Spin-Coated vs. Electrodeposited Mn Oxide Films as Water Oxidation Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Films Prepared by Spin-Coating
2.3. Films Prepared by Electrodeposition
2.4. Characterization Techniques
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Spin-Coated Samples
3.2. Electrodeposited Samples
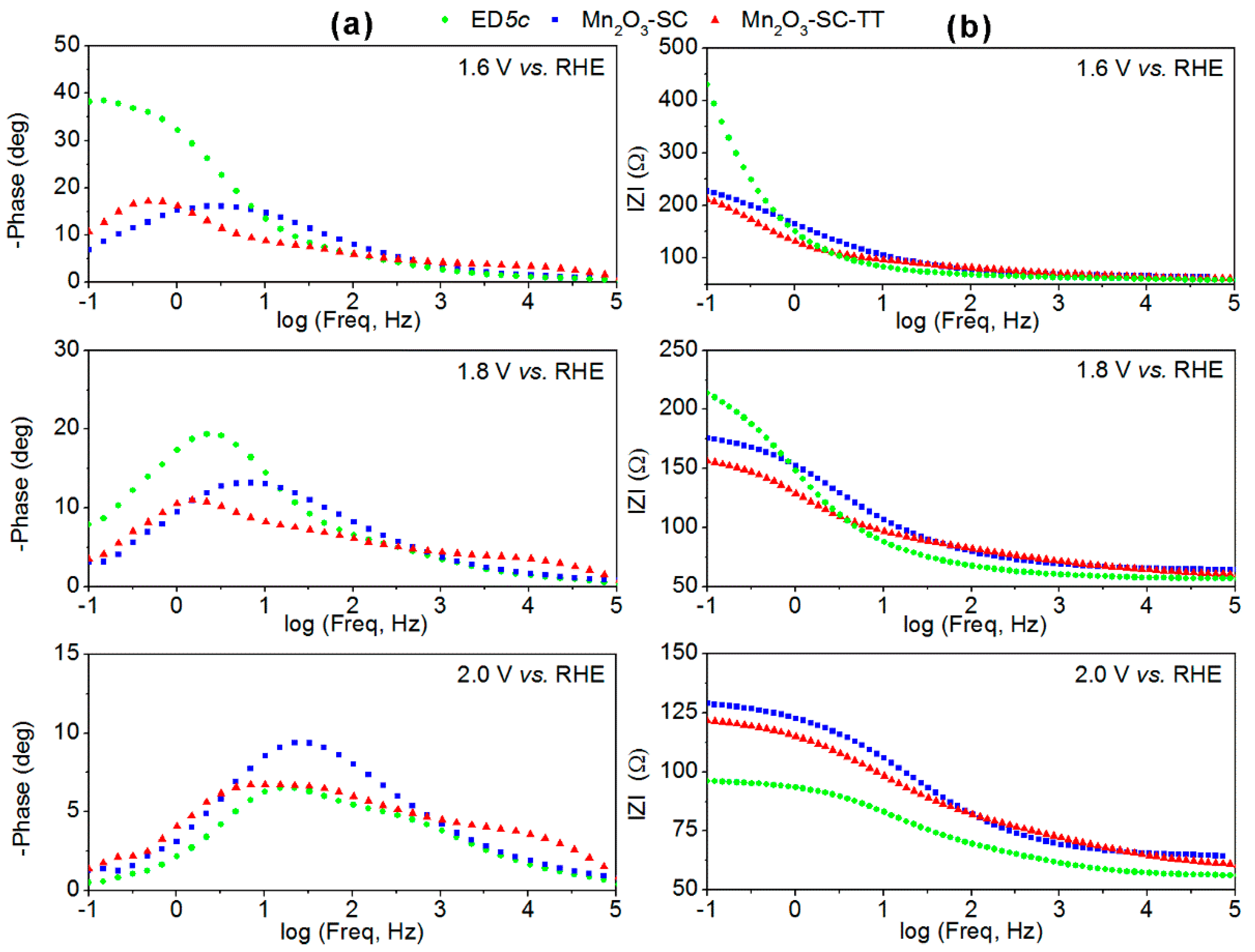
3.3. Charge Transfer and Transport Mechanisms in Mn2O3-Based Films
3.4. Oxygen/Hydrogen Evolution Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herrero, C.; Quaranta, A.; Leibl, W.; Rutherford, A.W.; Aukauloo, A. Artificial photosynthetic systems: Using light and water to provide electrons and protons for the synthesis of a fuel. Energy Environ. Sci. 2011, 4, 2353–2365. [Google Scholar] [CrossRef]
- Youngblood, W.J.; Lee, S.-H.A.; Kobayashi, Y.; Hernandez-Pagan, E.A.; Hoertz, P.G.; Moore, T.A.; Moore, A.L.; Gust, D.; Mallouk, T.E. Photoassisted overall water splitting in a visible light-absorbing dye-sensitized photoelectrochemical cell. J. Am. Chem. Soc. 2009, 131, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Wiechen, M.; Spiccia, L. Manganese oxides as efficient water oxidation catalysts. Chem. Cat. Chem. 2014, 6, 439–441. [Google Scholar] [CrossRef]
- Robinson, D.M.; Go, Y.B.; Mui, M.; Gardner, G.; Zhang, Z.; Mastrogiovanni, D.; Garfunkel, E.; Li, J.; Greenblatt, M.; Dismukes, G.C. Photochemical water oxidation by crystalline polymorphs of manganese oxides: Structural requirements for catalysis. J. Am. Chem. Soc. 2013, 135, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Del-Pilar, J.; King’ondu, C.K.; Kissel, E.; Garces, H.F.; Huang, H.; El-Sawy, A.M.; Dutta, P.K.; Suib, S.L. Water oxidation catalysis using amorphous manganese oxides, octahedral molecular sieves (OMS-2), and octahedral layered (OL-1) manganese oxide structures. J. Phys. Chem. C 2012, 116, 6474–6483. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Zhang, Y.; Du, J.; Han, X.; Chen, J. Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies. Angew. Chem. Int. Ed. 2013, 52, 2474–2477. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M.; Sedigh, D.J. Water oxidation by manganese oxides, a new step towards a complete picture: Simplicity is the ultimate sophistication. Dalton Trans. 2013, 42, 12173–12178. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Mandal, S.K.; Bhaduri, S.; Armstrong, W.H. Manganese clusters with relevance to photosystem ii. Chem. Rev. 2004, 104, 3981–4026. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M.; Ehrenberg, T.; Wiechen, M.; Kurz, P. Calcium manganese(III) oxides (CaMn2O4·xH2O) as biomimetic oxygen-evolving catalysts. Angew. Chem. Int. Ed. 2010, 49, 2233–2237. [Google Scholar] [CrossRef] [PubMed]
- Baktash, E.; Zaharieva, I.; Schroder, M.; Goebel, C.; Dau, H.; Thomas, A. Cyanamide route to calcium-manganese oxide foams for water oxidation. Dalton Trans. 2013, 42, 16920–16929. [Google Scholar] [CrossRef] [PubMed]
- Swierk, J.R.; Mallouk, T.E. Design and development of photoanodes for water-splitting dye-sensitized photoelectrochemical cells. Chem. Soc. Rev. 2013, 42, 2357–2387. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.; Hidalgo, D.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Cauda, V.; Tresso, E.; Saracco, G. Comparison of photocatalytic and transport properties of TiO2 and ZnO nanostructures for solar-driven water splitting. Phys. Chem. Chem. Phys. 2015, 17, 7775–7786. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wolcott, A.; Wang, G.; Sobo, A.; Fitzmorris, R.C.; Qian, F.; Zhang, J.Z.; Li, Y. Nitrogen-doped ZnO nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2009, 9, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Turner, J.A. Characterization of hematite thin films for photoelectrochemical water splitting in a dual photoelectrode device. J. Electrochem. Soc. 2010, 157, F173–F178. [Google Scholar] [CrossRef]
- Hernández, S.; Thalluri, S.M.; Sacco, A.; Bensaid, S.; Saracco, G.; Russo, N. Photo-catalytic activity of BiVO4 thin-film electrodes for solar-driven water splitting. Appl. Catal. A Gen. 2015, 504, 266–271. [Google Scholar] [CrossRef]
- Boppana, V.B.R.; Yusuf, S.; Hutchings, G.S.; Jiao, F. Nanostructured alkaline-cation-containing δ-MnO2 for photocatalytic water oxidation. Adv. Funct. Mater. 2013, 23, 878–884. [Google Scholar] [CrossRef]
- Jiao, F.; Frei, H. Nanostructured cobalt and manganese oxide clusters as efficient water oxidation catalysts. Energy Environ. Sci. 2010, 3, 1018–1027. [Google Scholar] [CrossRef]
- Hernández, S.; Bensaid, S.; Armandi, M.; Sacco, A.; Chiodoni, A.; Bonelli, B.; Garrone, E.; Pirri, C.F.; Saracco, G. A new method for studying activity and reaction kinetics of photocatalytic water oxidation systems using a bubbling reactor. Chem. Eng. J. 2014, 238, 17–26. [Google Scholar] [CrossRef]
- Armandi, M.; Hernandez, S.; Vankova, S.; Zanarini, S.; Bonelli, B.; Garrone, E. Visible-light driven oxidation of water as catalyzed by Co-APO-5 in the presence of Ru sensitizer. ACS Catal. 2013, 3, 1272–1278. [Google Scholar] [CrossRef]
- Chou, S.; Cheng, F.; Chen, J. Electrodeposition synthesis and electrochemical properties of nanostructured γ-MnO2 films. J. Power Sources 2006, 162, 727–734. [Google Scholar] [CrossRef]
- Zhou, F.; Izgorodin, A.; Hocking, R.K.; Spiccia, L.; MacFarlane, D.R. Electrodeposited MnOx films from ionic liquid for electrocatalytic water oxidation. Adv. Energy Mater. 2012, 2, 1013–1021. [Google Scholar] [CrossRef]
- Wu, M.-S. Electrochemical capacitance from manganese oxide nanowire structure synthesized by cyclic voltammetric electrodeposition. Appl. Phys. Lett. 2005, 87, 153102. [Google Scholar] [CrossRef]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 electrodeposited films for the oxygen evolution reaction of water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Lee, S.Y.; González-Flores, D.; Ohms, J.; Trost, T.; Dau, H.; Zaharieva, I.; Kurz, P. Screen-printed calcium-birnessite electrodes for water oxidation at neutral pH and an “electrochemical harriman series”. Chem. Sus. Chem. 2014, 7, 3442–3451. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Hocking, R.K.; Chang, S.L.Y.; Italiano, C.; Patti, A.F.; Arena, F.; Spiccia, L. Highly active screen-printed electrocatalysts for water oxidation based on β-manganese oxide. Energy Environ. Sci. 2013, 6, 2222–2232. [Google Scholar] [CrossRef]
- Natsume, Y.; Sakata, H. Zinc oxide films prepared by sol-gel spin-coating. Thin Solid Films 2000, 372, 30–36. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Kosbar, L.L.; Murray, C.E.; Copel, M.; Afzali, A. High-mobility ultrathin semiconducting films prepared by spin coating. Nature 2004, 428, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.; Messina, R.; Sacco, A.; Manfredi, D.; Vankova, S.; Garrone, E.; Saracco, G.; Hernández, S. Thick mesoporous TiO2 films through a sol–gel method involving a non-ionic surfactant: Characterization and enhanced performance for water photo-electrolysis. Int. J. Hydrog. Energy 2014, 39, 21512–21522. [Google Scholar] [CrossRef]
- Bagshaw, S.A.; Prouzet, E.; Pinnavaia, T.J. Templating of mesoporous molecular sieves by nonionic polyethylene oxide surfactants. Science 1995, 269, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Ottone, C.; Armandi, M.; Hernández, S.; Bensaid, S.; Fontana, M.; Pirri, C.F.; Saracco, G.; Garrone, E.; Bonelli, B. Effect of surface area on the rate of photocatalytic water oxidation as promoted by different manganese oxides. Chem. Eng. J. 2015, 278, 36–45. [Google Scholar] [CrossRef]
- Pugliese, D.; Bella, F.; Cauda, V.; Lamberti, A.; Sacco, A.; Tresso, E.; Bianco, S. A chemometric approach for the sensitization procedure of ZnO flowerlike microstructures for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 11288–11295. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Deibert, B.J.; Kaushik, S.; Gardner, G.; Hwang, S.; Wang, H.; Al-Sharab, J.F.; Garfunkel, E.; Fabris, L.; Li, J.; et al. Coordination geometry and oxidation state requirements of corner-sharing MnO6 octahedra for water oxidation catalysis: An investigation of manganite (γ-MnOOH). ACS Catal. 2016, 6, 2089–2099. [Google Scholar] [CrossRef]
- Zaharieva, I.; Chernev, P.; Risch, M.; Klingan, K.; Kohlhoff, M.; Fischer, A.; Dau, H. Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide. Energy Environ. Sci. 2012, 5, 7081–7089. [Google Scholar] [CrossRef]
- Pang, S.C.; Anderson, M.A.; Chapman, T.W. Novel electrode materials for thin-film ultracapacitors: Comparison of electrochemical properties of sol-gel-derived and electrodeposited manganese dioxide. J. Electrochem. Soc. 2000, 147, 444–450. [Google Scholar] [CrossRef]
- Hernández, S.; Tortello, M.; Sacco, A.; Quaglio, M.; Meyer, T.; Bianco, S.; Saracco, G.; Pirri, C.F.; Tresso, E. New transparent laser-drilled fluorine-doped tin oxide covered quartz electrodes for photo-electrochemical water splitting. Electrochim. Acta 2014, 131, 184–194. [Google Scholar] [CrossRef]
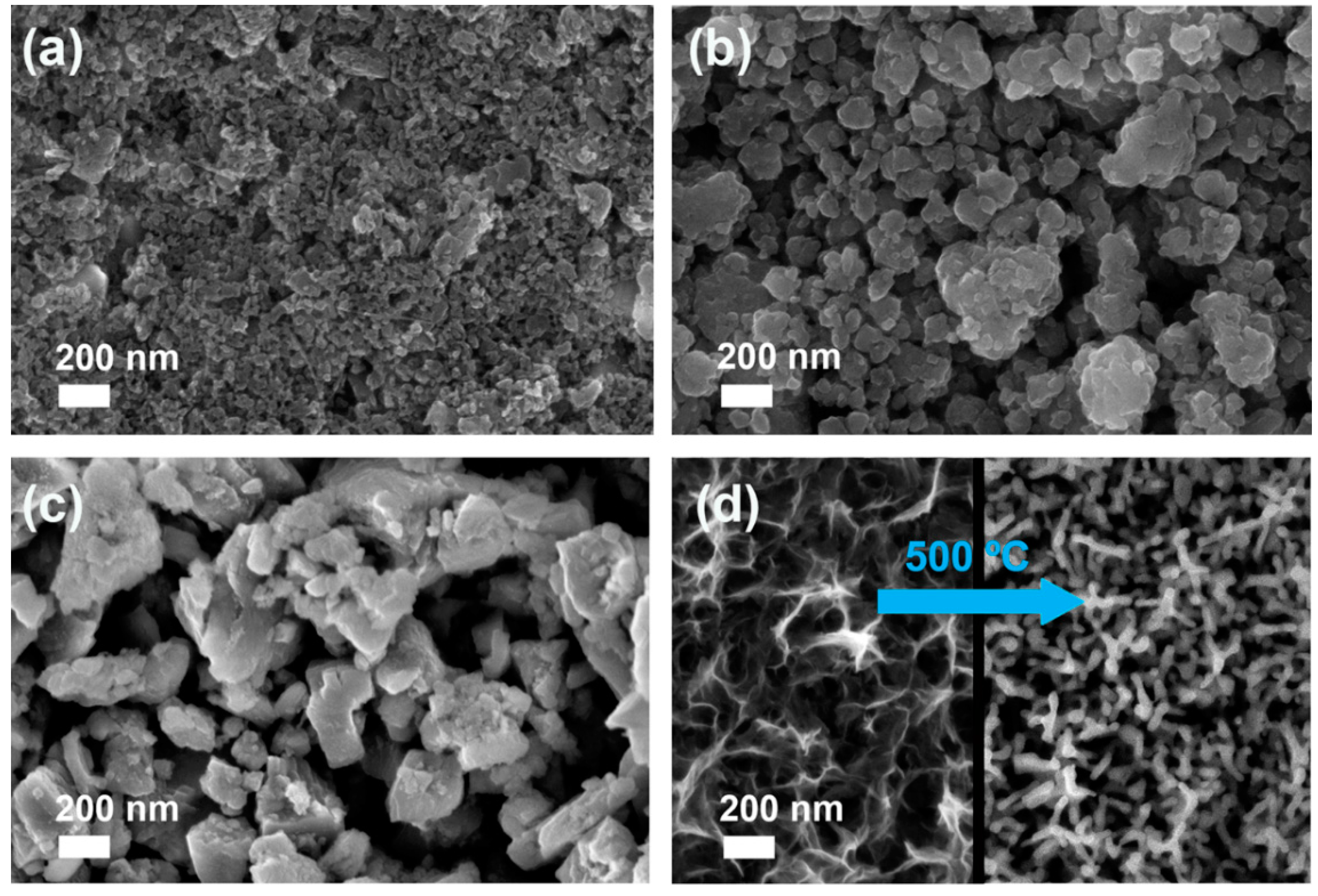
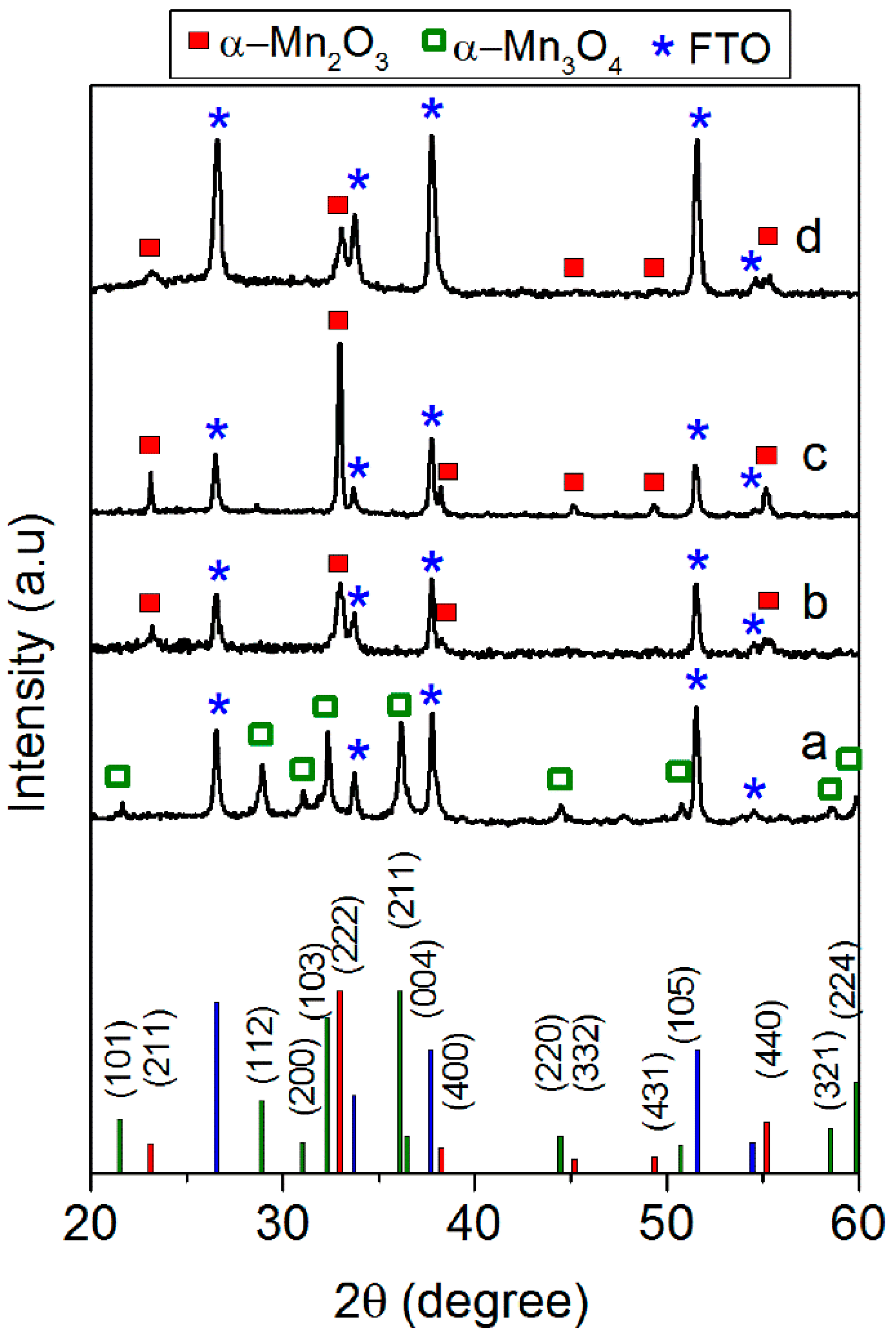
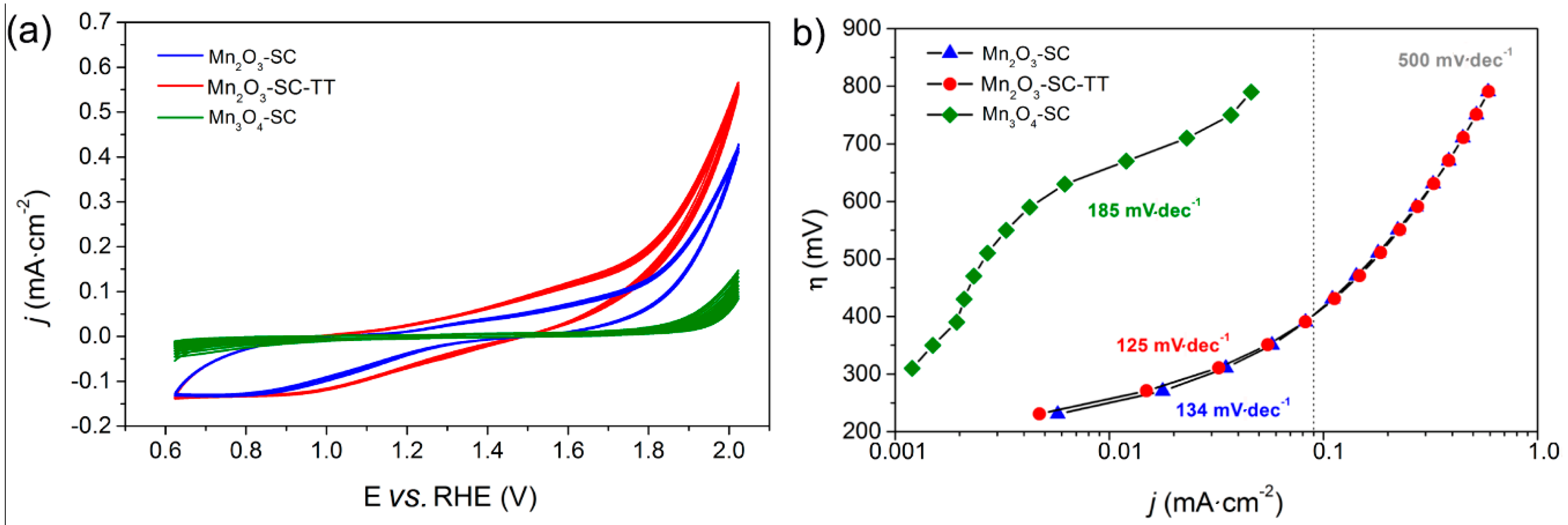
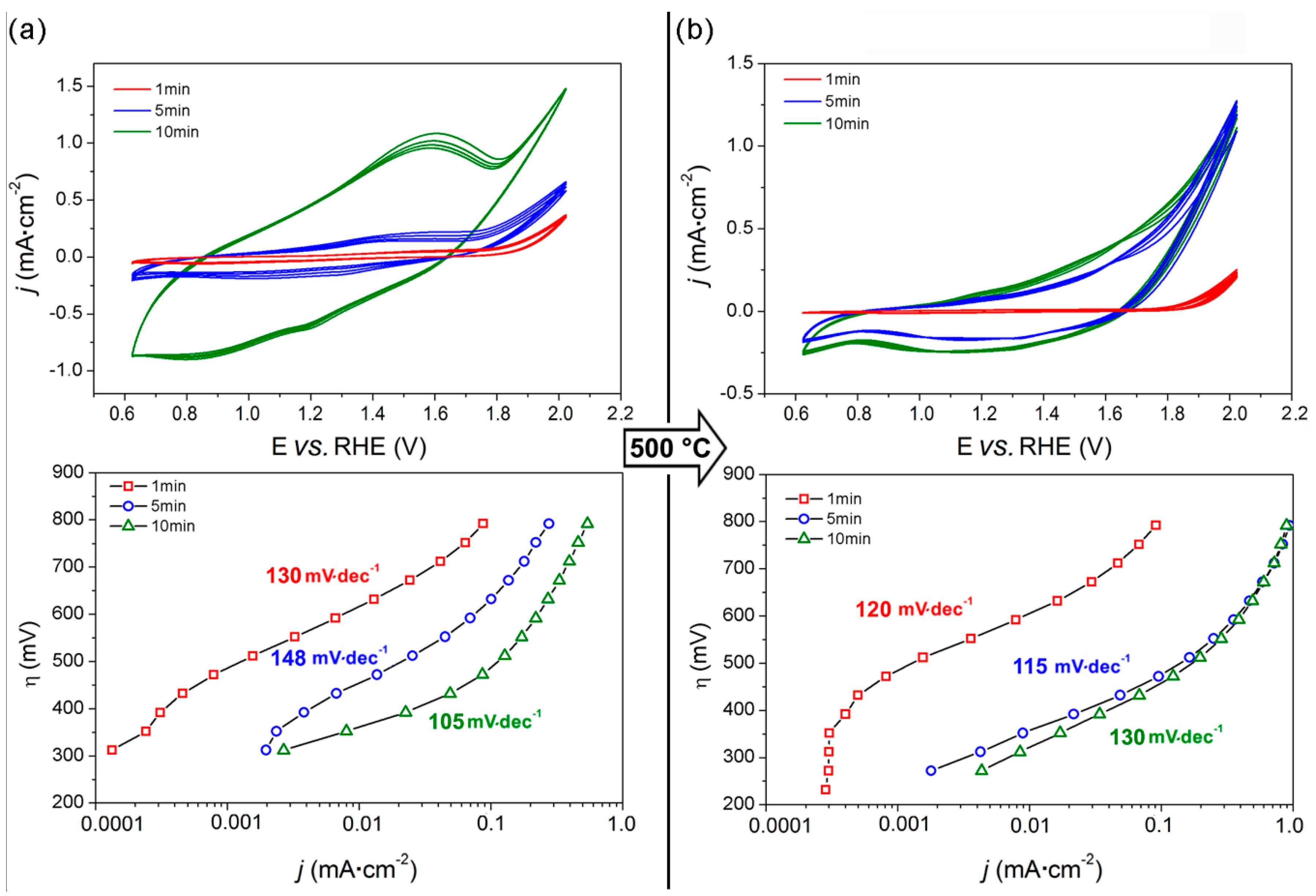
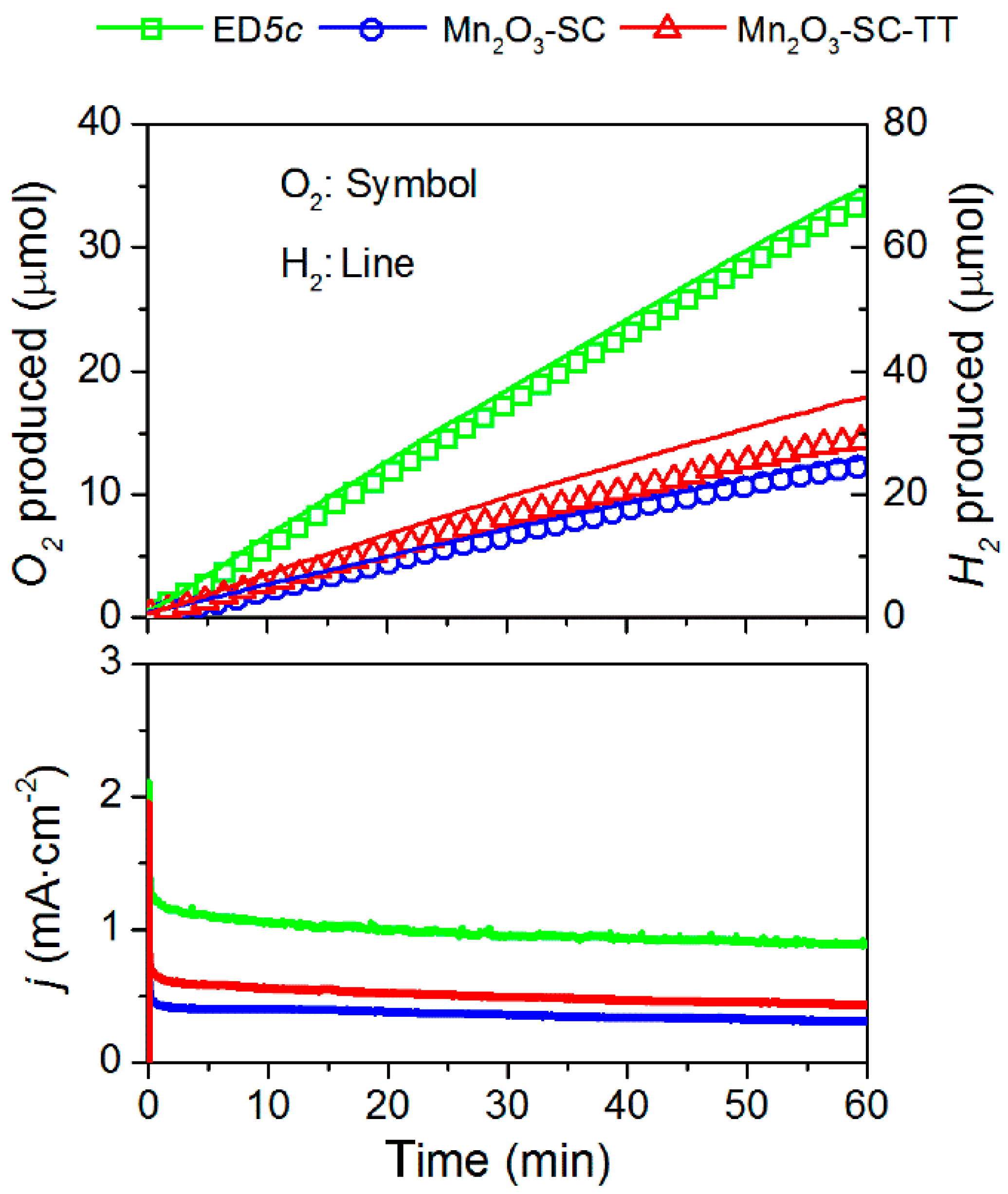
| Sample | On-set Potential a (V vs. RHE) | Tafel Slope (mV·dec−1) | Current Density at 2 V vs. RHE b (mA·cm−2) | O2 Production c (μmol) | Faradaic Efficiency for O2 Production (%) | Faradaic Efficiency for H2 Production (%) |
|---|---|---|---|---|---|---|
| Mn2O3-SC-TT | 1.61 | 125 | 0.58 | 14 | 78 | 86 |
| Mn2O3-SC | 1.58 | 134 | 0.44 | 12 | 95 | 97 |
| Mn3O4-SC | 1.75 | 185 | 0.11 | - | - | - |
| ED5c | 1.58 | 115 | 1.25 | 48 | 85 | 85 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, S.; Ottone, C.; Varetti, S.; Fontana, M.; Pugliese, D.; Saracco, G.; Bonelli, B.; Armandi, M. Spin-Coated vs. Electrodeposited Mn Oxide Films as Water Oxidation Catalysts. Materials 2016, 9, 296. https://doi.org/10.3390/ma9040296
Hernández S, Ottone C, Varetti S, Fontana M, Pugliese D, Saracco G, Bonelli B, Armandi M. Spin-Coated vs. Electrodeposited Mn Oxide Films as Water Oxidation Catalysts. Materials. 2016; 9(4):296. https://doi.org/10.3390/ma9040296
Chicago/Turabian StyleHernández, Simelys, Carminna Ottone, Sara Varetti, Marco Fontana, Diego Pugliese, Guido Saracco, Barbara Bonelli, and Marco Armandi. 2016. "Spin-Coated vs. Electrodeposited Mn Oxide Films as Water Oxidation Catalysts" Materials 9, no. 4: 296. https://doi.org/10.3390/ma9040296
APA StyleHernández, S., Ottone, C., Varetti, S., Fontana, M., Pugliese, D., Saracco, G., Bonelli, B., & Armandi, M. (2016). Spin-Coated vs. Electrodeposited Mn Oxide Films as Water Oxidation Catalysts. Materials, 9(4), 296. https://doi.org/10.3390/ma9040296











