Electronic Structure and Optical Property Analysis of Al/Ga-Codoped ZnO through First-Principles Calculations
Abstract
:1. Introduction
2. Calculation Models and Methods
3. Results and Discussion
3.1. Geometric Structure
3.2. Charge Density
3.3. Electronic Structure
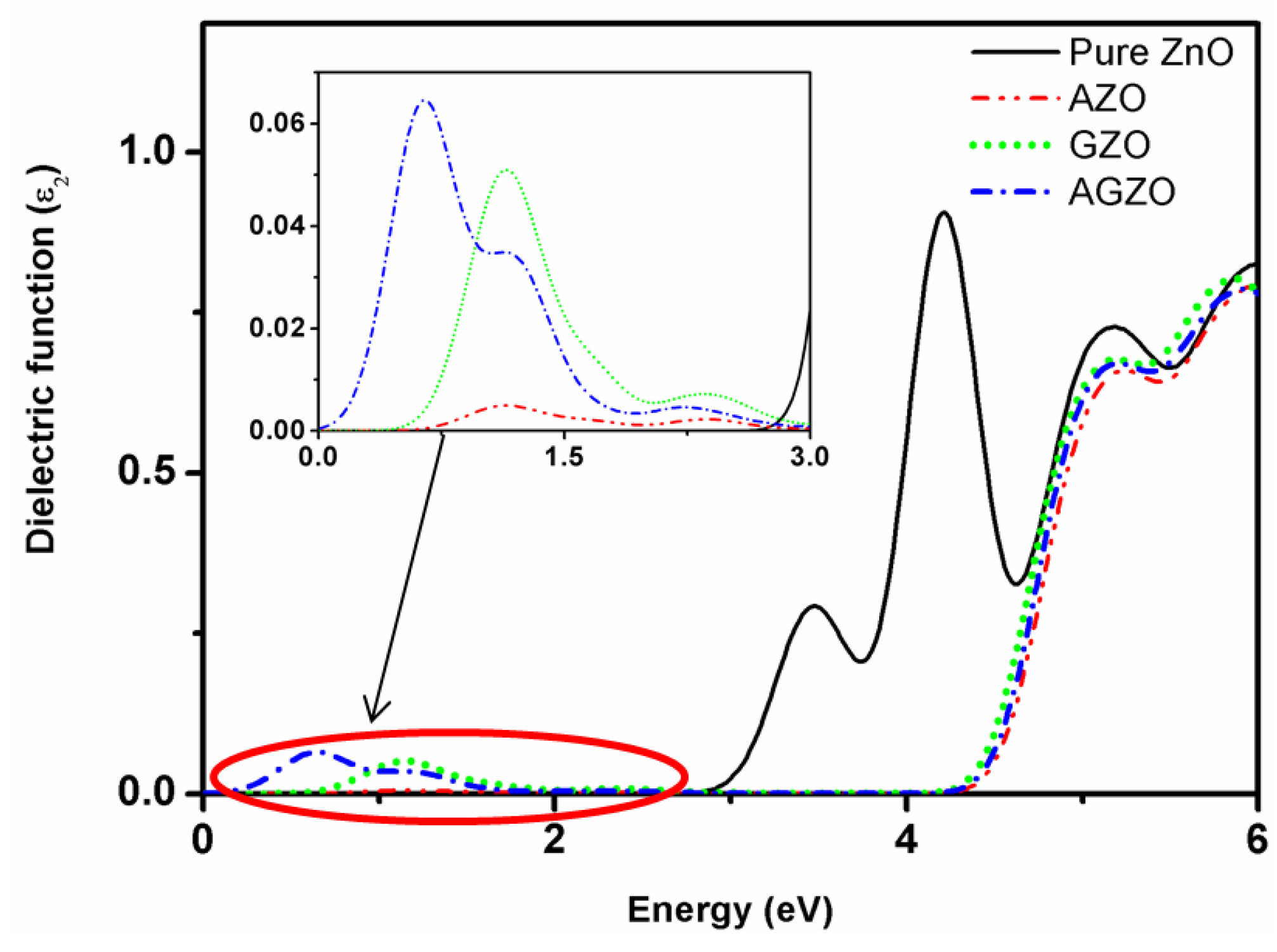
3.4. Optical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interests
References
- Choi, Y.J.; Gong, S.C.; Johnson, D.C.; Golledge, S.; Yeom, G.Y.; Park, H.H. Characteristics of the electromagnetic interference shielding effectiveness of Al-doped ZnO thin films deposited by atomic layer deposition. Appl. Surf. Sci. 2013, 269, 92–97. [Google Scholar] [CrossRef]
- Li, Z.Z.; Chen, Z.Z.; Huang, W.; Chang, S.H.; Ma, X.M. The transparence comparison of Ga- and Al-doped ZnO thin films. Appl. Surf. Sci. 2011, 257, 8486–8489. [Google Scholar] [CrossRef]
- Shin, S.W.; Agawane, G.L.; Kim, I.Y.; Kwon, Y.B.; Jung, I.O.; Gang, M.G.; Moholkar, A.V.; Moon, J.H.; Kim, J.H.; Lee, J.Y. Low temperature epitaxial growth and characterization of Ga-doped ZnO thin films on Al2O3 (0001) substrates prepared with different buffer layers. Appl. Surf. Sci. 2012, 258, 5073–5079. [Google Scholar] [CrossRef]
- Chang, S.C. In-line sputtered gallium and aluminum codoped zinc oxide films for organic solar cells. Int. J. Photoenergy 2014, 2014. [Google Scholar] [CrossRef]
- Ebrahimifard, R.; Golobostanfard, M.R.; Abdizadeh, H. Sol-gel derived Al and Ga co-doped ZnO thin films: An optoelectronic study. Appl. Surf. Sci. 2014, 290, 252–259. [Google Scholar] [CrossRef]
- Lee, W.; Shin, S.; Jung, D.R.; Kim, J.; Nahm, C.; Moon, T.; Park, B. Investigation of electronic and optical properties in Al-Ga codoped ZnO thin films. Curr. Appl. Phys. 2012, 12, 628–631. [Google Scholar] [CrossRef]
- Shin, J.H.; Shin, D.K.; Lee, H.Y.; Lee, J.Y.; Cho, N.I.; Lee, S.J. Characteristics of gallium and aluminum co-doped ZnO (GAZO) transparent thin films deposited by using the PLD process. J. Korean Phys. Soc. 2009, 55, 947–951. [Google Scholar]
- Liu, J.; Zhang, W.J.; Song, D.Y.; Ma, Q.; Zhang, L.; Zhang, H.; Ma, X.B.; Song, H.Y. Comparative study of the sintering process and thin film sputtering of AZO, GZO and AGZO ceramics targets. Ceram. Int. 2014, 40, 12905–12915. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Bao, C.G.; Ma, S.Q.; Hou, S.Z. Effect of crystallinity of ZnO buffer layer on the properties of epitaxial (ZnO:Al)/(ZnO:Ga) bi-layer films deposited on c-sapphire substrate. Appl. Surf. Sci. 2011, 257, 7893–7899. [Google Scholar] [CrossRef]
- Bazzani, M.; Neroni, A.; Calzolari, A.; Catellani, A. Optoelectronic properties of Al:ZnO: Critical dosage for an optimal transparent conductive oxide. Appl. Phys. Lett. 2011, 98. [Google Scholar] [CrossRef]
- Gabás, M.; Torelli, P.; Barrett, N.T.; Sacchi, M.; Bruneval, F.; Cui, Y.; Simonelli, L.; Díaz-Carrasco, P.; Barrado, J.R.R. Direct observation of Al-doping-induced electronic states in the valence band and band gap of ZnO films. Phys. Rev. B Condens. Matter. 2011, 84. [Google Scholar] [CrossRef]
- Palacios, P.; Sánchez, K.; Wahnón, K. Ab-initio valence band spectra of Al, In doped ZnO. Thin Solid Films 2009, 517, 2448–2451. [Google Scholar] [CrossRef]
- Lee, M.H.; Peng, Y.C.; Wu, H.C. Effects of intrinsic defects on electronic structure and optical properties of Ga-doped ZnO. J. Alloys Compd. 2014, 616, 122–127. [Google Scholar] [CrossRef]
- Saniz, R.; Xu, Y.; Matsubara, M.; Amini, M.N.; Dixit, H.; Lamoen, D.; Partoens, B. A simplified approach to the band gap correction of defect formation energies: Al, Ga, and In-doped ZnO. J. Phys. Chem. Solids 2013, 74, 45–50. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Mat. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Wu, H.C.; Peng, Y.C.; Chen, C.C. Effects of Ga concentration on electronic and optical properties of Ga-doped ZnO from first principles calculations. Opt. Mater. 2013, 35, 509–515. [Google Scholar] [CrossRef]
- Kisi, E.H.; Elcombe, M.M. u parameters for the wurtzite structure of ZnS and ZnO using powder neutron diffraction. Acta Crystallogr. Sec. C 1989, 45, 1867–1870. [Google Scholar] [CrossRef]
- Macdonald, F.; Lide, D.R. CRC handbook of chemistry and physics: From paper to web. Abstr. Pap. Am. Chem. S 2003, 225, U552. [Google Scholar]
- Lin, Y.C.; Chen, T.Y.; Wang, L.C.; Lien, S.Y. Comparison of AZO, GZO, and AGZO THIN FILMS TCOs applied for a-Si solar cells. J. Electrochem. Soc. 2012, 159, H599–H604. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Shin, J.H.; Shin, D.K.; Lee, H.Y.; Lee, J.Y. Properties of multilayer gallium and aluminum doped ZnO(GZO/AZO) transparent thin films deposited by pulsed laser deposition process. T. Nonferr. Metal. Soc. 2011, 21, S96–S99. [Google Scholar] [CrossRef]
- Wu, H.C.; Peng, Y.C.; Shen, T.P. Electronic and optical properties of substitutional and interstitial Si-doped ZnO. Materials 2012, 5, 2088–2100. [Google Scholar] [CrossRef]
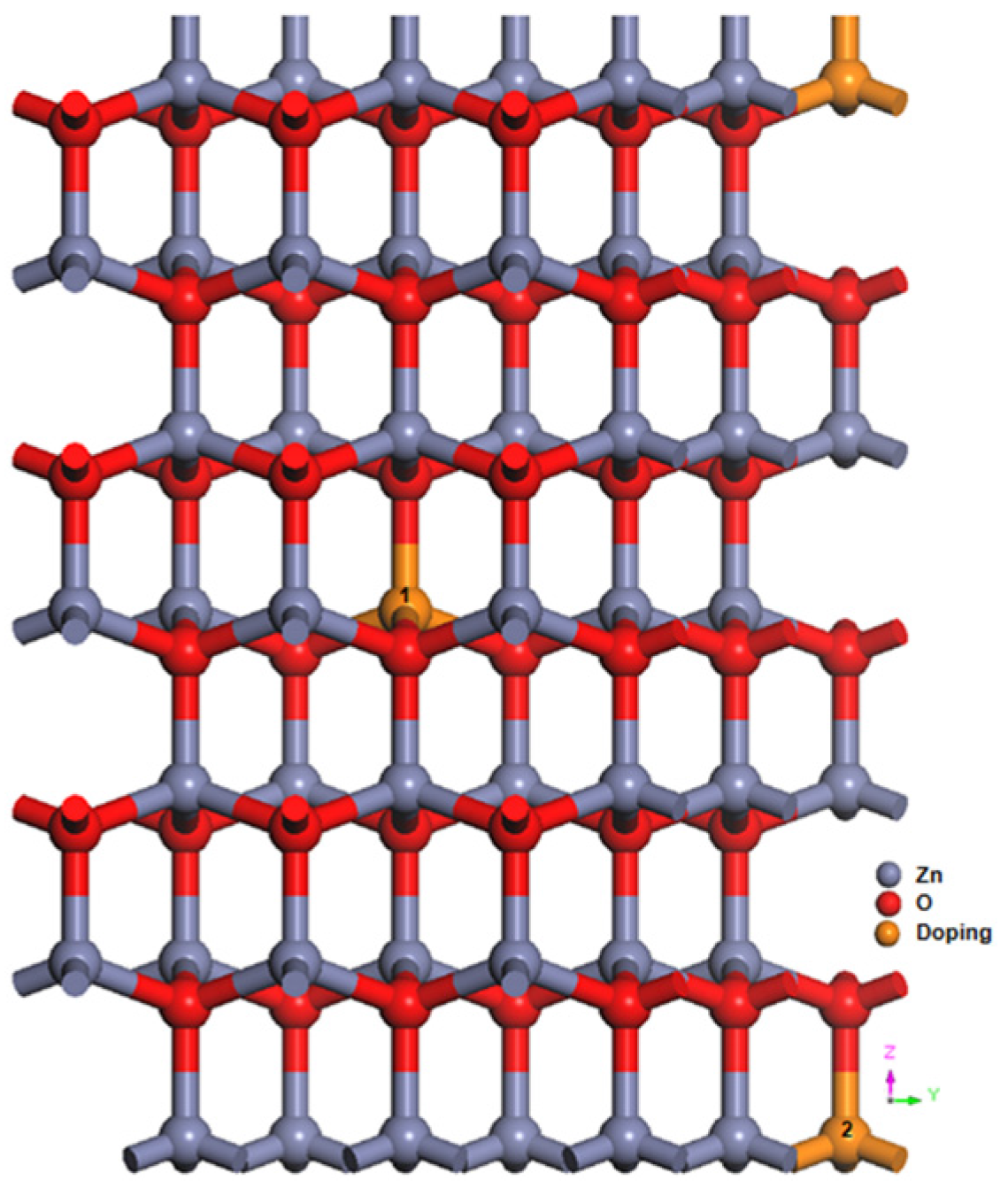
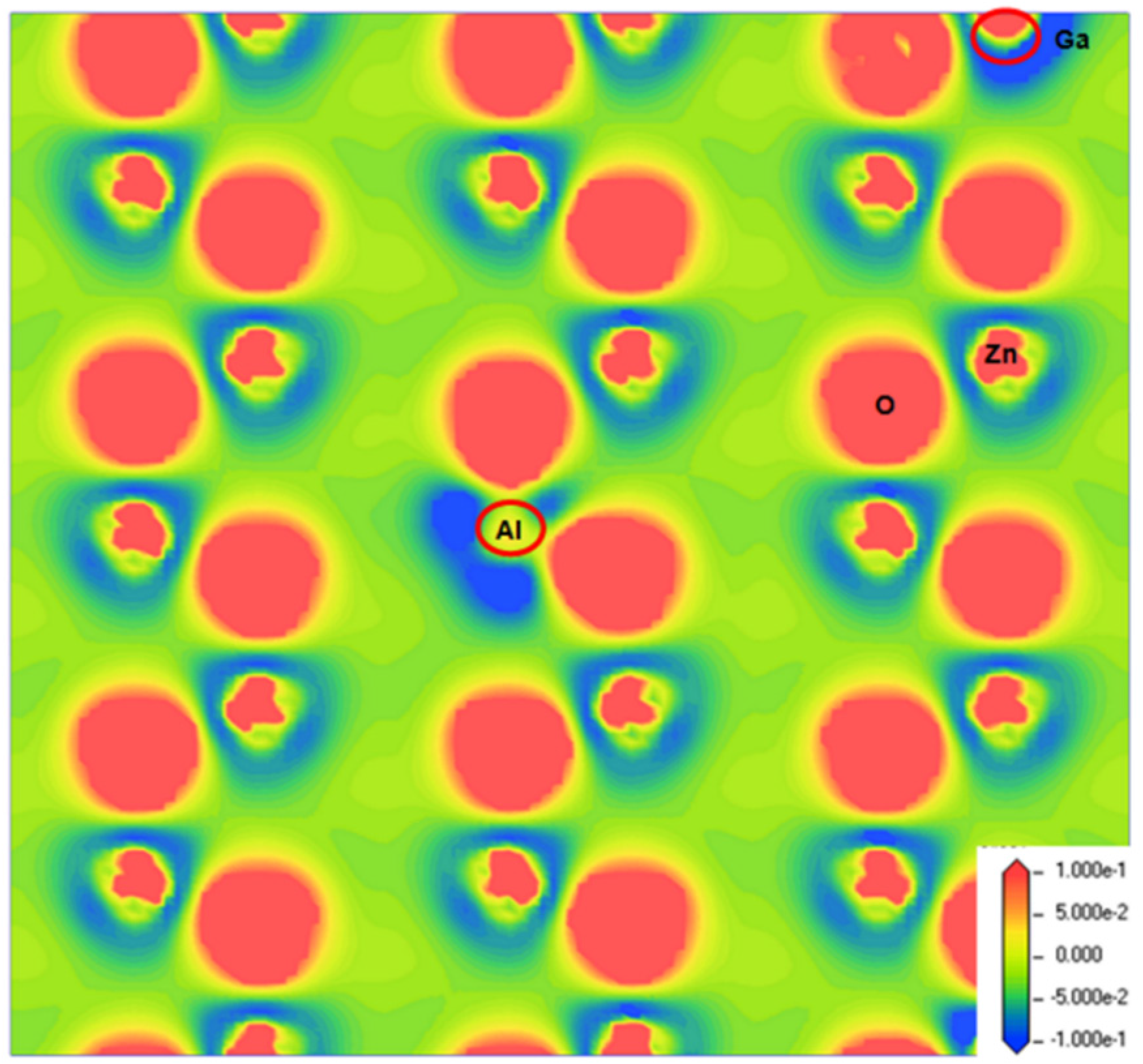
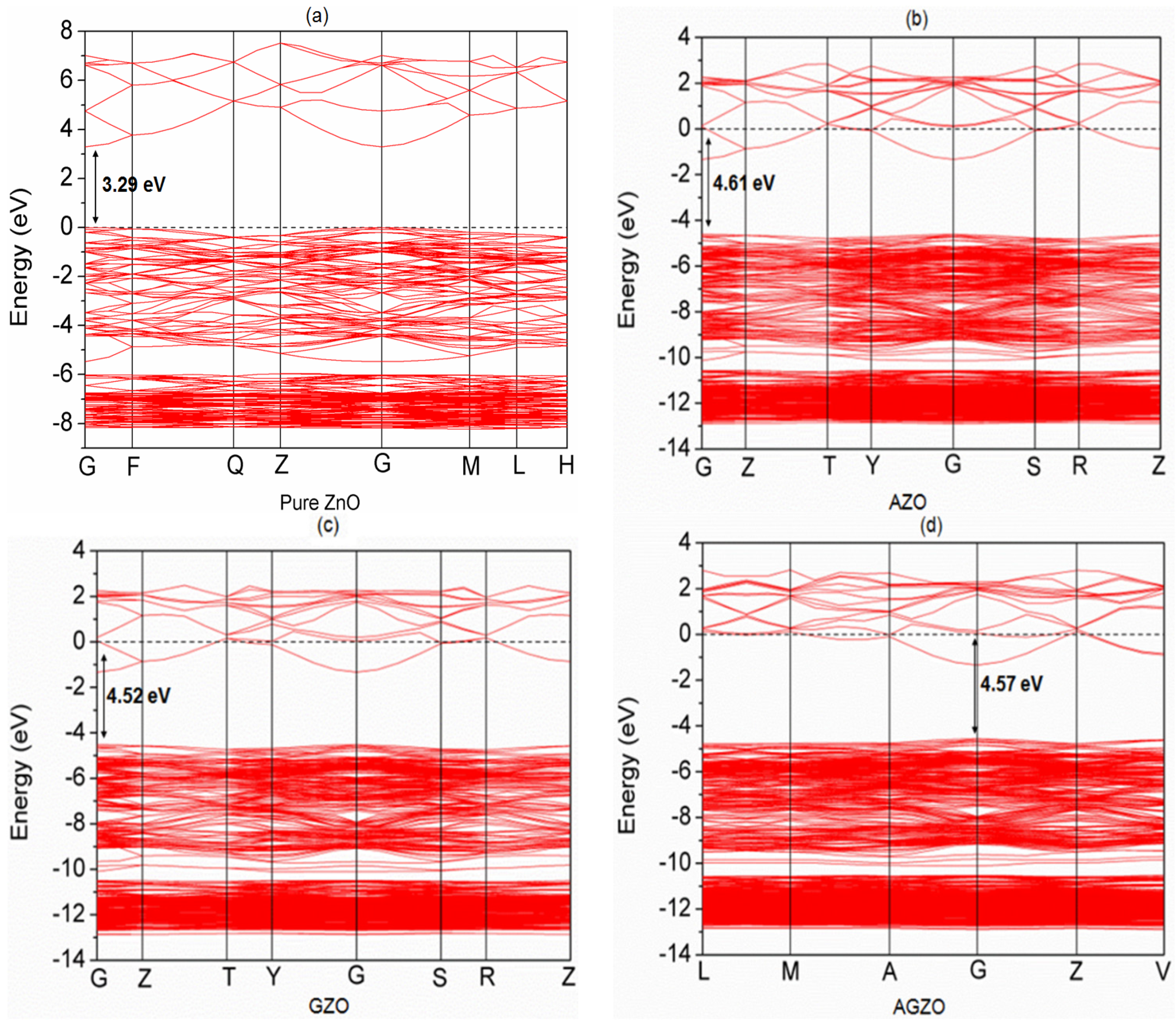
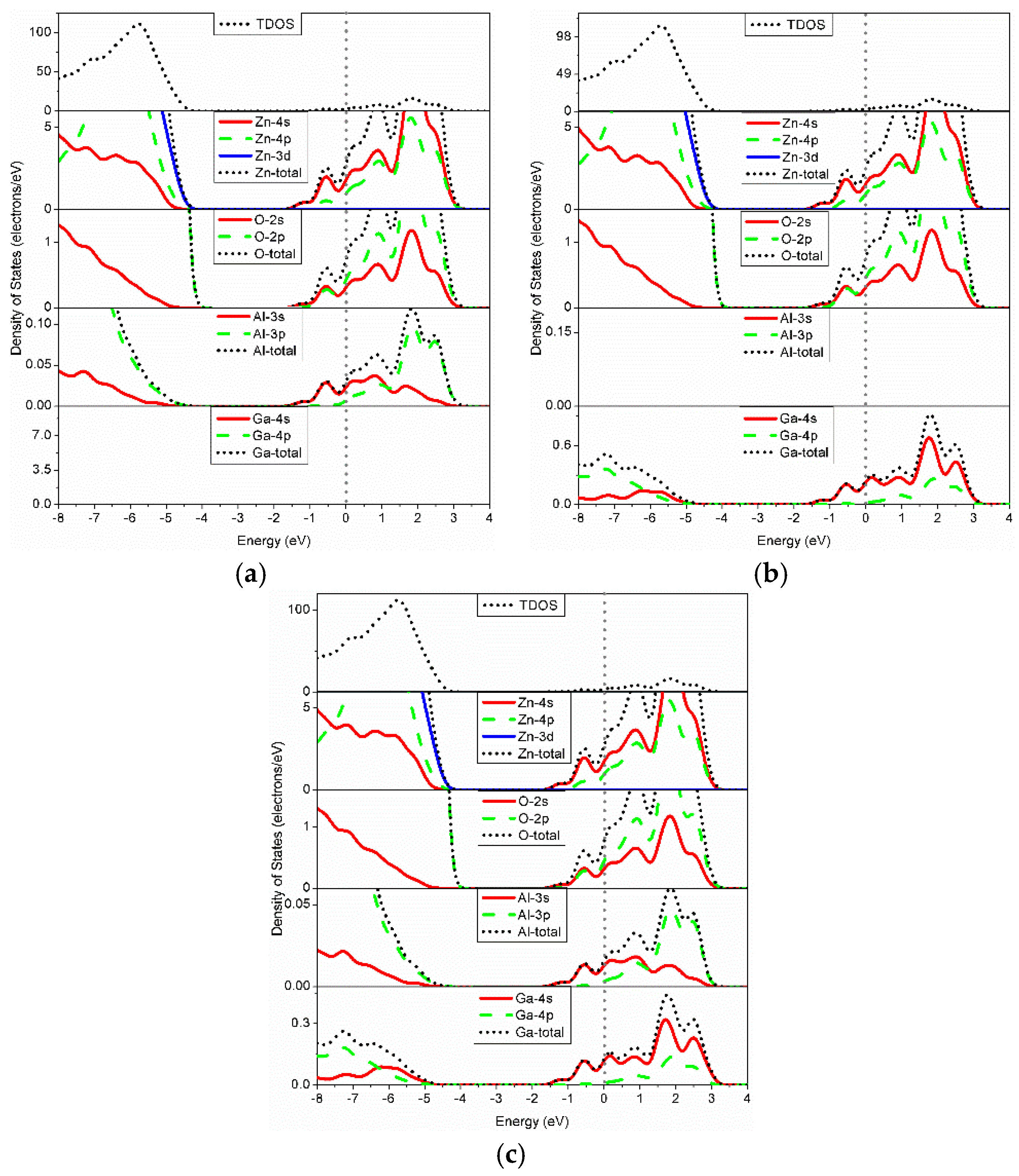
| Structure | a (Å) | c (Å) | c/a | Bond Length (Å) | ||
|---|---|---|---|---|---|---|
| Zn–O | Al–O | Ga–O | ||||
| Pure ZnO | 3.281 | 5.296 | 1.614 | 2.002 | – | – |
| AZO | 3.277 | 5.301 | 1.618 | 2.009 | 1.810 | – |
| GZO | 3.284 | 5.312 | 1.618 | 2.009 | – | 1.908 |
| AGZO | 3.281 | 5.306 | 1.617 | 2.009 | 1.811 | 1.907 |
| Structure | Atomic Population (│e│) | Bond Population | |||||
|---|---|---|---|---|---|---|---|
| Zn | O | Al | Ga | Zn–O | Al–O | Ga–O | |
| Pure ZnO | 0.940 | −0.940 | – | – | 0.400 | – | – |
| AZO | 0.921 | −0.944 | 1.610 | – | 0.384 | 0.498 | – |
| GZO | 0.919 | −0.932 | – | 1.370 | 0.386 | – | 0.475 |
| AGZO | 0.920 | −0.938 | 1.600 | 1.360 | 0.385 | 0.498 | 0.473 |
| Carrier Concentration (#/cm3) | |||
|---|---|---|---|
| Structure | PDOS-Al | PDOS-Ga | TDOS |
| AZO | 1.779 × 1019 | – | 1.733 × 1021 |
| GZO | – | 1.347 × 1020 | 1.753 × 1021 |
| AGZO | 8.557 × 1018 | 7.505 × 1019 | 1.734 × 1021 |
| Structure | 200–400 nm (%) | 400–800 nm (%) |
|---|---|---|
| Pure | 64.9 | 88.4 |
| AZO | 73.4 | 90.9 |
| GZO | 71.2 | 90.6 |
| AGZO | 72.8 | 90.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Wu, H.-C. Electronic Structure and Optical Property Analysis of Al/Ga-Codoped ZnO through First-Principles Calculations. Materials 2016, 9, 164. https://doi.org/10.3390/ma9030164
Chen C-C, Wu H-C. Electronic Structure and Optical Property Analysis of Al/Ga-Codoped ZnO through First-Principles Calculations. Materials. 2016; 9(3):164. https://doi.org/10.3390/ma9030164
Chicago/Turabian StyleChen, Chieh-Cheng, and Hsuan-Chung Wu. 2016. "Electronic Structure and Optical Property Analysis of Al/Ga-Codoped ZnO through First-Principles Calculations" Materials 9, no. 3: 164. https://doi.org/10.3390/ma9030164
APA StyleChen, C.-C., & Wu, H.-C. (2016). Electronic Structure and Optical Property Analysis of Al/Ga-Codoped ZnO through First-Principles Calculations. Materials, 9(3), 164. https://doi.org/10.3390/ma9030164






