Nanoparticles Suitable for BCAA Isolation Can Serve for Use in Magnetic Lipoplex-Based Delivery System for L, I, V, or R-rich Antimicrobial Peptides
Abstract
:1. Introduction
2. Results and Discussion
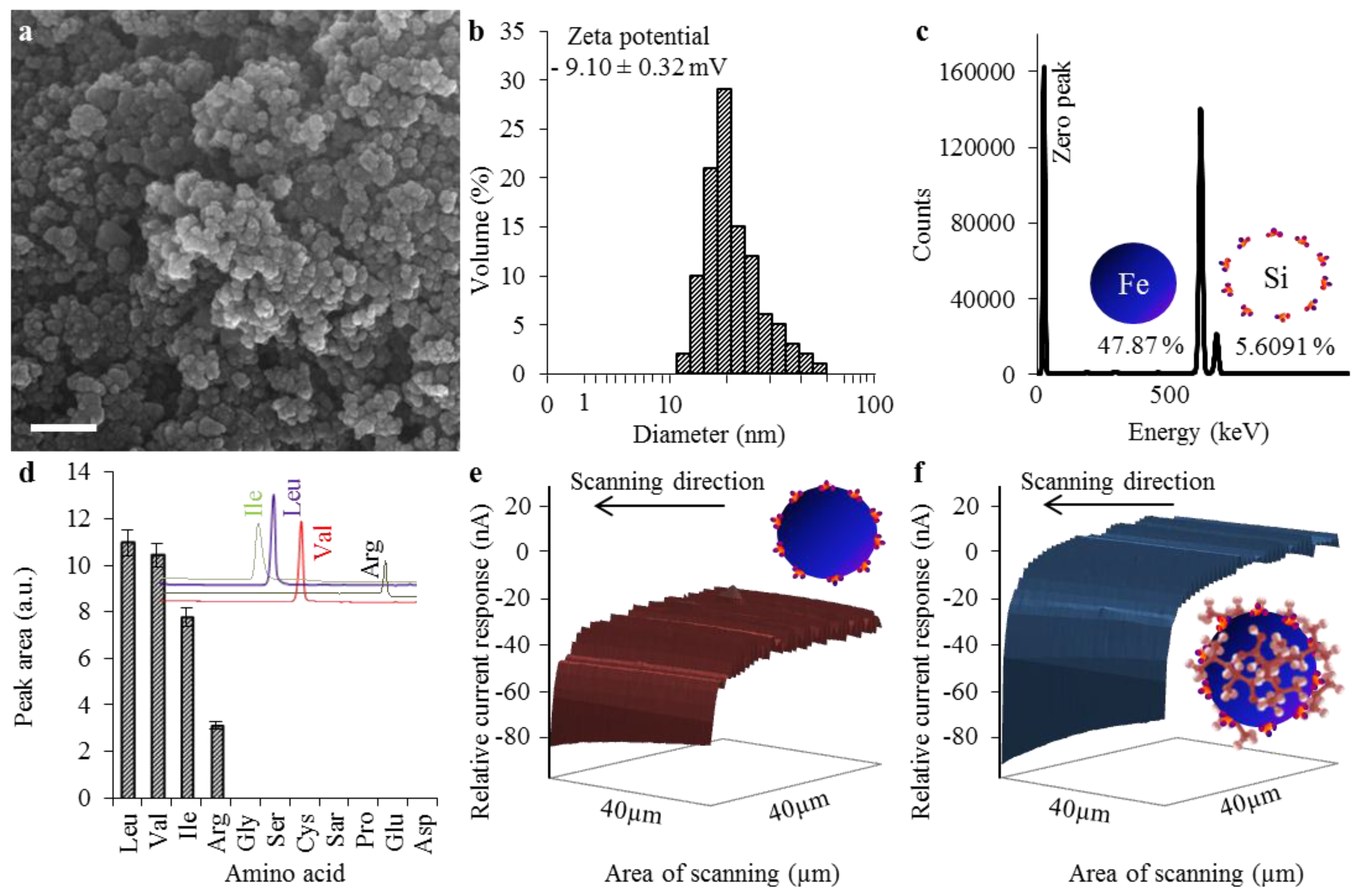
2.1. Characterization of Nanomaghemite@APTES Conjugate’s Physicochemical Properties
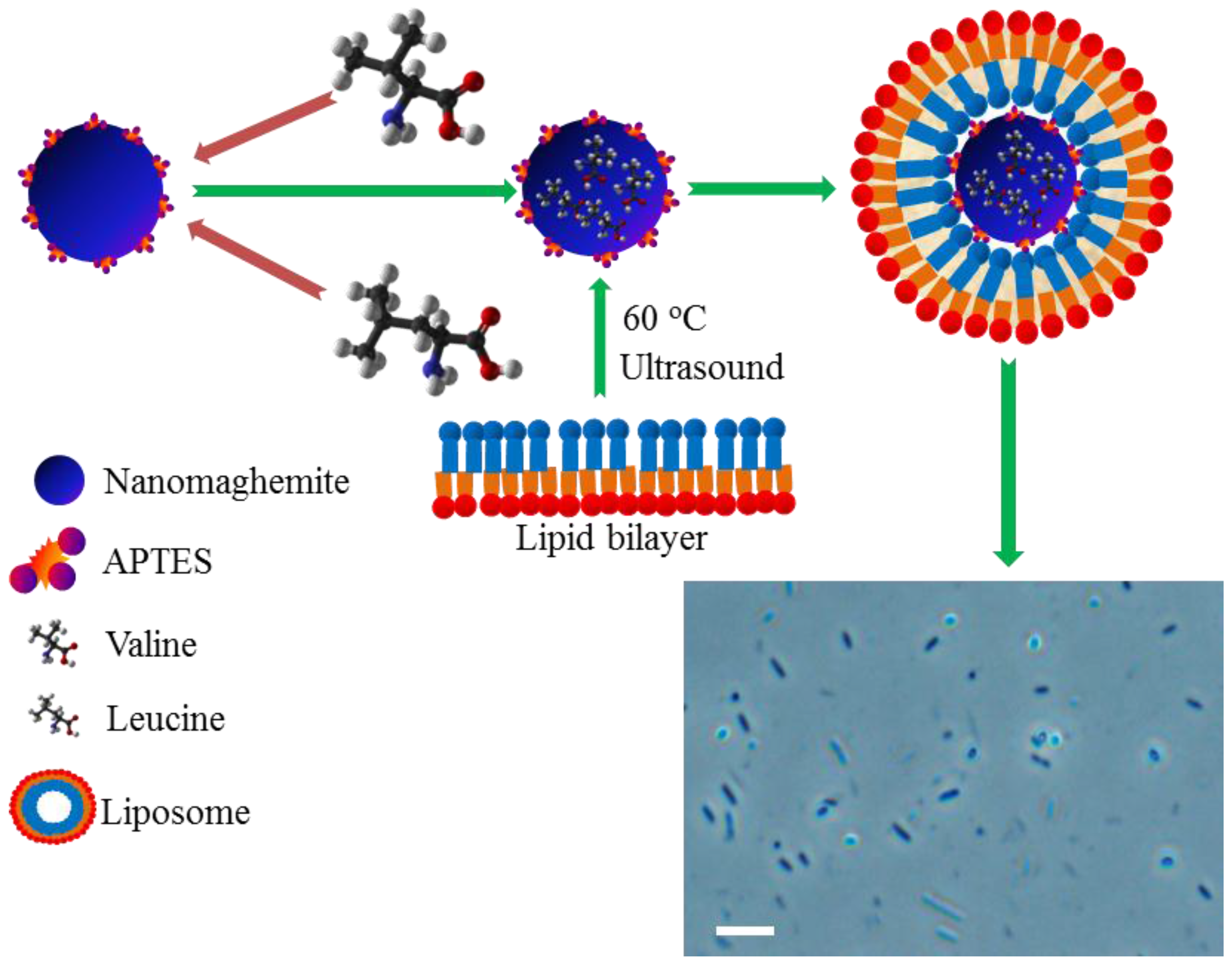
2.2. Encapsulation of Paramagnetic Nanoparticles into Liposome Inner Cavity
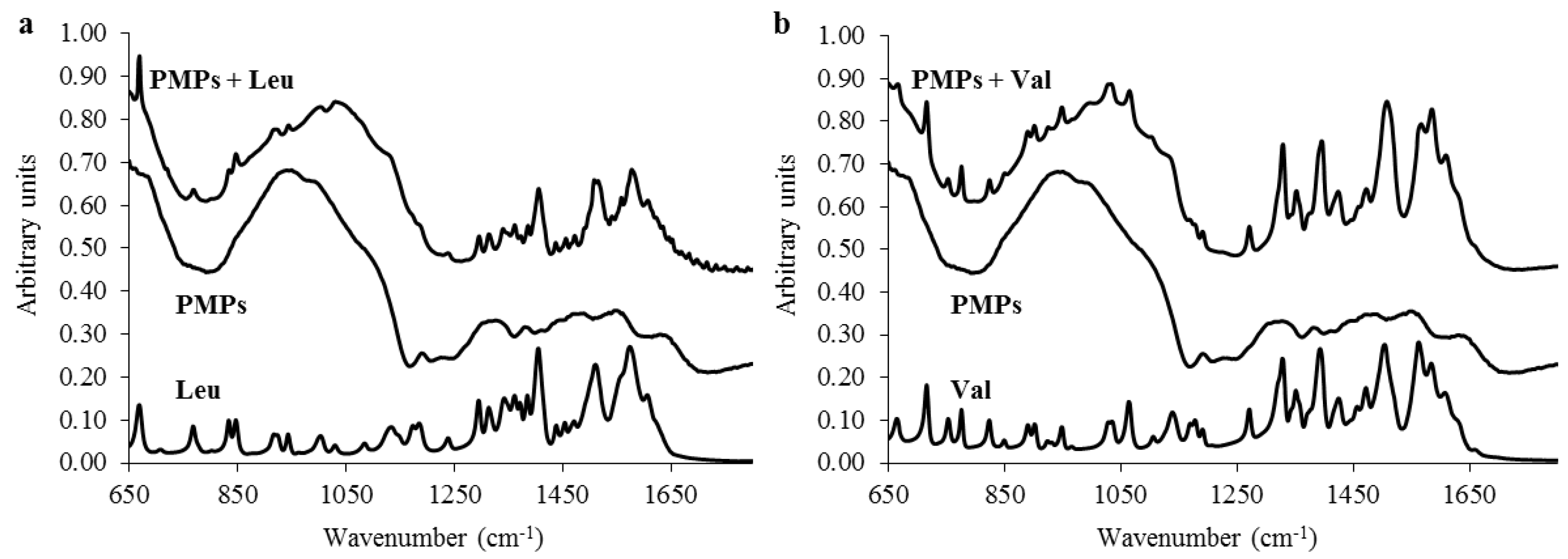
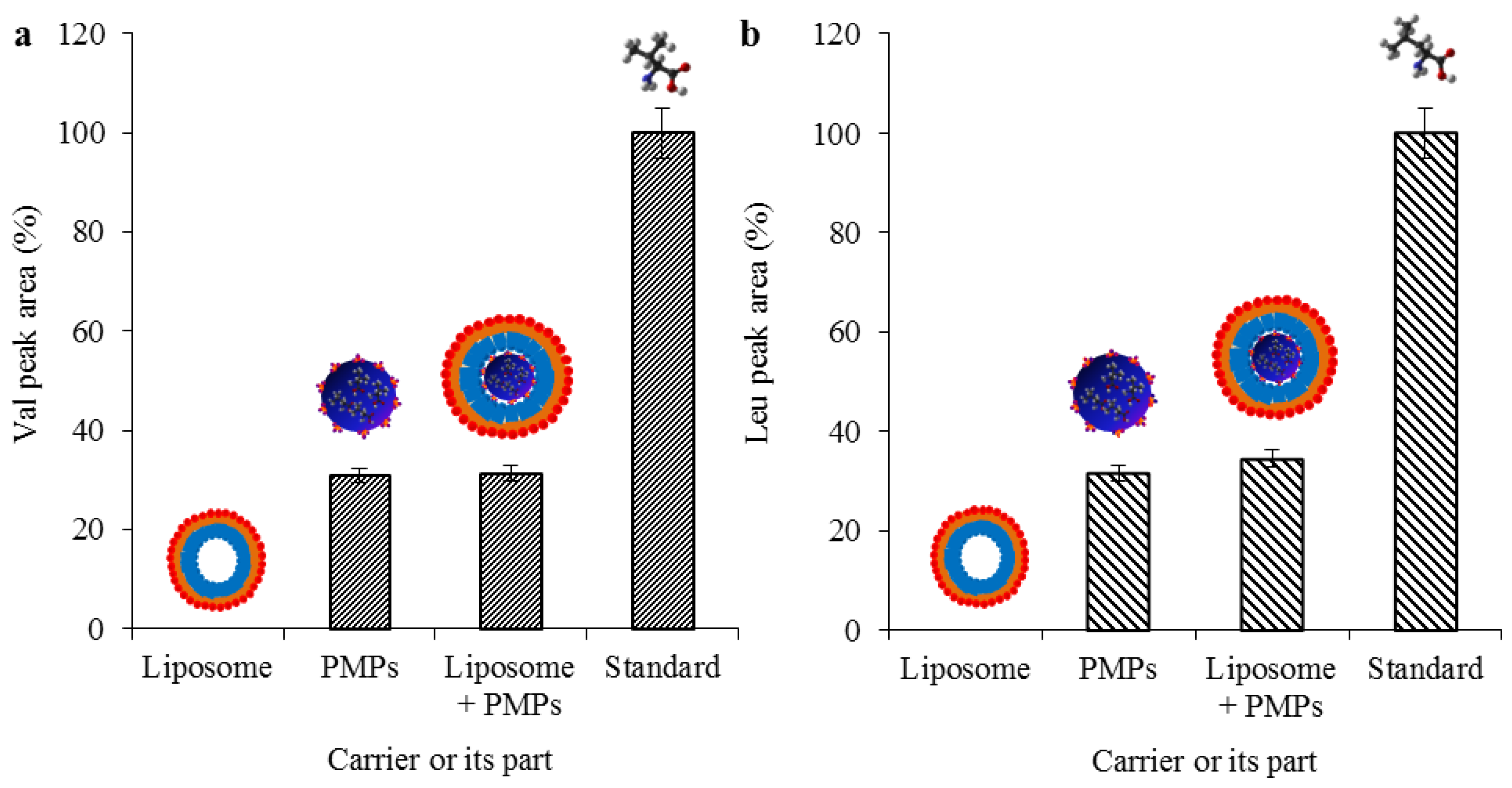
2.3. Evaluation of Transporter Complex Individual Parts Binding Affinity towards BCAAs
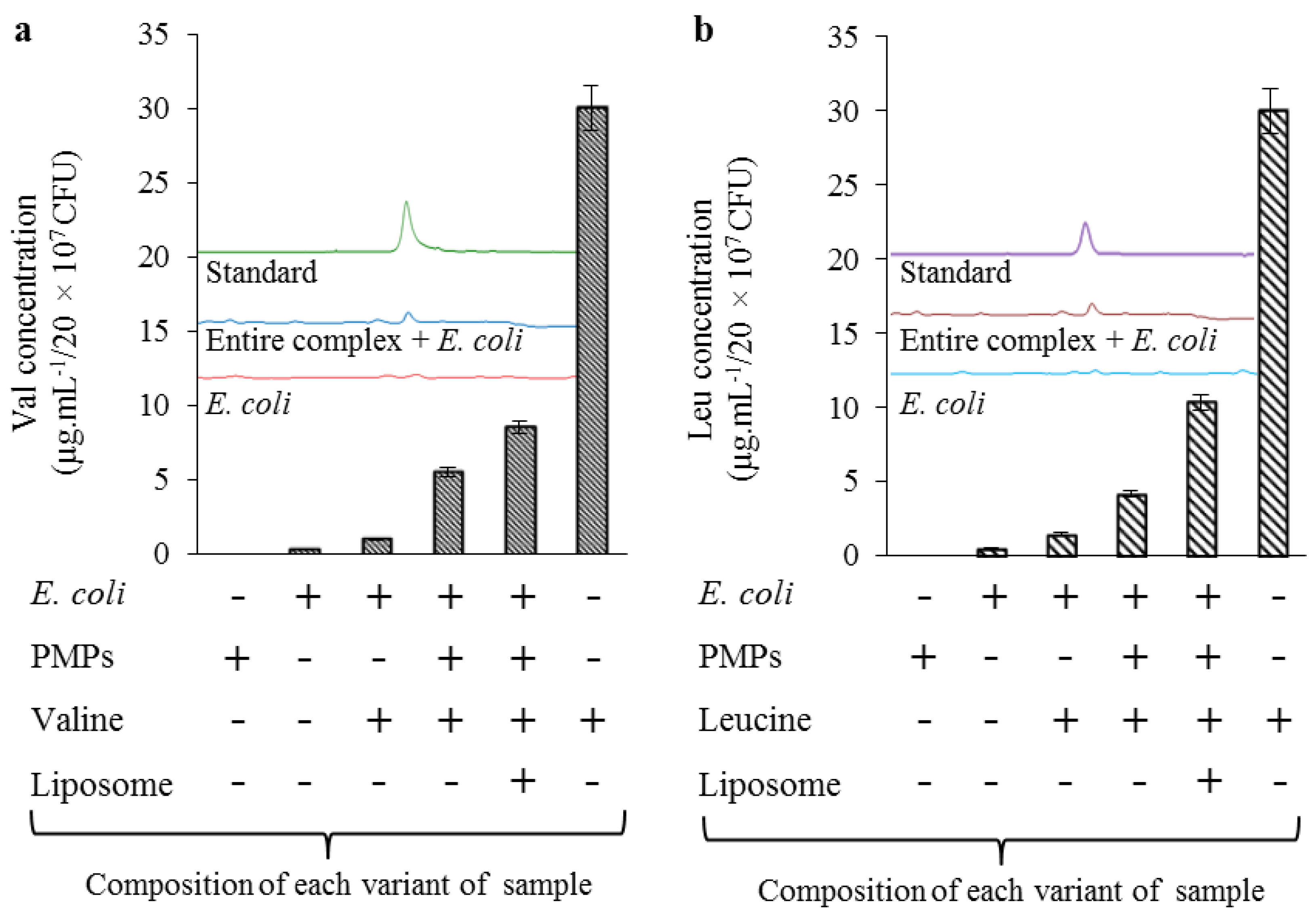
2.4. Uptake Efficiency of Lipoplex-Based Magnetic Nanotransporter Tested on E. Coli Strain
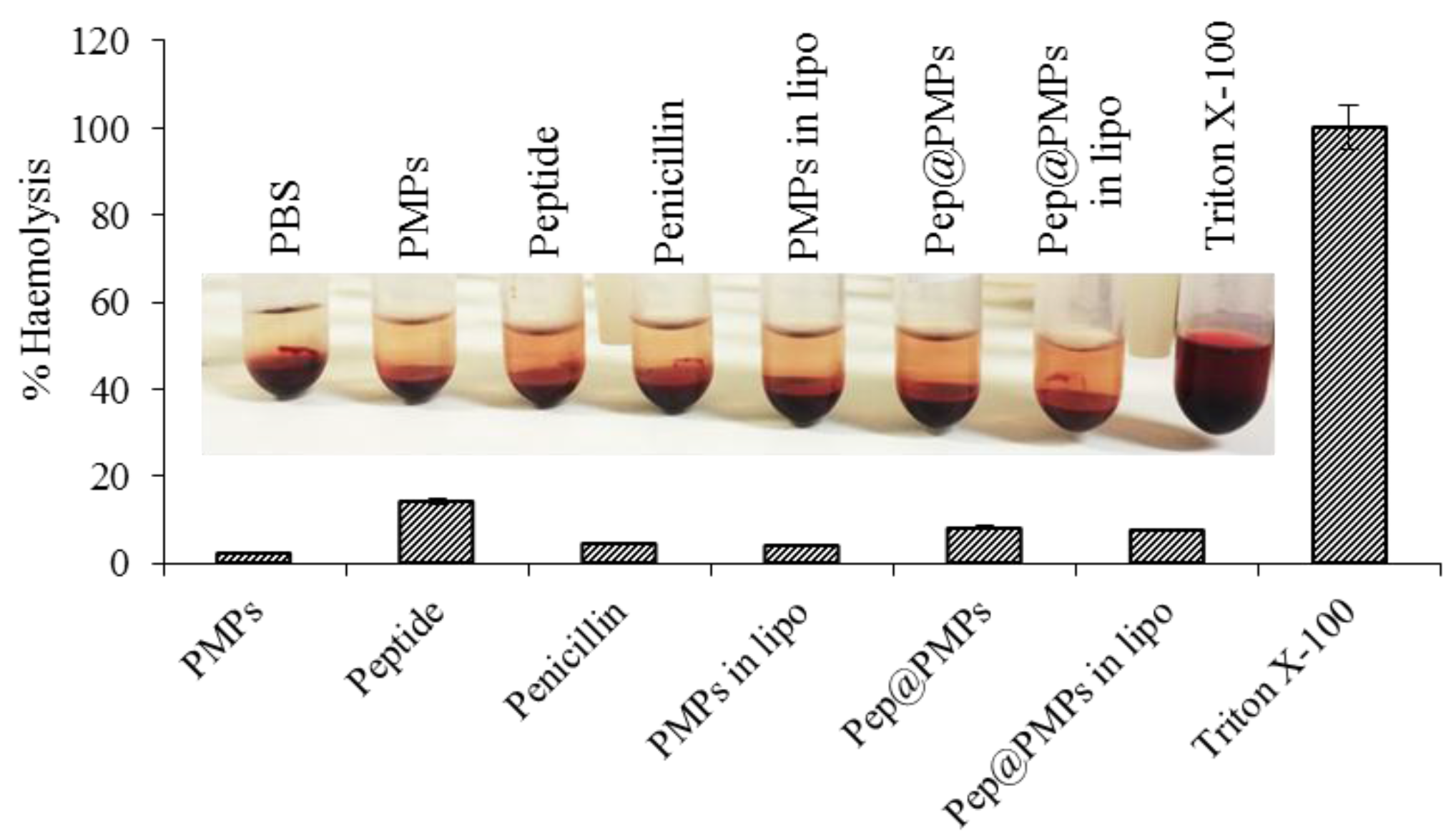
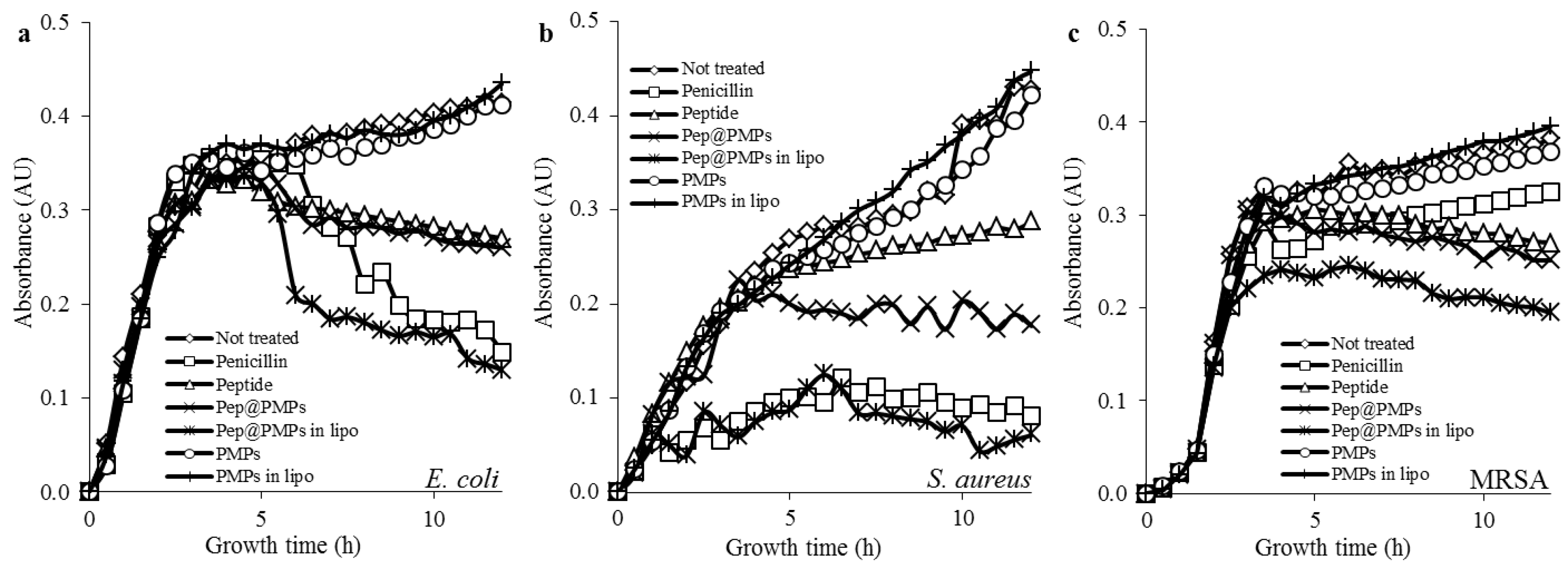
2.5. Testing of Magnetic Lipoplex System Bearing Antimicrobial Peptide
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Nanomaghemite and Peptide
3.3. Modification of Nanomaghemite Core with APTES
3.4. Characterization of Nanomahemite@APTES Particles Morphology
3.5. Determination of Size Distribution and Zeta Potential
3.6. X-Ray Fluorescence Analysis of Paramagnetic Particles
3.7. Workflow Process of Isolation of Amino Acids/Peptide Using APTES@Nanomaghemite
3.8. Determination of Relative Current Response of Particles/Liquid Interface
3.9. Ion-Exchange Liquid Chromatography Analyses of PMPs Chemical Moieties Specifity
3.10. ATR-FT-IR
3.11. Preparation of Liposomes
3.12. Encapsulation of Particles Bearing BCAAs/Peptide into Liposome Inner Cavity
3.13. Escherichia Coli, Staphylococcus Aureus, and MRSA Cultivation, Treatment, and Harvest
3.14. HPLC-ESI QTOF Mass Spectrometry
3.15. Hemocompatibility Assay
3.16. Determination of Growth Curves
3.17 Descriptive Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BCAAs: | Branched chain amino acids |
| Val: | Valine |
| Leu: | Leucine |
| Ile: | Isoleucine |
| Arg: | Arginine |
| APTES: | (3-aminopropyl)triethoxysilane |
| mTOR: | mammalian target of rapamycin |
| MRI: | Magnetic resonance imaging |
| E. coli : | Escherichia coli |
| S. aureus : | Staphylococcus aureus |
| MRSA: | Methicilin-resistant S. aureus |
| HPLC-ESI-QTOF: | High performance liquid chromatography electrospray ionization quadrupole time of flight |
| RBCs: | Red blood cells |
| SEM: | Scanning electron microscopy |
| IEC: | Ion-exchange chromatography |
| SECM: | Scanning electrochemical microscope |
| ATR-FT-IR: | Attenuated total reflectance Fourier transform infrared spectroscopy |
| PMPs: | Paramagnetic particles |
| AMPs: | Antimicrobial peptides |
| PBS: | Phosphate buffered saline |
| XRF: | X-ray fluorescence |
References
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Seo, S.W.; Jung, G.Y.; Lee, J. Easy access to efficient magnetically recyclable separation of histidine-tagged proteins using superparamagnetic nickel ferrite nanoparticle clusters. J. Mater. Chem. 2011, 21, 6713–6717. [Google Scholar] [CrossRef]
- Pompa, P.P.; Martiradonna, L.; Torre, A.D.; Sala, F.D.; Manna, L.; De Vittorio, M.; Calabi, F.; Cingolani, R.; Rinaldi, R. Metal-enhanced fluorescence of colloidal nanocrystals with nanoscale control. Nat. Nano 2006, 1, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Liu, F.-K.; Ko, F.-H. Potential role of gold nanoparticles for improved analytical methods: An introduction to characterizations and applications. Anal. Bioanal. Chem. 2011, 399, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-K. Analysis and applications of nanoparticles in the separation sciences: A case of gold nanoparticles. J. Chromatogr. A 2009, 1216, 9034–9047. [Google Scholar] [CrossRef] [PubMed]
- Pinzon-Daza, M.L.; Campia, I.; Kopecka, J.; Garzon, R.; Ghigo, D.; Riganti, C. Nanoparticle- and liposome-carried drugs: New strategies for active targeting and drug delivery across blood-brain barrier. Current Drug Metab. 2013, 14, 625–640. [Google Scholar] [CrossRef]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.G. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002, 269, 5338–5349. [Google Scholar] [CrossRef] [PubMed]
- Katta, A.; Kundla, S.; Kakarla, S.K.; Wu, M.Z.; Fannin, J.; Paturi, S.; Liu, H.; Addagarla, H.S.; Blough, E.R. Impaired overload-induced hypertrophy is associated with diminished mtor signaling in insulin-resistant skeletal muscle of the obese zucker rat. Am. J. Physiol. Regul. Integr. Compar. Physiol. 2010, 299, R1666–R1675. [Google Scholar] [CrossRef] [PubMed]
- She, P.X.; Olson, K.C.; Kadota, Y.; Inukai, A.; Shimomura, Y.; Hoppel, C.L.; Adams, S.H.; Kawamata, Y.; Matsumoto, H.; Sakai, R.; et al. Leucine and protein metabolism in obese zucker rats. PLos ONE 2013, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Q.; Lv, Y.F.; Gu, Y.; Dong, N.; Li, D.S.; Shan, A.S. Rational design of cationic antimicrobial peptides by the tandem of leucine-rich repeat. Amino Acids 2013, 44, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Ma, Q.Q.; Shan, A.S.; Lv, Y.F.; Hu, W.N.; Gu, Y.; Li, Y.Z. Strand length-dependent antimicrobial activity and membrane-active mechanism of arginine- and valine-rich beta-hairpin-like antimicrobial peptides. Antimicrob. Agents Chemother. 2012, 56, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Pauser, S.; Reszka, R.; Wagner, S.; Wolf, K.J.; Buhr, H.J.; Berger, G. Liposome-encapsulated superparamagnetic iron oxide particles as markers in an mri-guided search for tumor-specific drug carriers. Anti Cancer Drug Des. 1997, 12, 125–135. [Google Scholar]
- Pauser, S.; Reszka, R.; Wagner, S.; Wolf, K.J.; Buhr, H.J.; Berger, G. Superparamagnetic Iron Oxide Particles as Marker Substances for Searching Tumor Specific Liposomes with Magnetic Resonance Imaging; Plenum Press Div Plenum Publishing Corp: New York, NY, USA, 1997; pp. 561–568. [Google Scholar]
- Bakkerwoudenberg, I.; Lokerse, A.F.; Tenkate, M.T.; Melissen, P.M.B.; Vanvianen, W.; Vanetten, E.W.M. Liposomes as carriers of antimicrobial agents or immunomodulatory agents in the treatment of infections. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 61–67. [Google Scholar] [CrossRef]
- Moribe, K.; Maruyama, K. Pharmaceutical design of the liposomal antimicrobial agents for infectious disease. Curr. Pharm. Des. 2002, 8, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.W.; Gitter, B.; Ruger, R.; Wieland, G.D.; Chen, M.; Liu, X.L.; Albrecht, V.; Fahr, A. Antimicrobial peptide-modified liposomes for bacteria targeted delivery of temoporfin in photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2011, 10, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Goli, K.K.; Gera, N.; Liu, X.M.; Rao, B.M.; Rojas, O.J.; Genzer, J. Generation and properties of antibacterial coatings based on electrostatic attachment of silver nanoparticles to protein-coated polypropylene fibers. ACS Appl. Mater. Interfaces 2013, 5, 5298–5306. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.L. The penicillins: A review and update. J. Midwifery Women Health 2002, 47, 426–434. [Google Scholar] [CrossRef]
- Yao, Z.W.; Hu, W.; Yin, S.; Huang, Z.; Zhu, Q.; Chen, J.N.; Zang, Y.H.; Dong, L.; Zhang, J.F. 3,3′-diindolymethane ameliorates adriamycin-induced cardiac fibrosis via activation of a brca1-dependent anti-oxidant pathway. Pharmacol. Res. 2013, 70, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Schwegmann, H.; Feitz, A.J.; Frimmel, F.H. Influence of the zeta potential on the sorption and toxicity of iron oxide nanoparticles on S. cerevisiae and E. coli. J. Colloid Interface Sci. 2010, 347, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.J.; Xiang, H.; Kim, T.; Chun, M.S.; Lee, K. Surface properties of submicrometer silica spheres modified with aminopropyltriethoxysilane and phenyltriethoxysilane. J. Colloid Interface Sci. 2006, 304, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Xu, X.; Lin, Z.J.; Rai, D.K. Conformational search for zwitterionic leucine and hydrated conformers of both the canonical and zwitterionic leucine using the DFT-CPCM model. Vib. Spectrosc. 2011, 56, 74–81. [Google Scholar] [CrossRef]
- Zitka, O.; Cernei, N.; Heger, Z.; Matousek, M.; Kopel, P.; Kynicky, J.; Masarik, M.; Kizek, R.; Adam, V. Microfluidic chip coupled with modified paramagnetic particles for sarcosine isolation in urine. Electrophoresis 2013, 34, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Ostergaard, J.; Sturup, S.; Gammelgaard, B. Determination of platinum drug release and liposome stability in human plasma by CE-ICP-MS. Int. J. Pharm. 2013, 449, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Arouri, A.; Hansen, A.H.; Rasmussen, T.E.; Mouritsen, O.G. Lipases, liposomes and lipid-prodrugs. Curr. Opin. Colloid Interfaces 2013, 18, 419–431. [Google Scholar] [CrossRef]
- Yeung, T.; Gilbert, G.E.; Shi, J.; Silvius, J.; Kapus, A.; Grinstein, S. Membrane phosphatidylserine regulates surface charge and protein localization. Science 2008, 319, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Lutwyche, P.; Cordeiro, C.; Wiseman, D.J.; St-Louis, M.; Uh, M.; Hope, M.J.; Webb, M.S.; Finlay, B.B. Intracellular delivery and antibacterial activity of gentamicin encapsulated in ph-sensitive liposomes. Antimicrob. Agents Chemother. 1998, 42, 2511–2520. [Google Scholar] [PubMed]
- Meng, Y.S.; Hou, X.C.; Lei, J.X.; Chen, M.M.; Cong, S.C.; Zhang, Y.Y.; Ding, W.M.; Li, G.L.; Li, X.R. Multi-functional liposomes enhancing target and antibacterial immunity for antimicrobial and anti-biofilm against methicillin-resistant staphylococcus aureus. Pharm. Res. 2016, 33, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Campanha, M.T.N.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Interactions between cationic liposomes and bacteria: The physical-chemistry of the bactericidal action. J. Lipid Res. 1999, 40, 1495–1500. [Google Scholar] [PubMed]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, C.; Liang, G.Z.; Zhang, M.Z.; Zheng, J. Engineering antimicrobial peptides with improved antimicrobial and hemolytic activities. J. Chem Inf. Model. 2013, 53, 3280–3296. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Talan, D.A. Management of skin abscesses in the era of methicillin-resistant Staphylococcus Aureus. N. Engl. J. Med. 2014, 370, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Levy, M.; Bacri, J.C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and mri contrast agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, E.; Gil, L.; Israel, L.L.; Passoni, L.; Naddaka, M.; Pucci, A.; Reese, T.; Gomez-Vallejo, V.; Milani, P.; Matteoli, M.; et al. Biocompatible nanocomposite for PET/MRI hybrid imaging. Int. J. Nanomed. 2012, 7, 6021–6033. [Google Scholar]
- Daniele, M.A.; Shaughnessy, M.L.; Roeder, R.; Childress, A.; Bandera, Y.P.; Foulger, S. Magnetic nanoclusters exhibiting protein-activated near-infrared fluorescence. ACS Nano 2013, 7, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Dinu-Pirvu, C.; Ferdes, M.; Butu, A.; Ortan, A.; Ghica, M.V. Physicochemical investigation of low soluble biocompounds entrapped in lipid carriers. Farmacia 2013, 61, 182–192. [Google Scholar]
- Zitka, O.; Heger, Z.; Kominkova, M.; Skalickova, S.; Krizkova, S.; Adam, V.; Kizek, R. Preconcentration based on paramagnetic microparticles for the separation of sarcosine using hydrophilic interaction liquid chromatography coupled with coulometric detection. J. Sep. Sci. 2014, 37, 465–475. [Google Scholar] [CrossRef] [PubMed]
| Cargo | Concentration Used for Encapsulation | Concentration Determined (Recovery) | Complex Size Distribution | Complex Zeta Potential |
|---|---|---|---|---|
| Unit | µg·mL−1 | µg·mL−1 | nm | mV |
| Val | 100 | 31.3 | 131 ± 24 | 28.88 ± 0.38 |
| Leu | 100 | 32.6 | 142 ± 26 | 33.05 ± 0.54 |
| Treatment Agent | Concentration Applied | Concentration Analyzed in E. Coli * | Repeatability ** (n = 5) | Uptake Efficiency | Reproducibility *** (n = 5) |
|---|---|---|---|---|---|
| Unit | µg·mL−1 | µg·mL−1 | (%) | (%) | (%) |
| Val | 30 | 1.0 | 5.8 | 3.3 | 9.8 |
| Val@PMPs * | 30 | 5.5 | 8.5 | 18.3 | 10.0 |
| Val@PMPs in lipo * | 30 | 8.6 | 7.1 | 28.7 | 8.2 |
| Leu | 30 | 1.5 | 6.2 | 5.0 | 4.1 |
| Leu@PMPs * | 30 | 4.1 | 6.9 | 13.7 | 11.2 |
| Leu@PMPs in lipo * | 30 | 10.4 | 7.6 | 34.7 | 6.1 |
| Cargo | Concentration Used for Encapsulation | Concentration Determined (Recovery) | Complex Size Distribution | Complex zeta Potential |
|---|---|---|---|---|
| Unit | µg·mL−1 | µg·mL−1 | nm | mV |
| Peptide | 50 | 15.1 | 168 ± 49 | 39.11 ± 4.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesely, R.; Jelinkova, P.; Hegerova, D.; Cernei, N.; Kopel, P.; Moulick, A.; Richtera, L.; Heger, Z.; Adam, V.; Zitka, O. Nanoparticles Suitable for BCAA Isolation Can Serve for Use in Magnetic Lipoplex-Based Delivery System for L, I, V, or R-rich Antimicrobial Peptides. Materials 2016, 9, 260. https://doi.org/10.3390/ma9040260
Vesely R, Jelinkova P, Hegerova D, Cernei N, Kopel P, Moulick A, Richtera L, Heger Z, Adam V, Zitka O. Nanoparticles Suitable for BCAA Isolation Can Serve for Use in Magnetic Lipoplex-Based Delivery System for L, I, V, or R-rich Antimicrobial Peptides. Materials. 2016; 9(4):260. https://doi.org/10.3390/ma9040260
Chicago/Turabian StyleVesely, Radek, Pavlina Jelinkova, Dagmar Hegerova, Natalia Cernei, Pavel Kopel, Amitava Moulick, Lukas Richtera, Zbynek Heger, Vojtech Adam, and Ondrej Zitka. 2016. "Nanoparticles Suitable for BCAA Isolation Can Serve for Use in Magnetic Lipoplex-Based Delivery System for L, I, V, or R-rich Antimicrobial Peptides" Materials 9, no. 4: 260. https://doi.org/10.3390/ma9040260
APA StyleVesely, R., Jelinkova, P., Hegerova, D., Cernei, N., Kopel, P., Moulick, A., Richtera, L., Heger, Z., Adam, V., & Zitka, O. (2016). Nanoparticles Suitable for BCAA Isolation Can Serve for Use in Magnetic Lipoplex-Based Delivery System for L, I, V, or R-rich Antimicrobial Peptides. Materials, 9(4), 260. https://doi.org/10.3390/ma9040260










