous flow in a bulk metallic glass with low glass transition temperature. Appl. Phys. Lett. 2011, 98, 141913. [Google Scholar] [CrossRef]
- Zhao, M.; Li, M. Local heating in shear banding of bulk metallic glasses. Scr. Mater. 2011, 65, 493–496. [Google Scholar] [CrossRef]
- Zhang, H.W.; Subhash, G.; Maiti, S. Local heating and viscosity drop during shear band evolution in bulk metallic glasses under quasistatic loading. J. Appl. Phys. 2007, 102, 043519. [Google Scholar] [CrossRef]
- Yang, B.; Liu, C.T.; Nieh, T.G.; Morrison, M.L.; Liaw, P.K.; Buchanan, R.A. Localized heating and fracture criterion for bulk metallic glasses. J. Mater. Res. 2006, 21, 915–922. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Greer, A.L. Temperature rise at shear bands in metallic glasses. Nat. Mater. 2006, 5, 15–18. [Google Scholar] [CrossRef]
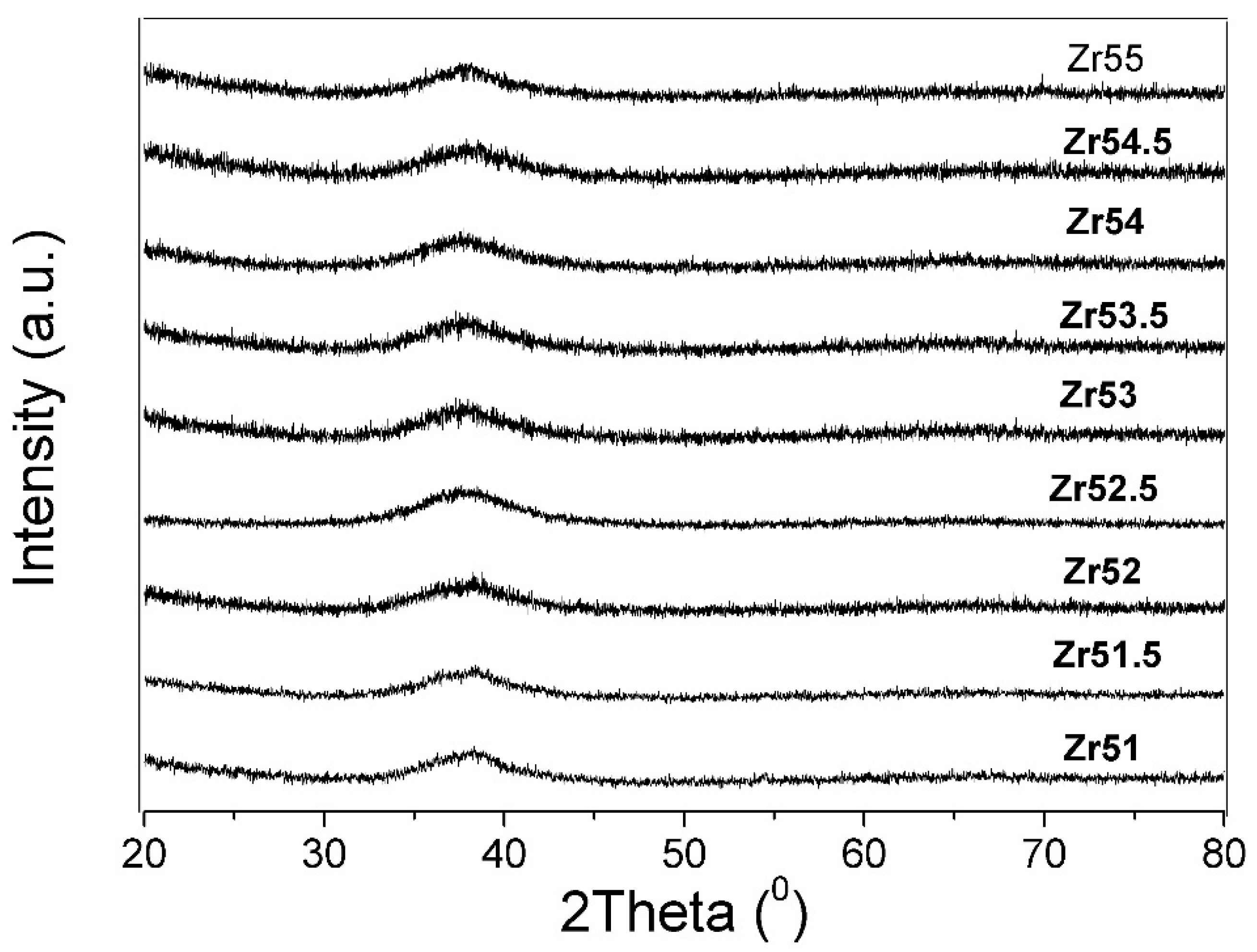
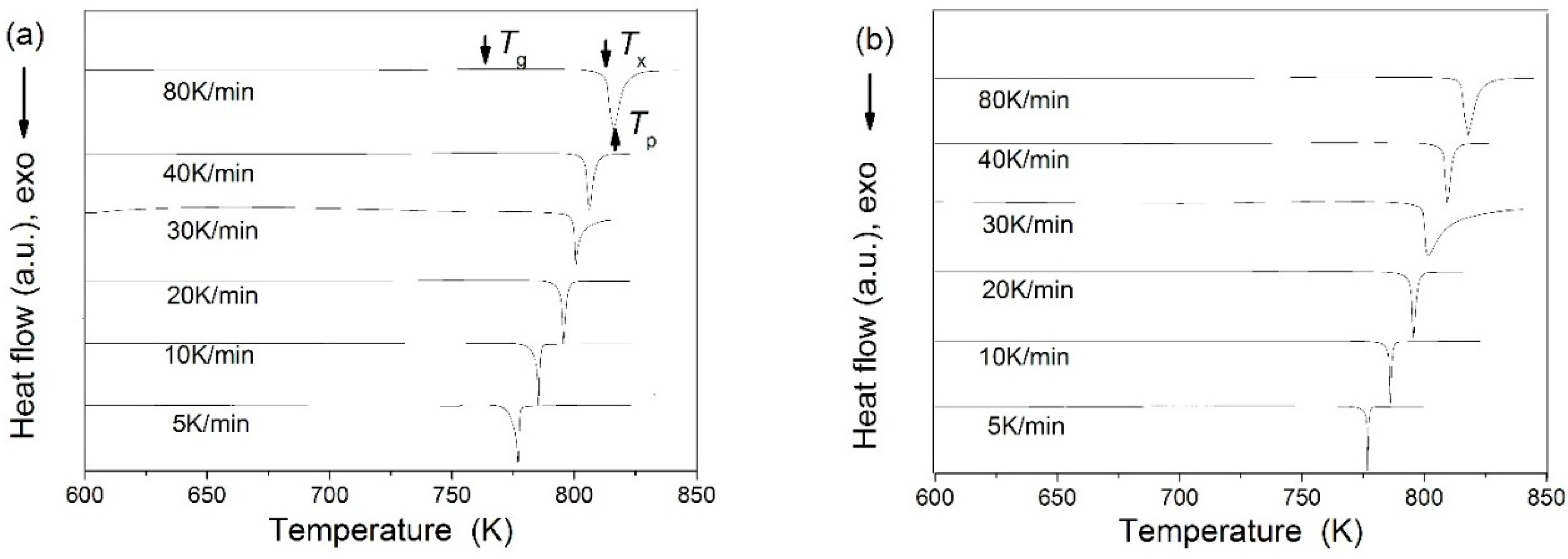

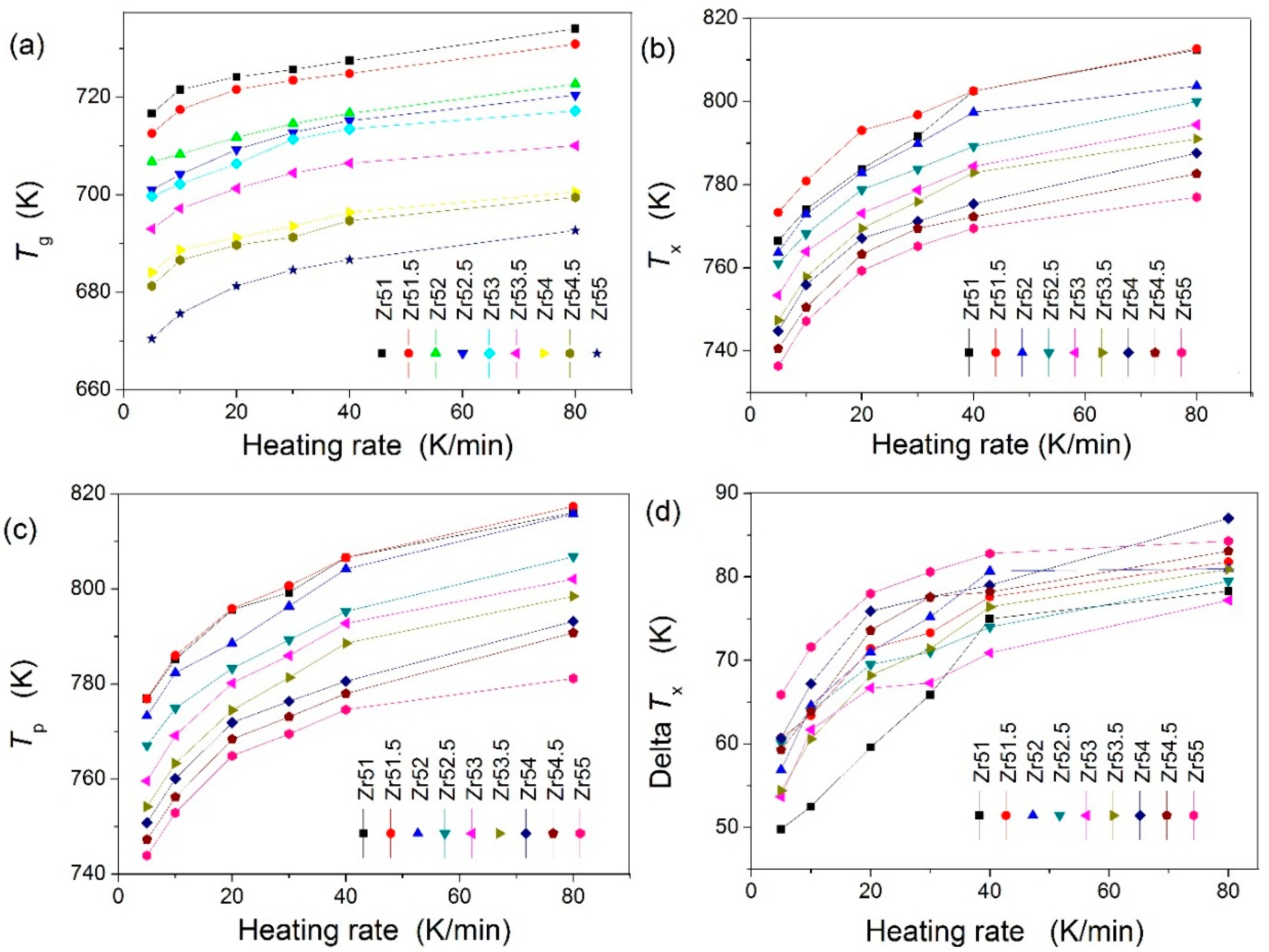
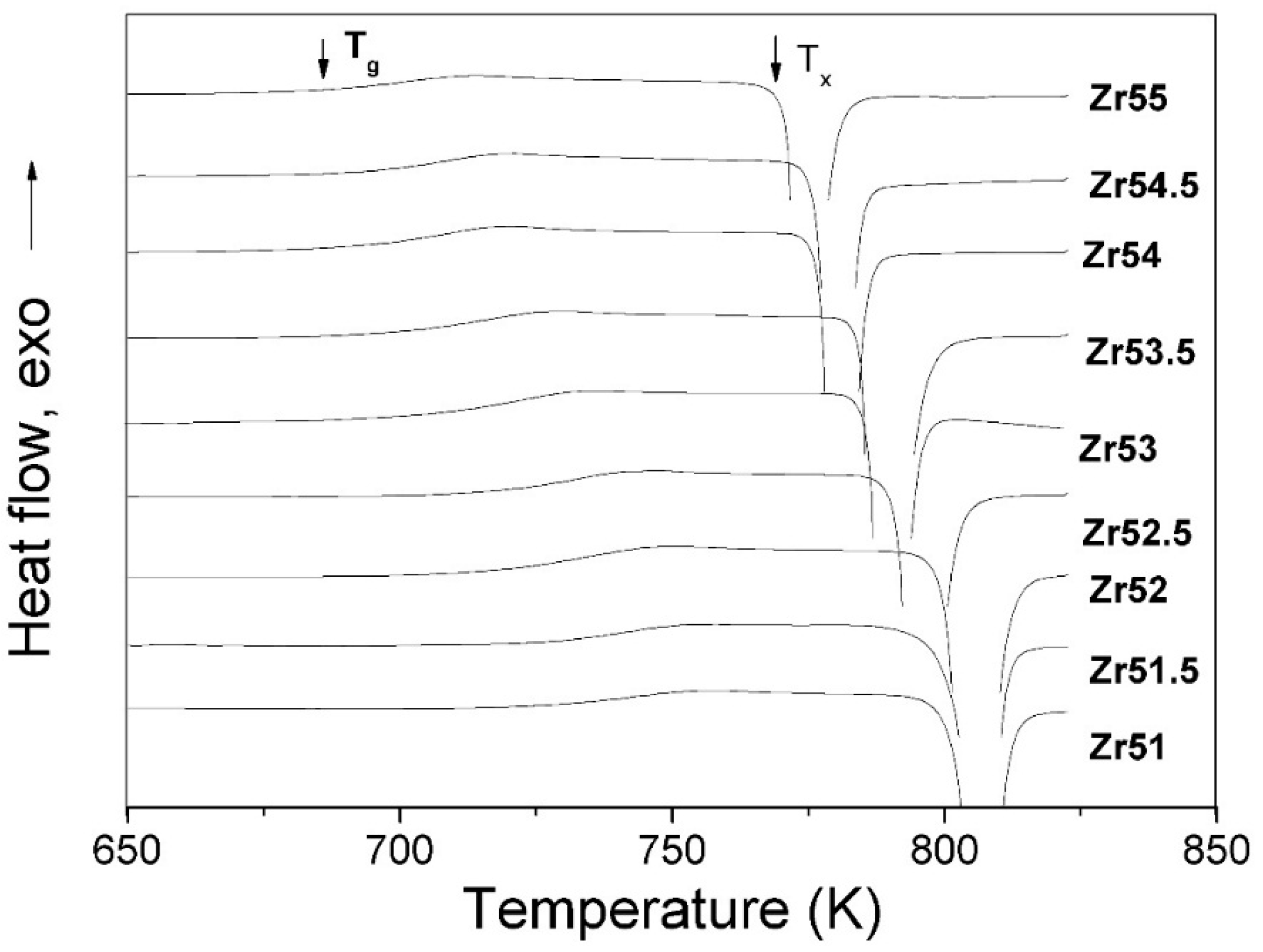

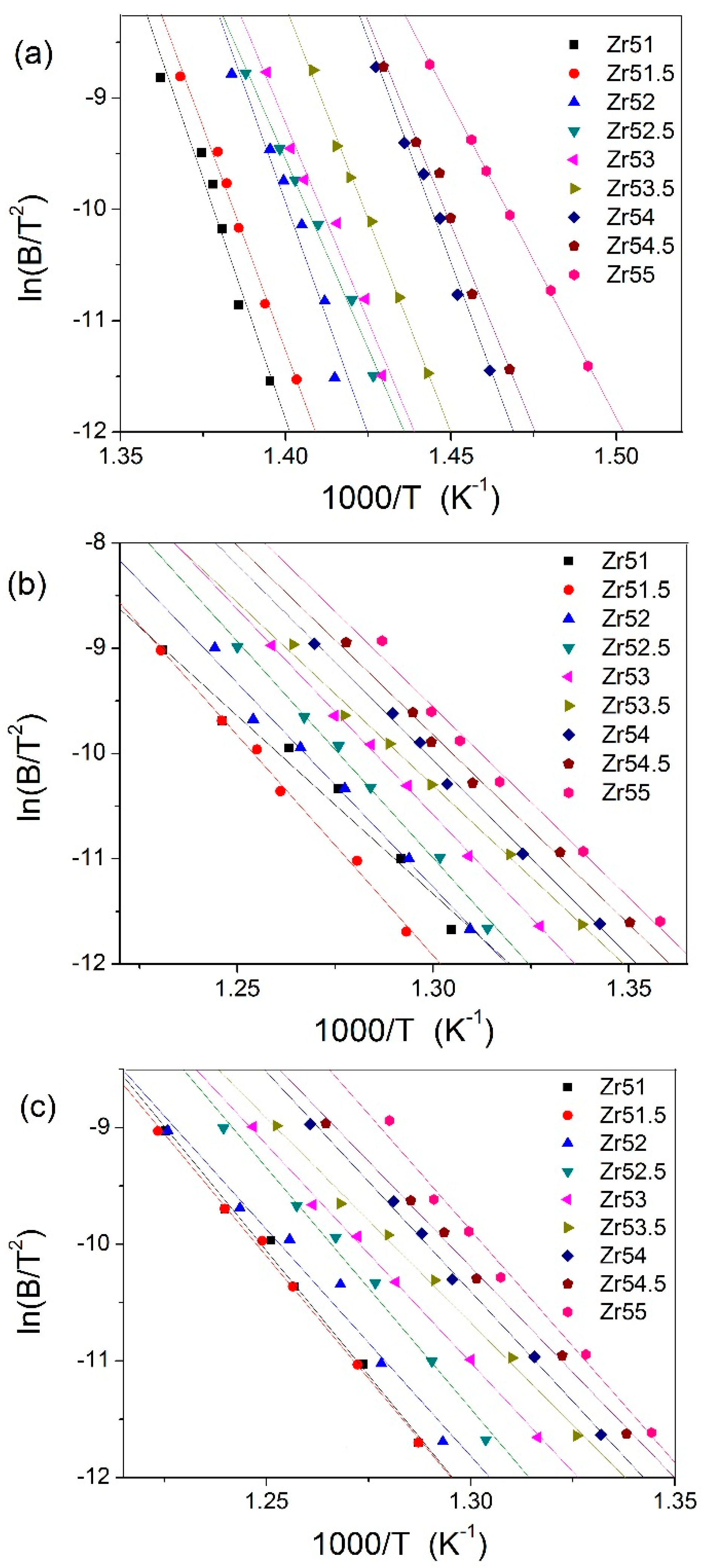
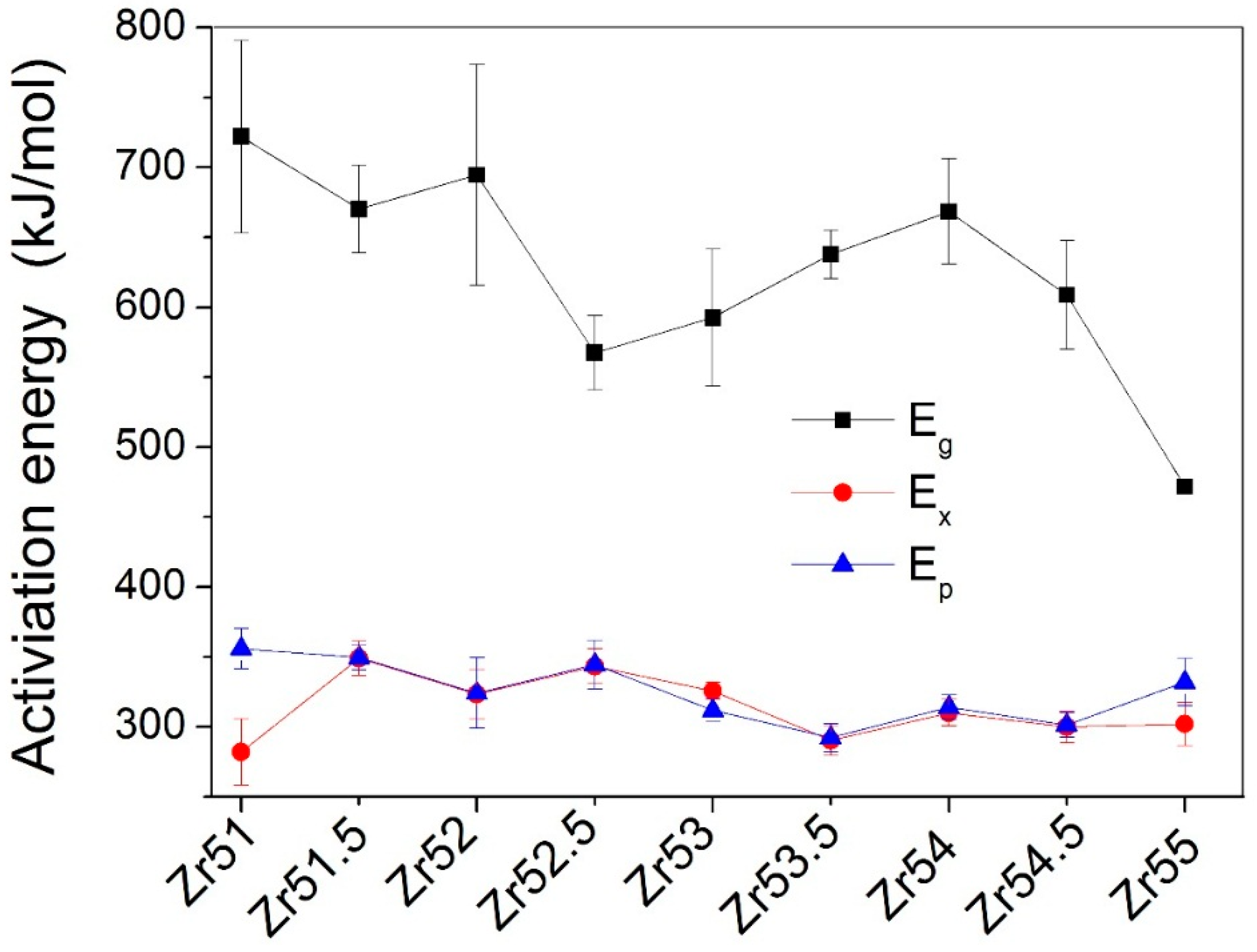
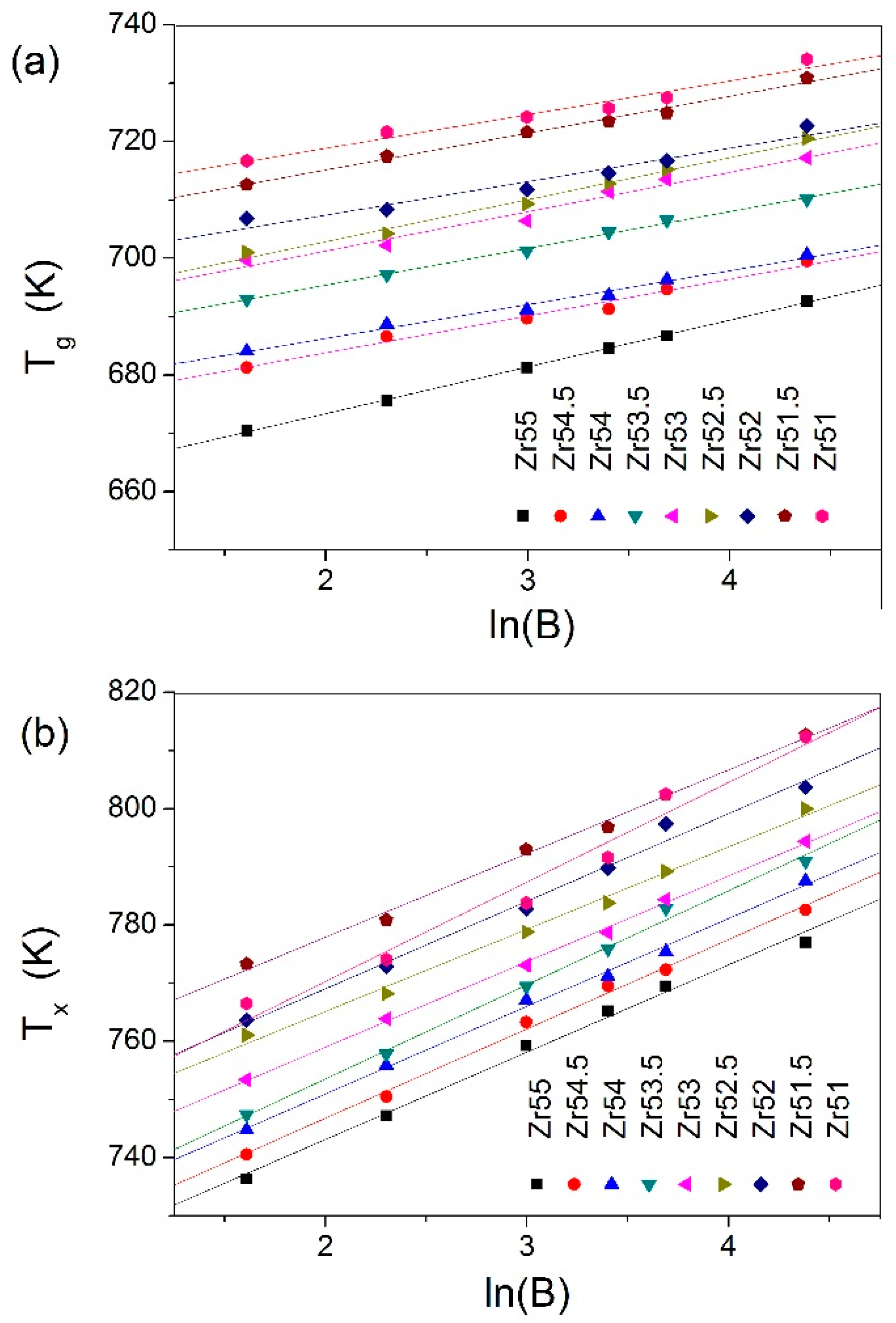
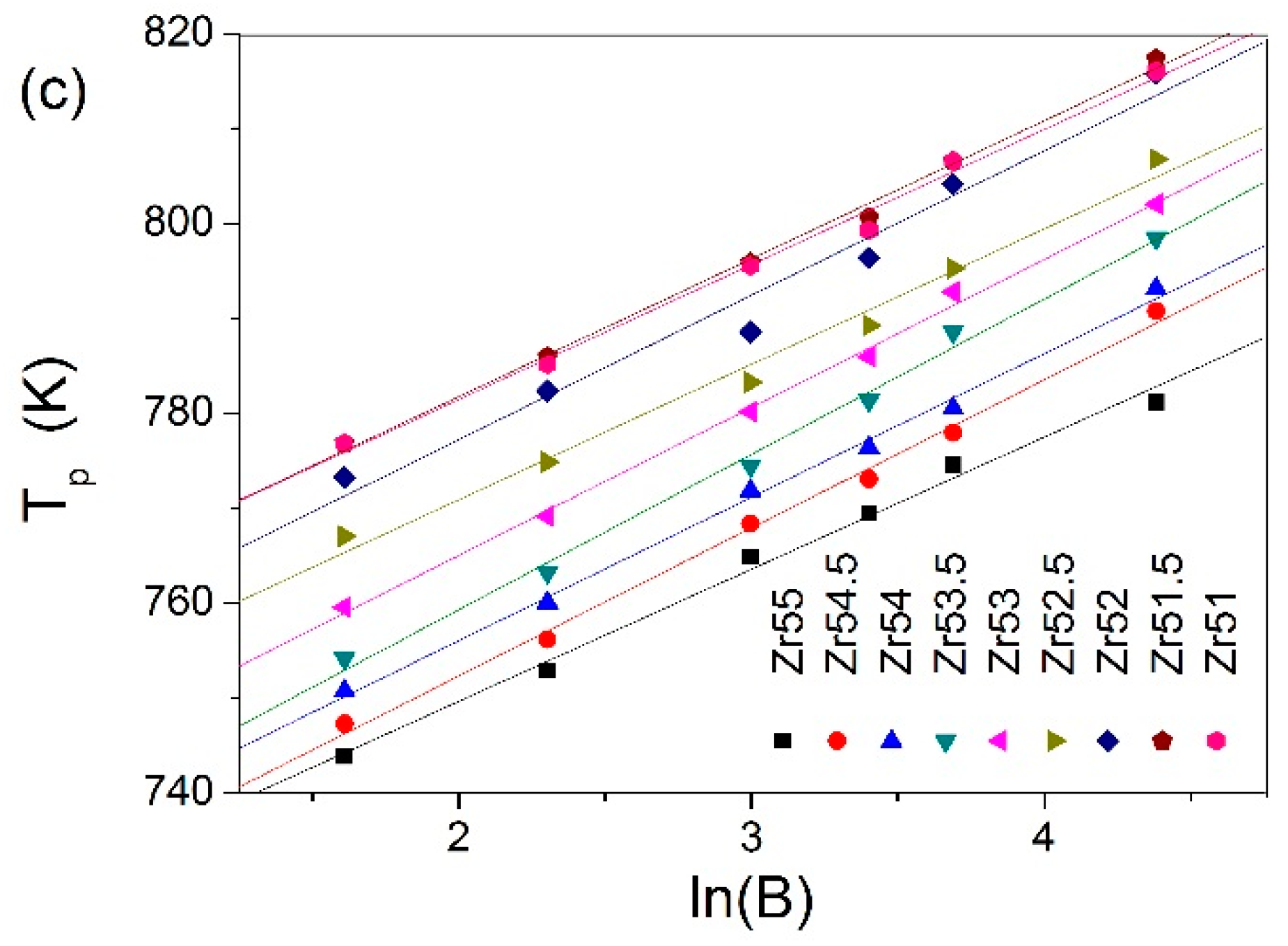

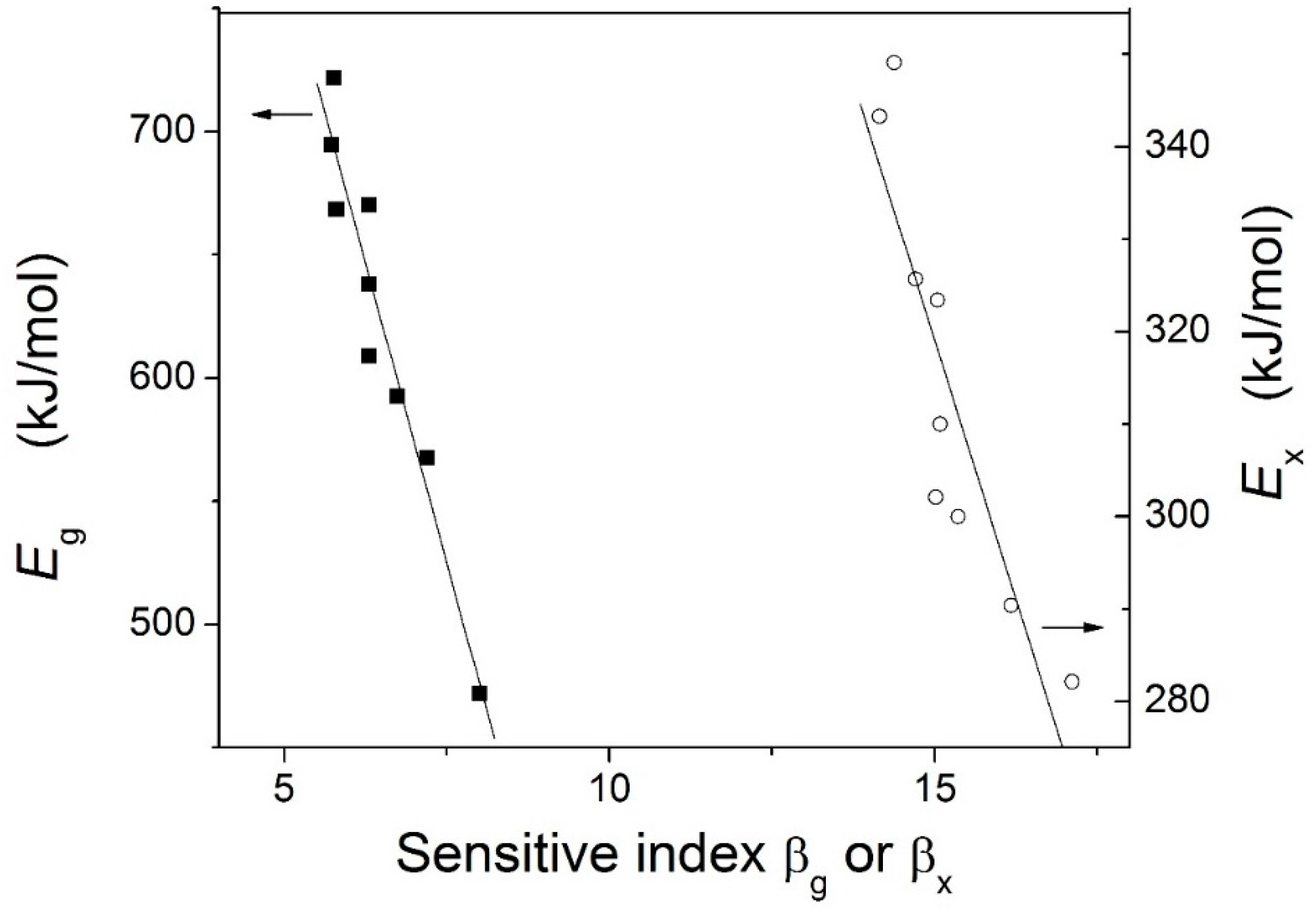
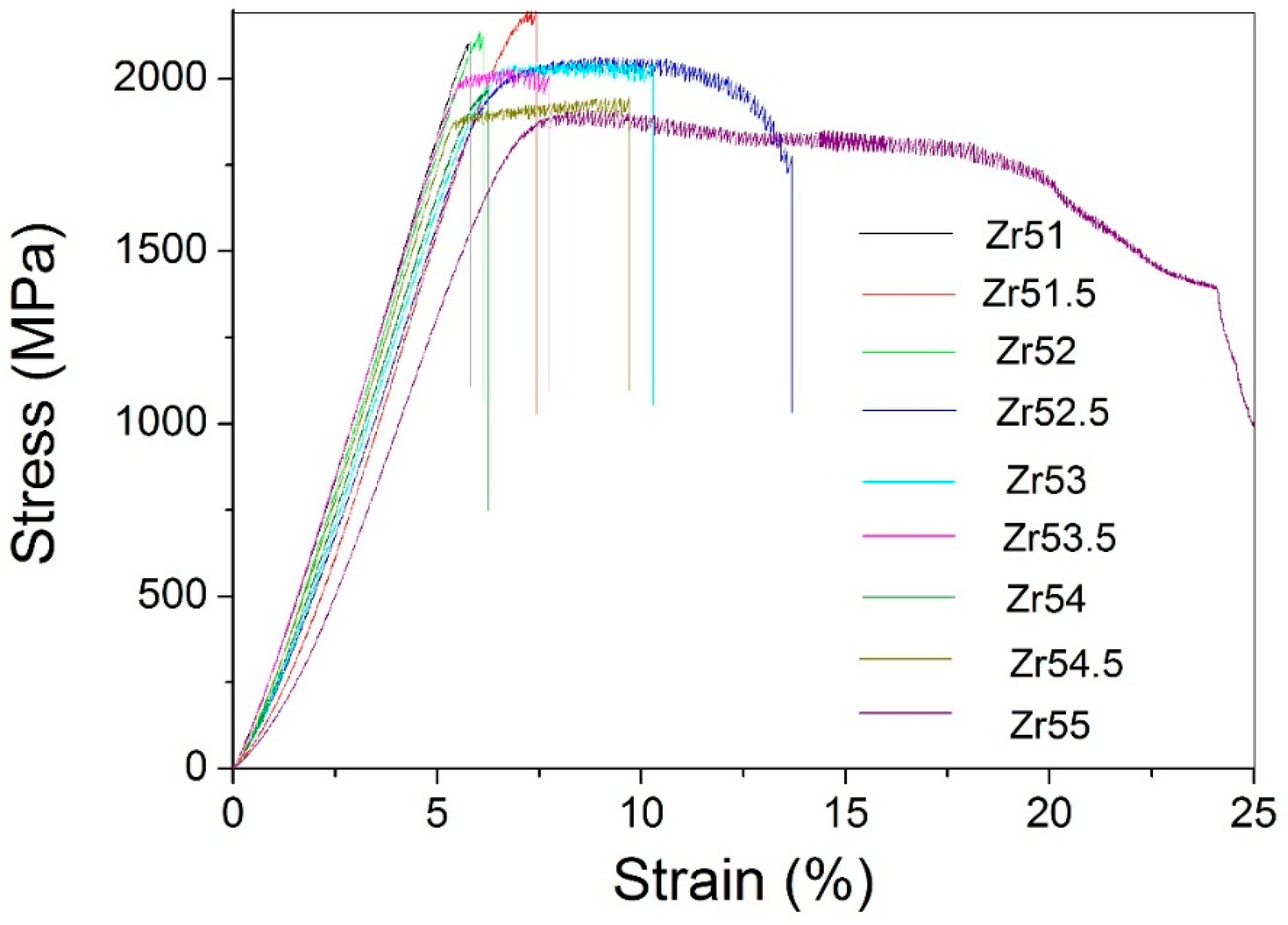

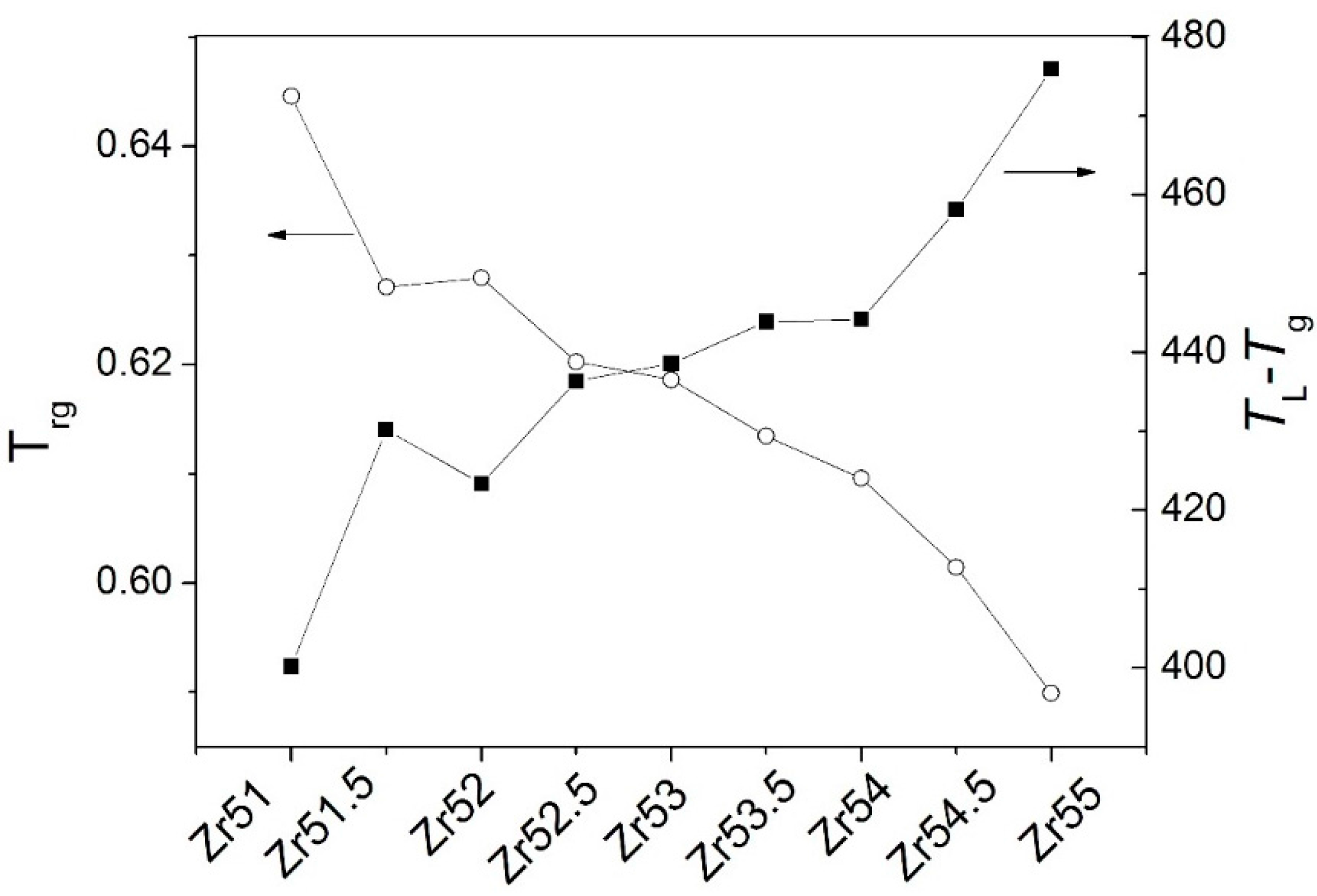
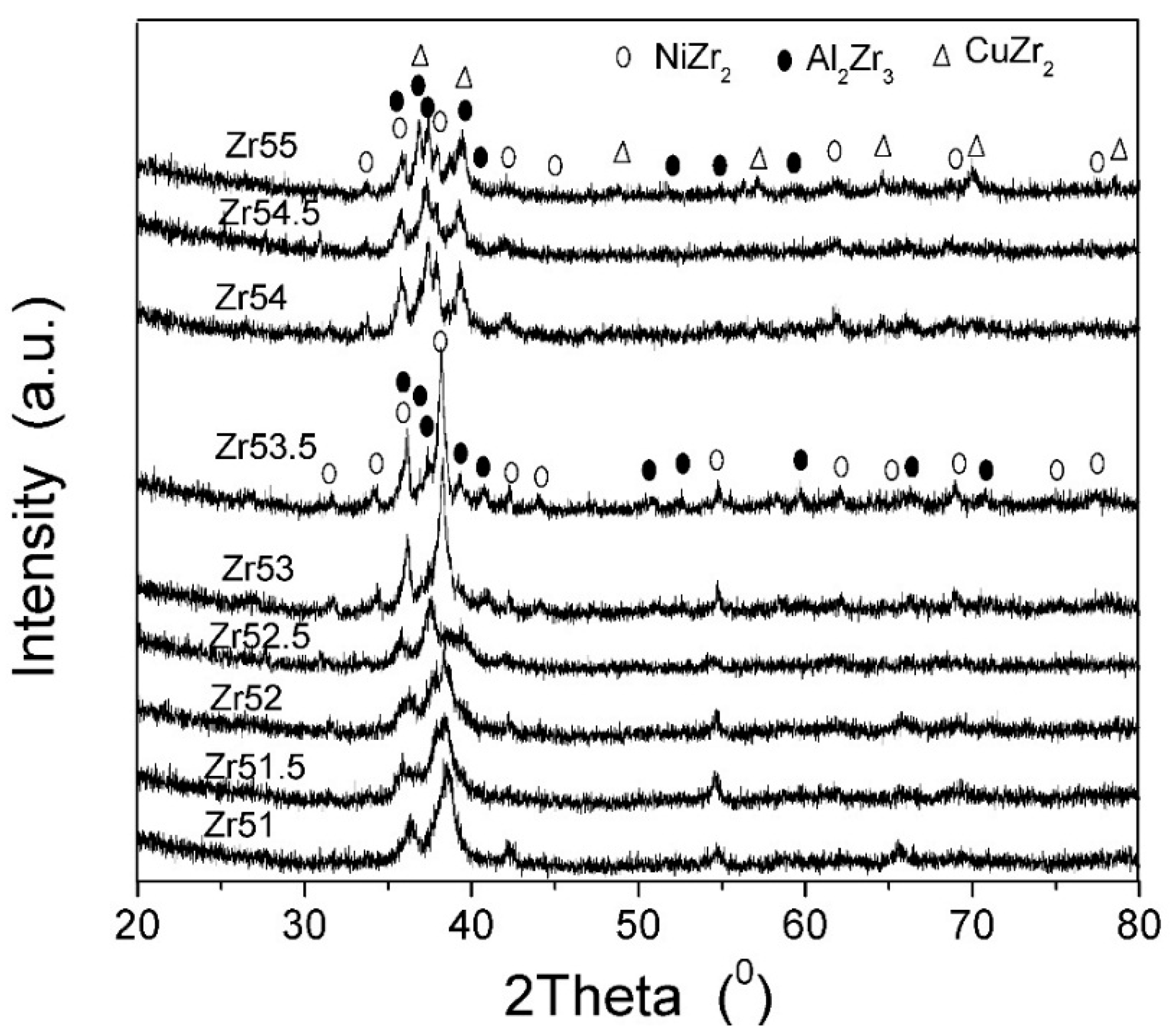
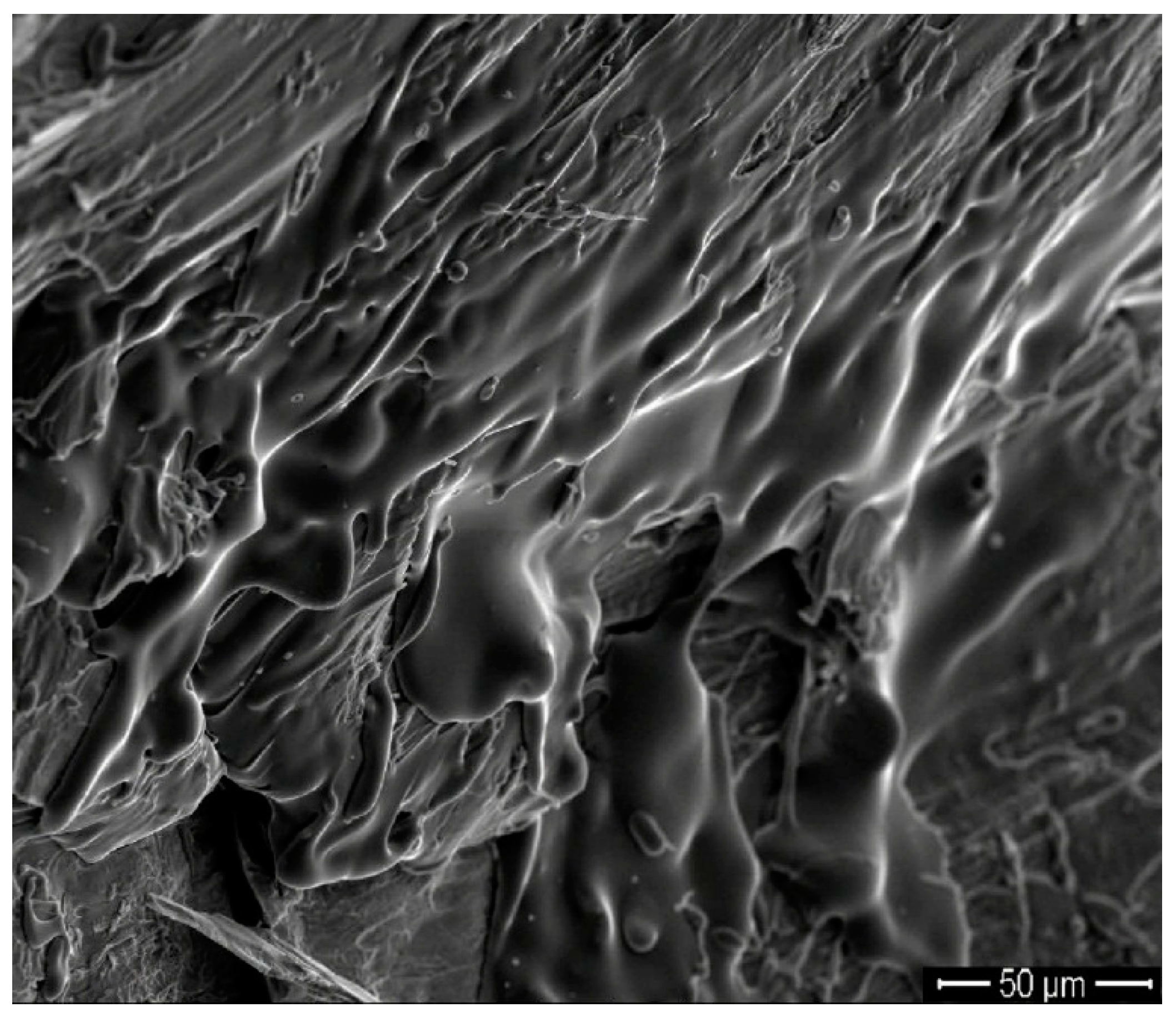
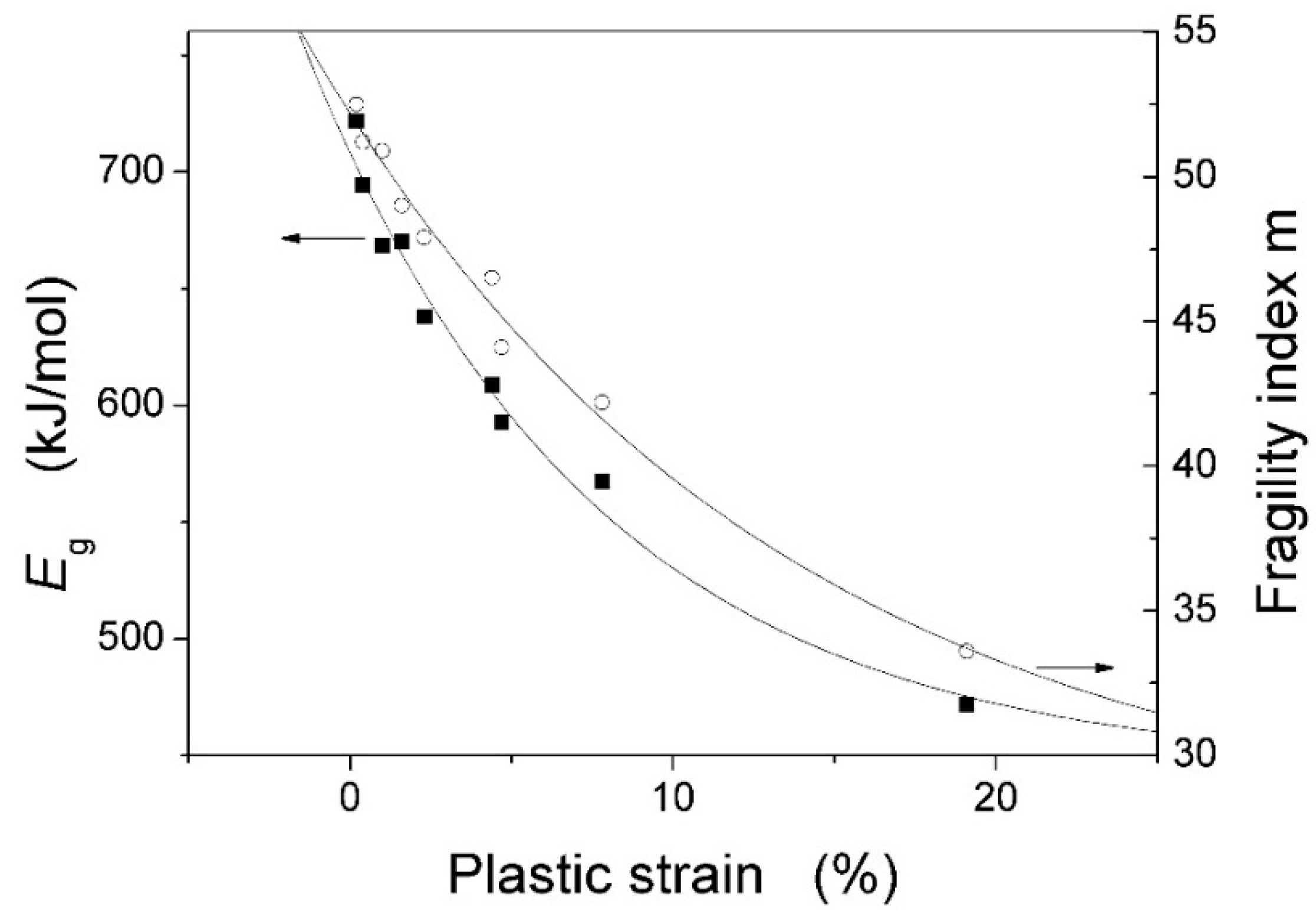
| Metallic Glasses | Tg (K) | Tx (K) | ΔTx (K) | Tm (K) | TL (K) | ΔHx (J/g) | Eg (kJ/mol) | Ex (kJ/mol) | Trg | ΔT (K) | m | βg | βx | σy (MPa) | σf (MPa) | εp (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr51Al14.2Ni15.9Cu18.9 | 725.7 | 791.6 | 65.9 | 1021.3 | 1125.9 | 54.6 | 721.9 | 282.1 | 0.6446 | 400.2 | 52.5 | 5.7692 | 17.1162 | 2072.5 | 2104.9 | 0.2 |
| Zr51.5Al13.6Ni14.9Cu20 | 723.5 | 796.8 | 73.3 | 1015.0 | 1153.7 | 48.4 | 670.2 | 349.1 | 0.6271 | 430.2 | 49.0 | 6.3086 | 14.3785 | 1838.0 | 2184.6 | 1.6 |
| Zr52Al12.9Ni13.8Cu21.3 | 714.6 | 789.8 | 75.2 | 1060.6 | 1138.0 | 51.2 | 694.6 | 323.4 | 0.6279 | 423.4 | 51.2 | 5.7305 | 15.0479 | 2041.9 | 2114.5 | 0.4 |
| Zr52.5Al12.2Ni12.6Cu22.7 | 712.8 | 783.8 | 71.0 | 1048.7 | 1149.2 | 56.7 | 567.4 | 343.3 | 0.6203 | 436.4 | 42.2 | 7.2022 | 14.1554 | 1870.1 | 2040.1 | 7.8 |
| Zr53Al11.6Ni11.7Cu23.7 | 711.4 | 778.7 | 67.3 | 1044.7 | 1150.0 | 50.8 | 592.7 | 325.7 | 0.6168 | 438.6 | 44.1 | 6.7406 | 14.7100 | 1840.6 | 2015.5 | 4.7 |
| Zr53.5Al10.9Ni10.6Cu25 | 704.5 | 775.9 | 71.4 | 1041.2 | 1148.4 | 52.9 | 637.9 | 290.4 | 0.6135 | 443.9 | 47.9 | 6.2987 | 16.1749 | 1974.0 | 1995.4 | 2.3 |
| Zr54Al10.2Ni9.4Cu26.4 | 693.6 | 771.2 | 77.6 | 1028.2 | 1137.8 | 49.6 | 668.4 | 310.0 | 0.6096 | 444.2 | 50.9 | 5.8047 | 15.0870 | 1781.6 | 1959.3 | 1.0 |
| Zr54.5Al9.6Ni8.4Cu27.5 | 691.3 | 769.5 | 78.2 | 996.4 | 1149.4 | 55.4 | 608.8 | 300.0 | 0.6014 | 458.1 | 46.5 | 6.3063 | 15.3629 | 1863.1 | 1907.3 | 4.4 |
| Zr55Al8.9Ni7.3Cu28.8 | 684.6 | 765.2 | 80.6 | 992.0 | 1160.5 | 44.8 | 471.9 | 302.1 | 0.5899 | 475.9 | 33.6 | 8.0065 | 15.0184 | 1737.5 | 1892.6 | 19.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, A.; Ding, D.; Liu, Y.; Wu, H.; An, W.; Zhou, G.; Luo, Y.; Peng, Y. A Series of Zr-Based Bulk Metallic Glasses with Room Temperature Plasticity. Materials 2016, 9, 408. https://doi.org/10.3390/ma9060408
Cai A, Ding D, Liu Y, Wu H, An W, Zhou G, Luo Y, Peng Y. A Series of Zr-Based Bulk Metallic Glasses with Room Temperature Plasticity. Materials. 2016; 9(6):408. https://doi.org/10.3390/ma9060408
Chicago/Turabian StyleCai, Anhui, Dawei Ding, Yong Liu, Hong Wu, Weike An, Guojun Zhou, Yun Luo, and Yongyi Peng. 2016. "A Series of Zr-Based Bulk Metallic Glasses with Room Temperature Plasticity" Materials 9, no. 6: 408. https://doi.org/10.3390/ma9060408
APA StyleCai, A., Ding, D., Liu, Y., Wu, H., An, W., Zhou, G., Luo, Y., & Peng, Y. (2016). A Series of Zr-Based Bulk Metallic Glasses with Room Temperature Plasticity. Materials, 9(6), 408. https://doi.org/10.3390/ma9060408






