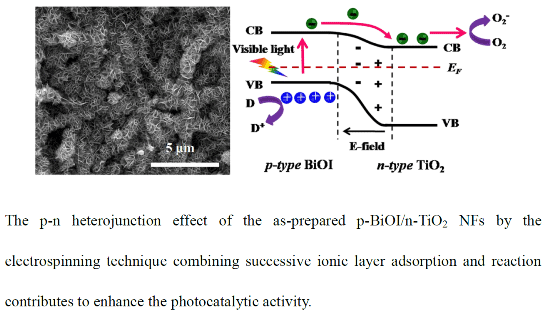
Heterojunctions of p-BiOI Nanosheets/n-TiO2 Nanofibers: Preparation and Enhanced Visible-Light Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
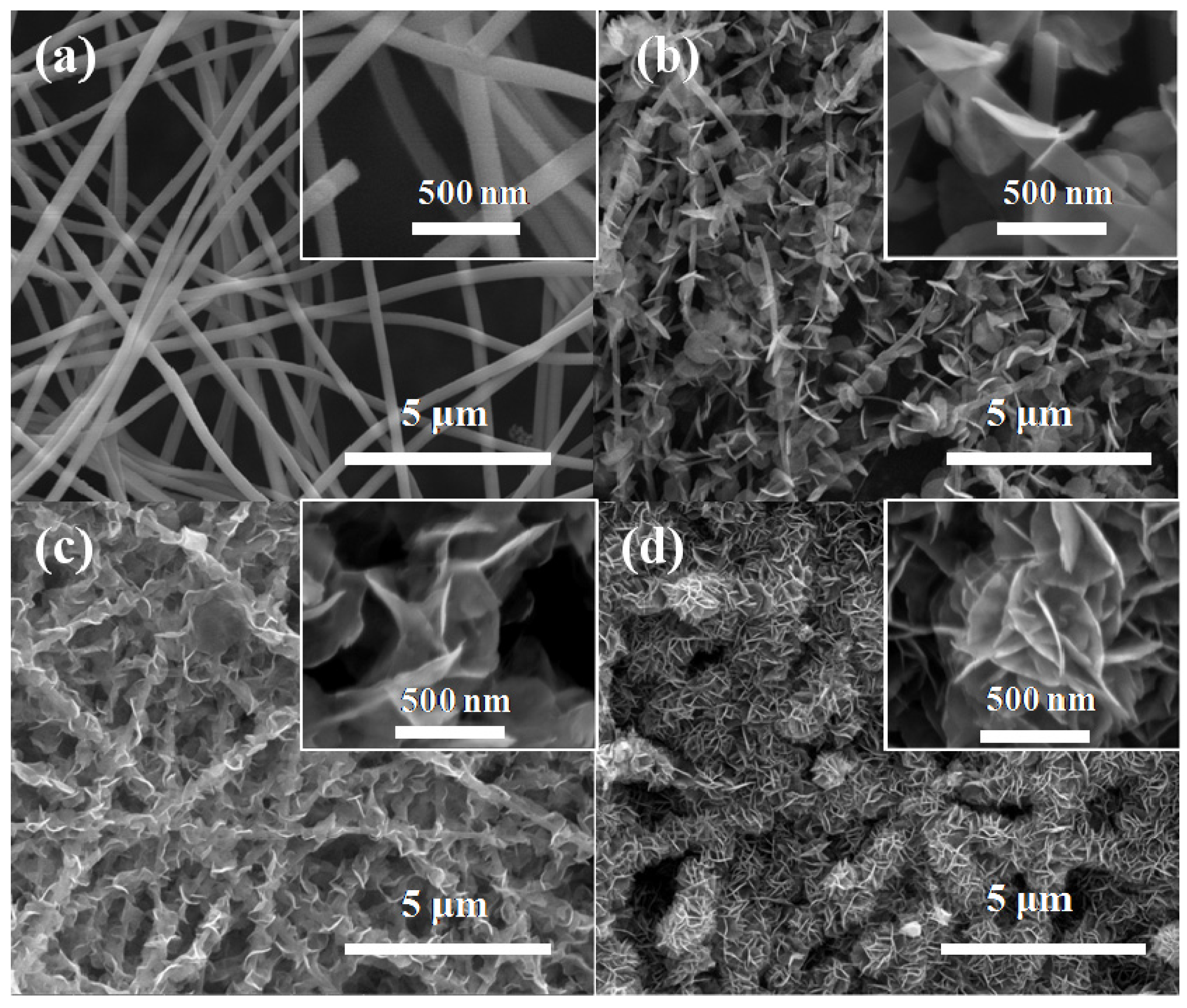
2.1. Morphologies
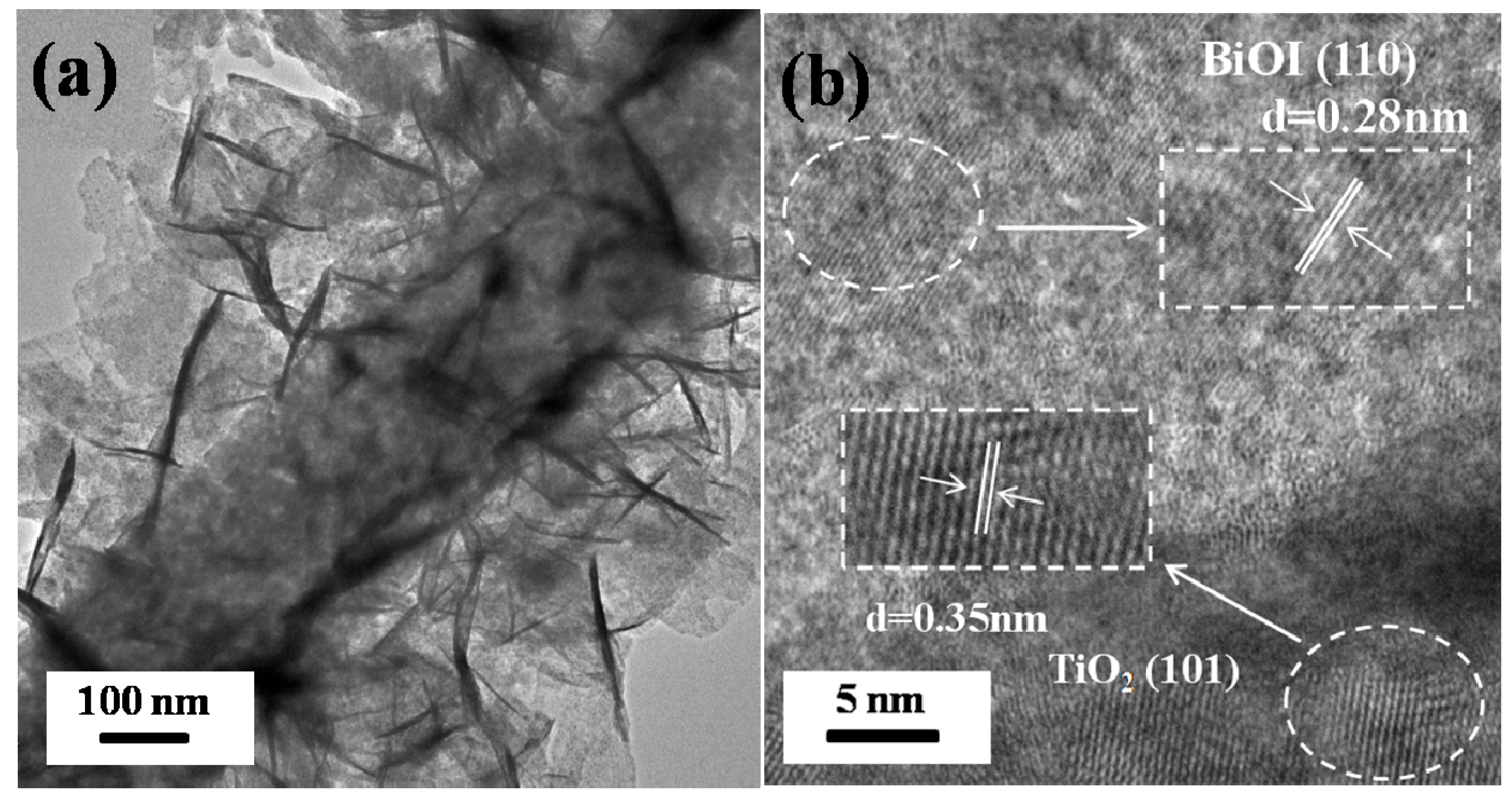
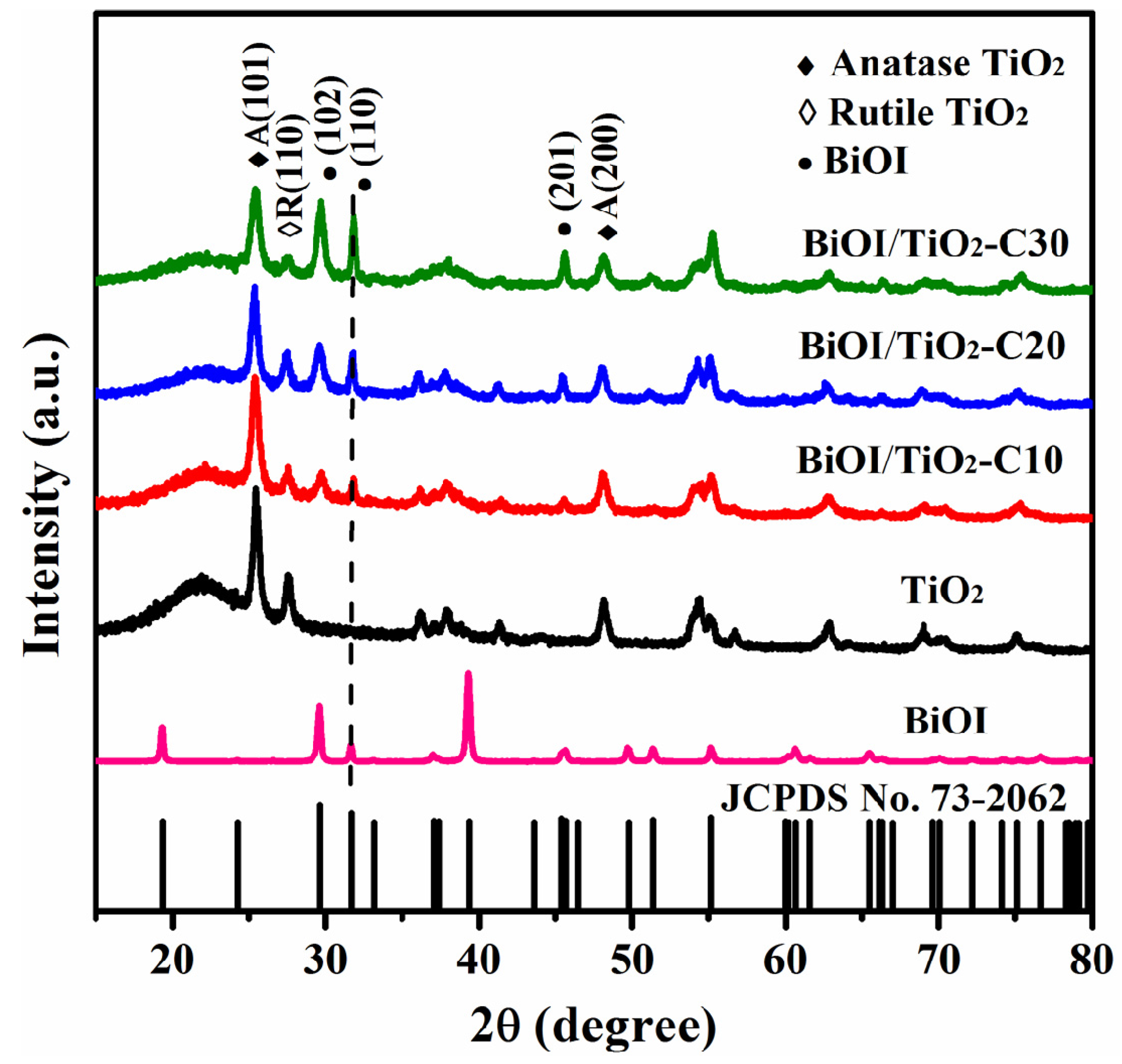
2.2. Structure Characterization
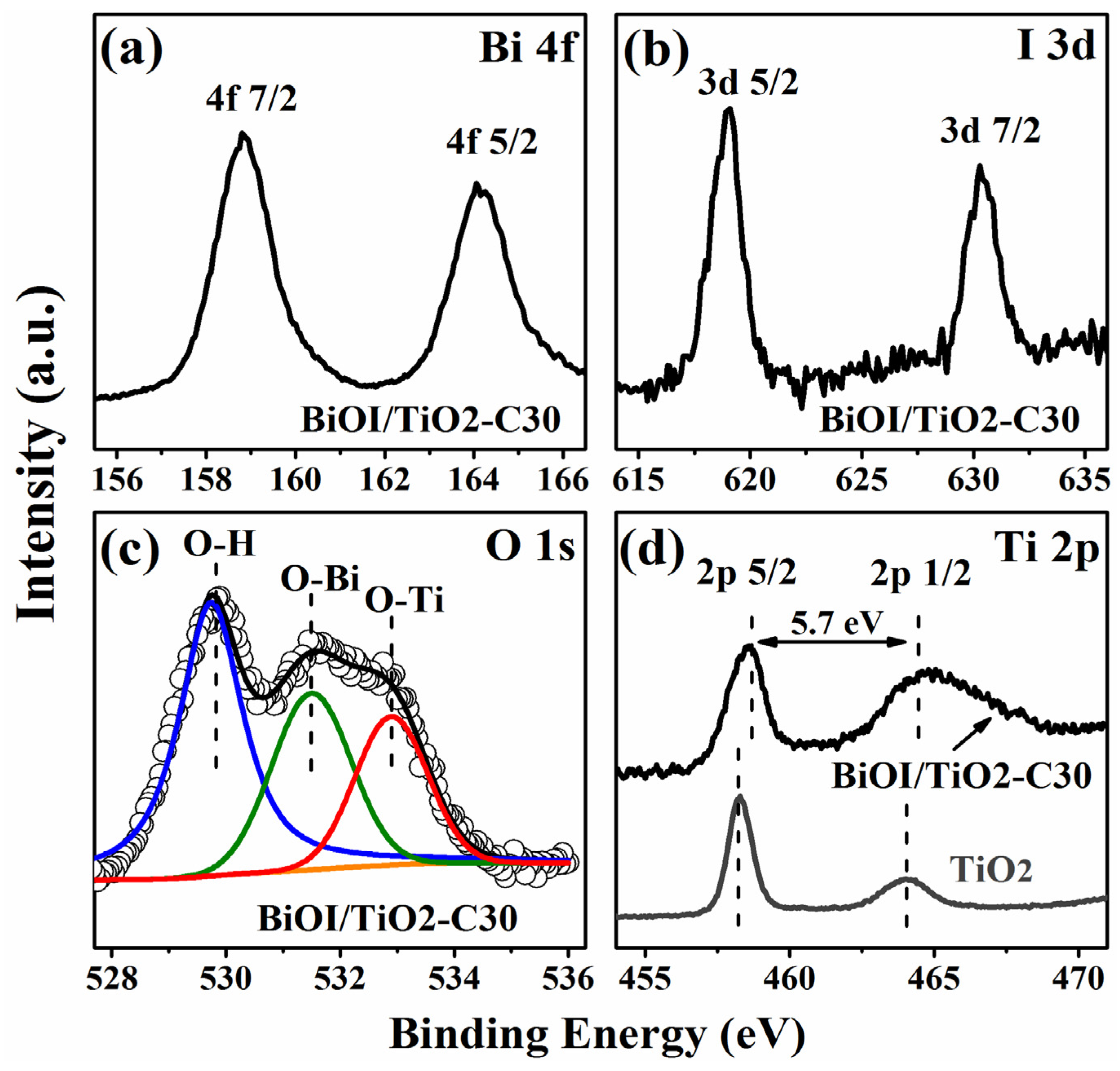
2.3. Composition and Chemical States
2.4. Nitrogen Adsorption

| Samples | Cycles | BET Specific Surface Area (m2/g) | Kapp (h−1) |
|---|---|---|---|
| TiO2 NFs | 0 | 15.88 | 0.000 ± 0.000 |
| BiOI/TiO2-C10 | 10 | 7.27 | 0.141 ± 0.002 |
| BiOI/TiO2-C20 | 20 | 19.19 | 0.197 ± 0.009 |
| BiOI/TiO2-C30 | 30 | 38.44 | 0.724 ± 0.095 |
| M-BT (Bi:Ti = 0.4:1) | - | - | 0.267 ± 0.024 |
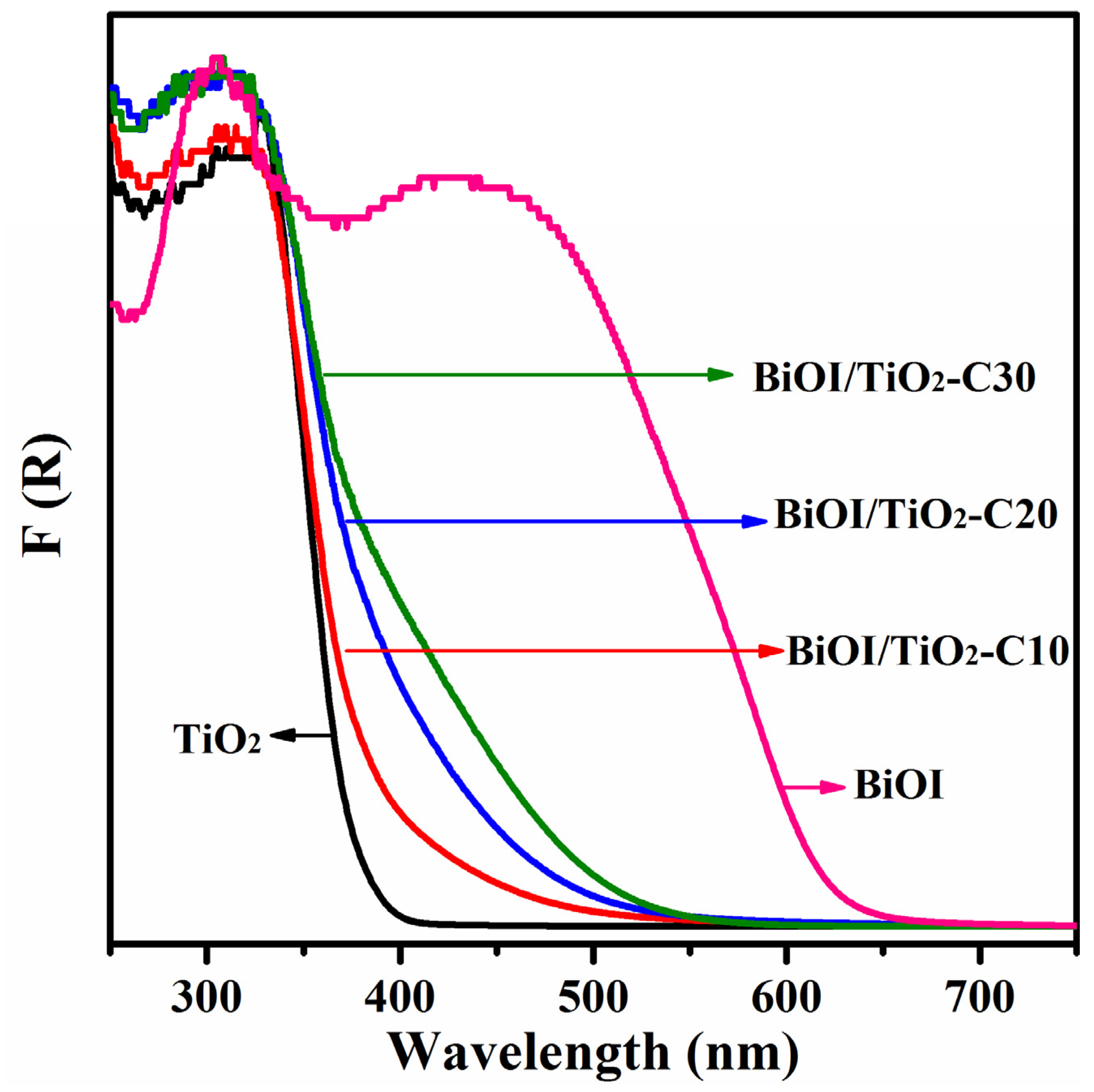
2.5. Optical Properties
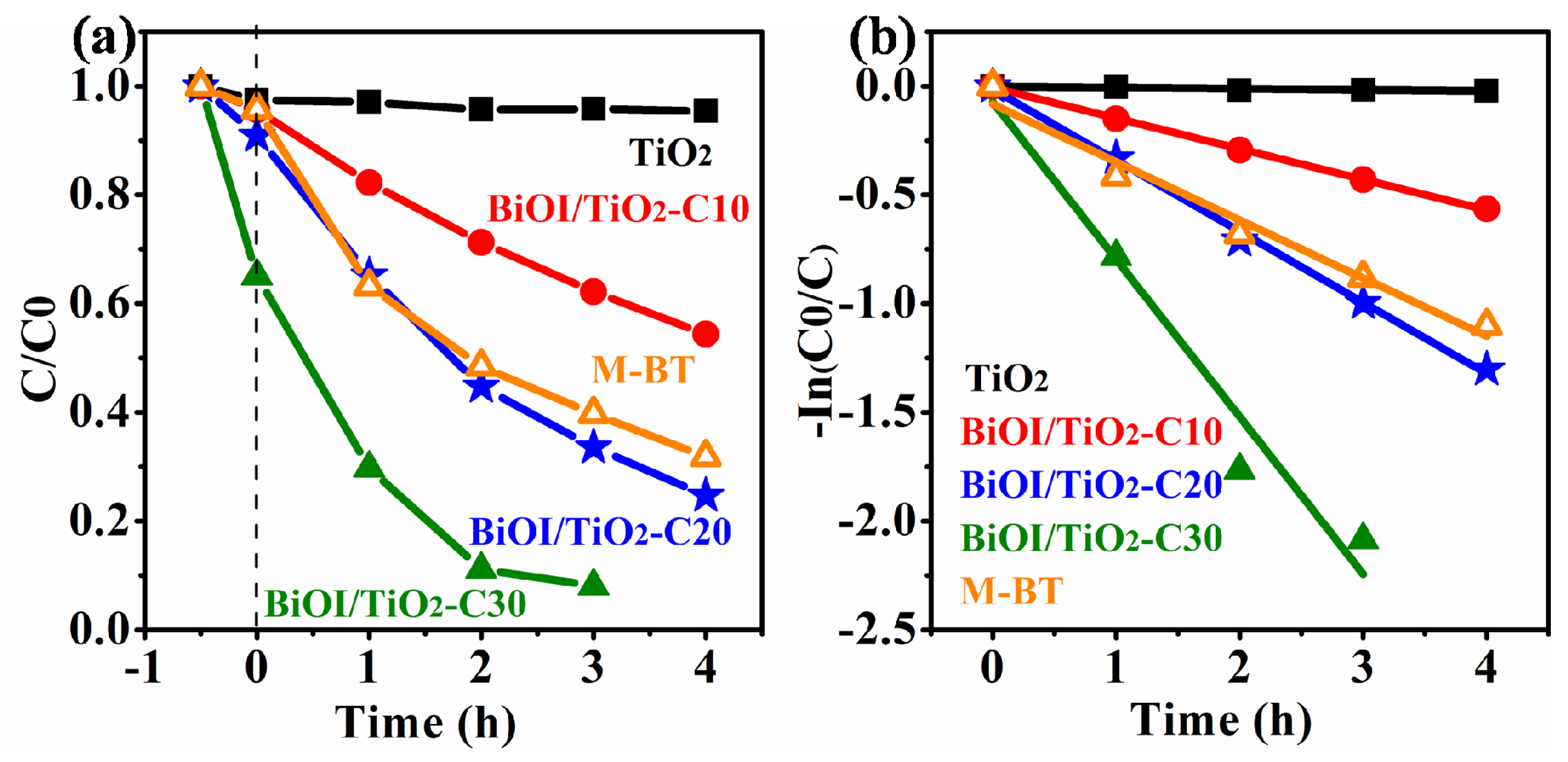
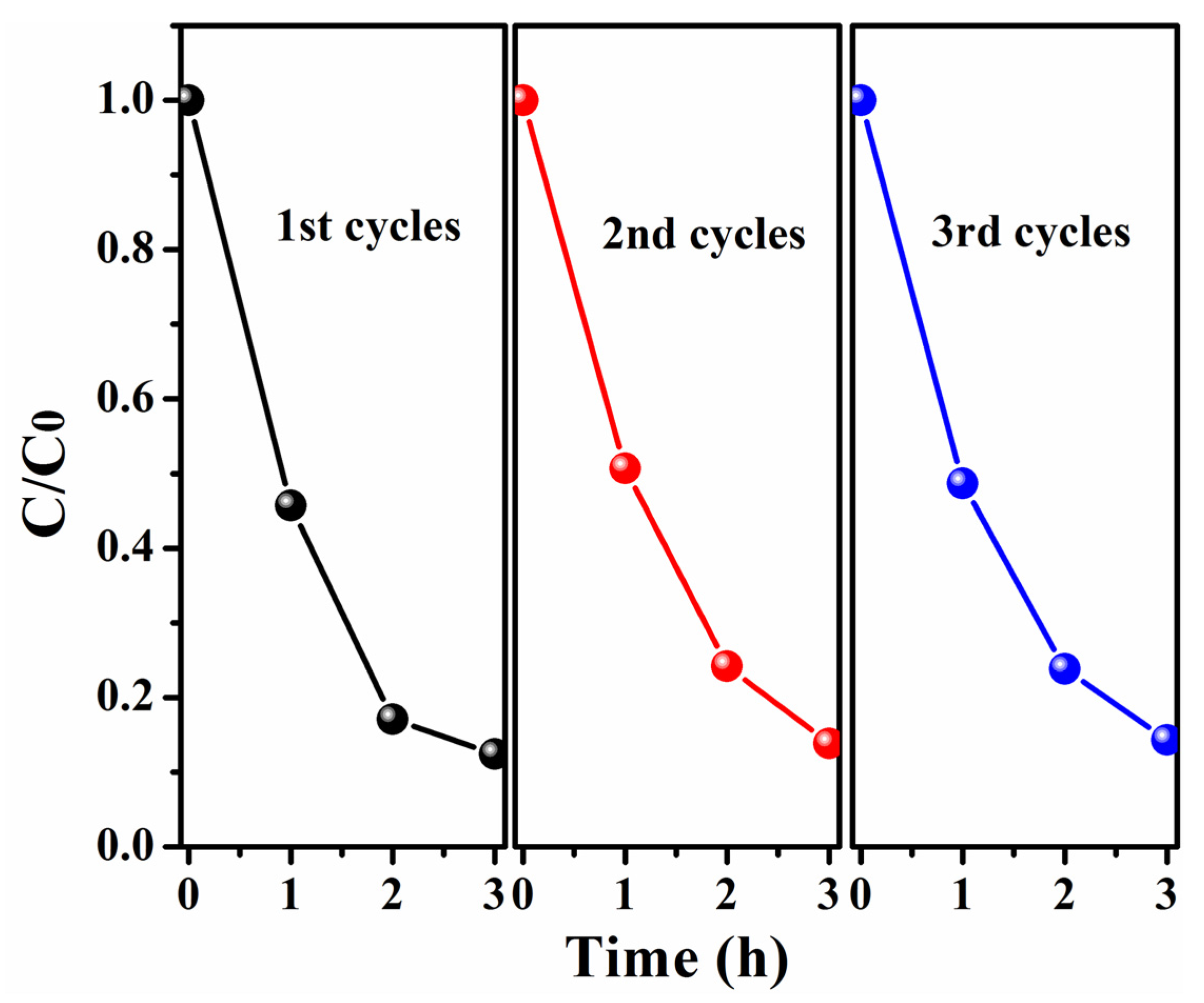
2.6. Photocatalytic Properties
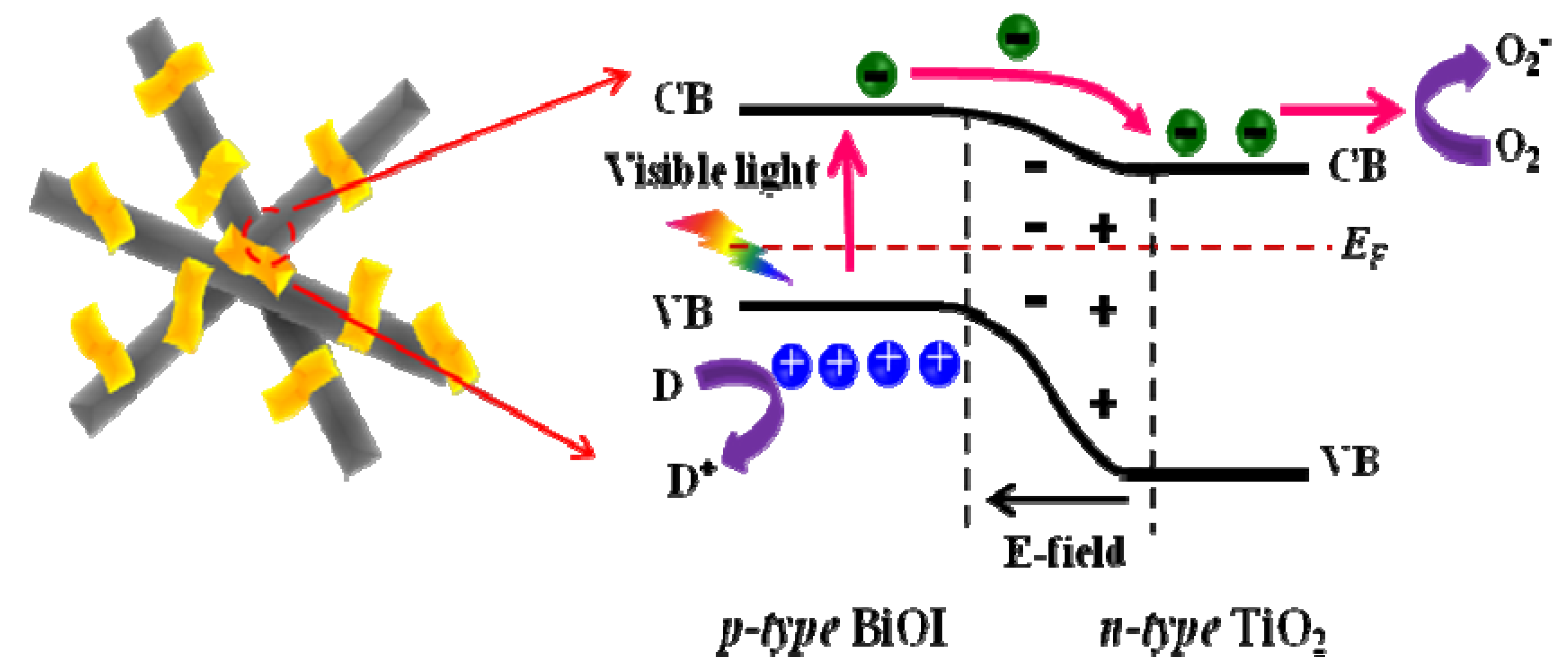
3. Experimental
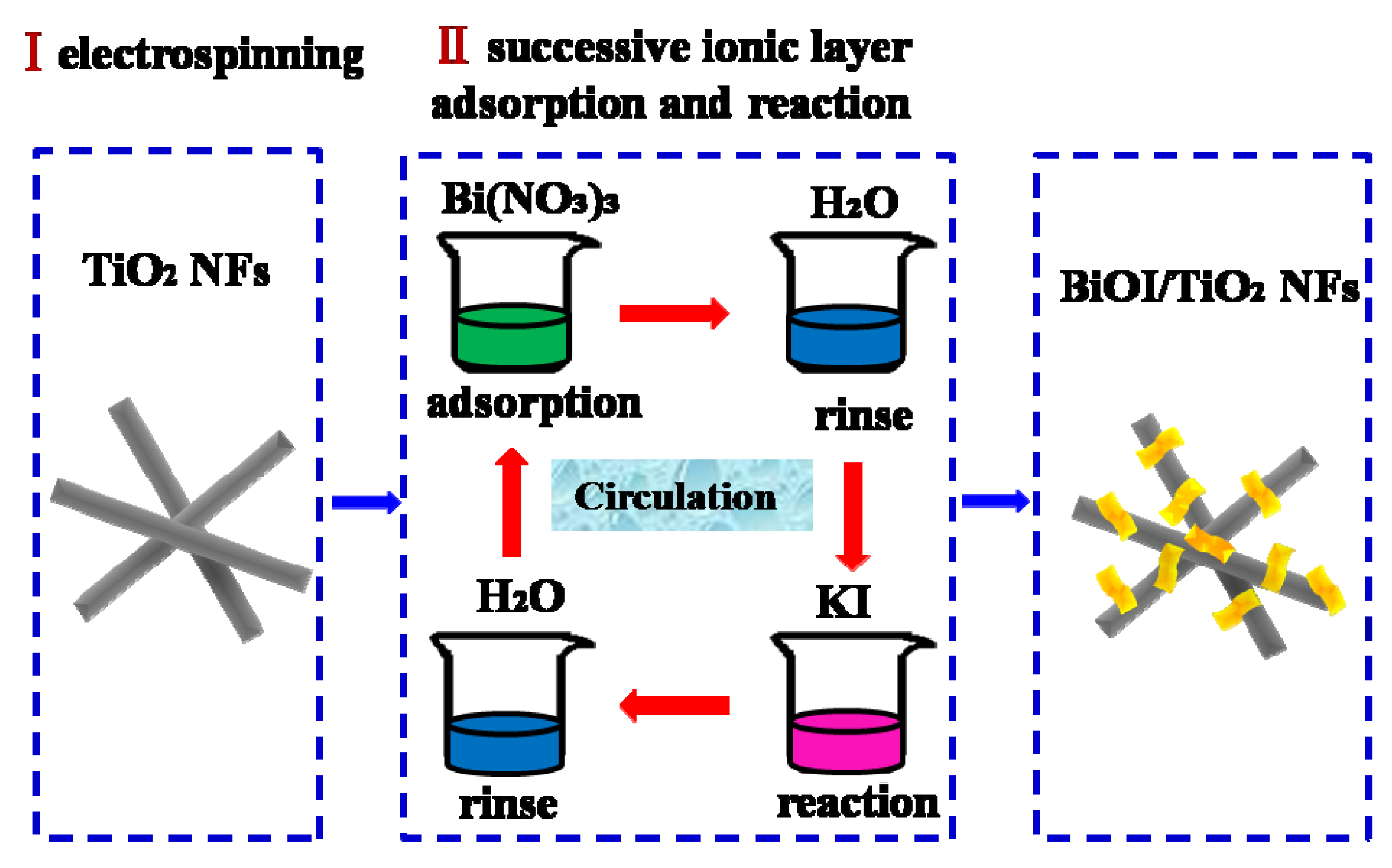
3.1. Fabrication of TiO2 Nanofibers
3.2. Fabrication of BiOI/TiO2 Nanofibers
3.3. Characterizations
3.4. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Huang, F.; Zheng, C.; Wang, W. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Fan, C.; Wang, Y.; Wang, Y.; Liang, Z. A novel BiOCl thin film prepared by electrochemical method and its application in photocatalysis. Appl. Catal. B Environ. 2013, 132–133, 332–341. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X = Cl, Br, I) nanoplate microspheres. J. Phys. Chem. C 2008, 112, 747–753. [Google Scholar] [CrossRef]
- Wang, D.H.; Gao, G.Q.; Zhang, Y.W.; Zhou, L.S.; Xu, A.W.; Chen, W. Nanosheet-constructed porous BiOCl with dominant {001} facets for superior photosensitized degradation. Nanoscale 2012, 4, 7780–7785. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Zhang, J.; Miao, M.; Jin, Y. Solvothermal synthesis of flower-like BiOBr microspheres with highly visible-light photocatalytic performances. Appl. Catal. B Environ. 2012, 111–112, 334–341. [Google Scholar] [CrossRef]
- Ye, L.Q.; Chen, J.N.; Tian, L.H.; Liu, J.Y.; Peng, T.Y.; Deng, K.J.; Zan, L. BiOI thin film via chemical vapor transport: Photocatalytic activity, durability, selectivity and mechanism. Appl. Catal. B Environ. 2013, 130, 1–7. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, W.; Huang, B.; Wang, Z.; Zhan, J.; Qin, X.; Zhang, X.; Dai, Y. Tailoring AgI nanoparticles for the assembly of AgI/BiOI hierarchical hybrids with size-dependent photocatalytic activities. J. Mater. Chem. A 2013, 1, 7131–7136. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.-D. Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity. J. Mater. Chem. 2010, 20, 5866–5870. [Google Scholar] [CrossRef]
- Cao, S.; Chen, C.; Liu, T.; Tsang, Y.; Zhang, X.; Yu, W.; Chen, W. Synthesis of reduced graphene oxide/alpha-Bi2Mo3O12@beta-Bi2O3 heterojunctions by organic electrolytes assisted UV-excited method. Chem. Eng. J. 2014, 257, 309–316. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, M.; Chen, Q.G.; Fan, C.M.; Zhou, H.Y.; Xu, A.W. Novel onedimensional Bi2O3-Bi2WO6 p-n hierarchical heterojunction with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 8517–8524. [Google Scholar] [CrossRef]
- Chang, X.X.; Wang, T.; Zhang, P.; Zhang, J.J.; Li, A.; Gong, J.L. Enhanced surface reaction kinetics and charge separation of p-n heterojunction Co3O4/BiVO4 photoanodes. J. Am. Chem. Soc. 2015, 137, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Li, L.; Zhang, X. One-dimensional visible-light-driven bifunctional photocatalysts based on Bi4Ti3O12 nanofiber frameworks and Bi2XO6 (X=Mo, W) nanosheets. Appl. Catal. B Environ. 2014, 160–161, 757–766. [Google Scholar] [CrossRef]
- Reddy, K.H.; Martha, S.; Parida, K.M. Fabrication of novel p-BiOI/n-ZnTiO3 heterojunction for degradation of rhodamine 6G under visible light irradiation. Inorg. Chem. 2013, 52, 6390–6401. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, Z.; Sun, H.; Sun, L.; Li, Q.; Zhao, X.; Jia, C.-J.; Fan, W. Facile fabrication of p-BiOI/n-Zn2SnO4 heterostructures with highly enhanced visible light photocatalytic performances. Mater. Res. Bull. 2014, 55, 196–204. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; Sun, P.; Zhang, L. ZnO/BiOI heterostructures: Photoinduced charge-transfer property and enhanced visible-light photocatalytic activity. J. Phys. Chem. C 2011, 115, 20555–20564. [Google Scholar] [CrossRef]
- Hou, D.; Hu, X.; Hu, P.; Zhang, W.; Zhang, M.; Huang, Y. Bi4Ti3O12 nanofibers-BiOI nanosheets p-n junction: Facile synthesis and enhanced visible-light photocatalytic activity. Nanoscale 2013, 5, 9764–9772. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Yamashita, Y.; Tamura, H. Heterogeneous photocatalytic reduction of dichromate on n-type semiconductor catalysts. Nature 1979, 282, 817–818. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, H.; Zeng, Y.; Huang, T. Anatase TiO2 single crystals with dominant {001} facets: Facile fabrication from Ti powders and enhanced photocatalytical activity. Appl. Surf. Sci. 2013, 274, 117–123. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Ju, S.; Lu, W.; Zhao, L.; Zhou, C.; Wang, W. Fabrication of micrometer-scale spherical titanate nanotube assemblies with high specific surface area. Mater. Lett. 2010, 64, 1204–1207. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, M.; Li, H.; Piao, L.; Wang, W. Facile synthesis of hollow TiO2 single nanocrystals with improved photocatalytic and photoelectrochemical activities. ChemPlusChem 2015, 80, 688–696. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Wang, W. Hierarchical TiO2 spheres: Facile fabrication and enhanced photocatalysis. Rare Metals 2011, 30, 153–156. [Google Scholar] [CrossRef]
- Liu, M.; Sunada, K.; Hashimoto, K.; Miyauchi, M. Visible-light sensitive Cu(ii)-TiO2 with sustained anti-viral activity for efficient indoor environmental remediation. J. Mater. Chem. A 2015, 3, 17312–17319. [Google Scholar] [CrossRef]
- Wu, D.Y.; Wang, H.Y.; Li, C.L.; Xia, J.; Song, X.J.; Huang, W.S. Photocatalytic self-cleaning properties of cotton fabrics functionalized with p-BiOI/n-TiO2 heterojunction. Surf. Coat. Technol. 2014, 258, 672–676. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, B.; Dang, L.; Yao, H.; Li, Z. BiOI-sensitized TiO2 in phenol degradation: A novel efficient semiconductor sensitizer. Chem. Phys. Lett. 2011, 508, 102–106. [Google Scholar] [CrossRef]
- Zhang, D. Heterostructural BiOI/TiO2 composite with highly enhanced visible light photocatalytic performance. Russ. J. Phys. Chem. A 2014, 88, 2476–2485. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Li, X.; Sun, Y.; Zhang, M.; Mu, J.; Zhang, P.; Guo, Z.; Liu, Y. Hierarchical assembly of ultrathin hexagonal SnS2 nanosheets onto electrospun TiO2 nanofibers: Enhanced photocatalytic activity based on photoinduced interfacial charge transfer. Nanoscale 2013, 5, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, L.; Tang, Y.; Luo, S.; Liu, Y.; Zhang, S.; Zeng, Y.; Xu, Y. Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2015, 164, 1–9. [Google Scholar] [CrossRef]
- Wang, K.X.; Shao, C.L.; Li, X.H.; Zhang, X.; Lu, N.; Miao, F.J.; Liu, Y.C. Hierarchical heterostructures of p-type BiOCl nanosheets on electrospun n-type TiO2 nanofibers with enhanced photocatalytic activity. Catal. Commun. 2015, 67, 6–10. [Google Scholar] [CrossRef]
- Lu, M.X.; Shao, C.L.; Wang, K.X.; Lu, N.; Zhang, X.; Zhang, P.; Zhang, M.Y.; Li, X.H.; Liu, Y.C. p-MoO3 nanostructures/n-TiO2 nanofiber heterojunctions: Controlled fabrication and enhanced photocatalytic properties. ACS Appl. Mater. Inter. 2014, 6, 9004–9012. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Tian, L.; Peng, T.; Zan, L. Synthesis of highly symmetrical BiOI single-crystal nanosheets and their {001} facet-dependent photoactivity. J. Mater. Chem. 2011, 21, 12479–12484. [Google Scholar] [CrossRef]
- Wang, S.M.; Guan, Y.; Wang, L.P.; Zhao, W.; He, H.; Xiao, J.; Yang, S.G.; Sun, C. Fabrication of a novel bifunctional material of BiOI/Ag3VO4 with high adsorption-photocatalysis for efficient treatment of dye wastewater. Appl. Catal. B Environ. 2015, 168, 448–457. [Google Scholar] [CrossRef]
- Liu, H.; Cao, W.; Su, Y.; Wang, Y.; Wang, X. Synthesis, characterization and photocatalytic performance of novel visible-light-induced Ag/BiOI. Appl. Catal. B Environ. 2012, 111–112, 271–279. [Google Scholar] [CrossRef]
- Chu, M.-W.; Ganne, M.; Caldes, M.T.; Brohan, L. X-ray photoelectron spectroscopy and high resolution electron microscopy studies of Aurivillius compounds: Bi4−xLaxTi3O12(x = 0, 0.5, 0.75, 1.0, 1.5, and 2.0). J. Appl. Phys. 2002, 91, 3178. [Google Scholar] [CrossRef]
- Wei, X.X.; Chen, C.M.; Guo, S.Q.; Guo, F.; Li, X.M.; Wang, X.X.; Cui, H.T.; Zhao, L.F.; Li, W. Advanced visible-light-driven photocatalyst BiOBr-TiO2-graphene composite with graphene as a nano-filler. J. Mater. Chem. A 2014, 2, 4667–4675. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yang, M.; Zhang, G.S.; Dionysiou, D.D. HNO3-involved one-step low temperature solvothermal synthesis of N-doped TiO2 nanocrystals for efficient photocatalytic reduction of Cr(VI) in water. Appl. Catal. B Environ. 2013, 142, 249–258. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Shao, C.; Li, X.; Miao, F.; Lu, N.; Liu, Y. Heterojunctions of p-BiOI Nanosheets/n-TiO2 Nanofibers: Preparation and Enhanced Visible-Light Photocatalytic Activity. Materials 2016, 9, 90. https://doi.org/10.3390/ma9020090
Wang K, Shao C, Li X, Miao F, Lu N, Liu Y. Heterojunctions of p-BiOI Nanosheets/n-TiO2 Nanofibers: Preparation and Enhanced Visible-Light Photocatalytic Activity. Materials. 2016; 9(2):90. https://doi.org/10.3390/ma9020090
Chicago/Turabian StyleWang, Kexin, Changlu Shao, Xinghua Li, Fujun Miao, Na Lu, and Yichun Liu. 2016. "Heterojunctions of p-BiOI Nanosheets/n-TiO2 Nanofibers: Preparation and Enhanced Visible-Light Photocatalytic Activity" Materials 9, no. 2: 90. https://doi.org/10.3390/ma9020090
APA StyleWang, K., Shao, C., Li, X., Miao, F., Lu, N., & Liu, Y. (2016). Heterojunctions of p-BiOI Nanosheets/n-TiO2 Nanofibers: Preparation and Enhanced Visible-Light Photocatalytic Activity. Materials, 9(2), 90. https://doi.org/10.3390/ma9020090