Effect of Nano-TiC Dispersed Particles and Electro-Codeposition Parameters on Morphology and Structure of Hybrid Ni/TiC Nanocomposite Layers
Abstract
:1. Introduction
2. Experimental
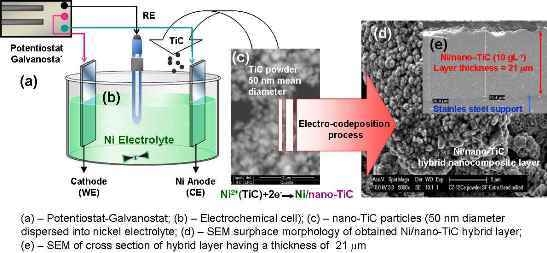
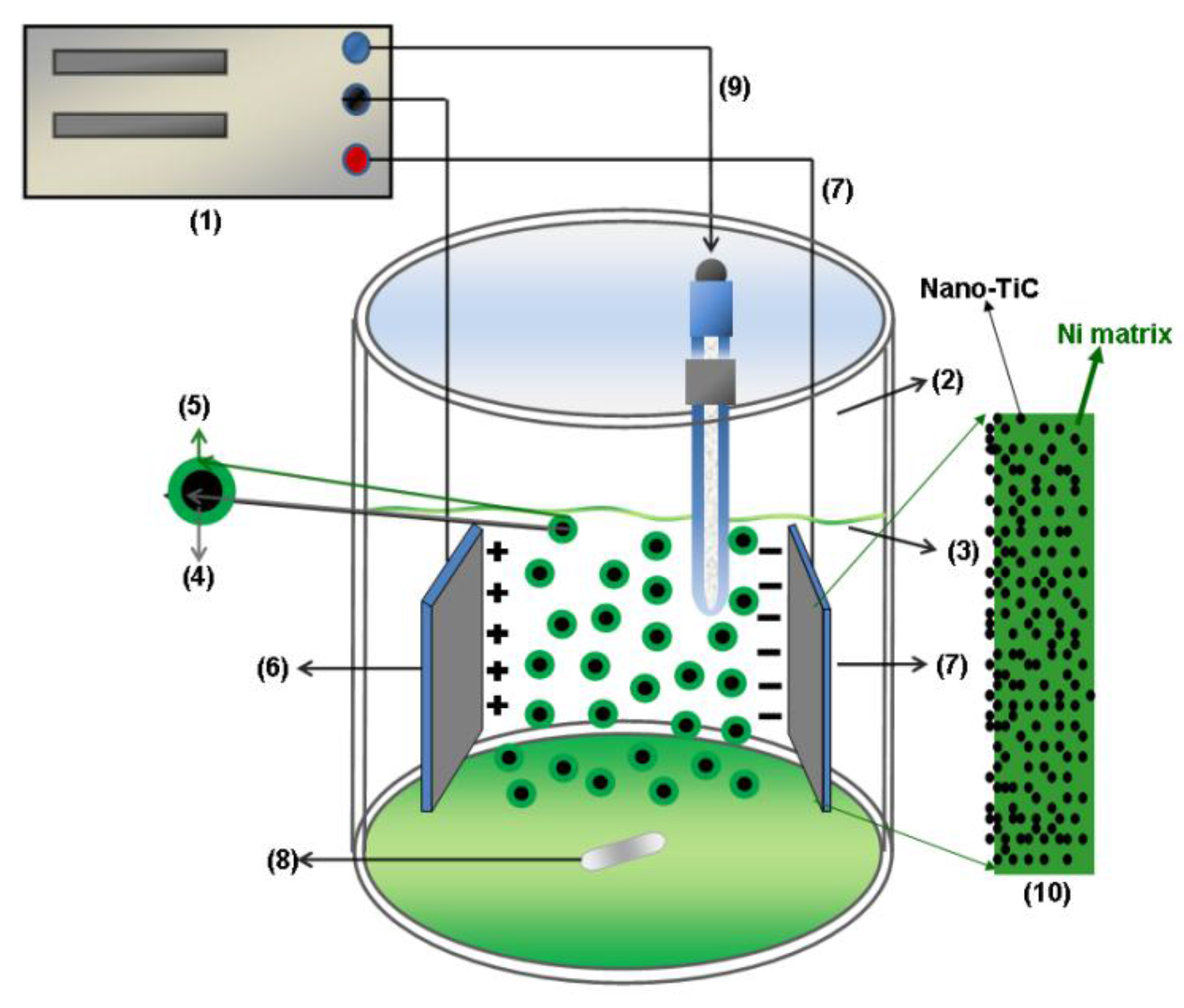
2.1. Preparation of Ni/TiC Nanostructured Layers
2.2. Characterization of the Electrodeposited Layers
3. Results and Discussion
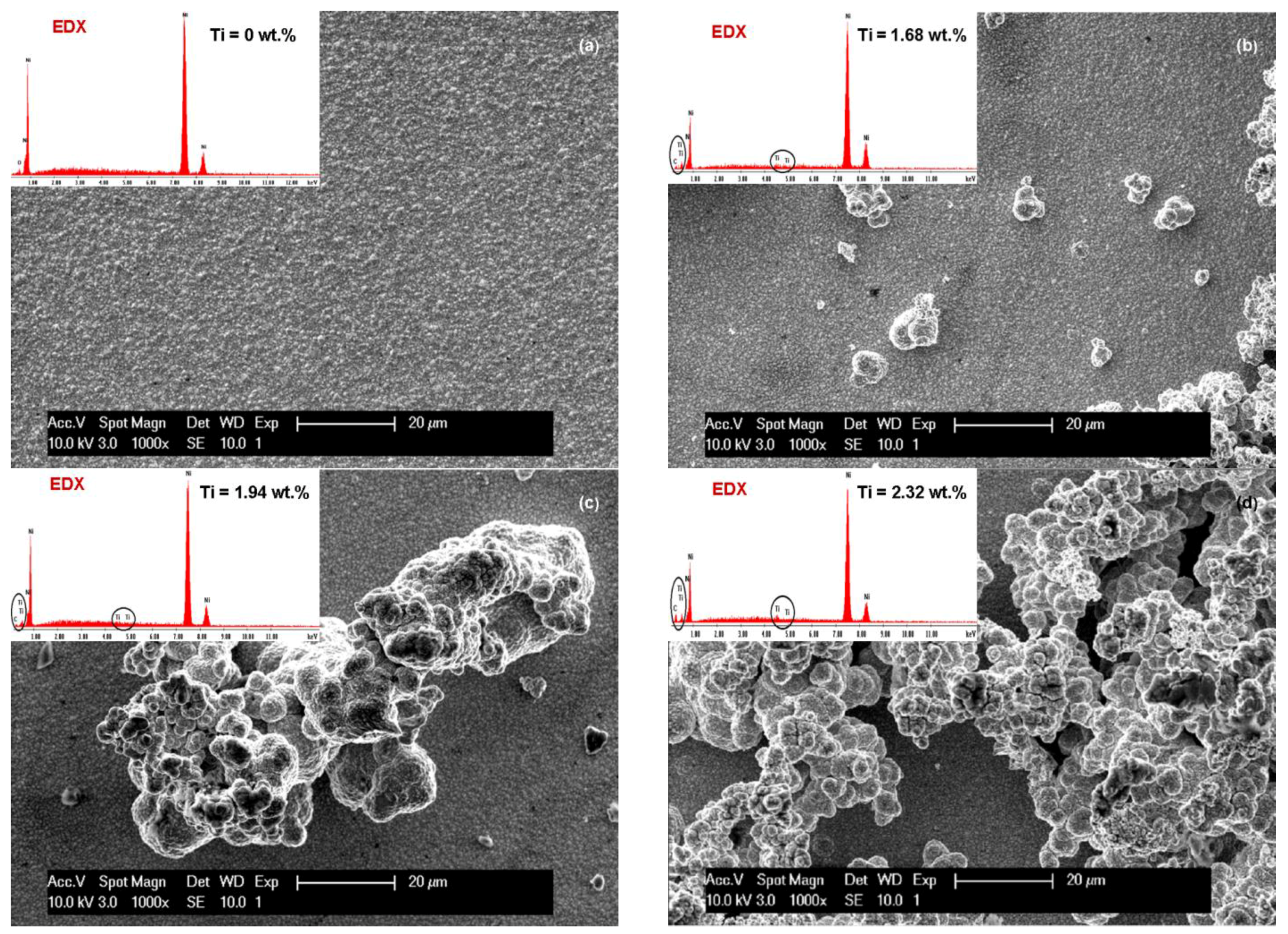
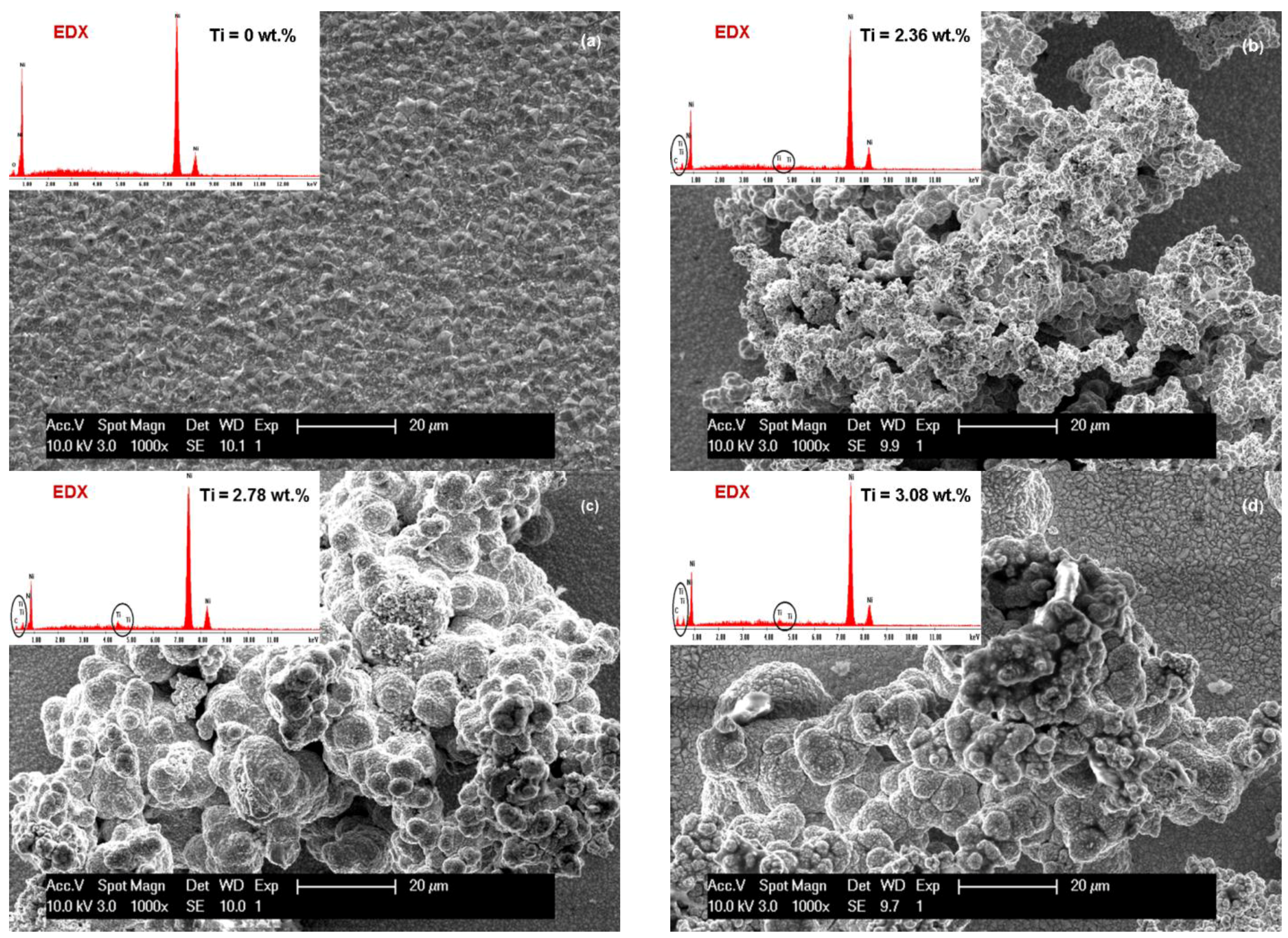
3.1. SEM Surface Morphology of Ni/Nano-TiC Hybrid Nanocomposite Layers
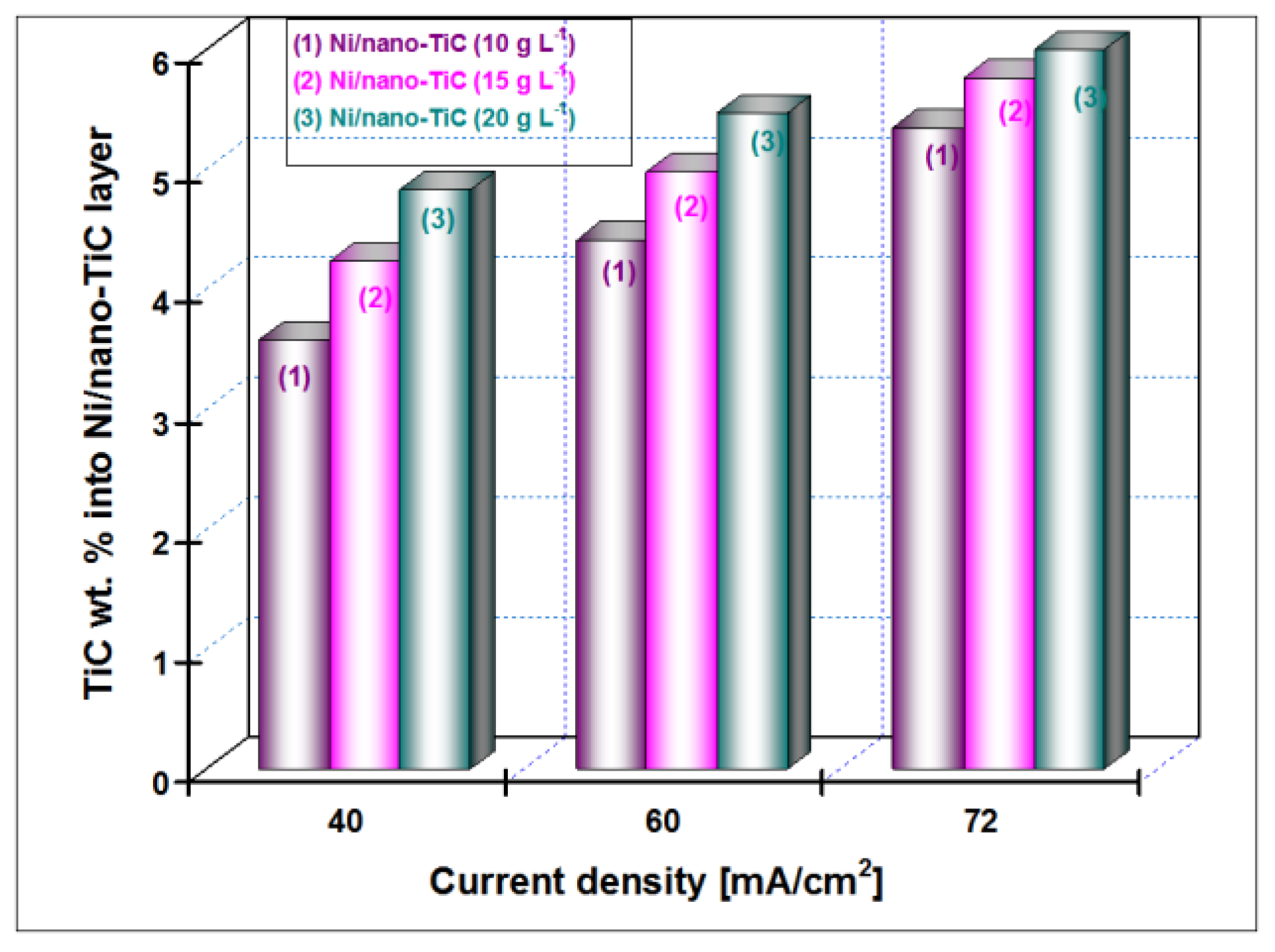
3.2. Nano TiC Particles Incorporation into Nickel Matrix
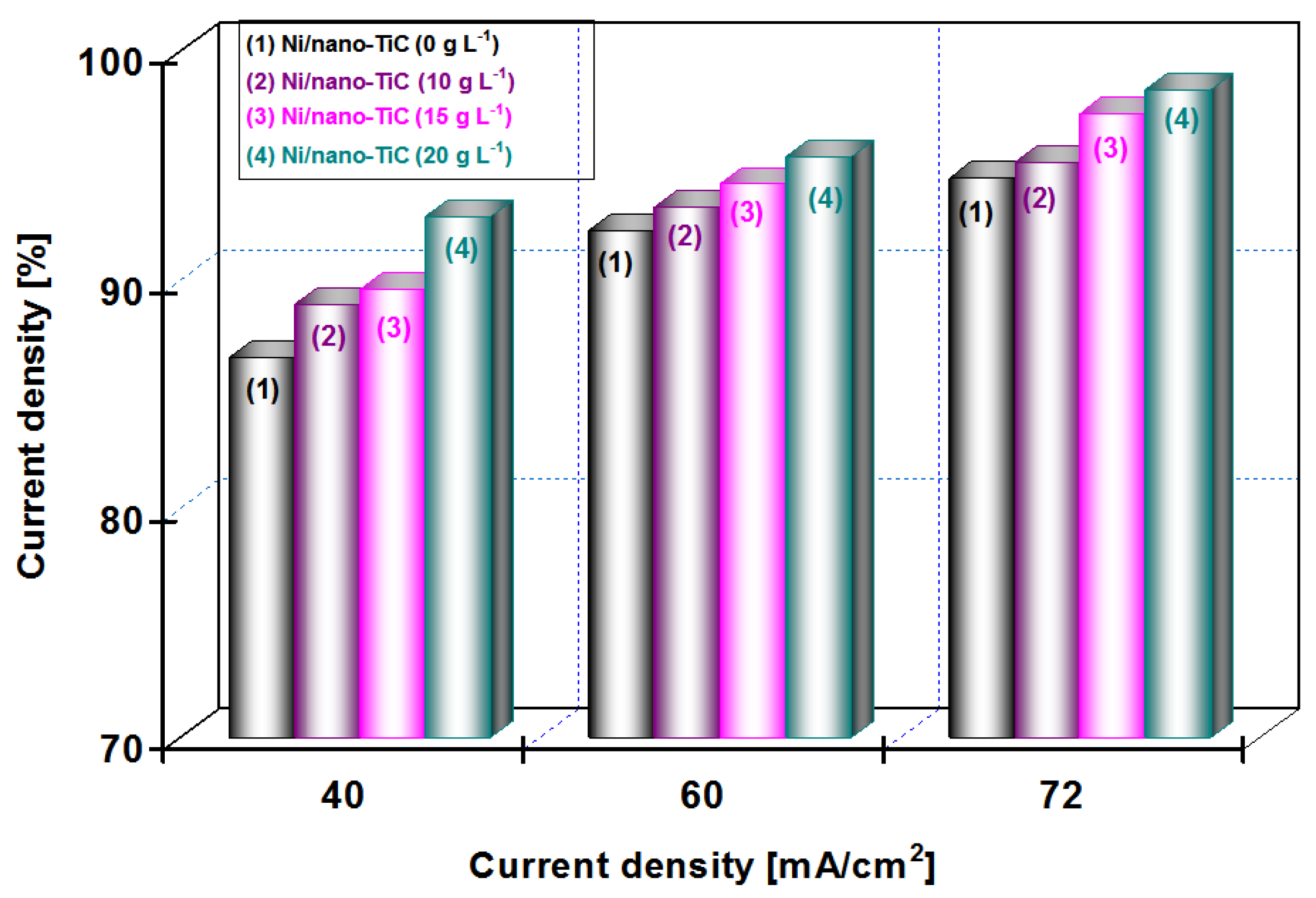
3.3. Current Efficiency During Electro-Codeposition
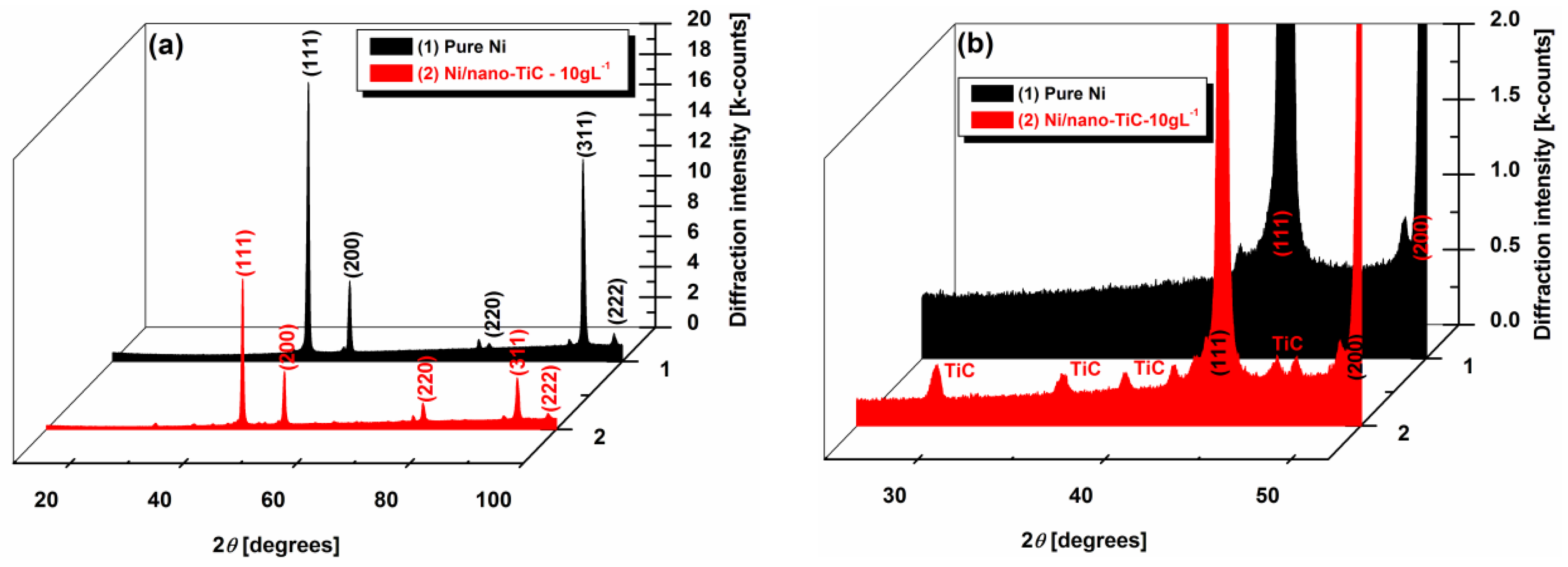
3.4. X-ray Diffraction Patterns of Ni/TiC Hybrid Nanocomposite Layers
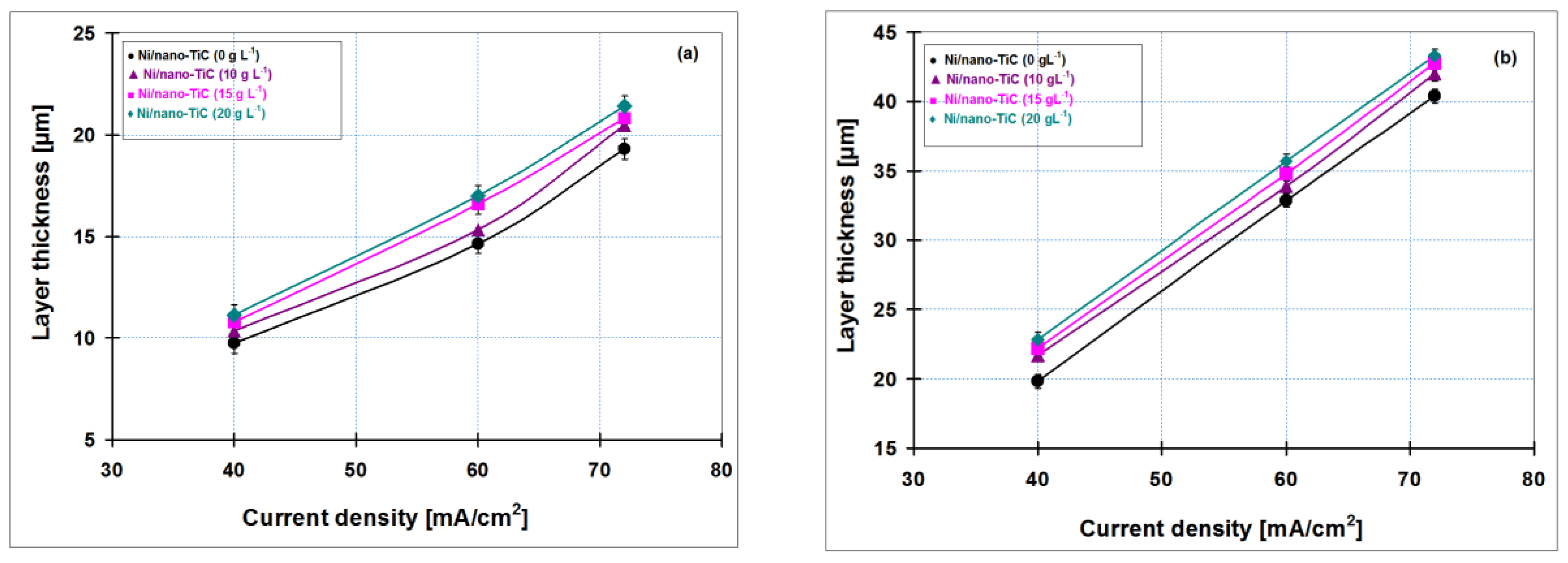
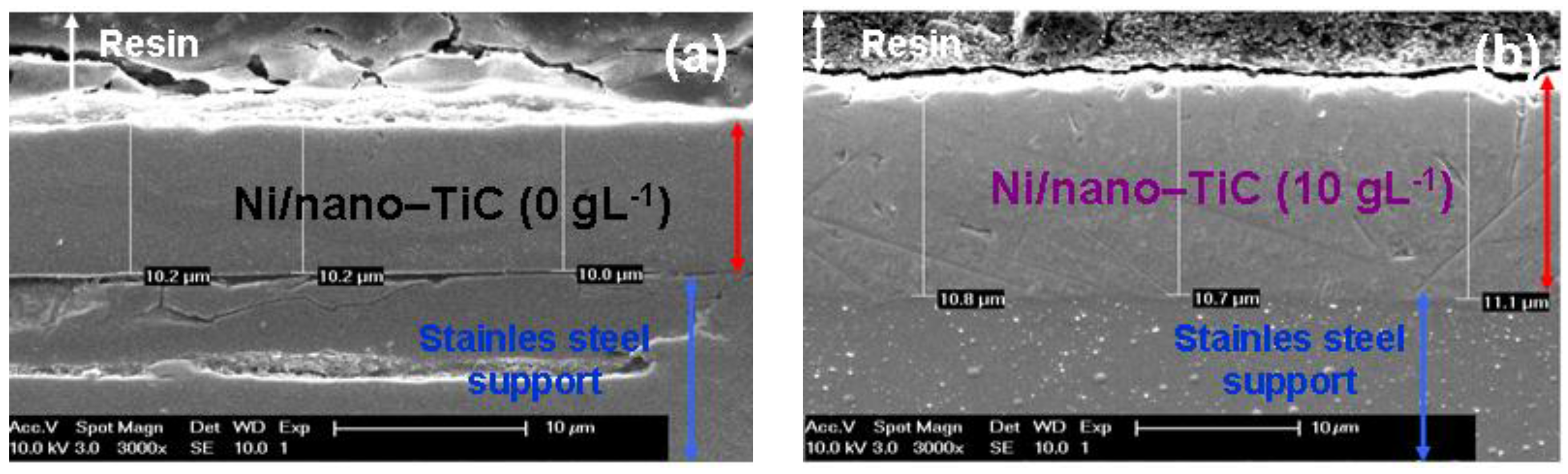
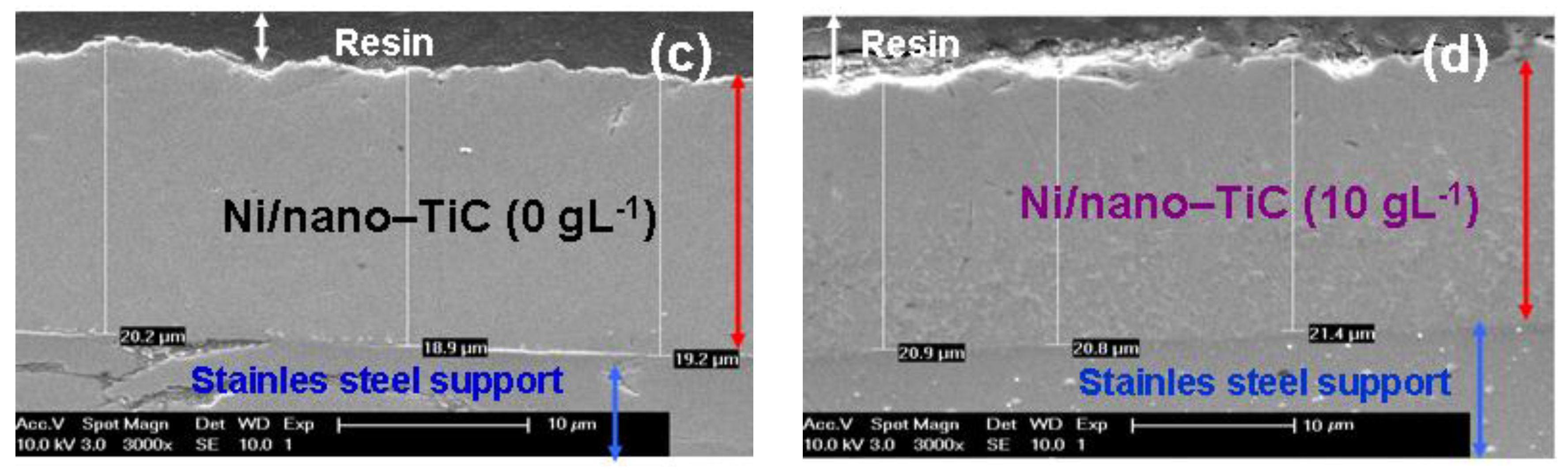
3.5. Thicknesses of Ni/Nano-TiC Hybrid Nanostructured Layers
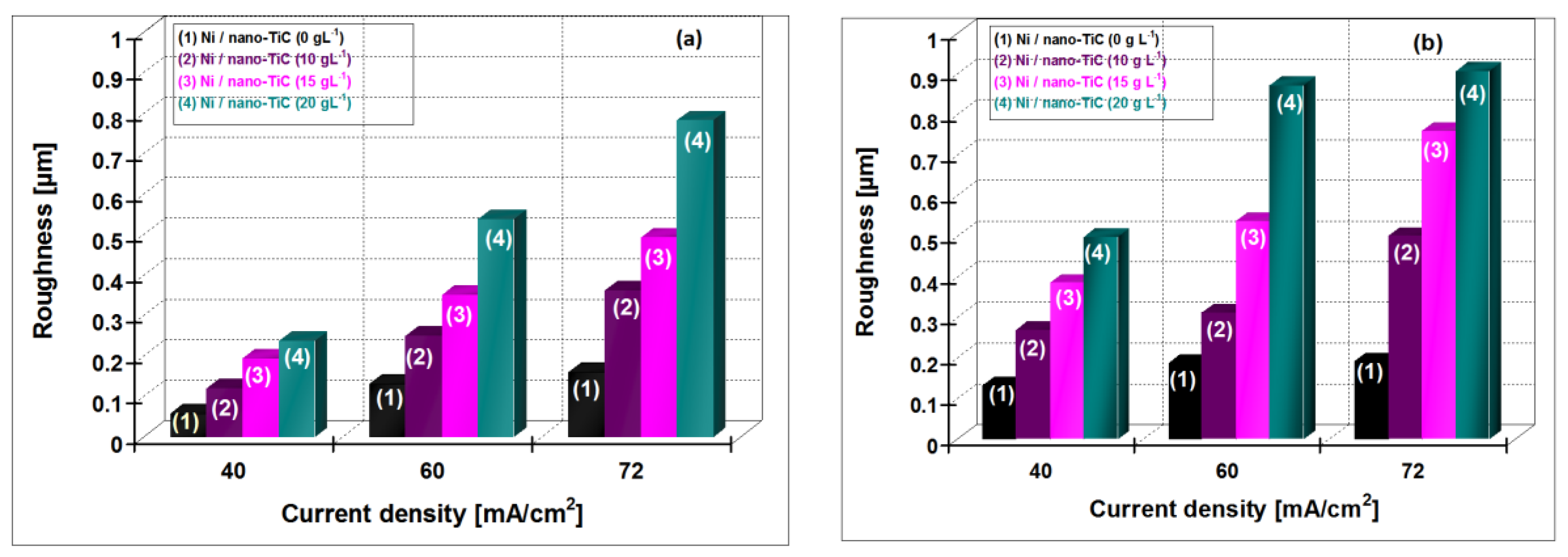
3.6. Roughness of Electrodeposited Surfaces
3.7. Nanoidentation of Surface Layers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fernando, R.H. Nanocomposite and nanostructured coatings: Recent advancements. In Nanotechnology Applications in Coatings; Fernando, R.H., Sung, L.P., Eds.; American Chemical Society: Washington, DC, USA, 2009; Volume 1008, pp. 2–21. [Google Scholar]
- Ahmad, Y.H.; Mohamed, A.M.A. Electrodeposition of nanostructured nickel-ceramic composite coatings: A review. Int. J. Electrochem. Sci. 2014, 9, 1942–1963. [Google Scholar]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Mohajeri, S.; Dolati, A.; Rezagholibeiki, S. Electrodeposition of Ni/WC nano composite in sulfate solution. Mater. Chem. Phys. 2011, 129, 746–750. [Google Scholar] [CrossRef]
- Bajwa, R.S.; Khan, Z.; Bakolas, V.; Braun, W. Effect of bath ionic strength on adhesion and tribological properties of pure nickel and Ni-based nanocomposite coatings. J. Adhes. Sci. Technol. 2015, 30, 653–665. [Google Scholar] [CrossRef]
- Bajwa, R.S.; Khan, Z.; Bakolas, V.; Braun, W. Water-lubricated nickel-based composite (Ni-Al2O3, Ni-SiC and Ni-ZrO2) thin film coatings for industrial applications. Acta Metall. Sin. Engl. Lett. 2016, 29, 8–16. [Google Scholar] [CrossRef]
- Beltowska-Lehman, E.; Indyka, P.; Bigos, A.; Szczerba, M.J.; Kot, M. Ni-W/ZrO2 nanocomposites obtained by ultrasonic DC electrodeposition. Mater. Des. 2015, 80, 1–11. [Google Scholar] [CrossRef]
- Benea, L.; Başa, S.-B.; Dănăilă, E.; Caron, N.; Raquet, O.; Ponthiaux, P.; Celis, J.-P. Fretting and wear behaviors of Ni/nano-WC composite coatings in dry and wet conditions. Mater. Des. 2015, 65, 550–558. [Google Scholar] [CrossRef]
- Trivedi, P.; Gupta, P.; Srivastava, S.; Jayaganthan, R.; Chandra, R.; Roy, P. Characterization and in vitro biocompatibility study of Ti-Si-N nanocomposite coatings developed by using physical vapor deposition. Appl. Surf. Sci. 2014, 293, 143–150. [Google Scholar] [CrossRef]
- Farrokhzad, M.A.; Saha, G.C.; Khan, T.I. Wear performance of co-electrodeposited cermet coatings. Surf. Coat. Technol. 2013, 235, 75–85. [Google Scholar] [CrossRef]
- Parida, G.; Chaira, D.; Chopkar, M.; Basu, A. Synthesis and characterization of Ni-TiO2 composite coatings by electro-co-deposition. Surf. Coat. Technol. 2011, 205, 4871–4879. [Google Scholar] [CrossRef]
- Gan, J.A.; Berndt, C.C. Nanocomposite coatings: Thermal spray processing, microstructure and performance. Int. Mater. Rev. 2015, 60, 195–244. [Google Scholar] [CrossRef]
- Pavlov, A.I.; Benea, L.; Celis, J.P.; Vazquez, L. Influence of nano-tio2 co-deposition on the morphology, microtopography and crystallinity of ni/nano-tio2 electrosynthesized nanocomposite coatings. Dig. J. Nanomater. Biostruct. 2013, 8, 1043–1050. [Google Scholar]
- Hovestad, A.; Janssen, L.J.J. Electrochemical codeposition of inert particles in a metallic matrix. J. Appl. Electrochem. 1995, 25, 519–527. [Google Scholar] [CrossRef]
- Tam, J.; Palumbo, G.; Erb, U. Recent advances in superhydrophobic electrodeposits. Materials 2016, 9, 2–27. [Google Scholar] [CrossRef]
- Akhtar, F.; Guo, S.J. Microstructure, mechanical and fretting wear properties of TiC-stainless steel composites. Mater. Charact. 2008, 59, 84–90. [Google Scholar] [CrossRef]
- Figiel, P.; Zimowski, S.; Klimczyk, P.; Dziwisz, T.; Jaworska, L. Mechanical and tribological properties of TiC-based composites for ED machining. Arch. Mater. Sci. Eng. 2008, 33, 83–88. [Google Scholar]
- Walsh, F.C.; Ponce de León, C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying coatings technology. Trans. Inst. Mater. Finish. 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Thiemig, D.; Bund, A. Characterization of electrodeposited Ni-TiO2 nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 2976–2984. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Ahmed, Z. The effect of current density on properties of electrodeposited nanocrystalline nickel. J. Appl. Electrochem. 2003, 33, 733–739. [Google Scholar] [CrossRef]
- Spanou, S.; Pavlatou, E.A.; Spyrellis, N. Ni/nano-TiO2 composite electrodeposits: Textural and structural modifications. Electrochim. Acta 2009, 54, 2547–2555. [Google Scholar] [CrossRef]
- Xue, Y.-J.; Jia, X.-Z.; Zhou, Y.-W.; Ma, W.; Li, J.-S. Tribological performance of Ni-CeO2 composite coatings by electrodeposition. Surf. Coat. Technol. 2006, 200, 5677–5681. [Google Scholar] [CrossRef]
- Saha, R.K.; Khan, T.I. Effect of applied current on the electrodeposited Ni-Al2O3 composite coatings. Surf. Coat. Technol. 2010, 205, 890–895. [Google Scholar] [CrossRef]
- Suzuki, Y.; Arai, S.; Endo, M. Ni-P alloy-carbon black composite films fabricated by electrodeposition. Appl. Surf. Sci. 2010, 256, 6914–6917. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Zuo, Y.; Zhao, X.H.; Zhao, J.M.; Xiong, J.P. The effects of electrodeposition current density on properties of Ni-CNTs composite coatings. Surf. Coat. Technol. 2008, 202, 3246–3250. [Google Scholar] [CrossRef]
- Baghery, P.; Farzam, M.; Mousavi, A.B.; Hosseini, M. Ni-TiO2 nanocomposite coating with high resistance to corrosion and wear. Surf. Coat. Technol. 2010, 204, 3804–3810. [Google Scholar] [CrossRef]
- Calderón, J.A.; Henao, J.E.; Gómez, M.A. Erosion-corrosion resistance of Ni composite coatings with embedded SiC nanoparticle. Electrochim. Acta 2014, 124, 190–198. [Google Scholar] [CrossRef]
- Singh, D.K.; Tripathi, M.K.; Singh, V.B. Preparation of Ni-TiC nanocomposites by electrolytic codeposition from a non-aqueous bath and their characterization. J. Electrochem. Soc. 2012, 159, 469–472. [Google Scholar] [CrossRef]
- Arghavanian, R.; Parvini-Ahmadi, N. The effect of co-electrodeposited ZrO2 particles on the microstructure and corrosion resistance of Ni coatings. J. Solid State Electrochem. 2011, 15, 2199–2204. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni–SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloid. Surface. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Kobayashi, M.; Doi, Y. TiN and TiC coating on cemented carbides by Ion plating. Thin Solid Films 1978, 54, 67–74. [Google Scholar] [CrossRef]
- Sue, J.A.; Troue, H.H. Effect of crystallographic orientation on erosion characteristics of arc evaporation titanium nitride coating. Surf. Coat. Technol. 1987, 33, 169–181. [Google Scholar] [CrossRef]

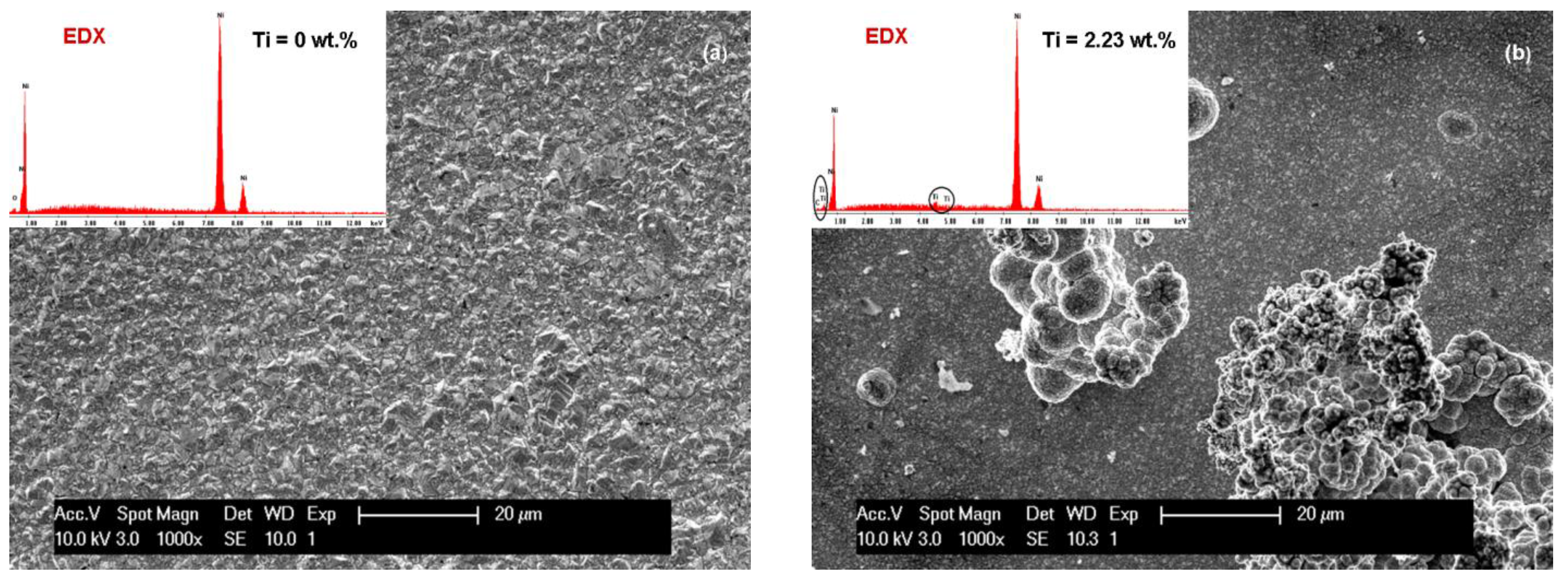
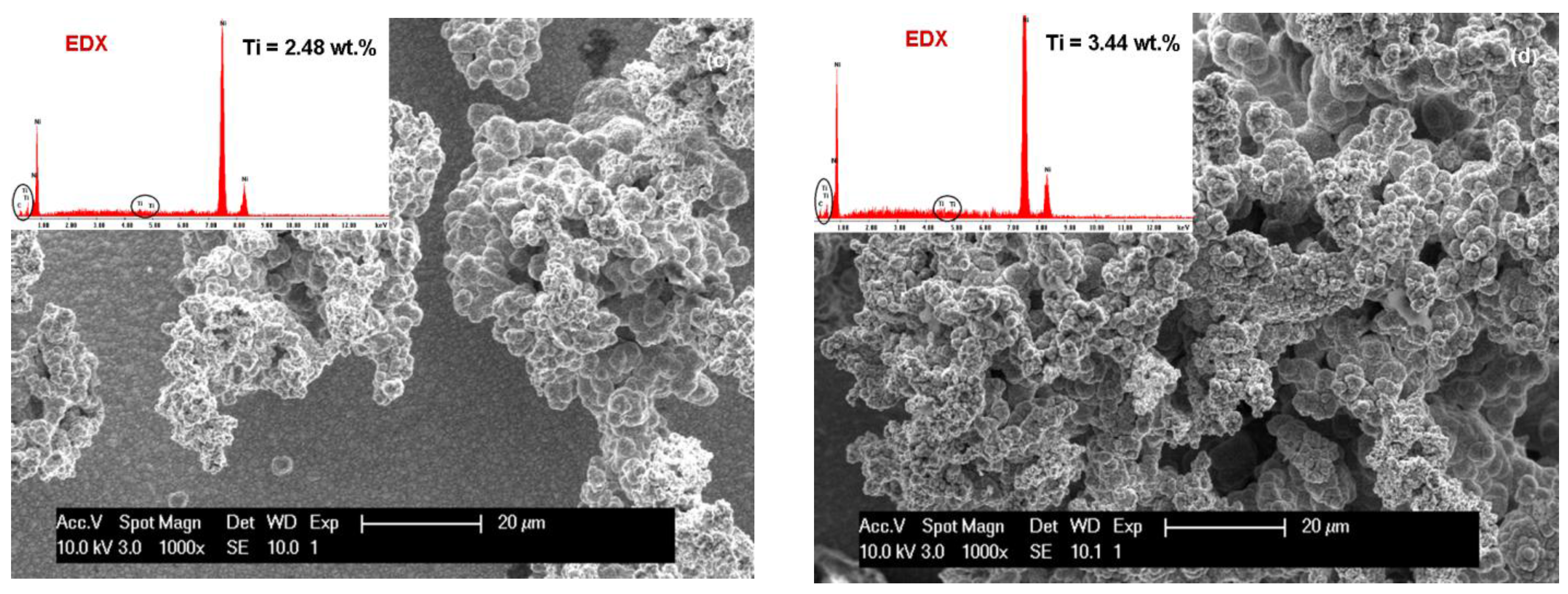
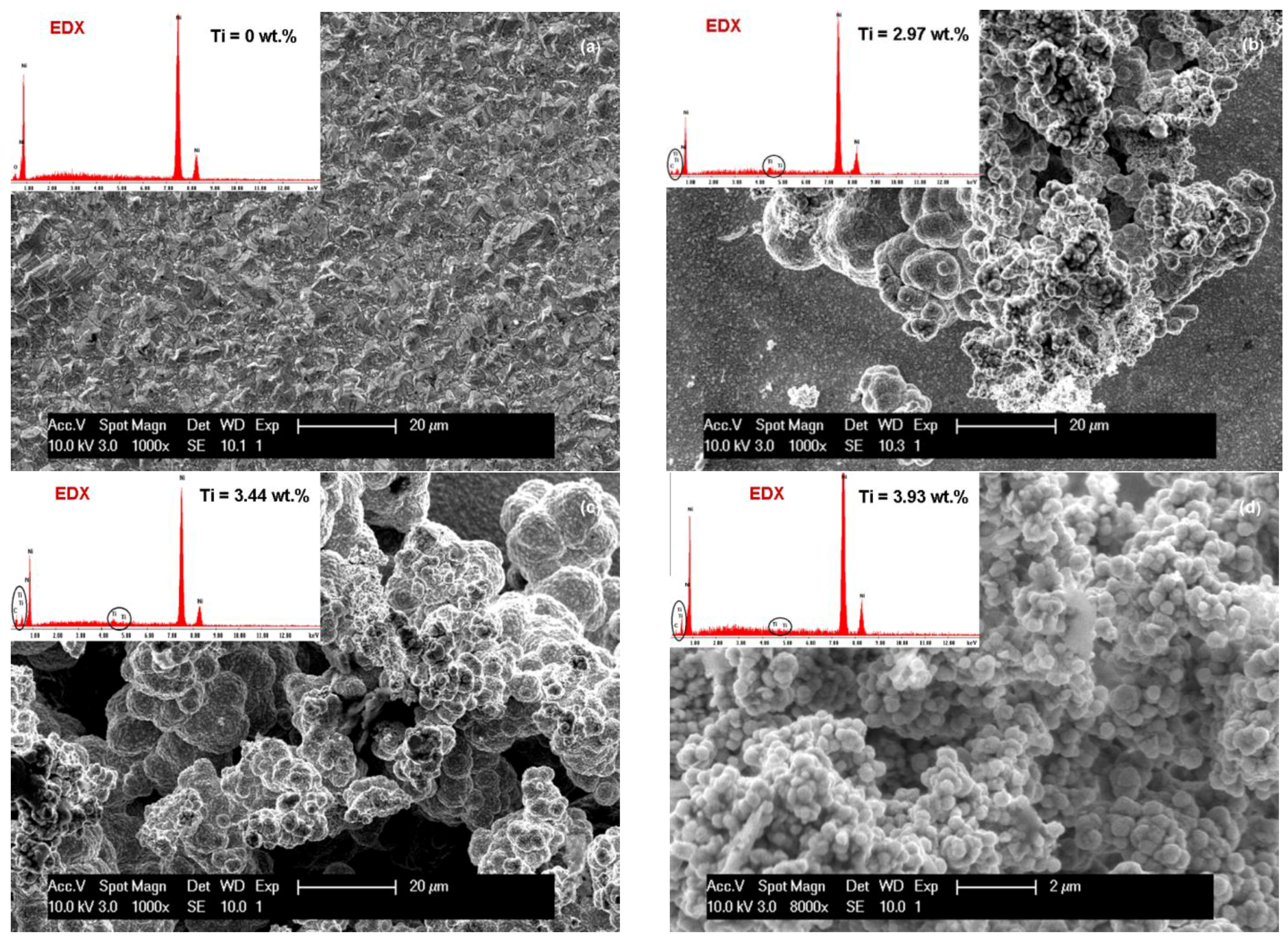
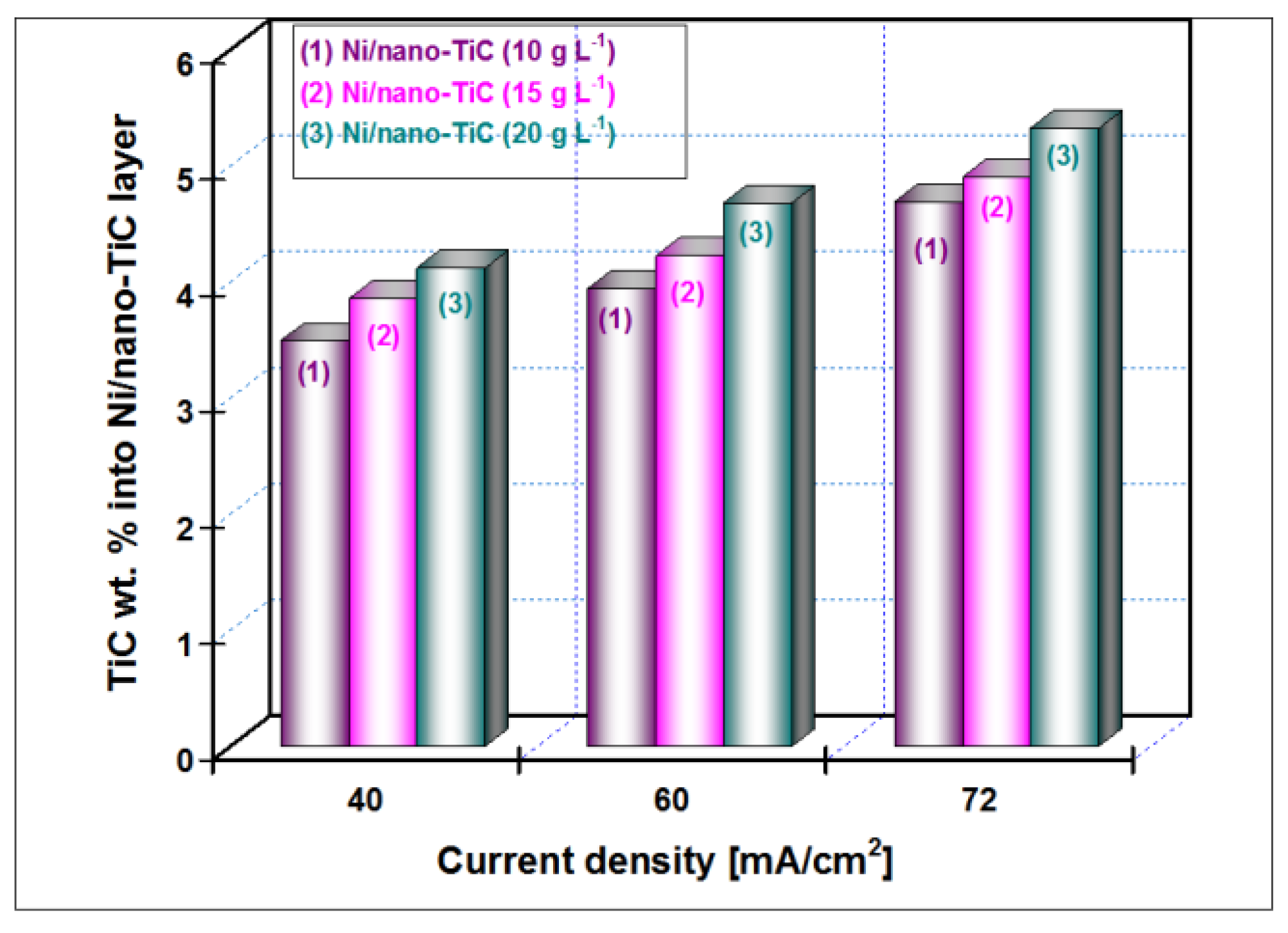

| Crystalline Planes | Diffraction Peak Intensity (K-Counts) | |
|---|---|---|
| Pure Ni Coating | Ni/Nano-TiC Coating (10 g·L−1) | |
| (111) | 18.34 | 9.88 |
| (200) | 5.29 | 3.81 |
| (311) | 13.29 | 3.38 |
| RTChkl | Pure Ni Coating | Ni/Nano-TiC Coating (10 g·L−1) |
|---|---|---|
| RTC111 | 14.81 | 16.76 |
| RTC200 | 10.17 | 15.40 |
| RTC311 | 53.65 | 28.73 |
| Type of Layer | Time of Deposition (min) | Identation Hardness (H) (GPa) | Vickers Hardness (Hv) | Elastic Modulus (E) (GPa) | H/E Ratio |
|---|---|---|---|---|---|
| Pure Ni layer | 15 | 3.64 | 337.92 | 201.66 | 0.0180 |
| Pure Ni layer | 30 | 3.87 | 351.37 | 205.32 | 0.0188 |
| Ni/nano-TiC layer (10 g·L−1) | 15 | 4.87 | 451.37 | 221.64 | 0.0220 |
| Ni/nano-TiC layer (10 g·L−1) | 30 | 5.87 | 543.92 | 216.45 | 0.0271 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benea, L.; Celis, J.-P. Effect of Nano-TiC Dispersed Particles and Electro-Codeposition Parameters on Morphology and Structure of Hybrid Ni/TiC Nanocomposite Layers. Materials 2016, 9, 269. https://doi.org/10.3390/ma9040269
Benea L, Celis J-P. Effect of Nano-TiC Dispersed Particles and Electro-Codeposition Parameters on Morphology and Structure of Hybrid Ni/TiC Nanocomposite Layers. Materials. 2016; 9(4):269. https://doi.org/10.3390/ma9040269
Chicago/Turabian StyleBenea, Lidia, and Jean-Pierre Celis. 2016. "Effect of Nano-TiC Dispersed Particles and Electro-Codeposition Parameters on Morphology and Structure of Hybrid Ni/TiC Nanocomposite Layers" Materials 9, no. 4: 269. https://doi.org/10.3390/ma9040269
APA StyleBenea, L., & Celis, J.-P. (2016). Effect of Nano-TiC Dispersed Particles and Electro-Codeposition Parameters on Morphology and Structure of Hybrid Ni/TiC Nanocomposite Layers. Materials, 9(4), 269. https://doi.org/10.3390/ma9040269