Deposition of Antimicrobial Copper-Rich Coatings on Polymers by Atmospheric Pressure Jet Plasmas
Abstract
:1. Introduction
2. Results and Discussion
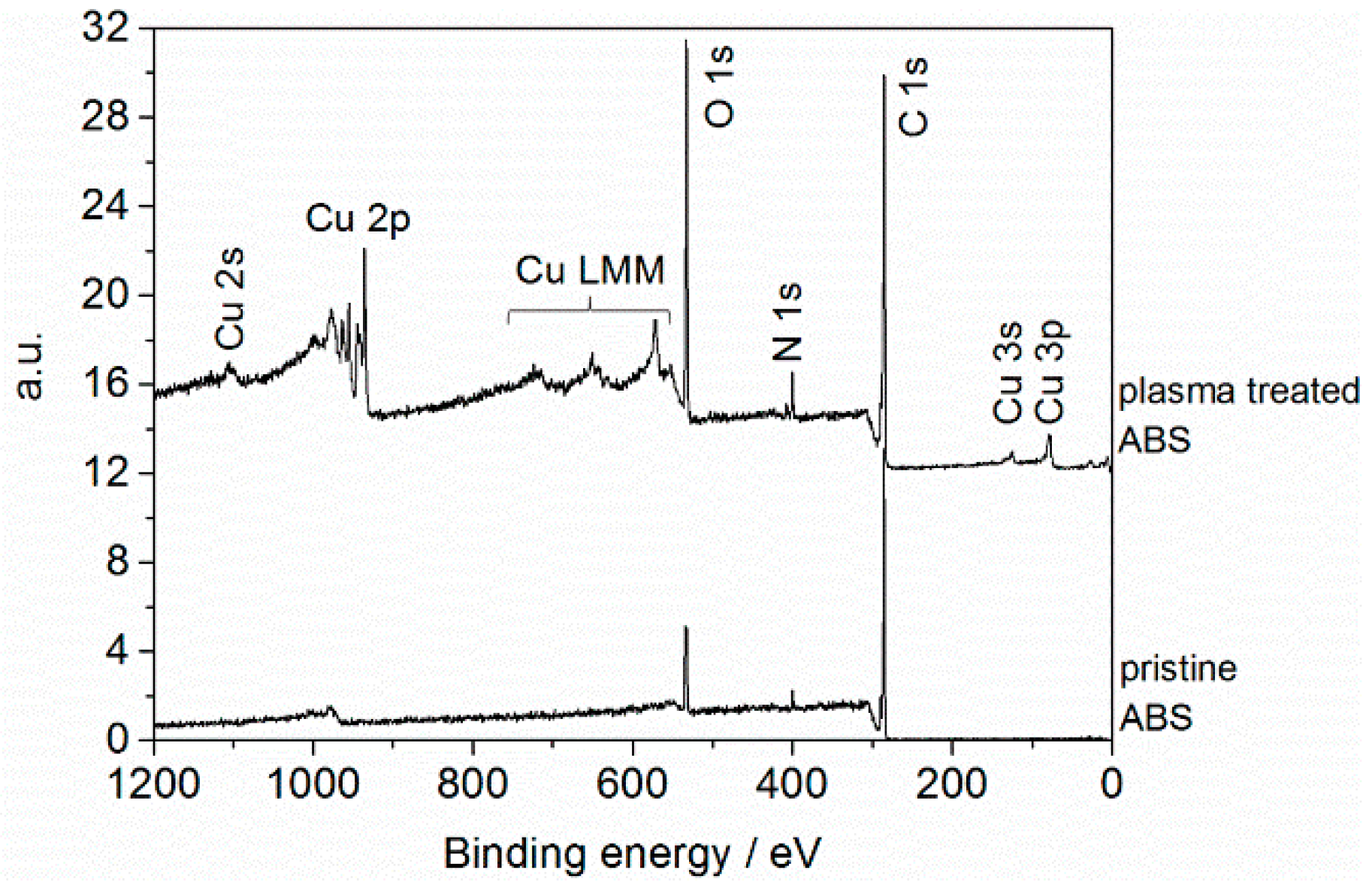
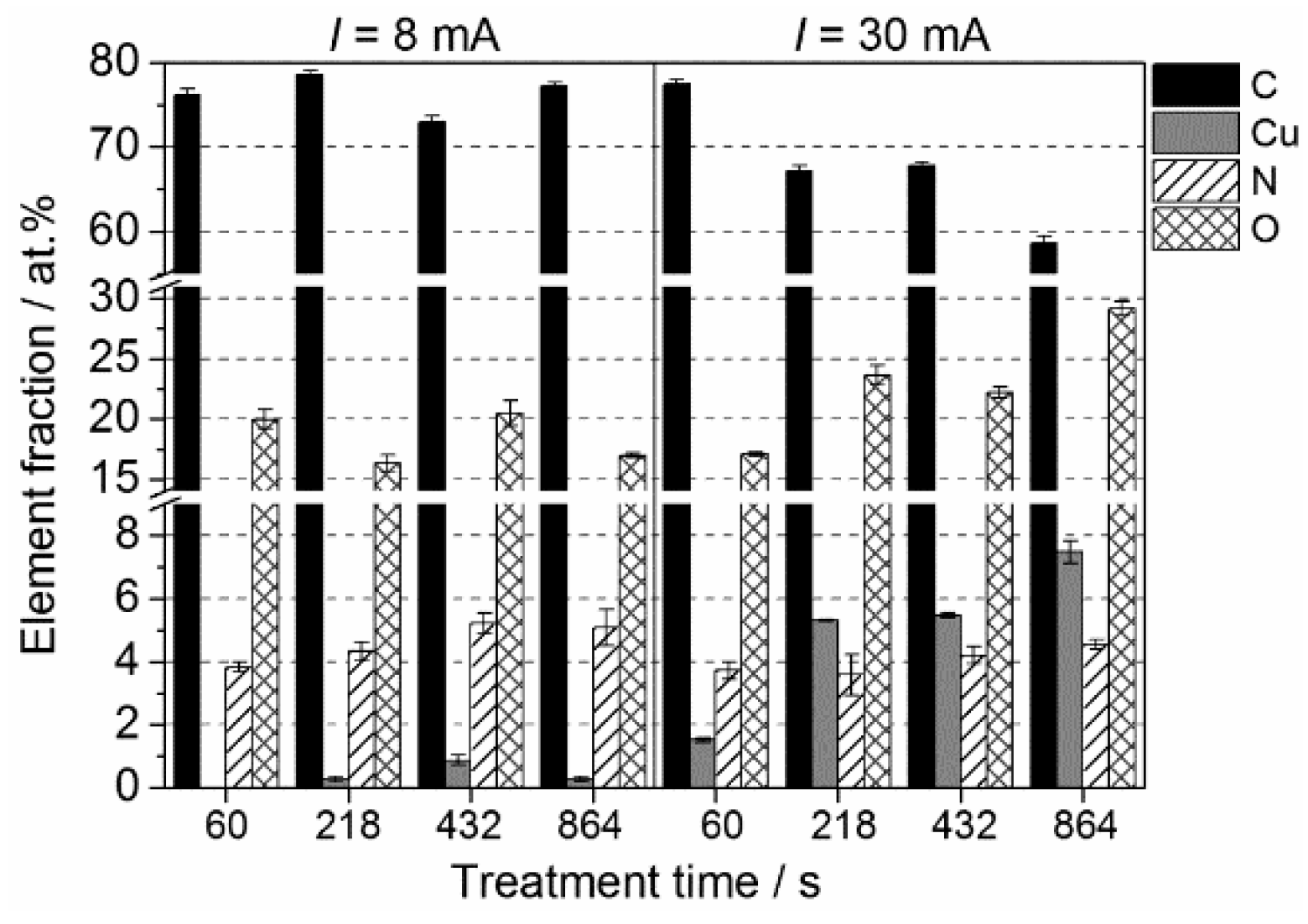
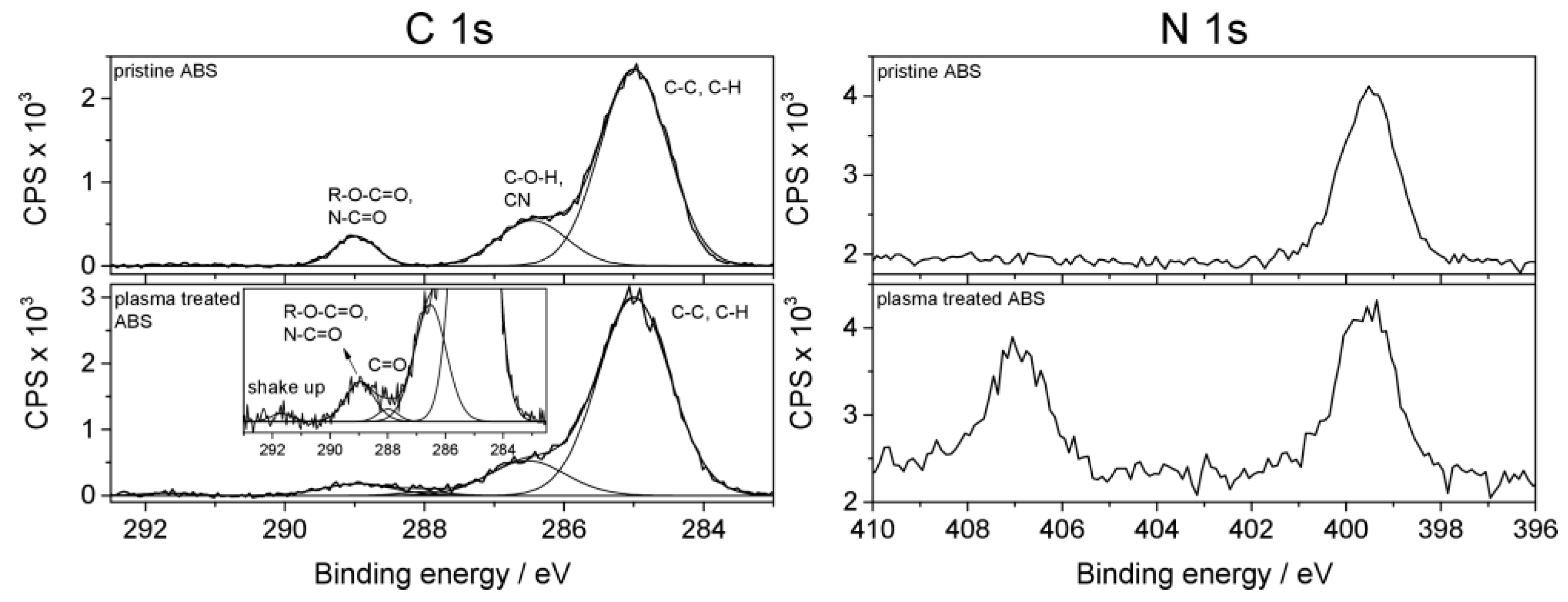
2.1. Chemical Characteristics
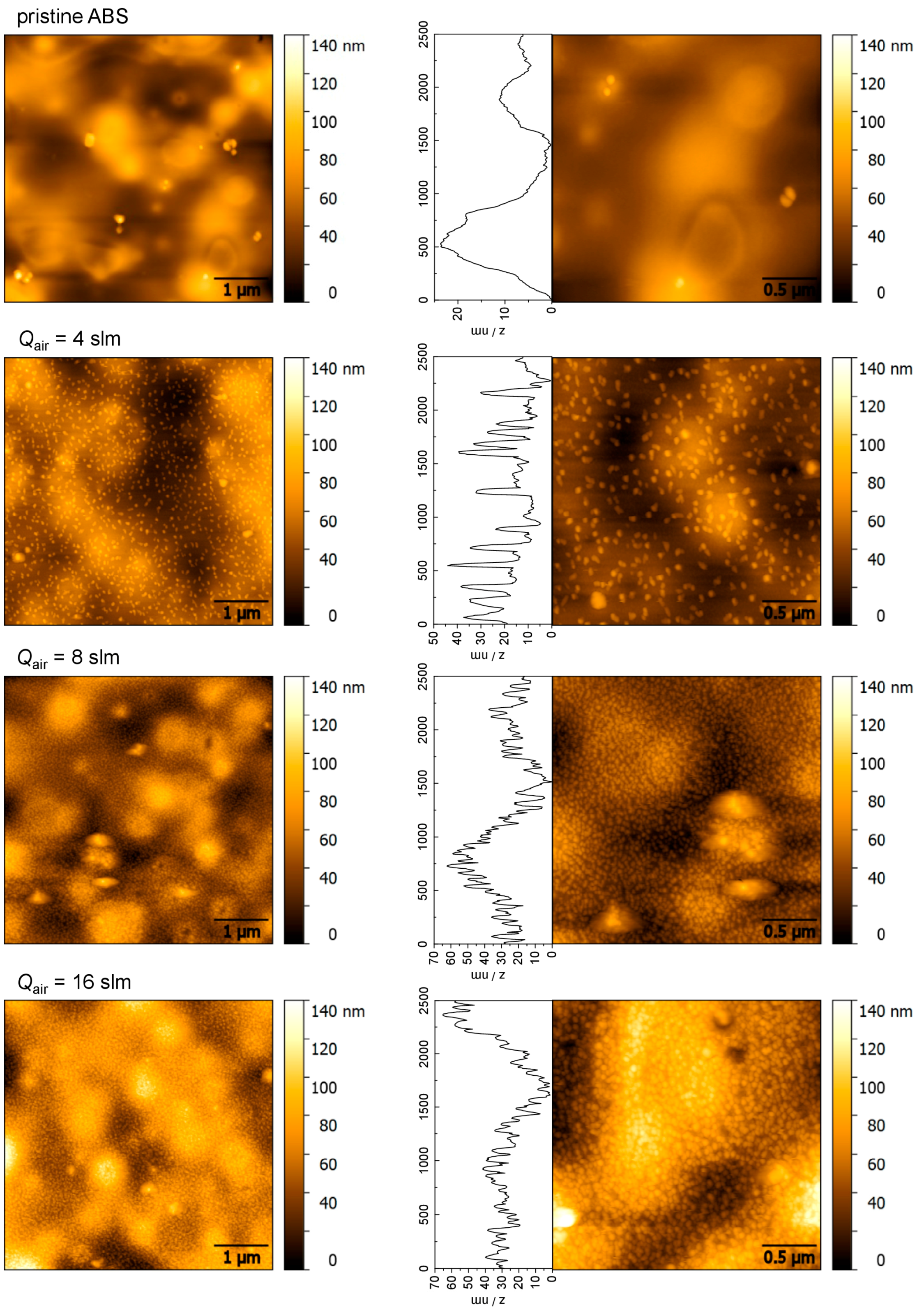
2.2. Topographical Characteristics
2.3. Antimicrobial Results
3. Materials and Methods
3.1. Polymeric Material
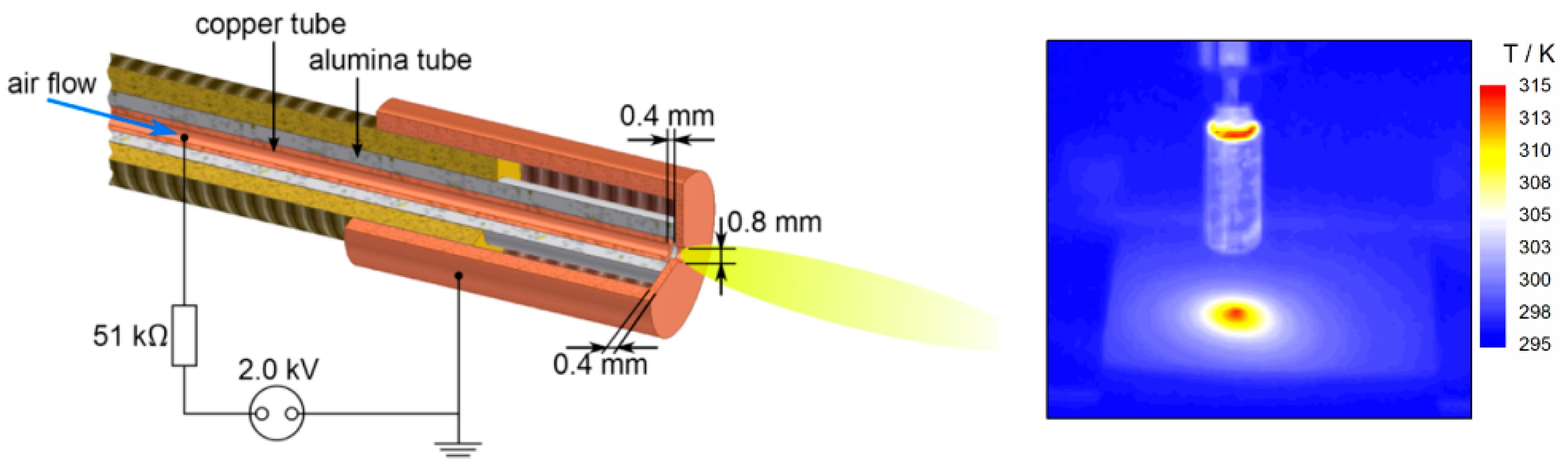
3.2. Plasma Source and Plasma Treatment
3.3. Surface Analysis
3.4. Antimicrobial Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABS | acrylonitrile butadiene styrene |
| DC | direct current |
| XPS | X-ray photoelectron spectroscopy |
| AFM | atomic force microscopy |
| EPA | Environmental Protection Agency |
| PVD | physical vapor deposition |
| CVD | chemical vapor deposition |
| slm | standard liter per minute |
| S. aureus | Staphylococcus aureus |
References
- Carpentier, B.; Cerf, O. Biofilms and their consequences, with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 1993, 75, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.S. Preventing health care—Associated infections. In Patient Safety and Quality: An Evidence-Based Handbook for Nurses; Hughes, R.G., Ed.; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. [Google Scholar]
- Kramer, A.; Assadian, O. Survival of microorganisms on inanimate surfaces. In Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections; Borkow, G., Ed.; Springer International Publishing: Berlin, Germany, 2014; pp. 7–26. [Google Scholar]
- Marchetti, A.; Rossiter, R. Economic burden of healthcare-associated infection in US acute care hospitals: Societal perspective. J. Med. Econ. 2013, 16, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the Antibiotic Era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Hedberg, Y.; Tornberg, M.; De Battice, L.; Svedhem, S.; Wallinder, I.O. Cell membrane damage and protein interaction induced by copper containing nanoparticles—Importance of the metal release process. Toxicology 2013, 313, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Pierson, J.F.; Wiederkehr, D.; Billard, A. Reactive magnetron sputtering of copper, silver, and gold. Thin Solid Films 2005, 478, 196–205. [Google Scholar] [CrossRef]
- Polak, M.; Ohl, A.; Quaas, M.; Lukowski, G.; Luthen, F.; Weltmann, K.D.; Schröder, K. Oxygen and water plasma-immersion ion implantation of copper into titanium for antibacterial surfaces of medical implants. Adv. Eng. Mater. 2010, 12, B511–B518. [Google Scholar] [CrossRef]
- Cohen, S.L.; Liehr, M.; Kasi, S. Mechanisms of copper chemical vapor-deposition. Appl. Phys. Lett. 1992, 60, 50–52. [Google Scholar] [CrossRef]
- Beier, O.; Pfuch, A.; Horn, K.; Weisser, J.; Schnabelrauch, M.; Schimanski, A. Low temperature deposition of antibacterially active silicon oxide layers containing silver nanoparticles, prepared by atmospheric pressure plasma chemical vapor deposition. Plasma Process. Polym. 2013, 10, 77–87. [Google Scholar] [CrossRef]
- Fanelli, F.; Fracassi, F. Aerosol-assisted atmospheric pressure cold plasma deposition of organic-inorganic nanocomposite coatings. Plasma Chem. Plasma Process. 2014, 34, 473–487. [Google Scholar] [CrossRef]
- Kredl, J.; Drache, S.; Quade, A.; Polak, M.; Muller, S.; Peglow, S.; Hippler, R.; Kolb, J.F. DC operated air plasma jet for antimicrobial copper coatings on temperature labile surfaces. IEEE Trans. Plasma Sci. 2014, 42, 2756–2757. [Google Scholar] [CrossRef]
- Lazea-Stoyanova, A.; Vlad, A.; Vlaicu, A.M.; Teodorescu, V.S.; Dinescu, G. Synthesis of copper particles by non-thermal atmospheric pressure plasma jet. Plasma Process. Polym. 2015, 12, 705–709. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mohamed, A.A.H.; Price, R.O.; Swanson, R.J.; Bowman, A.; Chiavarini, R.L.; Stacey, M.; Schoenbach, K.H. Cold atmospheric pressure air plasma jet for medical applications. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Modjarrad, K.; Ebnesajjad, S. Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier Inc.: Philadelphia, PA, USA, 2014. [Google Scholar]
- Oepen, S.; Gottschalk, A. Opportunities with specialties: Styrene copolymers (ABS, ASA, SAN, MABS, and ABS Blends). Kunstst. Int. 2011, 10, 22–26. [Google Scholar]
- Milionis, A.; Languasco, J.; Loth, E.; Bayer, I.S. Analysis of wear abrasion resistance of superhydrophobic acrylonitrile butadiene styrene rubber (ABS) nanocomposites. Chem. Eng. J. 2015, 281, 730–738. [Google Scholar] [CrossRef]
- Hong, N.; Zhan, J.; Wang, X.; Stec, A.A.; Richard Hull, T.; Ge, H.; Xing, W.; Song, L.; Hu, Y. Enhanced mechanical, thermal and flame retardant properties by combining graphene nanosheets and metal hydroxide nanorods for acrylonitrile-butadiene-styrene copolymer composite. Compos. Part A 2014, 64, 203–210. [Google Scholar] [CrossRef]
- Wang, G.L.; Duan, L.; Ding, K.H.; Zhang, M. Property reinforcement of acrylonitrile-butadiene-styrene by simultaneous incorporation of carbon nanotubes and self-prepared copper particles. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Chen, J.; Feng, Y.; Chen, N.N.; Li, B.; Chen, F.Y.; Zhang, X.B. Fabrication and properties of silver-doped acrylonitrile butadiene styrene (ABS) composites. Polym. Plast. Technol. Eng. 2013, 52, 1081–1088. [Google Scholar] [CrossRef]
- Zendehnam, A.; Robatmili, N.; Hosseini, S.M.; Arabzadegan, M.; Madaeni, S.S. Fabrication of novel (acrylonitrile butadiene styrene/activated carbon/silver nanoparticles) heterogeneous anion exchange membrane: Physico-chemical and antibacterial characteristics. J. Taiwan Inst. Chem. Eng. 2013, 44, 670–677. [Google Scholar] [CrossRef]
- Abenojar, J.; Torregrosa-Coque, R.; Martinez, M.A.; Martin-Martinez, J.M. Surface modifications of polycarbonate (PC) and acrylonitrile butadiene styrene (ABS) copolymer by treatment with atmospheric plasma. Surf. Coat. Technol. 2009, 203, 2173–2180. [Google Scholar] [CrossRef]
- Brocherieux, A.; Dessaux, O.; Goudmand, P.; Gengembre, L.; Grimblot, J.; Brunel, M.; Lazzaroni, R. Characterization of nickel films deposited by cold remote nitrogen plasma on acrylonitrile-utadiene-styrene copolymer. Appl. Surf. Sci. 1995, 90, 47–58. [Google Scholar] [CrossRef]
- Burkstrand, J.M. Electron spectroscopic study of oxygen-plasma-treated polymer surfaces. J. Vac. Sci. Technol. 1978, 15, 223–226. [Google Scholar] [CrossRef]
- Iijima, Y.; Niimura, N.; Hiraoka, K. Prevention of the reduction of CuO during X-ray photoelectron spectroscopy analysis. Surf. Interface Anal. 1996, 24, 193–197. [Google Scholar] [CrossRef]
- Robert, T.; Bartel, M.; Offergel, G. Characterization of oxygen species adsorbed on copper and nickel oxides by X-ray photoelectron spectroscopy. Surf. Sci. 1972, 33, 123–130. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Scrocco, M. Satellite structure in the X-ray photoelectron-spectra of CuO and Cu2O. Chem. Phys. Lett. 1979, 63, 52–56. [Google Scholar] [CrossRef]
- Xian, Y.; Wu, S.; Wang, Z.; Huang, Q.; Lu, X.; Kolb, J.F. Discharge dynamics and modes of an atmospheric pressure non-equilibrium air plasma jet. Plasma Process. Polym. 2013, 10, 372–378. [Google Scholar] [CrossRef]
- Hao, X.; Mattson, A.M.; Edelblute, C.M.; Malik, M.A.; Heller, L.C.; Kolb, J.F. Nitric oxide generation with an air operated non-thermal plasma jet and associated microbial inactivation mechanisms. Plasma Process. Polym. 2014, 11, 1044–1056. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers. The Scienta ESCA 300 Data Base; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Wilken, R.; Hollander, A.; Behnisch, J. Surface radical analysis on plasma-treated polymers. Surf. Coat. Technol. 1999, 116, 991–995. [Google Scholar] [CrossRef]
- Friedrich, J.F.; Mix, R.; Kühn, G. Adhesion of metals to plasma-induced functional groups at polymer surfaces. Surf. Coat. Technol. 2005, 200, 565–568. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.-J.; Seo, Y. Polyetheretherketone (PEEK) surface functionalization by low-energy ion-beam irradiation under a reactive O2 environment and its effect on the PEEK/copper adhesives. Langmuir 2004, 20, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Beil, S.; Horn, H.; Windisch, A.; Hilgers, C.; Pochner, K. Photochemical functionalization of polymer surfaces for subsequent metallization. Surf. Coat. Technol. 1999, 116–119, 1195–1203. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. Studies of copper nanoparticles effects on micro-organisms. Ann. Biol. Res. 2011, 2, 368–373. [Google Scholar]
- Chien, A.C.; Hill, N.S.; Levin, P.A. Cell size control in bacteria. Curr. Biol. 2012, 22, R340–R349. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.G.; Foster, S.J.; Richards, R.G. An introduction to Staphylococcus aureus, and techniques for identifying and quantifying S. aureus adhesins in relation to adhesion to biomaterials: Review. Eur. Cell Mater. 2002, 4, 39–60. [Google Scholar] [PubMed]
- Finke, B.; Polak, M.; Hempel, F.; Rebl, H.; Zietz, C.; Stranak, V.; Lukowski, G.; Hippler, R.; Bader, R.; Nebe, J.B.; et al. Antimicrobial potential of copper-containing titanium surfaces generated by ion implantation and dual high power impulse magnetron sputtering. Adv. Eng. Mater. 2012, 14, B224–B230. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mattson, A.M.; Edelblute, C.M.; Hao, X.L.; Malik, M.A.; Heller, L.C. Cold DC-operated air plasma jet for the inactivation of infectious microorganisms. IEEE Trans. Plasma Sci. 2012, 40, 3007–3026. [Google Scholar] [CrossRef]
- Mohamed, A.A.H.; Kolb, J.F.; Schoenbach, K.H. Low temperature, atmospheric pressure, direct current microplasma jet operated in air, nitrogen and oxygen. Eur. Phys. J. D 2010, 60, 517–522. [Google Scholar] [CrossRef]
- Block, R.; Toedter, O.; Schoenbach, K.H. Gas temperature measurements in high pressure glow discharges in air. In Proceedings of the 30th Plasmadynamic and Lasers Conference, Norfolk, VA, USA, 28 June–1 July 1999.
- Necas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kredl, J.; Kolb, J.F.; Schnabel, U.; Polak, M.; Weltmann, K.-D.; Fricke, K. Deposition of Antimicrobial Copper-Rich Coatings on Polymers by Atmospheric Pressure Jet Plasmas. Materials 2016, 9, 274. https://doi.org/10.3390/ma9040274
Kredl J, Kolb JF, Schnabel U, Polak M, Weltmann K-D, Fricke K. Deposition of Antimicrobial Copper-Rich Coatings on Polymers by Atmospheric Pressure Jet Plasmas. Materials. 2016; 9(4):274. https://doi.org/10.3390/ma9040274
Chicago/Turabian StyleKredl, Jana, Juergen F. Kolb, Uta Schnabel, Martin Polak, Klaus-Dieter Weltmann, and Katja Fricke. 2016. "Deposition of Antimicrobial Copper-Rich Coatings on Polymers by Atmospheric Pressure Jet Plasmas" Materials 9, no. 4: 274. https://doi.org/10.3390/ma9040274
APA StyleKredl, J., Kolb, J. F., Schnabel, U., Polak, M., Weltmann, K.-D., & Fricke, K. (2016). Deposition of Antimicrobial Copper-Rich Coatings on Polymers by Atmospheric Pressure Jet Plasmas. Materials, 9(4), 274. https://doi.org/10.3390/ma9040274






