Inhibited Bacterial Adhesion and Biofilm Formation on Quaternized Chitosan-Loaded Titania Nanotubes with Various Diameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Loading of HACC
2.3. Characterization of Drug Release from TNTs
2.4. Preparation and Characterization of Bacteria
2.5. Bacterial Adhesion Assay Using the Spread Plate Method
2.6. Biofilm Formation Assay Using the Tissue Culture Plate (TCP) Method
2.7. Observation of Bacterial Adhesion and Biofilm Formation Using Scanning Electron Microscopy (SEM)
2.8 Observation of Bacterial Adhesion and Biofilms Formation Using Confocal Laser Scanning Microscopy (CLSM)
2.9 Statistical Analysis
3. Results
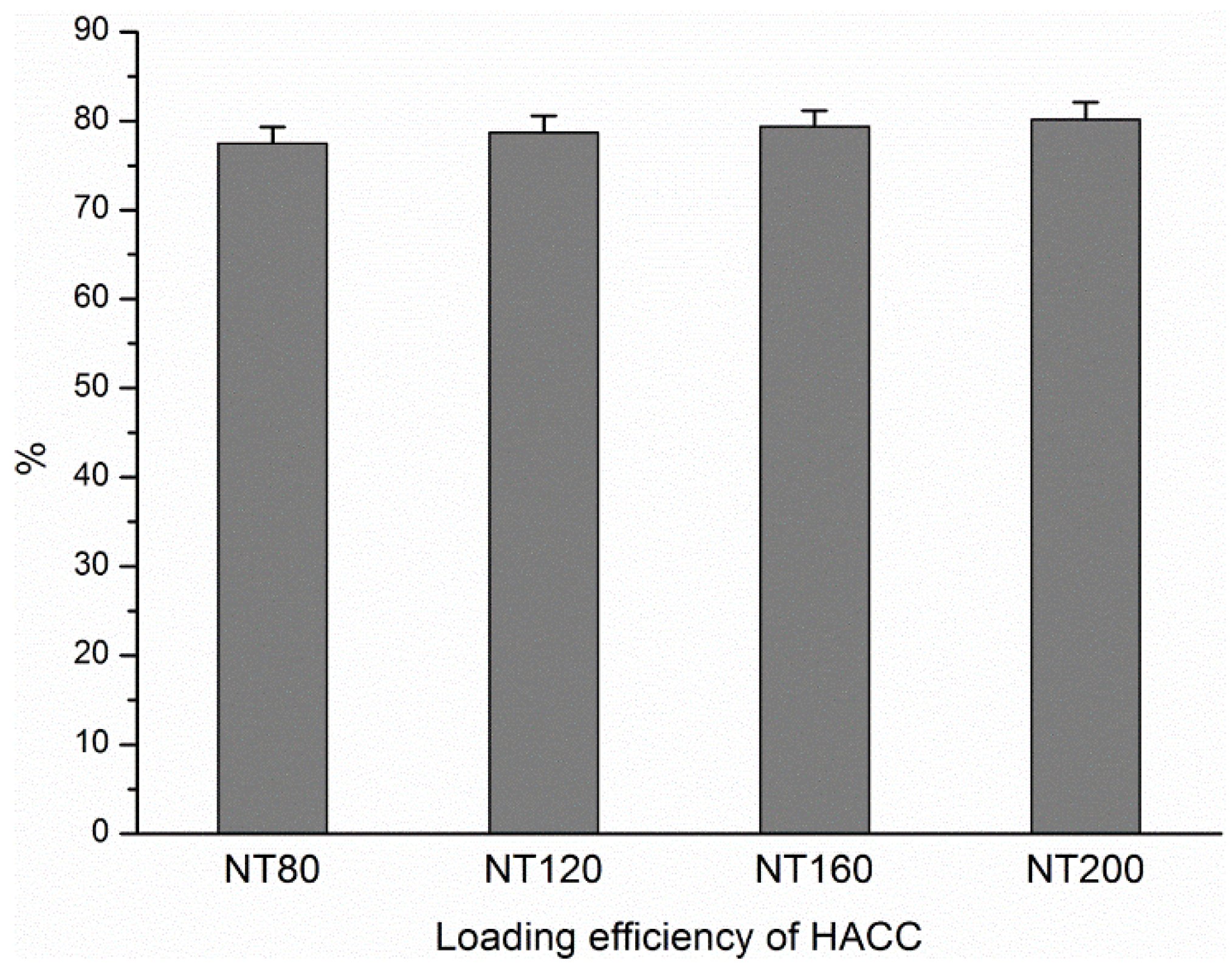
3.1. Loading Efficiency of HACC in Different Diameter Nanotubes
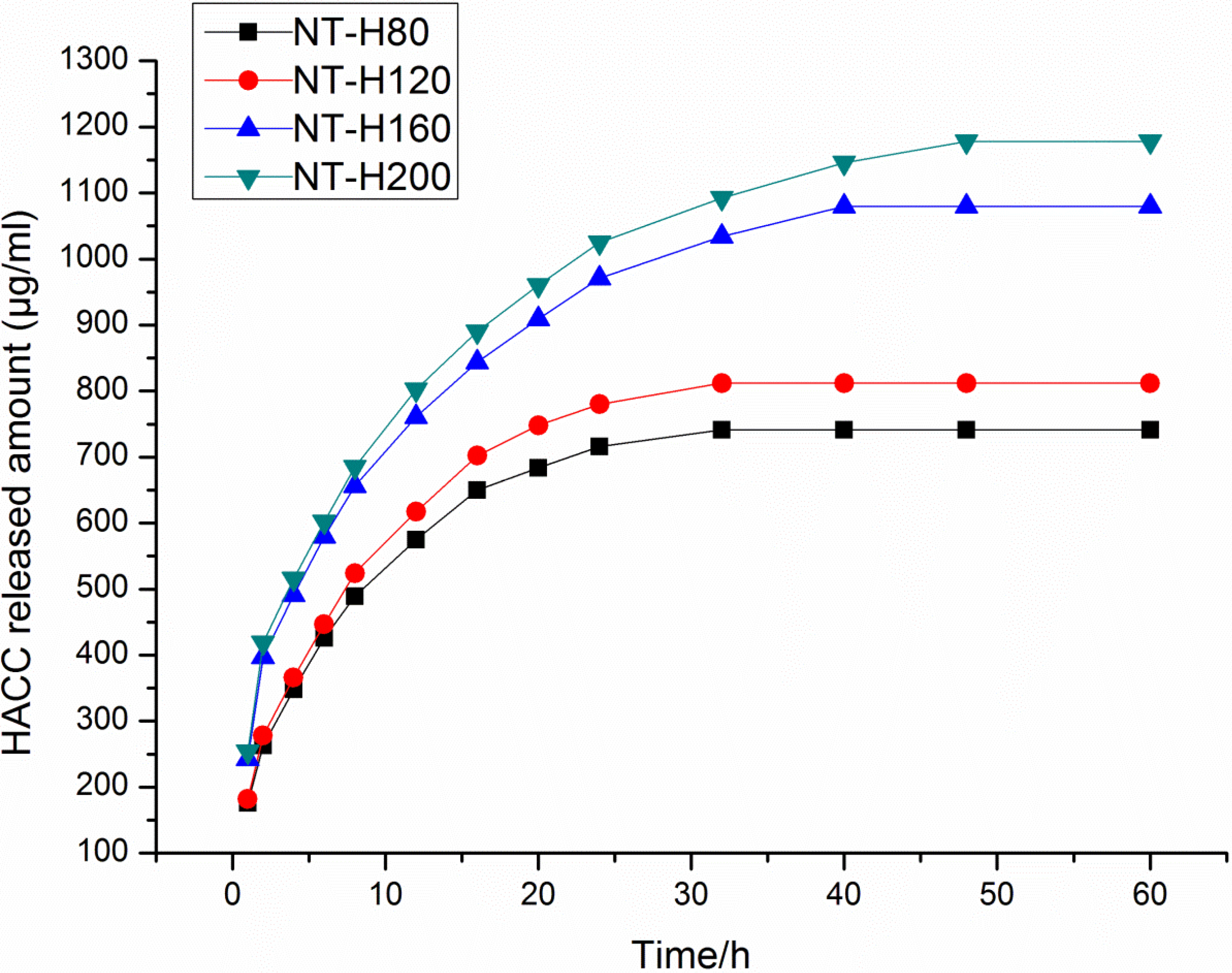
3.2. HACC Release from the Nanotubes
3.3. The MICs of the Bacterial Strains
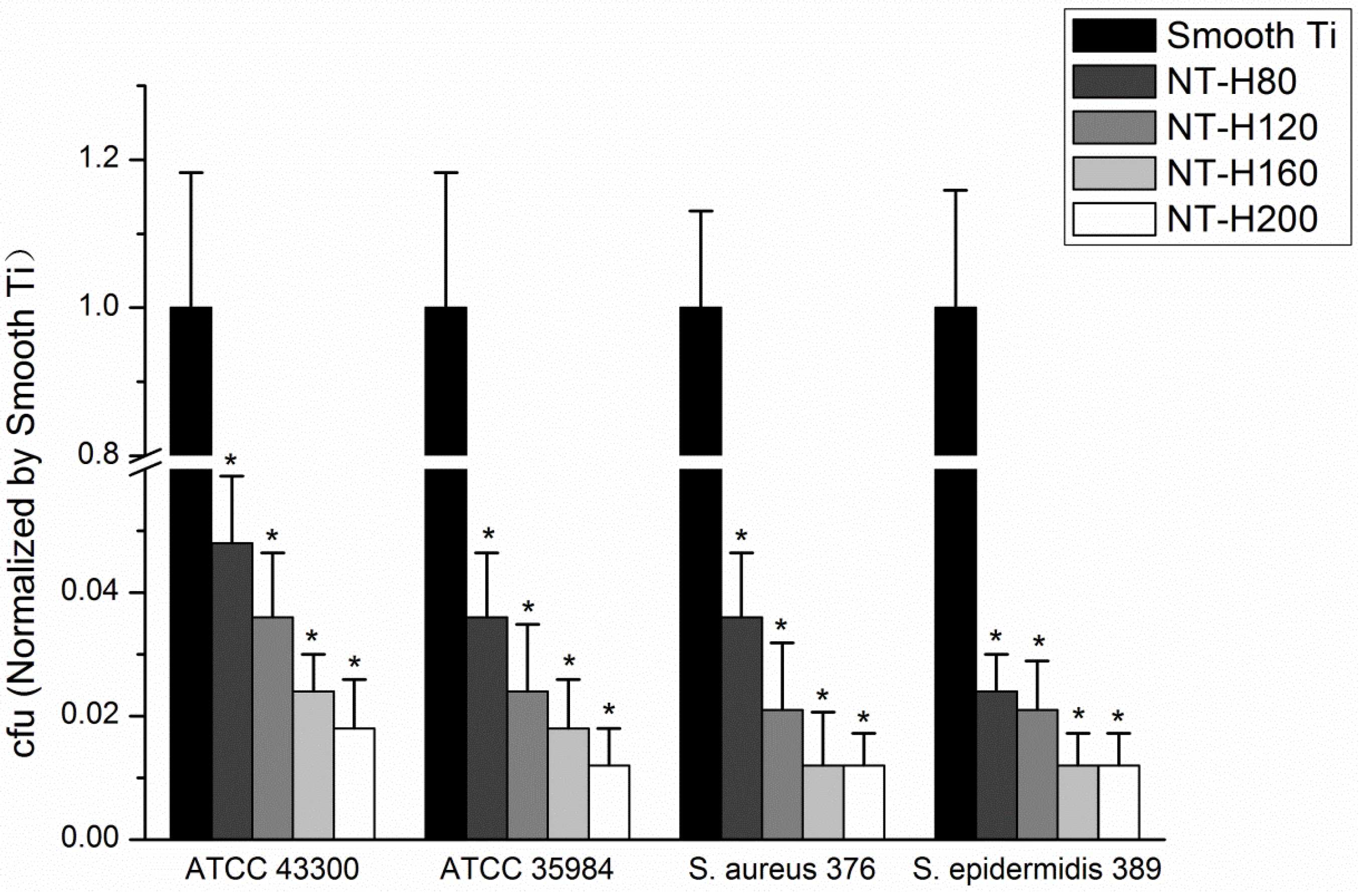
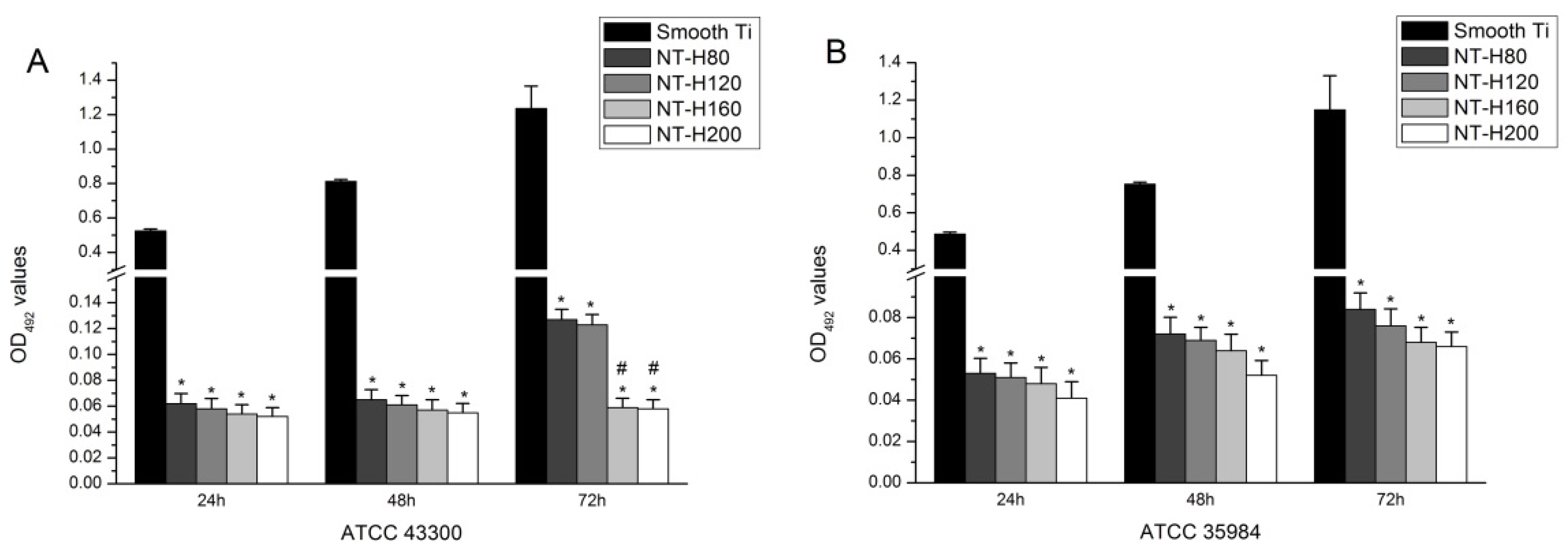
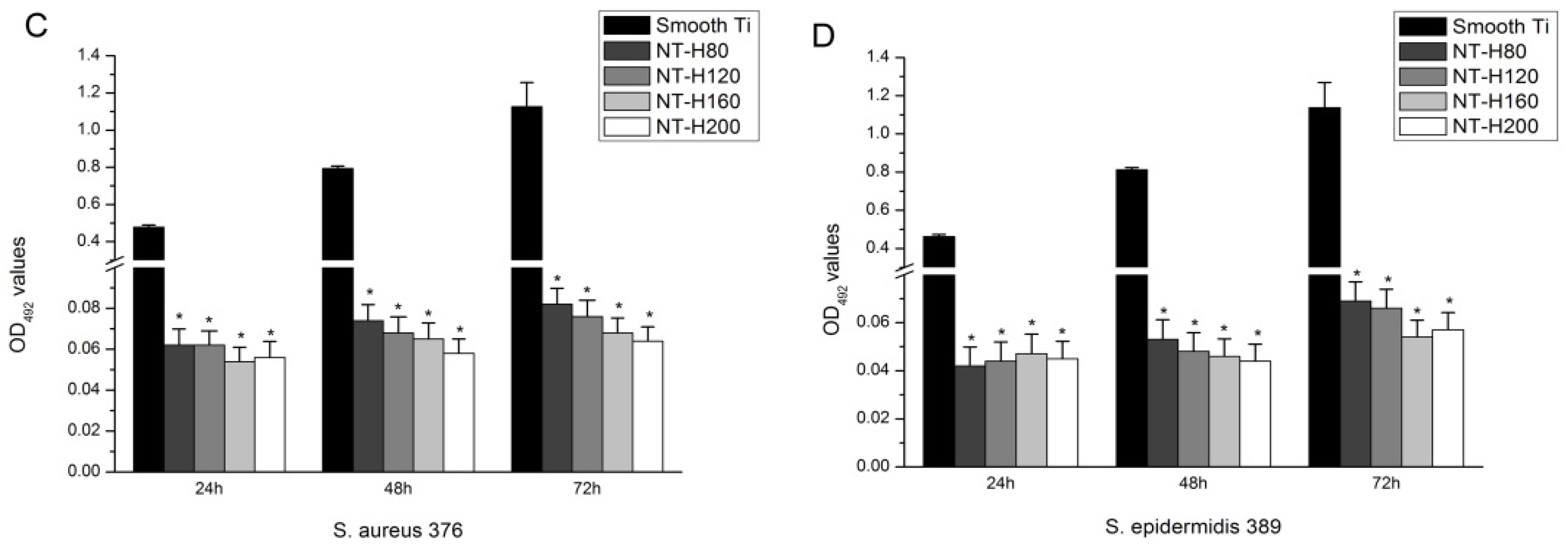
3.4. Inhibition of Bacterial Adhesion and Biofilm Formation on the NT-H
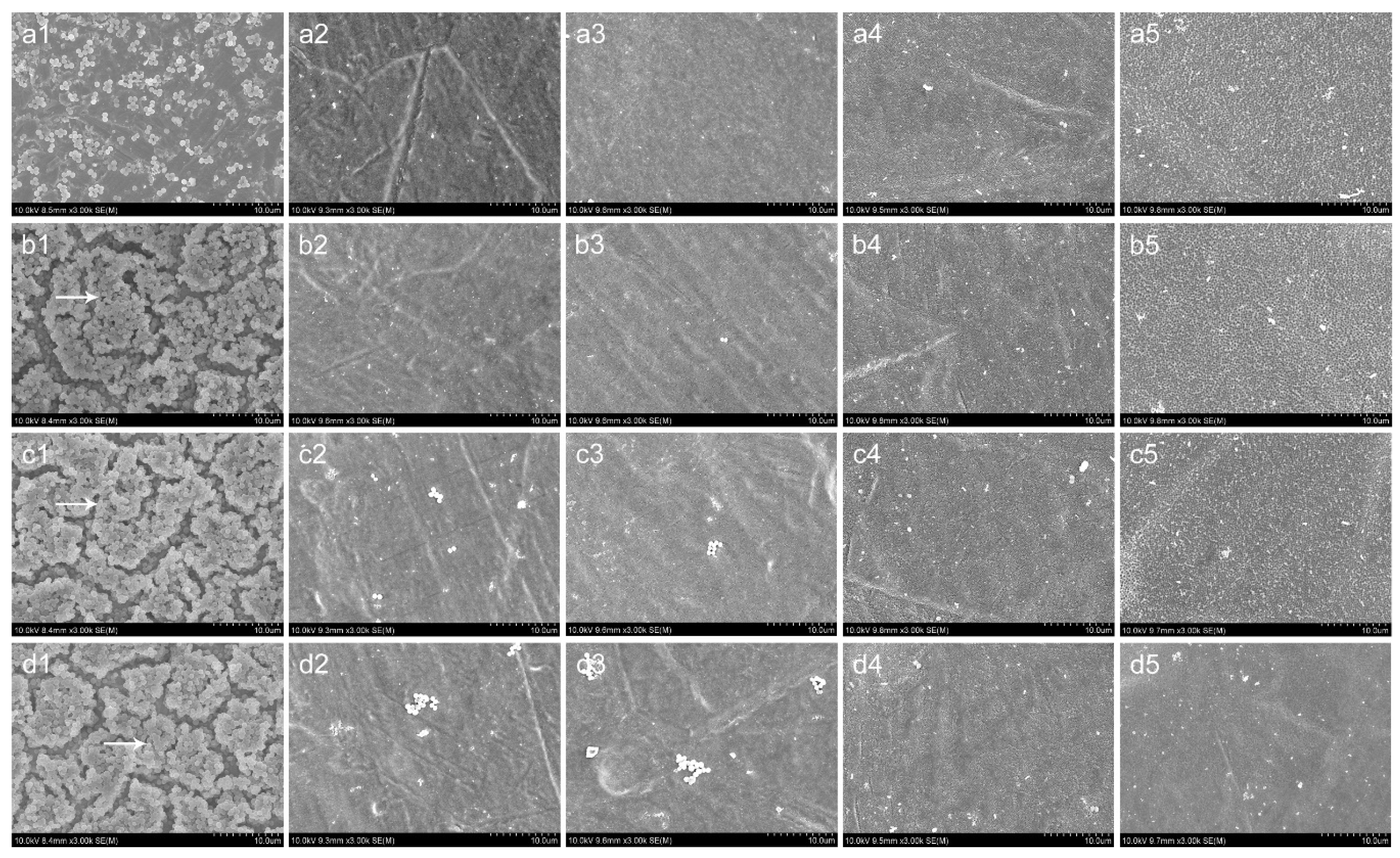
3.5. SEM and CLSM Observation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, K.-J.; Goodman, S.B. Identification of periprosthetic joint infection after total hip arthroplasty. J. Orthop. Translat. 2015, 3, 21–25. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Schmier, J.; Ong, K.L.; Zhao, K.; Parvizi, J. Infection burden for hip and knee arthroplasty in the United States. J. Arthroplast. 2008, 23, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Aw, M.S.; Findlay, D.; Losic, D. Local drug delivery to the bone by drug-releasing implants: Perspectives of nano-engineered titania nanotube arrays. Ther. Deliv. 2012, 3, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Crawford, G.A.; Chawla, N.; Das, K.; Bose, S.; Bandyopadhyay, A. Microstructure and deformation behavior of biocompatible TiO2 nanotubes on titanium substrate. Acta Biomater. 2007, 3, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-W.; Wang, H.; Ni, M.; Rui, Y.-F.; Cheng, T.-Y.; Cheng, C.-K.; Pan, X.-H.; Li, G.; Lin, C.-J. Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J. Orthop. Translat. 2014, 2, 35–42. [Google Scholar] [CrossRef]
- Kumeria, T.; Mon, H.; Aw, M.S.; Gulati, K.; Santos, A.; Griesser, H.J.; Losic, D. Advanced biopolymer-coated drug-releasing titania nanotubes (TNTs) implants with simultaneously enhanced osteoblast adhesion and antibacterial properties. Colloids Surf. B Biointerfaces 2015, 130, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Su, P.; Chen, S.; Wang, N.; Ma, Y.; Liu, Y.; Wang, J.; Zhang, Z.; Li, H.; Webster, T.J. Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale 2014, 6, 9050–9062. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Hang, R.; Huang, X.; Zhao, L.; Zhang, X.; Wang, L.; Tang, B.; Ma, S.; Chu, P.K. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials 2007, 28, 4880–4888. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-T.; Tan, H.-L.; Duan, Z.-L.; Yue, B.; Ma, R.; He, G.; Tang, T.-T. Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters. Int. J. Nanomedicine 2014, 9, 1215–1230. [Google Scholar] [PubMed]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.D. Prophylactic use of antibiotic bone cement: An emerging standard-in opposition. J. Arthroplast. 2004, 19, 73–77. [Google Scholar] [CrossRef]
- Zhilong, S.; Koon Gee, N.; En-Tang, K.; Chyekhoon, P.; Wilson, W. Titanium with surface-grafted dextran and immobilized bone morphogenetic protein-2 for inhibition of bacterial adhesion and enhancement of osteoblast functions. Tissue. Eng. Part A. 2009, 15, 417–426. [Google Scholar]
- Ghasemzadeh-Moghaddam, H.; Ghaznavi-Rad, E.; Sekawi, Z.; Yun-Khoon, L.; Nazri Aziz, M.; Hamat, R.A.; Melles, D.C.; van Belkum, A.; Shamsudin, M.N.; Neela, V. Methicillin-susceptible Staphylococcus aureus from clinical and community sources are genetically diverse. Int. J. Med. Microbiol. 2011, 301, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ince, A.; Schütze, N.; Karl, N.; Löhr, J.F.; Eulert, J. Gentamicin negatively influenced osteogenic function in vitro. Int. Orthop. 2007, 31, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Duewelhenke, N.; Krut, O.; Eysel, P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob. Agents Chemother. 2007, 51, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Goldberg, V.M.; Caplan, A.I. Toxic effects of gentamicin on marrow-derived human mesenchymal stem cells. Clin. Orthop. Relat. Res. 2006, 452, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Georgouli, T.; Bird, H.; Kontakis, G.; Giannoudis, P.V. The effect of antibiotics on bone healing: Current evidence. Expert. Opin. Drug Saf. 2011, 10, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Ma, R.; Lin, C.-C.; Liu, Z.-W.; Tang, T.-T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-X.; Wang, L.; Du, L.; Guo, S.-R.; Wang, X.-Q.; Tang, T.-T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Tan, H.-L.; Peng, Z.-X.; Li, Q.-T.; Xu, X.; Guo, S.-R.; Tang, T.-T. The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus. Biomaterials 2012, 33, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.X.; Tu, B.; Shen, Y.; Du, L.; Wang, L.; Guo, S.R.; Tang, T.-T. Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface. Antimicrob. Agents Chemother. 2011, 55, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Foraker, A.B.; Walczak, R.J.; Cohen, M.H.; Boiarski, T.A.; Grove, C.F.; Swaan, P.W. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal Caco-2 cell monolayers. Pharm. Res. 2003, 20, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.; Laitinen, L.; Kaukonen, A.M.; Tuura, J.; Björkqvist, M.; Heikkilä, T.; Vähä-Heikkilä, K.; Hirvonen, J.; Lehto, V.P. Mesoporous silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Control. Release. 2005, 108, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef]
- Zou, Q.; Li, Y.; Zhang, L.; Zuo, Y.; Li, J.; Li, J. Antibiotic delivery system using nano-hydroxyapatite/chitosan bone cement consisting of berberine. J. Biomed. Mater. Res. A 2009, 89, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Guo, S.-R.; Yang, S.-B.; Xu, X.; Tang, T.-T. Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement. Acta Biomater. 2012, 8, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Weis, P.; Diamond, G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997, 272, 12008–12013. [Google Scholar] [CrossRef] [PubMed]
- Beckloff, N.; Laube, D.; Castro, T.; Furgang, D.; Park, S.; Perlin, D.; Clements, D.; Tang, H.; Scott, R.W.; Tew, G.N.; Diamond, G. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob. Agents Chemother. 2007, 51, 4125–4132. [Google Scholar] [CrossRef] [PubMed]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials 2001, 22, 1607–1611. [Google Scholar] [CrossRef]
- Dunne, N.; Hill, J.; Mcafee, P.; Todd, K.; Kirkpatrick, R.; Tunney, M.; Patrick, S. In vitro study of the efficacy of acrylic bone cement loaded with supplementary amounts of gentamicin: Effect on mechanical properties, antibiotic release, and biofilm formation. Acta Orthop. 2007, 78, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Sherertz, R.J.; Raad, I.I.; Belani, A.; Koo, L.C.; Rand, K.H.; Pickett, D.L.; Straub, S.A.; Fauerbach, L.L. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J. Clin. Microbiol. 1990, 28, 76–82. [Google Scholar] [PubMed]
- Bjerkan, G.; Witsø, E.; Bergh, K. Sonication is superior to scraping for retrieval of bacteria in biofilm on titanium and steel surfaces in vitro. Acta Orthop. 2009, 80, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [PubMed]
- Mathur, T.; Singhal, S.; Khan, S.; Upadhyay, D.; Fatma, T.; Rattan, A. Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J. Med. Microbiol. 2006, 24, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Martincic, I.; Gusinjac, A.; Kalab, M.; Yang, A.F.; Ramirez-Arcos, S. Staphylococcus epidermidis forms biofilms under simulated platelet storage conditions. Transfusion 2007, 47, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Malcata, F.X. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Chen, X.-G.; Liu, C.-S.; Cha, D.-S.; Park, H.J. Oleoyl-chitosan nanoparticles inhibits escherichia coli and staphylococcus aureus by damaging the cell membrane and putative binding to extracellular or intracellular targets. Int. J. Food Microbiol. 2009, 132, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Cerca, N.; Martins, S.; Cerca, F.; Jefferson, K.K.; Pier, G.B.; Oliveira, R.; Azeredo, J. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J. Antimicrob. Chemother. 2005, 56, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Hobgood, C.D.; Webb, L.X.; Myrvik, Q.N. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials 1987, 8, 423–426. [Google Scholar] [CrossRef]
- Neoh, K.G.; Hu, X.; Zheng, D.; Kang, E.T. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Furneri, P.M.; Garozzo, A.; Musumarra, M.P.; Scuderi, A.C.; Russo, A.; Bonfiglio, G. Effects on adhesiveness and hydrophobicity of sub-inhibitory concentrations of netilmicin. Int. J. Antimicrob. Agents 2003, 22, 164–167. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.-C.; Webster, T.J. Increased osteoblast and decreased staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. A 2006, 78, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.-D.; Taylor, E.; Raimondo, T.; Webster, T.-J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-X.; Ni, J.-H.; Zheng, K.; Shen, Y.-D.; Wang, X.-Q.; He, G.; Jin, S.-H.; Tang, T.-T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar]

| Specimen | Total HACC Loaded (μg a/one disc) | Initial Release (μg b) | Total Release (μg) |
|---|---|---|---|
| NT-H80 | 1549 | 575 | 741 |
| NT-H120 | 1573 | 617 | 812 |
| NT-H160 | 1587 | 971 | 1080 |
| NT-H200 | 1603 | 1025 | 1178 |
| Microorganism | MIC (μg/mL) |
|---|---|
| ATCC43300 | 64 |
| ATCC35984 | 32 |
| S. aureus 376 | 32 |
| S. epidermidis 389 | 32 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-t.; Zhang, Y.-y.; Tan, H.-l.; Ao, H.-y.; Duan, Z.-l.; He, G.; Tang, T.-t. Inhibited Bacterial Adhesion and Biofilm Formation on Quaternized Chitosan-Loaded Titania Nanotubes with Various Diameters. Materials 2016, 9, 155. https://doi.org/10.3390/ma9030155
Lin W-t, Zhang Y-y, Tan H-l, Ao H-y, Duan Z-l, He G, Tang T-t. Inhibited Bacterial Adhesion and Biofilm Formation on Quaternized Chitosan-Loaded Titania Nanotubes with Various Diameters. Materials. 2016; 9(3):155. https://doi.org/10.3390/ma9030155
Chicago/Turabian StyleLin, Wen-tao, Yi-yuan Zhang, Hong-lue Tan, Hai-yong Ao, Zhao-ling Duan, Guo He, and Ting-ting Tang. 2016. "Inhibited Bacterial Adhesion and Biofilm Formation on Quaternized Chitosan-Loaded Titania Nanotubes with Various Diameters" Materials 9, no. 3: 155. https://doi.org/10.3390/ma9030155
APA StyleLin, W.-t., Zhang, Y.-y., Tan, H.-l., Ao, H.-y., Duan, Z.-l., He, G., & Tang, T.-t. (2016). Inhibited Bacterial Adhesion and Biofilm Formation on Quaternized Chitosan-Loaded Titania Nanotubes with Various Diameters. Materials, 9(3), 155. https://doi.org/10.3390/ma9030155






