Integrated Utilization of Sewage Sludge and Coal Gangue for Cement Clinker Products: Promoting Tricalcium Silicate Formation and Trace Elements Immobilization
Abstract
:1. Introduction
2. Experimental Section
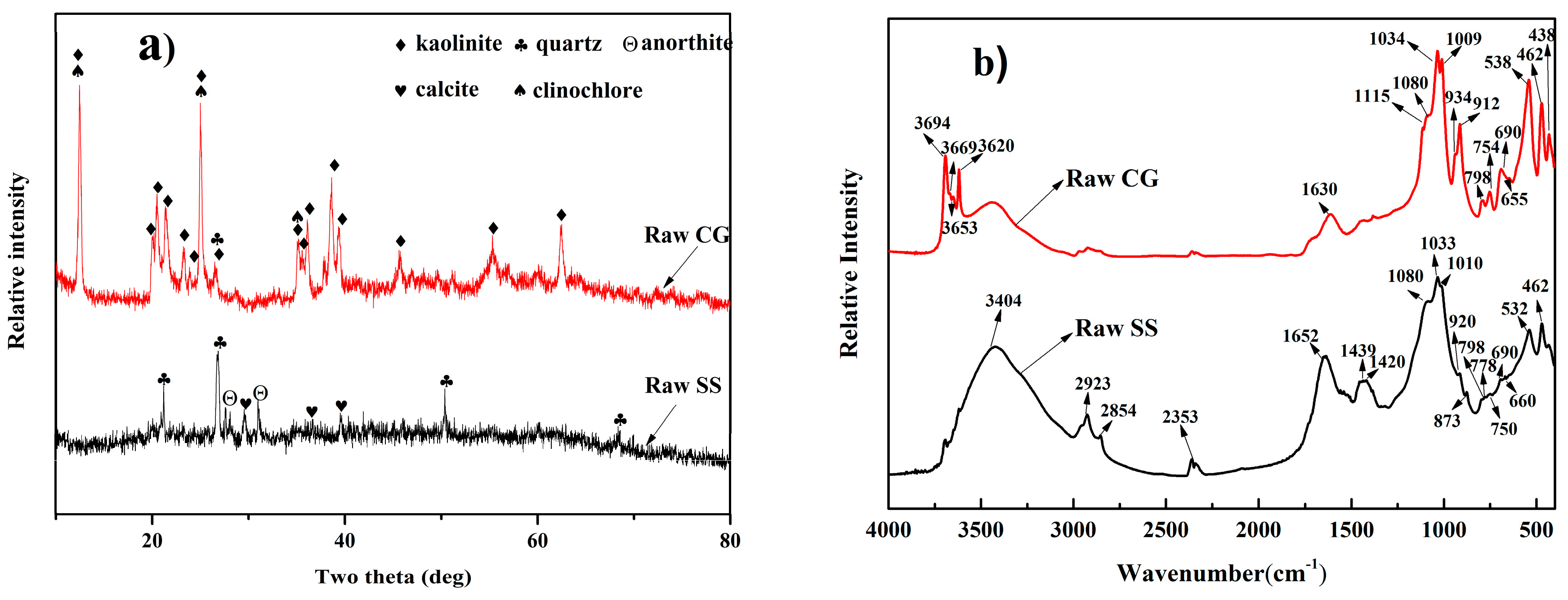
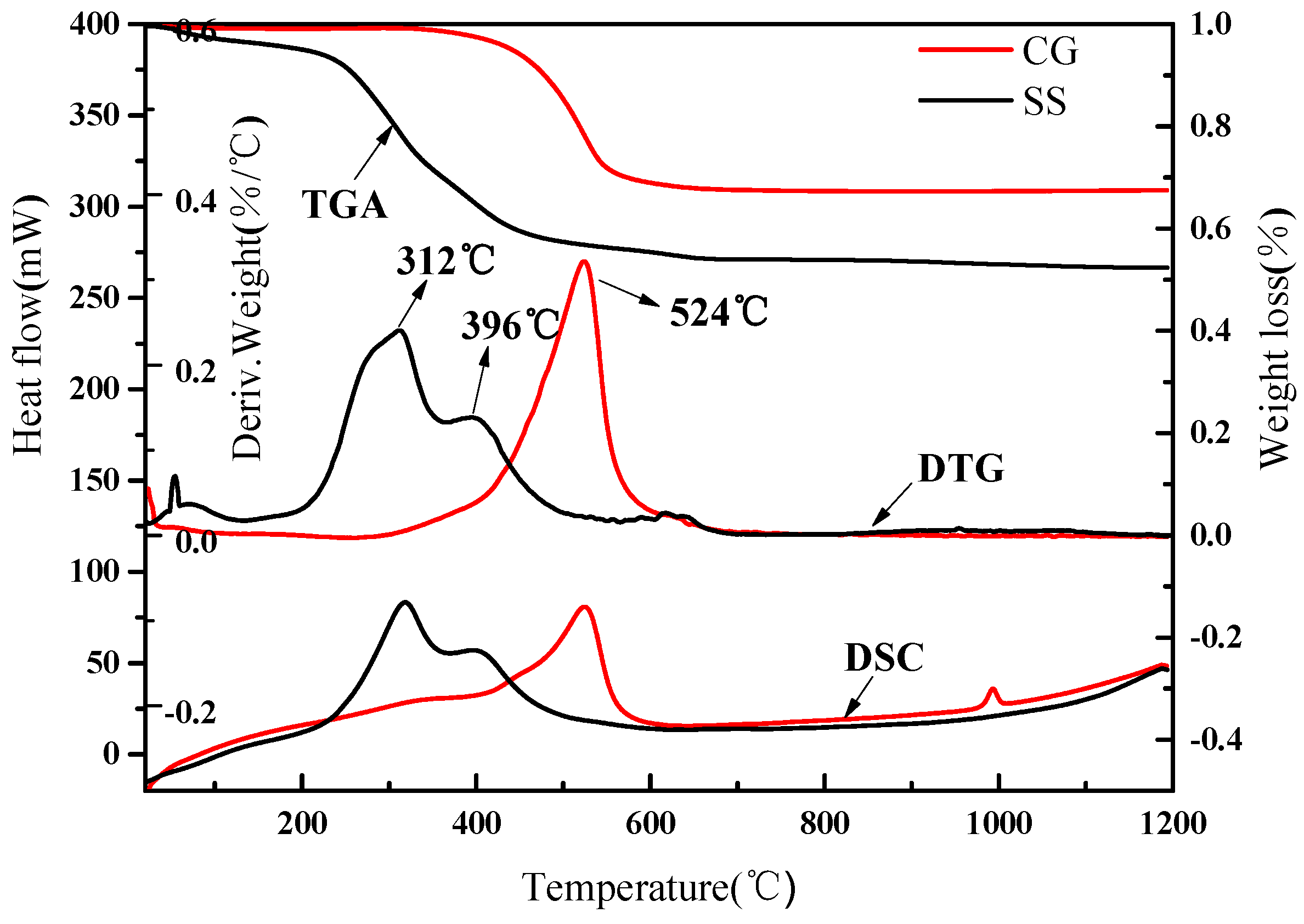
2.1. Materials Characterization
2.2. Eco-Cement Clinkers Preparation
2.3 Trace Element Immobilized Characterization
3. Results and Discussion
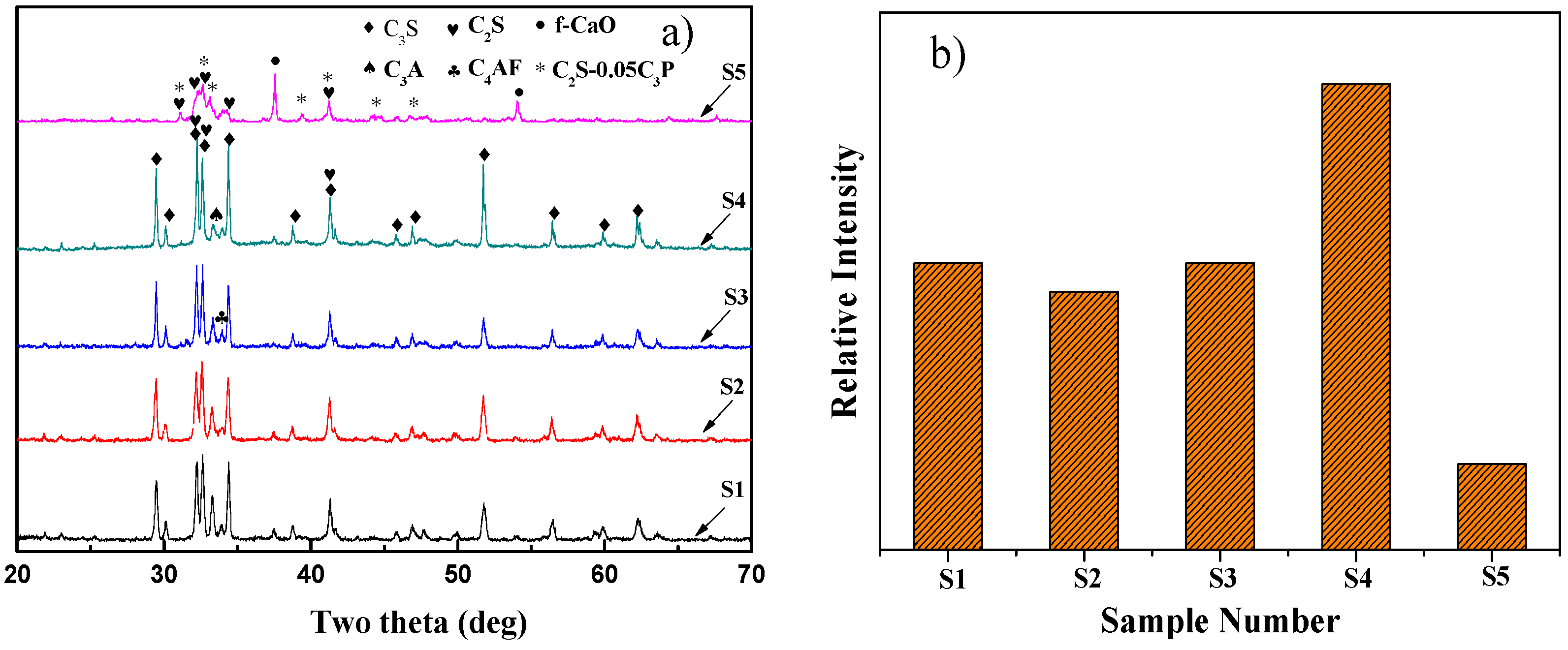
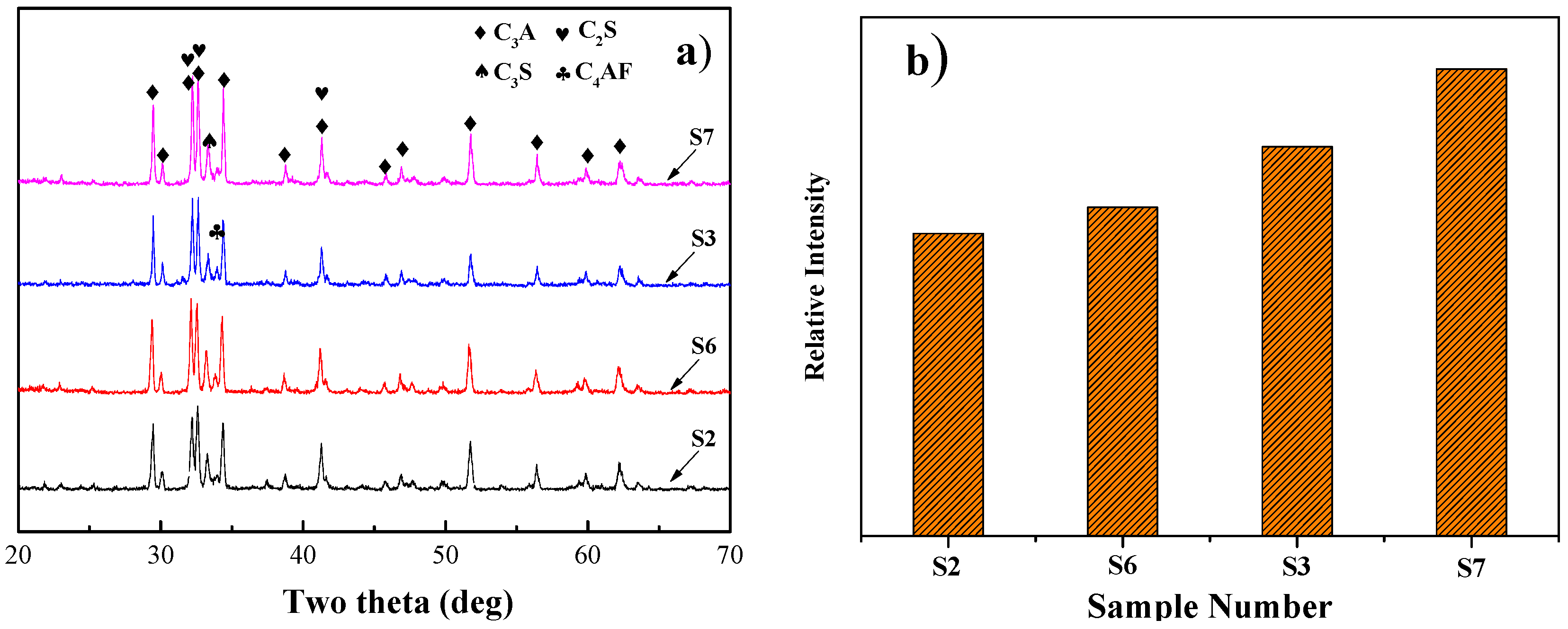
3.1. Mineralogical Characterization of the Eco-Cement Clinker
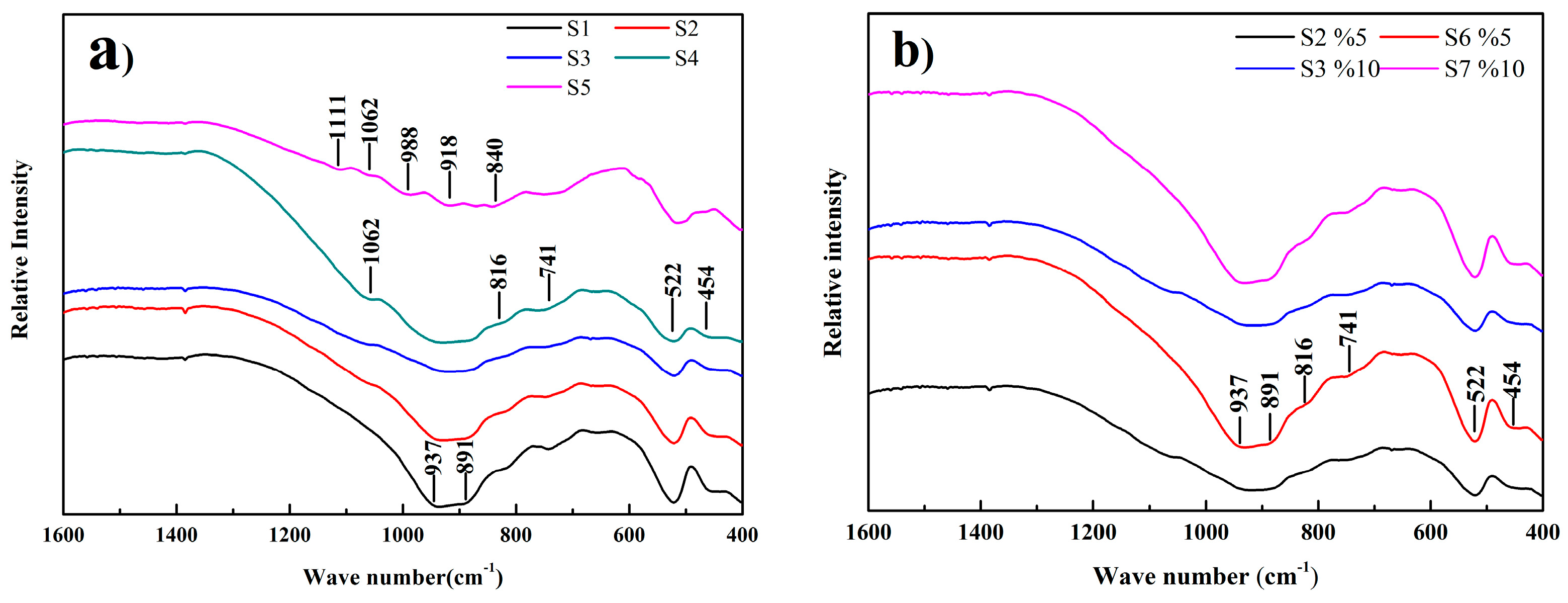
3.2. Structure Analysis of the Eco-Cement Clinker
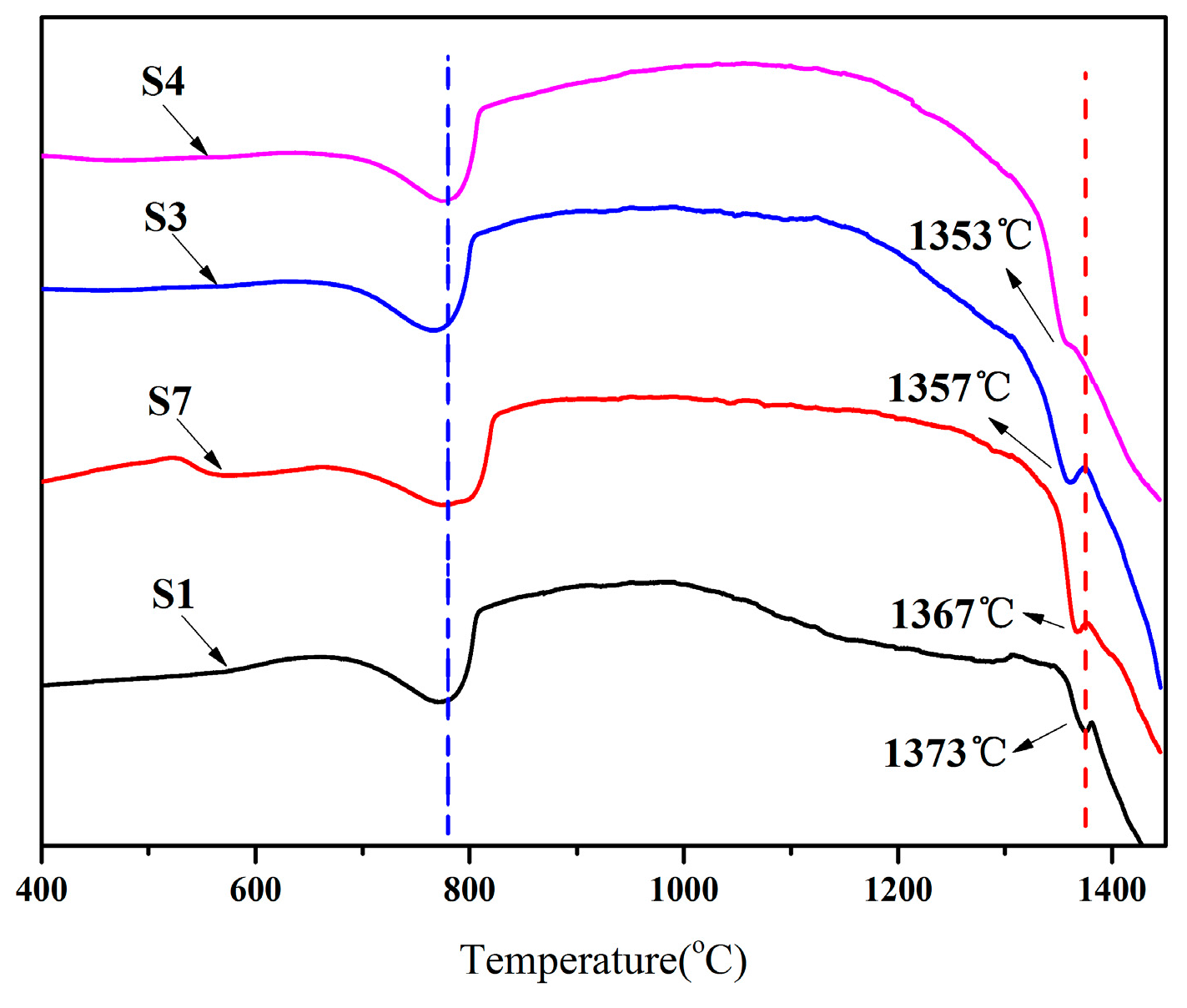
3.3. Liquid Phase Formation during Clinker Calcination Process
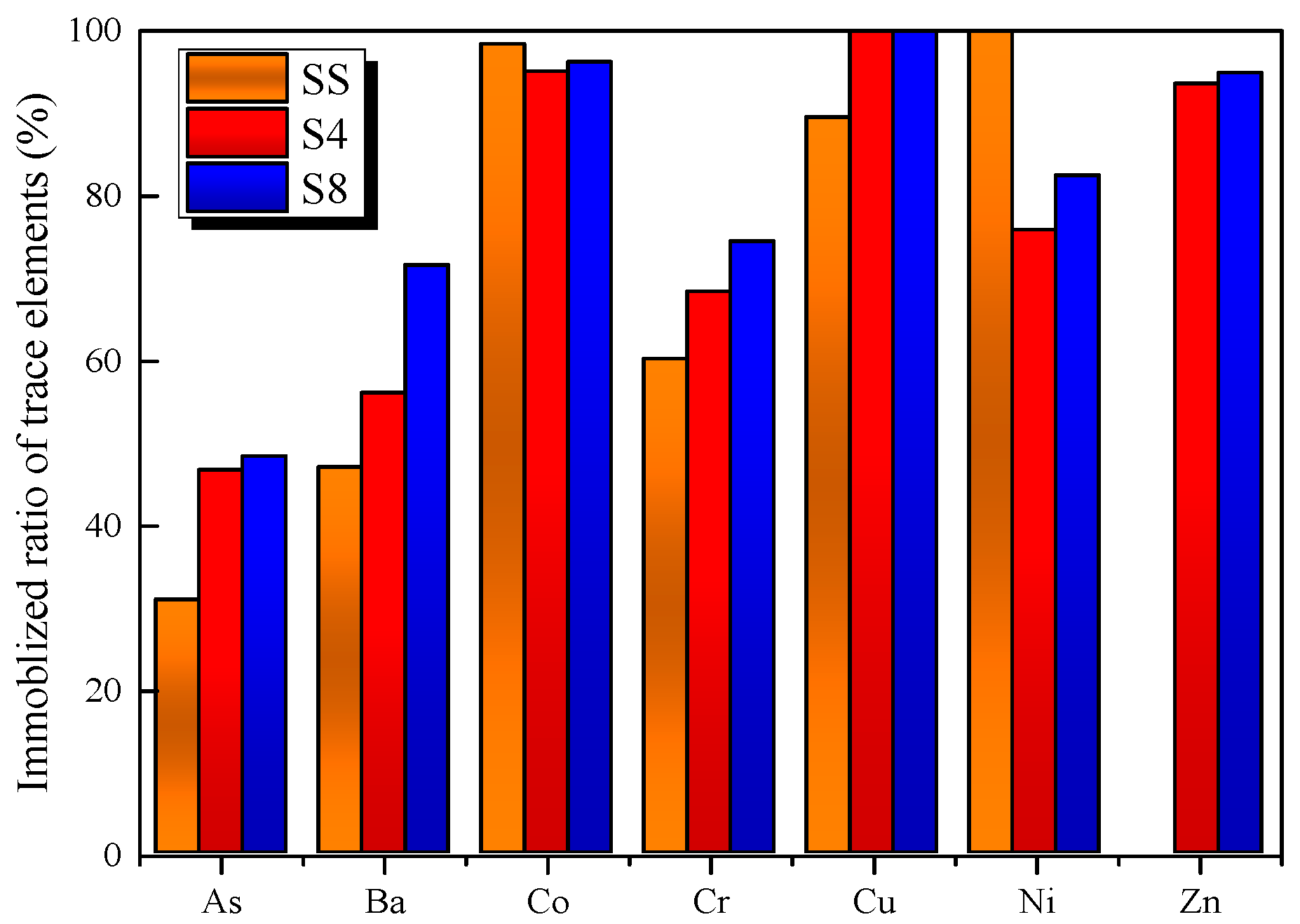
3.4. The Immobilization Behavior of Traces Elements
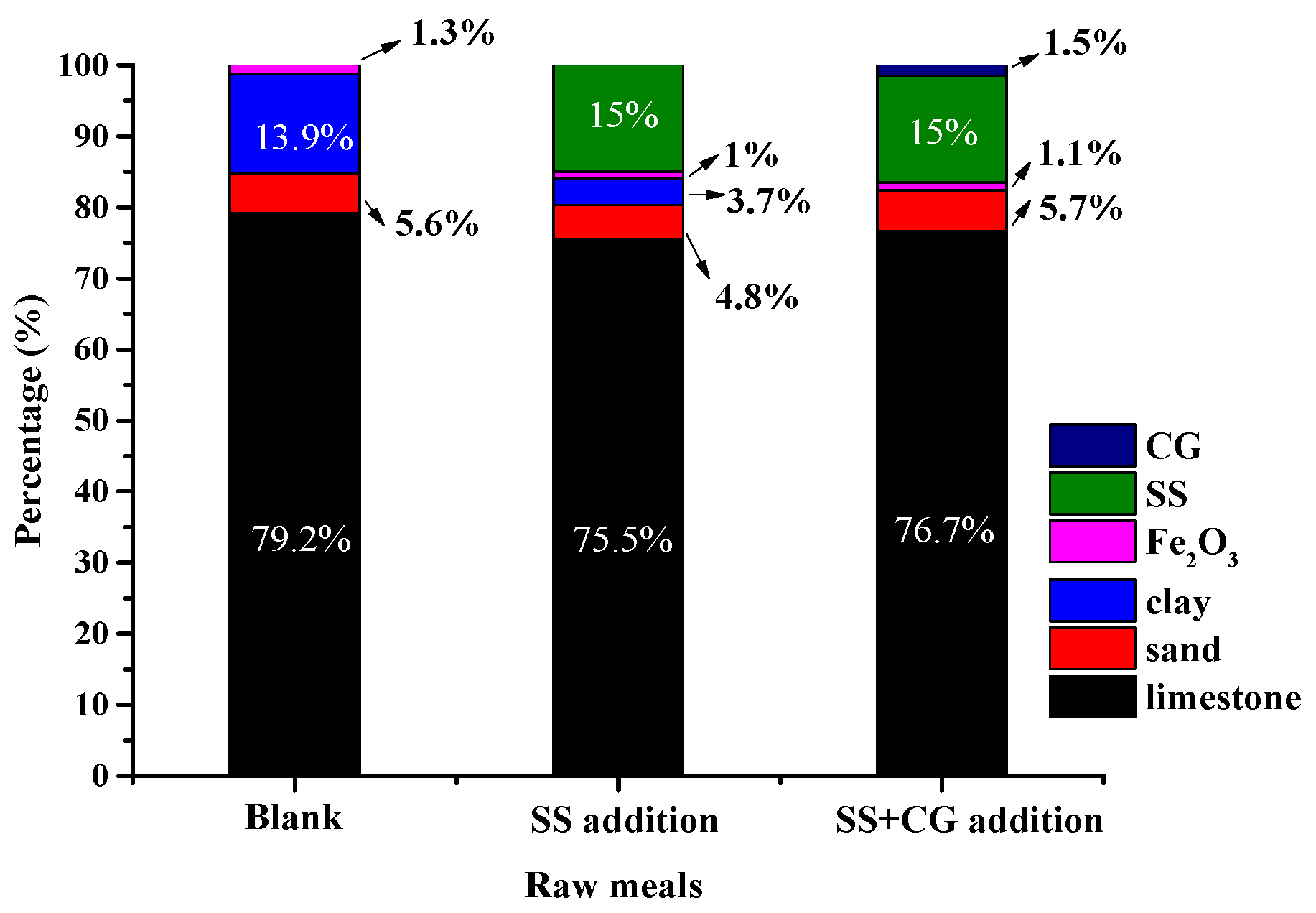
3.5. Energy and Material Balances with SS and CG Addition
4. Conclusions
- Appropriate SS and CG addition could be beneficial to C3S formation while excess SS addition had the negative effect. The decomposition of C3S into C2S and f-CaO was found to occur as the SS addition was increased to 30 wt % and the amount of SS blending in the raw meals should be strictly controlled. The positive effect of SS and CG additions for C3S formation can be attributed to different mechanisms. The former could be described by introducing a mass of impurities with low melting point, which could lead to decreasing the temperature of liquid phase formation while the special structure of CG could make it easier for the reaction between f-CaO and C2S. In addition, the raw meals with CG addition could boost more C3S appearance compared with SS.
- SS could lead to the modification of C3S polymorphism from the relatively asymmetric structure to more symmetrical structure (R) with increasing SS addition. As SS addition is increased to 15 wt %, the crystal structure of C3S becomes monoclinic (M).
- It was found that clinkers had a good effect on immobilizing most of the trace elements in SS, especially Zn. CG can also help stabilize trace elements during the calcinations process. The TCLP results show that the eco-cement clinkers met the standards of the current Chinese regulatory thresholds.
- Integrated utilization of SS and CG as alternative raw materials and fuel in cement manufacture could effectively reduce raw material and energy consumption. They are expected to reduce energy consumption by more than 60% as well as reduce a fair amount of the natural raw material consumption according to the present estimation.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hong, J.M.; Xu, C.Q.; Hong, J.L.; Tan, X.F.; Chen, W. Life cycle assessment of sewage sludge co-incineration in a coal-based power station. Waste Manag. 2013, 33, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Folgueras, M.B.; Alonso, M.; Diaz, R.M. Influence of sewage sludge treatment on pyrolysis and combustion of dry sludge. Energy 2013, 55, 426–435. [Google Scholar] [CrossRef]
- Murakami, T.; Suzuki, Y.; Nagasawa, H.; Yamamoto, T.; Koseki, T.; Hirose, H.; Okamoto, S. Combustion characteristics of sewage sludge in an incineration plant for energy recovery. Fuel Process Technol. 2009, 90, 778–783. [Google Scholar] [CrossRef]
- Zhou, H.B.; Ma, C.; Gao, D.; Chen, T.B.; Zheng, G.D.; Chen, J.; Pan, T.H. Application of a recyclable plastic bulking agent for sewage sludge composting. Bioresour. Technol. 2014, 152, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Querol, X.; Izquierdo, M.; Monfort, E.; Alvarez, E.; Font, O.; Moreno, T.; Alastuey, A.; Zhuang, X.; Lu, W.; Wang, Y. Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int. J. Coal Geol. 2008, 75, 93–104. [Google Scholar] [CrossRef]
- Zhou, C.C.; Liu, G.J.; Wu, D.; Fang, T.; Wang, R.W.; Fan, X. Mobility behavior and environmental implications of trace elements associated with coal gangue: A case study at the Huainan Coalfield in China. Chemosphere 2014, 95, 193–199. [Google Scholar]
- Zhou, C.C.; Liu, G.J.; Yan, Z.C.; Fang, T.; Wang, R.W. Transformation behavior of mineral composition and trace elements during coal gangue combustion. Fuel 2012, 97, 644–650. [Google Scholar] [CrossRef]
- Conesa, J.A.; Rey, L.; Egea, S.; Rey, M.D. Pollutant formation and emissions from cement kiln stack using a solid recovered fuel from municipal solid waste. Environ. Sci. Technol. 2011, 45, 5878–5884. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Zhou, S.Q.; Li, F.Z.; Lin, Y.X. Utilization of municipal sewage sludge as additives for the production of eco-cement. J. Hazard. Mater. 2012, 213, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Pavsic, P.; Mladenovic, A.; Mauko, A.; Kramar, S.; Dolenec, M.; Voncina, E.; Vrtac, K.P.; Bukovec, P. Sewage sludge/biomass ash based products for sustainable construction. J. Clean. Prod. 2014, 67, 117–124. [Google Scholar] [CrossRef]
- Saikia, N.; Kato, S.; Kojima, T. Production of cement clinkers from municipal solid waste incineration (MSWI) fly ash. Waste Manag. 2007, 27, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Tseng, D.H.; Lin, T.T. Characterization of eco-cement paste produced from waste sludges. Chemosphere 2011, 84, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.F.W. Cement chemistry. In Chemistry for Engineers; Academic Press: London, UK, 1990. [Google Scholar]
- Cui, S.P.; Lan, M.Z.; Zhang, J.; Wang, C.Y. Effect and incorporation mechanism of heavy metal elements in hazardous industrial wastes during clinker formation. J. Chin. Ceram. Soc. 2004, 32, 1264–1270. [Google Scholar]
- Fan, H.J.; Zhou, H.; Wang, J. Pyrolysis of municipal sewage sludges in a slowly heating and gas sweeping fixed-bed reactor. Energy Convers. Manag. 2014, 88, 1151–1158. [Google Scholar] [CrossRef]
- Yang, N.R.; Yue, W.H. The Handbook of Inorganic Matalloid Materials Atlas; Wuhan University of Technology Press: Wuhan, China, 2000. (In Chinese) [Google Scholar]
- Zhang, Y.Y.; Nakano, J.; Liu, L.L.; Wang, X.D.; Zhang, Z.T. Trace element partitioning behavior of coal gangue-fired CFB plant: Experimental and equilibrium calculation. Environ. Sci. Pollut. Res. 2015, 22, 15469–15478. [Google Scholar] [CrossRef] [PubMed]
- Percival, H.J.; Duncan, J.F.; Foster, P.K. Interpretation of the kaolinite-mullite reaction sequence from infrared absorption spectra. J. Am. Ceram. Soc. 2006, 57, 57–61. [Google Scholar] [CrossRef]
- Liu, G.R.; Song, H.J.; Wu, J.H. Thermogravimetric study and kinetic analysis of dried industrial sludge pyrolysis. Waste Manag. 2015, 41, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.H.; Martinez-Ramirez, S.; Blanco-Varela, M.T.; Donatello, S.; Guillem, M.; Puig, J.; Fos, C.; Larrotcha, E.; Flores, J. The effect of using thermally dried sewage sludge as an alternative fuel on Portland cement clinker production. J. Clean. Prod. 2013, 52, 94–102. [Google Scholar] [CrossRef]
- El-Rjoob, A.W.O.; Massadeh, A.M.; Omari, M.N. Evaluation of Pb, Cu, Zn, Cd, Ni and Fe levels in rosmarinus officinalis labaiatae (rosemary) medicinal plant and soils in selected zones in Jordan. Environ. Monit. Assess. 2008, 140, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Xu, W.; Yang, X.J.; Wu, J.G. Preparation of Portland cement with sugar filter mud as lime-based raw material. J. Clean. Prod. 2014, 66, 107–112. [Google Scholar] [CrossRef]
- Xu, W.; Xu, J.C.; Liu, J.; Li, H.X.; Cao, B.; Huang, X.F.; Li, G.M. The utilization of lime-dried sludge as resource for producing cement. J. Clean. Prod. 2014, 83, 286–293. [Google Scholar] [CrossRef]
- Bigare, M.; Guinier, A.; Mazieres, C.; Regourd, M.; Yannaqui, N.; Eysel, W.; Hahn, T.; Woermann, E. Polymorphism of tricalcium silicate and its solid solutions. J. Am. Ceram. Soc. 1967, 50, 609–619. [Google Scholar] [CrossRef]
- Urabe, K.; Nakano, H.; Morita, H. Structural modulations in monoclinic tricalcium silicate solid solutions doped with zinc oxide, M(i), M(ii), and M(iii). J. Am. Ceram. Soc. 2002, 85, 423–429. [Google Scholar] [CrossRef]
- Horvath, E.; Frost, R.L.; Mako, E.; Kristof, J.; Cseh, T. Thermal treatment of mechanochemically activated kaolinite. Thermochim. Acta 2003, 404, 227–234. [Google Scholar] [CrossRef]
- Qiu, G.H.; Luo, Z.Y.; Shi, Z.L.; Ni, M.J. Utilization of coal gangue and copper tailings as clay for cement clinker calcinations. J. Wuhan Univ. Technol. 2011, 26, 1205–1210. [Google Scholar] [CrossRef]
- Diouri, A.; Boukhari, A.; Aride, J.; Puertas, F.; Vazquez, T. Stable Ca3SiO5 solid solution containing manganese and phosphorus. Cem. Concr. Res. 1997, 27, 1203–1212. [Google Scholar] [CrossRef]
- Rodriguez, N.H.; Martinez-Ramirez, S.; Blanco-Varela, M.T.; Guillem, M.; Puig, J.; Larrotcha, E.; Flores, J. Evaluation of spray-dried sludge from drinking water treatment plants as a prime material for clinker manufacture. Cem. Concr. Comp. 2011, 33, 267–275. [Google Scholar] [CrossRef]
- Elkhadiri, I.; Diouri, A.; Boukhari, A.; Puertas, F.; Vazquez, T. Obtaining a sulfoaluminate belite cement by industrial waste. Mater. Construcc. 2003, 53, 57–69. [Google Scholar]
- Kakali, G.; Parissakis, G. Investigation of the effect of Zn oxide on the formation of Portland-cement clinker. Cem. Concr. Res. 1995, 25, 79–85. [Google Scholar] [CrossRef]
- Kakali, G.; Parissakis, G.; Bouras, D. A study on the burnability and the phase formation of PC clinker containing Cu oxide. Cem. Concr. Res. 1996, 26, 1473–1478. [Google Scholar] [CrossRef]
- Masaki, K.; Maki, I. Effect of prolonged heating at elevated temperatures on the phase composition and textures of Portland cement clinker. Cem. Concr. Res. 2002, 32, 931–934. [Google Scholar] [CrossRef]
- Tian, H.Z.; Liu, K.Y.; Zhou, J.R.; Lu, L.; Hao, J.M.; Qiu, P.P.; Gao, J.J.; Zhu, C.Y.; Wang, K.; Hua, S.B. Atmospheric emission inventory of hazardous trace elements from China’s coal-fired power plants-temporal trends and spatial variation characteristics. Environ. Sci. Technol. 2014, 48, 3575–3582. [Google Scholar] [CrossRef] [PubMed]
- Swaine, D.J. Why trace elements are important. Fuel Process. Technol. 2000, 65, 21–33. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, L.; Wang, X. Incorporation features of Cu~(2+),Zn~(2+),Cr~(6+),Cd~(2+) and Pb~(4+) ions in cement clinker. In Proceeding of the 8th International Symposium on Cement & Concrete (ISCC2013), Nanjing, China, 20 September 2013. (In Chinese)
- Folgueras, A.B.; Diaz, R.A.; Xiberta, J.; Prieto, I. Volatilisation of trace elements for coal-sewage sludge blends during their combustion. Fuel 2003, 82, 1939–1948. [Google Scholar] [CrossRef]
- Stephan, D.; Mallmann, R.; Knofel, D.; Hardtl, R. High intakes of Cr, Ni, and Zn in clinker Part I. Influence on burning process and formation of phases. Cem. Concr. Res. 1999, 29, 1949–1957. [Google Scholar] [CrossRef]
- Barros, A.M.; Tenorio, J.A.S.; Espinosa, D.C.R. Evaluation of the incorporation ratio of ZnO, PbO and CdO into cement clinker. J. Hazard. Mater. 2004, 112, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Yang, G. Promoting the adsorption of metal ions on kaolinite by defect sites: A molecular dynamics study. Sci. Rep. 2015, 5, 14377–14389. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Habert, G.; Billard, C.; Rossi, P.; Chen, C.; Roussel, N. Cement production technology improvement compared to factor 4 objectives. Cem. Concr. Res. 2010, 40, 820–826. [Google Scholar] [CrossRef]
| Proximate Analysis (wt %) | Ultimate Analysis (wt %) | Calorific Value (MJ/kg) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | moisture, ad a | ash, ad a | volatile matter, ad a | fixed carbon, ad a | Total | C, ad | H, ad | N, ad | S, ad | - | ||||||||||
| CG | 0.8 | 67.9 | 15 | 16.3 | 100 | 17.5 | 12.6 | 0.6 | 1.2 | 4.82 | ||||||||||
| SS | 2 | 53.3 | 35.8 | 8.9 | 100 | 23.2 | 4.0 | 3.0 | 1.3 | 9.35 | ||||||||||
| Chemical Composition (wt %) | ||||||||||||||||||||
| - | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | P2O5 | Others | LOI | Total | |||||||||
| CG | 32.8 | 28.8 | 1.2 | 0.1 | <0.1 | 0.1 | <0.1 | 0.1 | 2.5 | 34.4 | 100 | |||||||||
| SS | 25.2 | 5.6 | 4.2 | 6.4 | 1.5 | 1.2 | 0.6 | 4.9 | 1.4 | 48.6 | 100 | |||||||||
| Trace Elements Contents (mg/kg) | ||||||||||||||||||||
| - | As | Ba | Cd | Co | Cr | Cu | Mn | Ni | Pb | Zn | ||||||||||
| CG | 19 | ND | ND | 8 | 25 | 15 | 6 | 4 | 9 | 14 | ||||||||||
| SS | 50 | 256 | 2 | 11 | 124 | 358 | 543 | 41 | 48 | 1084 | ||||||||||
| Sample | Addition Amount (%) | |
|---|---|---|
| SS | CG | |
| S1(Blank sample) | 0 | 0 |
| S2 | 5 | 0 |
| S3 | 10 | 0 |
| S4 | 15 | 0 |
| S5 | 30 | 0 |
| S6 | 0 | 5 |
| S7 | 0 | 10 |
| S8 | 15 | 5 |
| Sample | Elements (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Ba | Cd | Co | Cr | Cu | Ni | Pb | Zn | |
| S4 | ND | 1 | ND | ND | ND | ND | ND | ND | 0.06 |
| S8 | ND | 2.13 | ND | ND | ND | ND | ND | ND | ND |
| GB 5085.3-2007 | 5 | 100 | 1 | ~ | 15 | 100 | 5 | 5 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhang, Y.; Liu, L.; Seetharaman, S.; Wang, X.; Zhang, Z. Integrated Utilization of Sewage Sludge and Coal Gangue for Cement Clinker Products: Promoting Tricalcium Silicate Formation and Trace Elements Immobilization. Materials 2016, 9, 275. https://doi.org/10.3390/ma9040275
Yang Z, Zhang Y, Liu L, Seetharaman S, Wang X, Zhang Z. Integrated Utilization of Sewage Sludge and Coal Gangue for Cement Clinker Products: Promoting Tricalcium Silicate Formation and Trace Elements Immobilization. Materials. 2016; 9(4):275. https://doi.org/10.3390/ma9040275
Chicago/Turabian StyleYang, Zhenzhou, Yingyi Zhang, Lili Liu, Seshadri Seetharaman, Xidong Wang, and Zuotai Zhang. 2016. "Integrated Utilization of Sewage Sludge and Coal Gangue for Cement Clinker Products: Promoting Tricalcium Silicate Formation and Trace Elements Immobilization" Materials 9, no. 4: 275. https://doi.org/10.3390/ma9040275
APA StyleYang, Z., Zhang, Y., Liu, L., Seetharaman, S., Wang, X., & Zhang, Z. (2016). Integrated Utilization of Sewage Sludge and Coal Gangue for Cement Clinker Products: Promoting Tricalcium Silicate Formation and Trace Elements Immobilization. Materials, 9(4), 275. https://doi.org/10.3390/ma9040275





