Metallurgical Parameters Controlling the Eutectic Silicon Charateristics in Be-Treated Al-Si-Mg Alloys
Abstract
:1. Introduction
2. Experimental Procedure
| Alloy Code | Element Concentration (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| Si | Fe | Mg | Ti | Sr | Be | Al | |
| A1 | 7.146 | 0.09 | 0.40 | 0.168 | 0.00 | 0.00 | Balance |
| A1B | 7.146 | 0.09 | 0.40 | 0.168 | 0.00 | 0.05 | Balance |
| A1S | 7.146 | 0.09 | 0.40 | 0.168 | 0.02 | 0.00 | Balance |
| A1BS | 7.146 | 0.09 | 0.40 | 0.168 | 0.02 | 0.05 | Balance |
| C3 | 7.146 | 0.60 | 0.80 | 0.168 | 0.00 | 0.00 | Balance |
| C3B | 7.146 | 0.60 | 0.80 | 0.168 | 0.00 | 0.05 | Balance |
| C3S | 7.146 | 0.60 | 0.80 | 0.168 | 0.02 | 0.00 | Balance |
| C3BS | 7.146 | 0.60 | 0.80 | 0.168 | 0.02 | 0.05 | Balance |
| Feature | Definition |
|---|---|
| Average Length (μm) | longest measurement of each particle |
| Average Area (μm2) | area of each particle |
| Roundness (percentage) | degree or percentage to which a particle is round or spherical. |
| Aspect Ratio | longest length measurement of a particle divided by the smallest length of the particle |
3. Results and Discussion
3.1. Effect of Solidification Rate
3.1.1. Low Solidification Rate (DAS ~ 65 μm)
| Alloy (Condition) | Area (μm2) | Length (μm) | Roundness (%) | Aspect Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| Av. | SD | Av. | SD | Av. | SD | Av. | SD | |
| A1 (non-modified) | 76.70 | 71.50 | 23.60 | 16.30 | 28.4 | 21.8 | 3.52 | 4.54 |
| C3 (non-modified) | 20.70 | 22.70 | 11.63 | 7.98 | 36.2 | 21.4 | 2.86 | 5.84 |
| A1B (Be-modified) | 49.60 | 52.40 | 15.20 | 12.00 | 33.8 | 21.8 | 2.85 | 3.45 |
| C3B (Be-modified) | 13.60 | 14.30 | 6.75 | 5.08 | 43.7 | 22.2 | 2.27 | 1.15 |
| A1S (Sr-modified) | 3.18 | 3.21 | 3.05 | 2.16 | 46.0 | 19.2 | 2.11 | 2.14 |
| C3S (Sr-modified) | 4.48 | 4.78 | 3.82 | 3.07 | 43.4 | 21.5 | 2.46 | 3.87 |
| A1BS (Be + Sr-modified) | 2.75 | 2.94 | 3.00 | 2.38 | 43.5 | 19.5 | 2.18 | 2.25 |
| C3BS (Be + Sr-modified) | 3.38 | 3.97 | 3.27 | 2.69 | 43.9 | 19.8 | 2.15 | 1.32 |
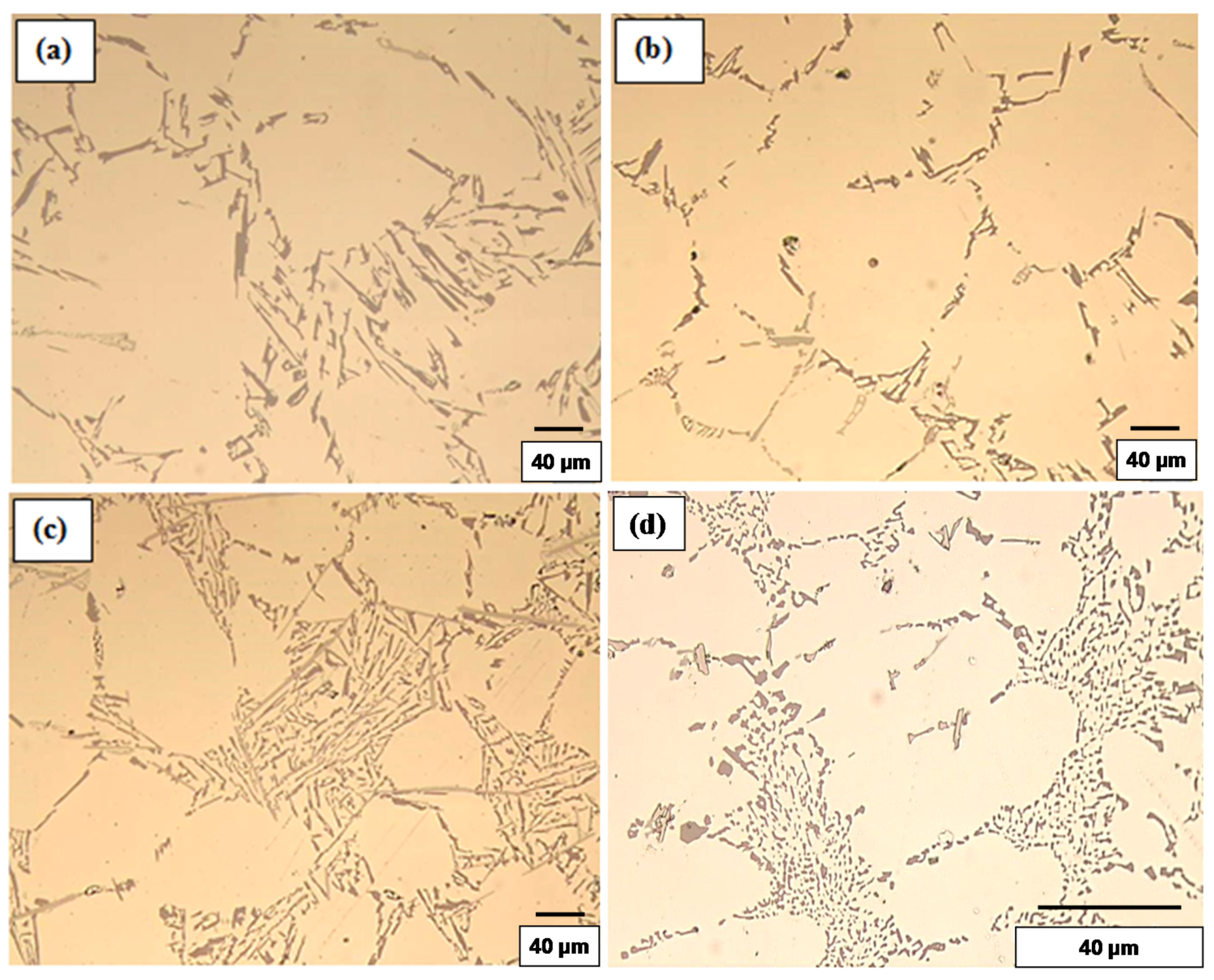
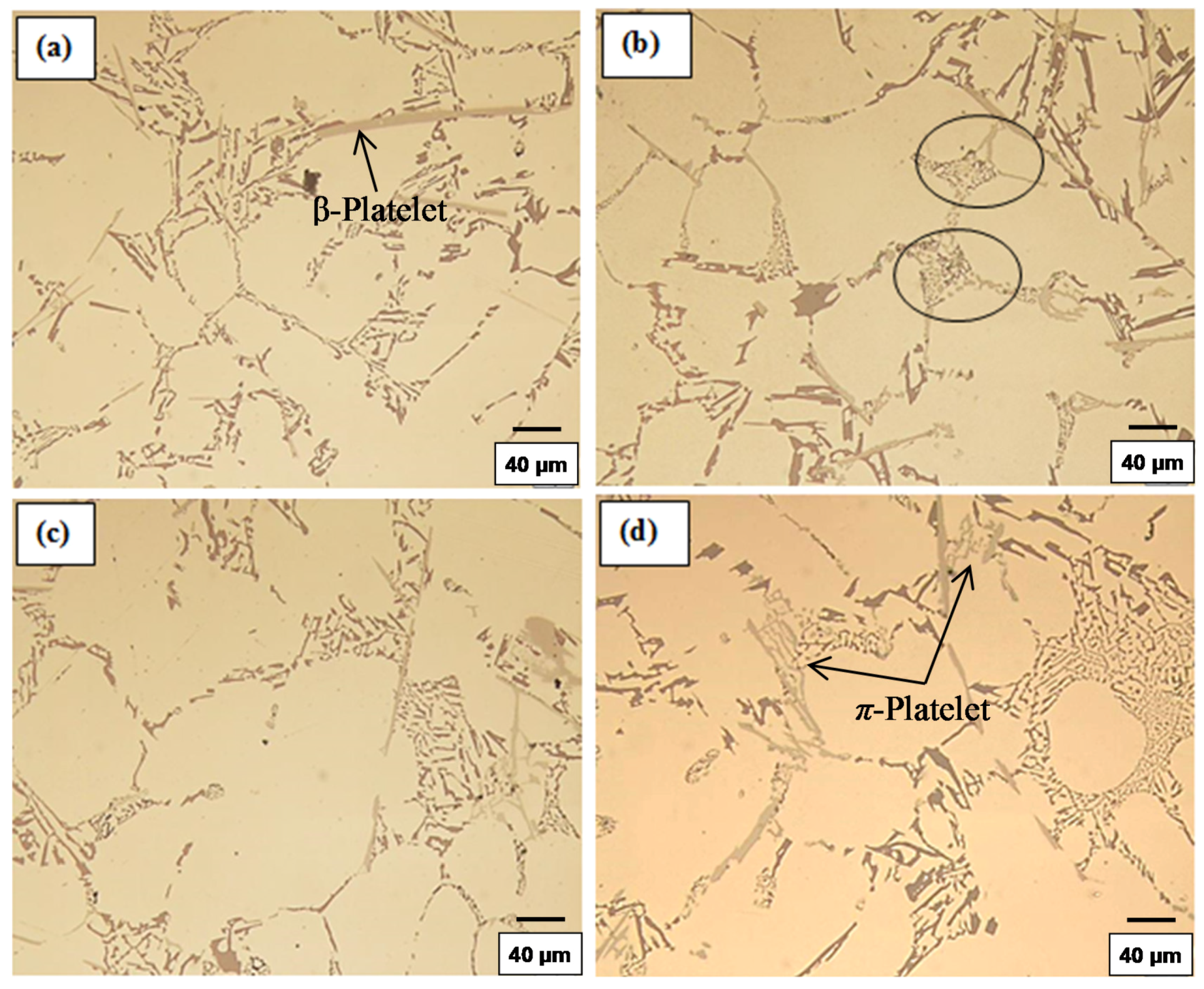
3.1.2. High Solidification Rate (DAS ~ 25 μm)
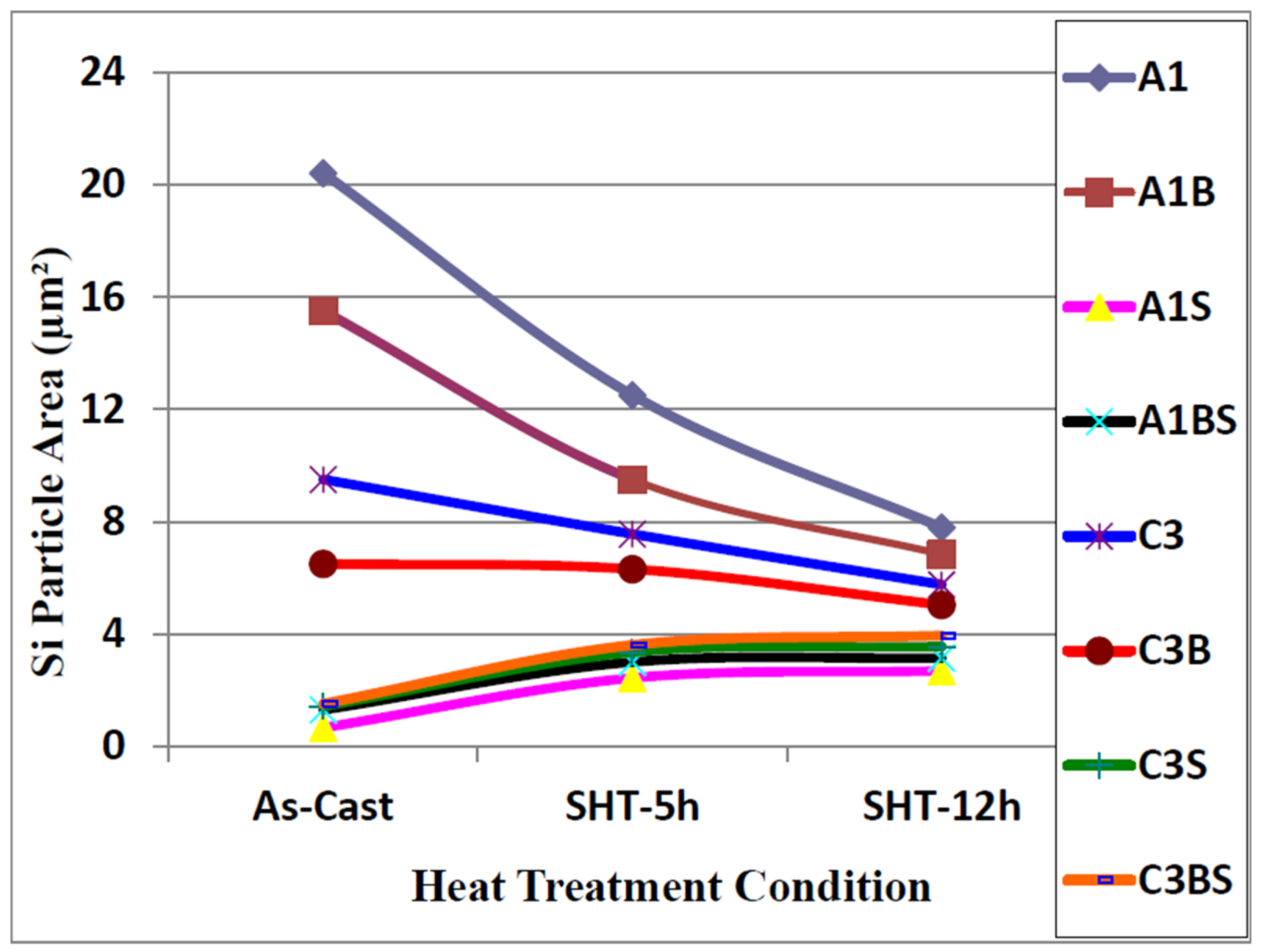
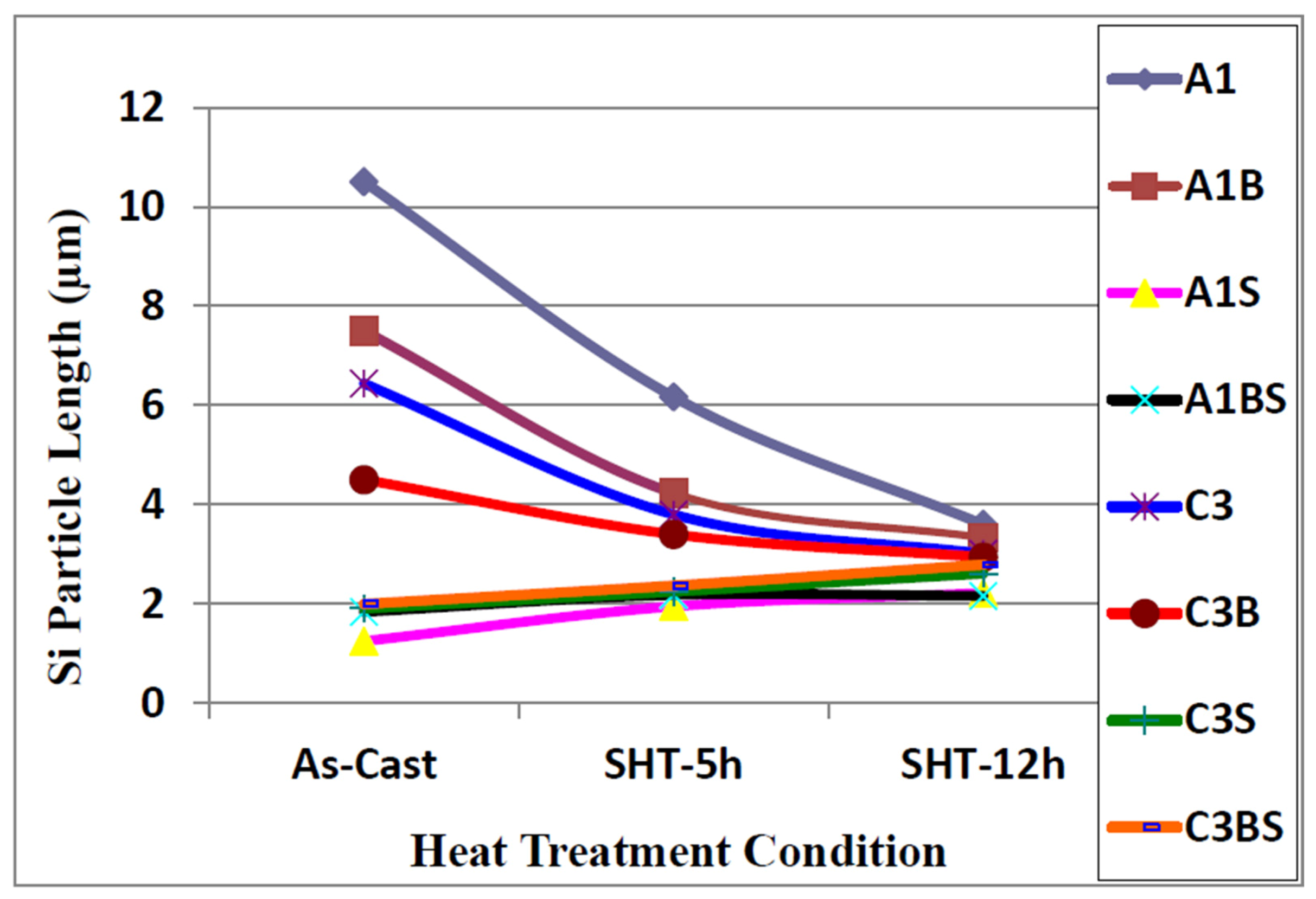

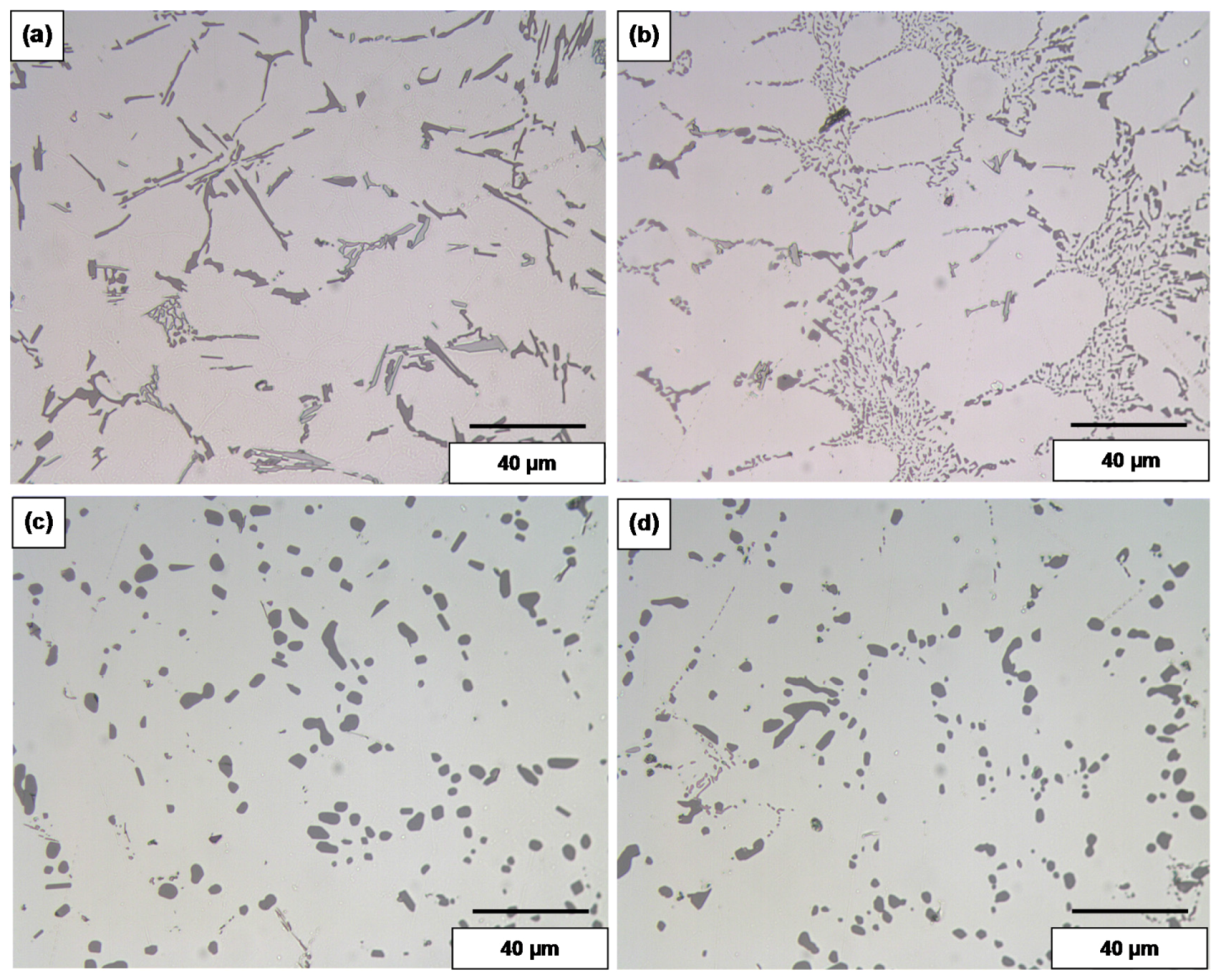
| Alloy (Condition) | Area (μm2) | Length (μm) | Roundness (%) | Aspect Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| Av. | SD | Av. | SD | Av. | SD | Av. | SD | |
| A1 (non-modified) | 28.40 | 9.50 | 11.50 | 6.46 | 18.9 | 8.9 | 3.78 | 1.79 |
| C3 (non-modified) | 15.30 | 7.90 | 9.60 | 6.50 | 35.7 | 21.1 | 2.73 | 1.66 |
| A1B (Be-modified) | 6.90 | 3.40 | 6.44 | 4.35 | 44.6 | 10.5 | 2.01 | 1.07 |
| C3B (Be-modified) | 3.17 | 2.21 | 2.98 | 1.40 | 56.9 | 12.4 | 1.99 | 0.93 |
| A1S (Sr-modified) | 1.67 | 0.40 | 1.25 | 1.07 | 50.3 | 7.9 | 1.84 | 0.70 |
| C3S (Sr-modified) | 2.30 | 1.26 | 1.91 | 0.88 | 59.2 | 9.7 | 1.99 | 0.92 |
| A1BS (Be + Sr-modified) | 1.61 | 1.04 | 1.84 | 1.66 | 47.6 | 8.8 | 1.94 | 0.81 |
| C3BS (Be + Sr-modified) | 1.53 | 0.40 | 2.00 | 1.76 | 46.7 | 8.9 | 1.99 | 0.93 |
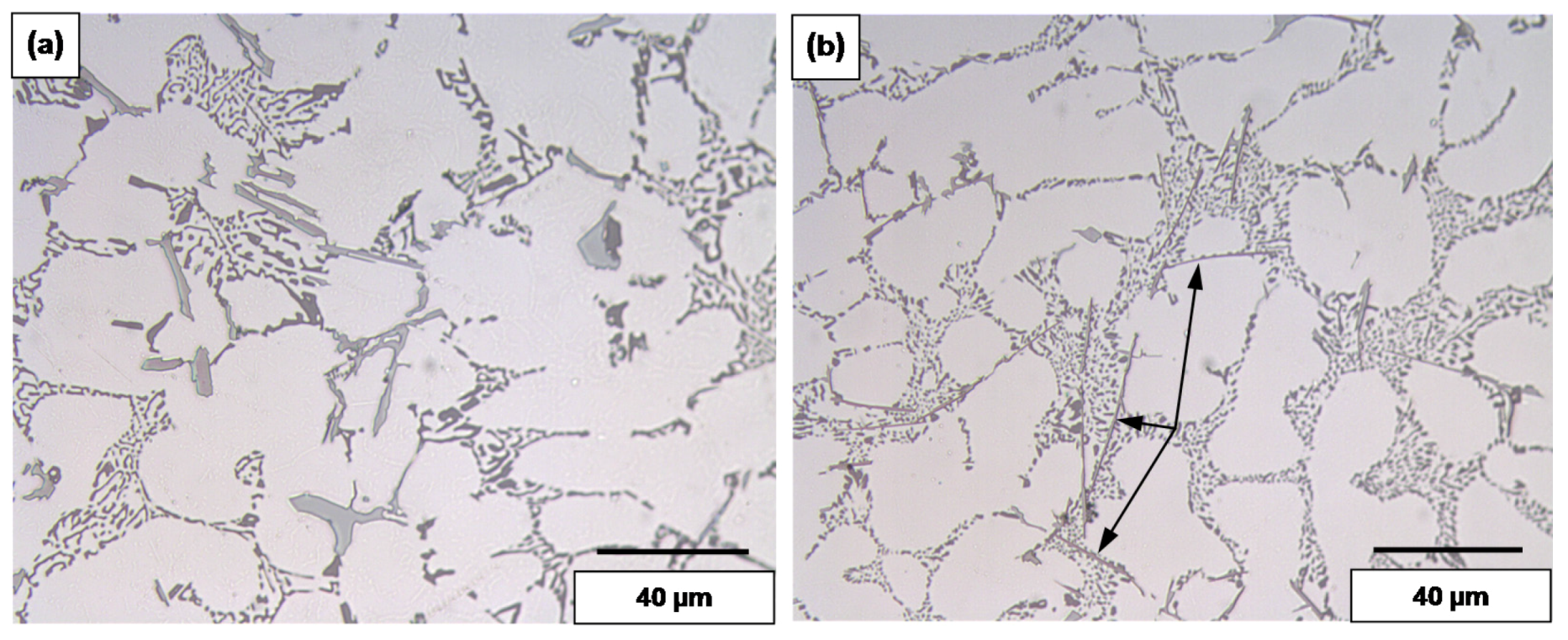
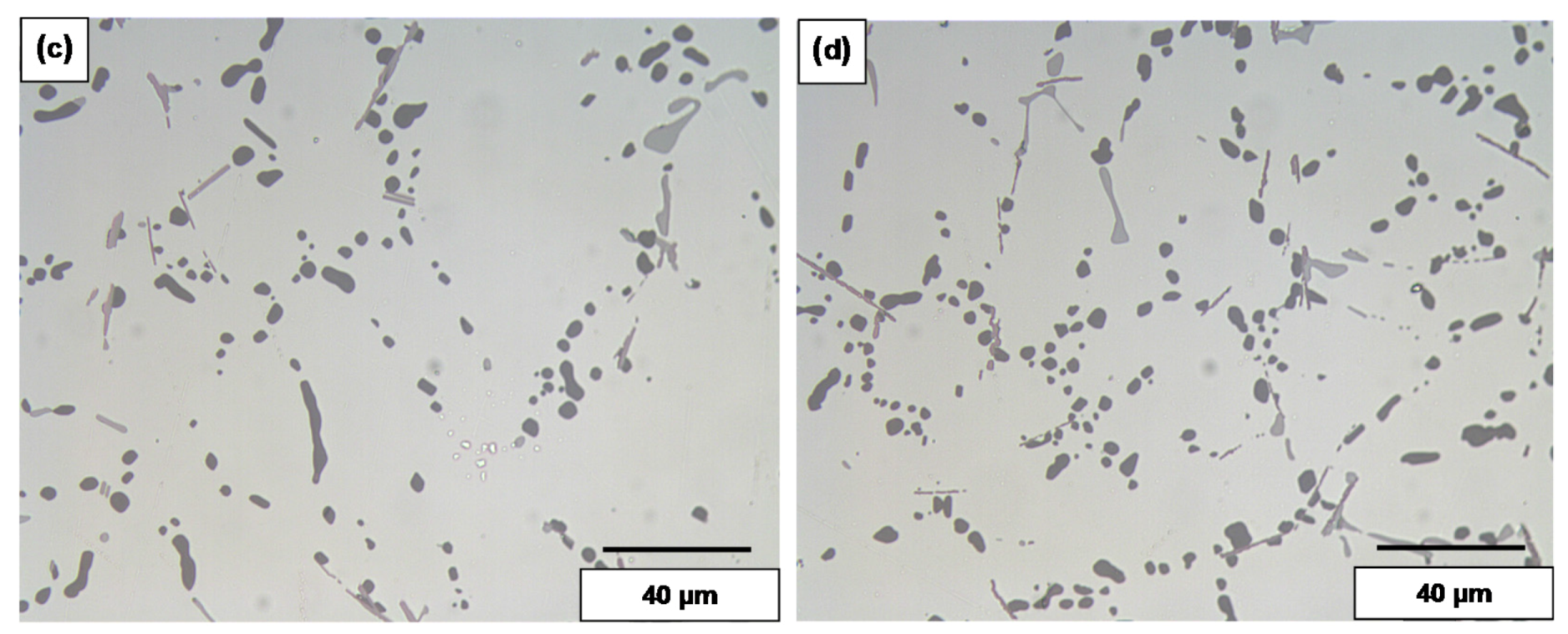
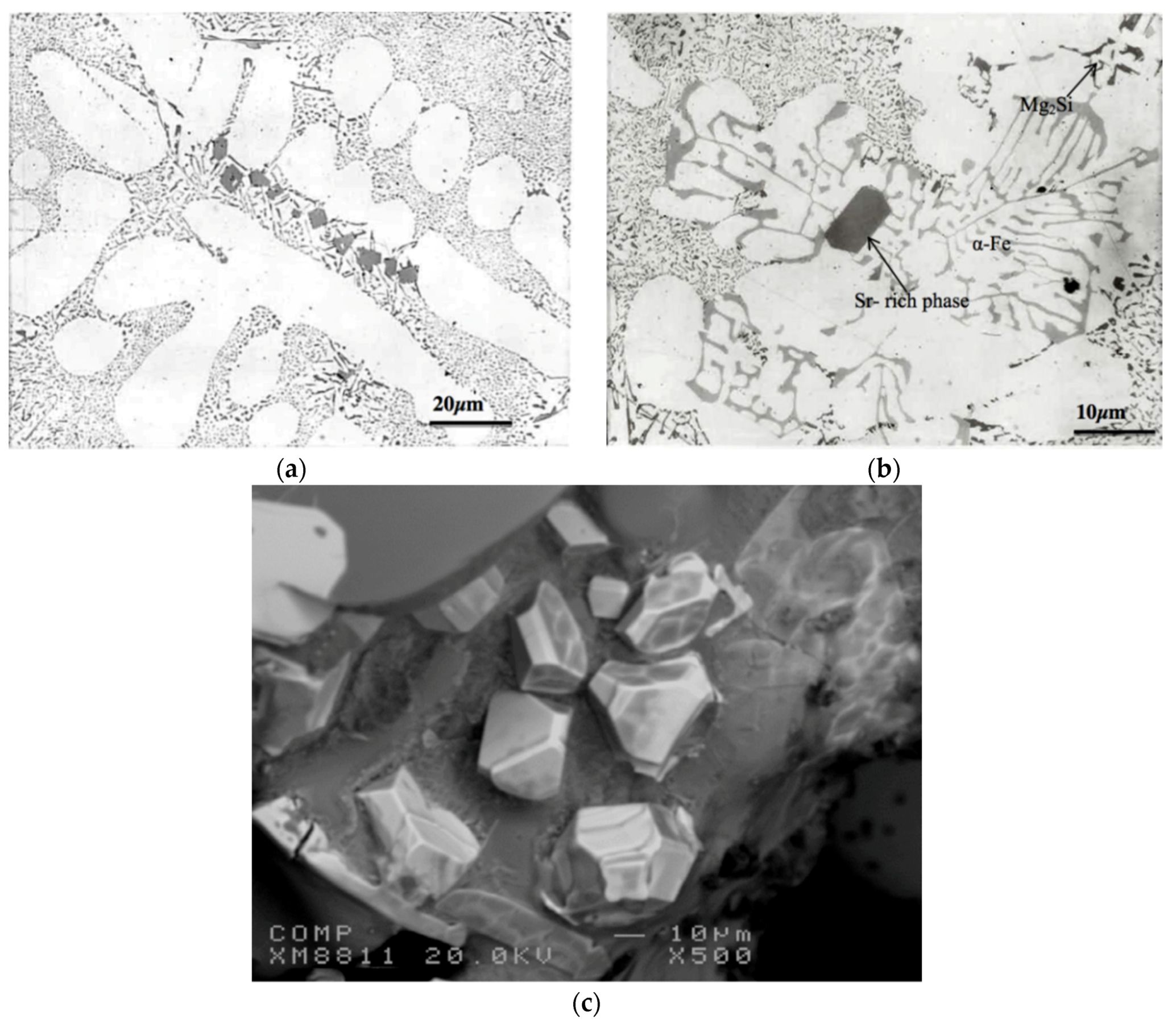
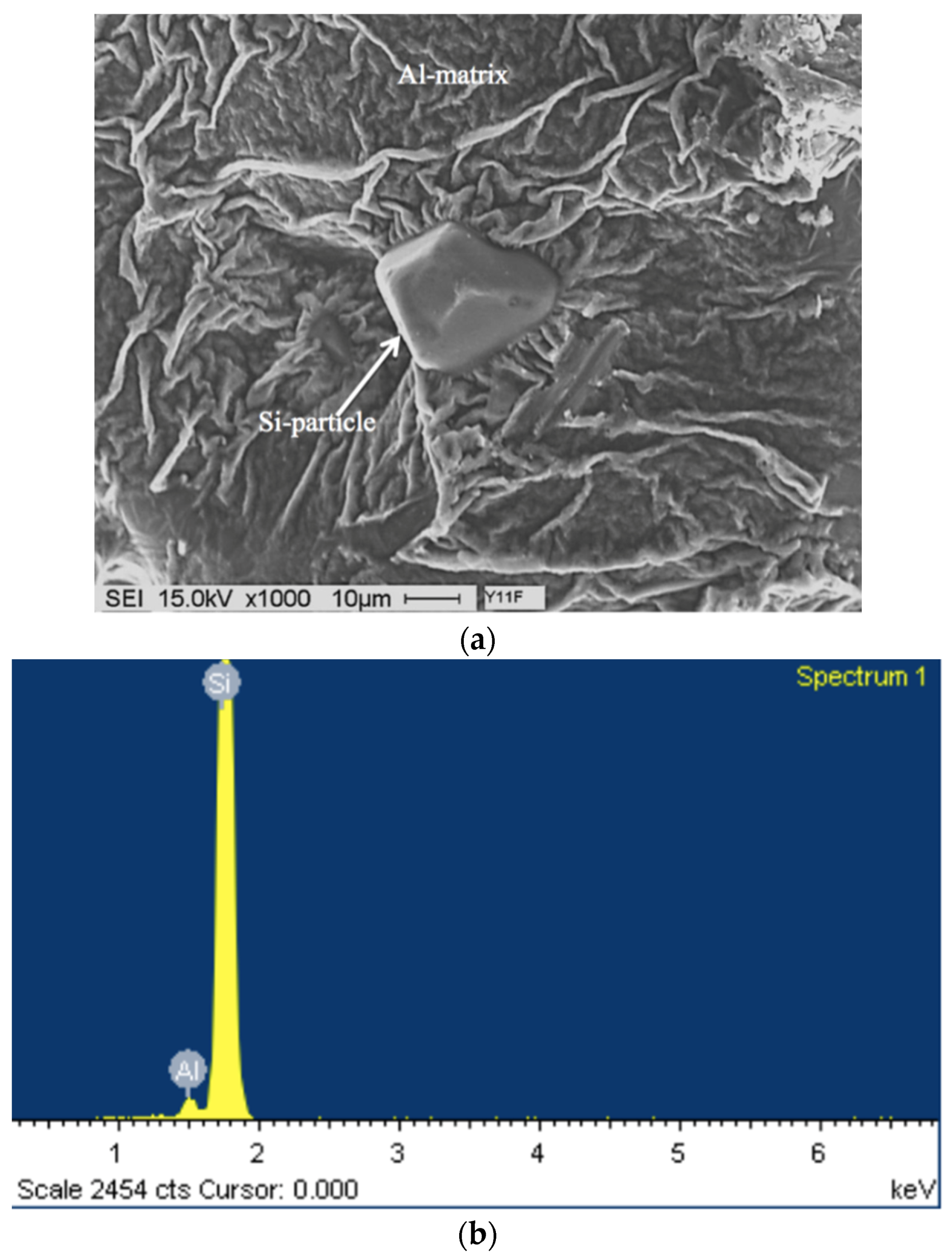
3.2. Overmodification and Overheating
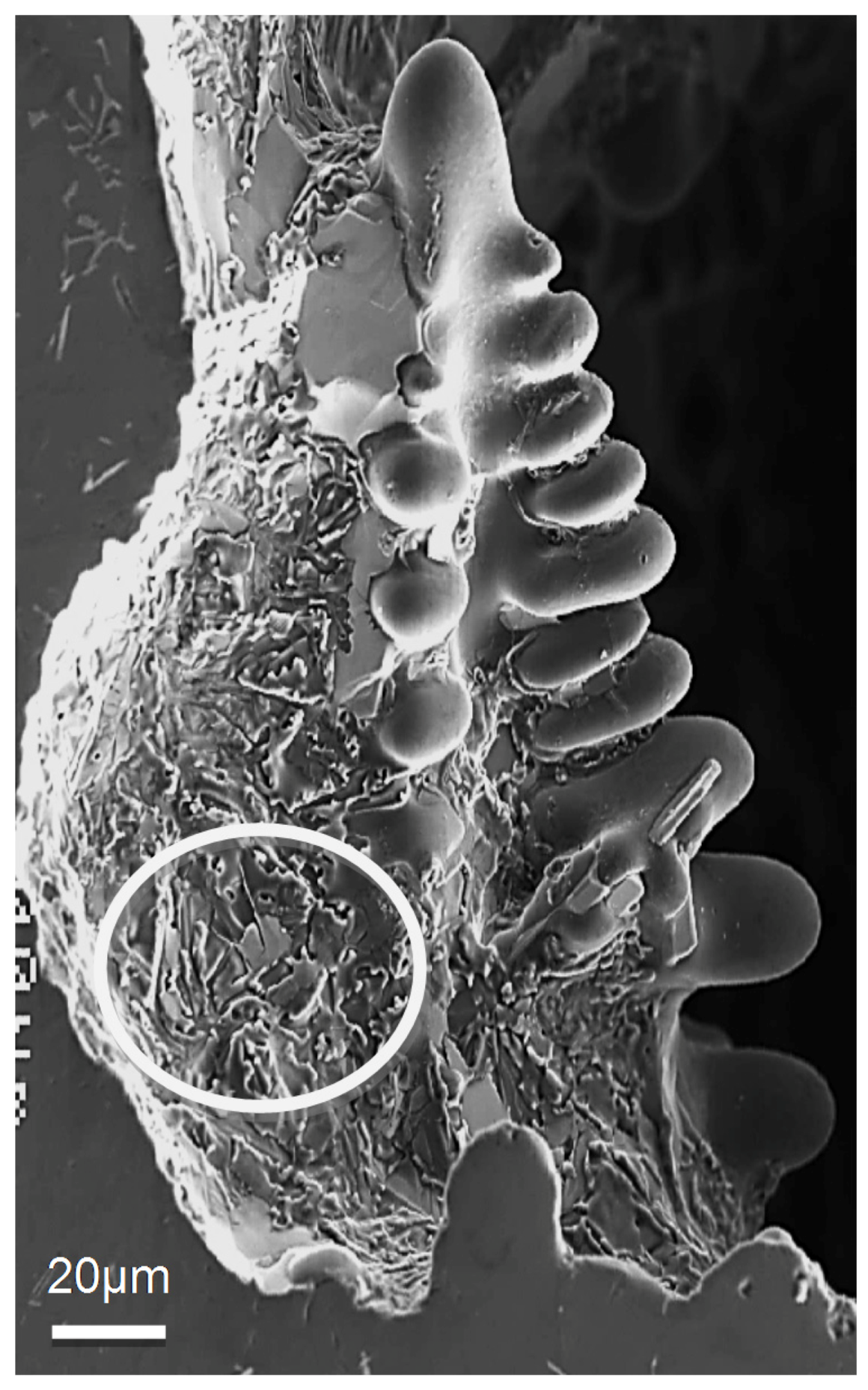
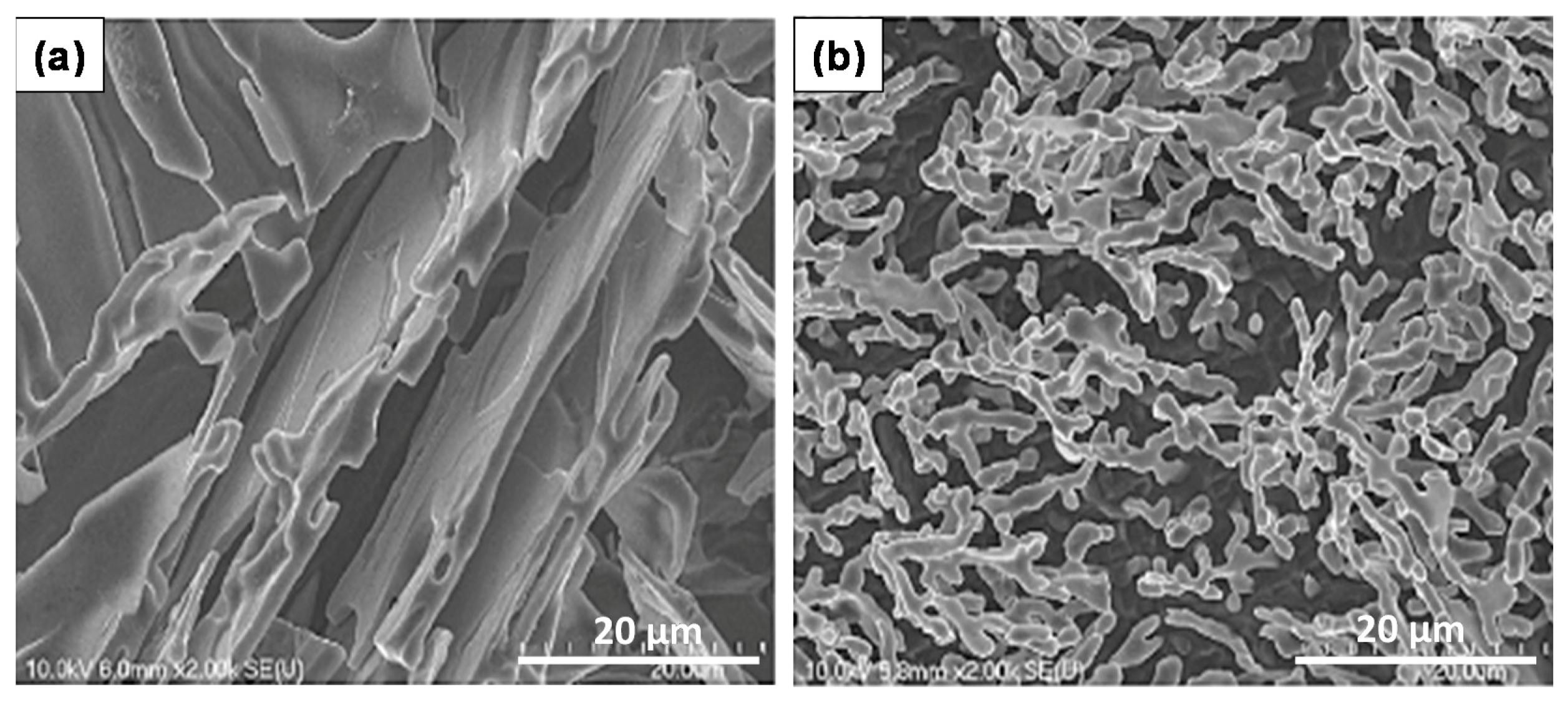
4. Fractography
| Alloy Code | Mechanical Property | As-cast | SHT 5 h | SHT 12 h |
|---|---|---|---|---|
| A1 | UTS (MPa) | 203.8 ± 8.3 | 262.2 ± 8.5 | 255.0 ± 8.6 |
| YS (MPa) | 97.1 ± 3.5 | 115.9 ± 5.8 | 107.5 ± 5.8 | |
| El (%) | 5.5 ± 0.65 | 14.9 ±1.2 | 15.6 ± 1.4 | |
| A1B | UTS (MPa) | 195.9 ± 9.2 | 252.0 ± 8.4 | 270.5 ± 9.2 |
| YS (MPa) | 98.0 ± 5.6 | 111.5 ± 6.8 | 120.5 ± 7.5 | |
| El (%) | 7.0 ± 0.82 | 17.8 ±1.4 | 21.6 ± 1.4 | |
| A1S | UTS (MPa) | 205.3 ± 9.3 | 251.9 ± 8.5 | 257.4 ± 8.5 |
| YS (MPa) | 96.7 ± 5.8 | 114.4 ± 4.5 | 116.3 ± 6.4 | |
| El (%) | 7.6 ± 0.67 | 16.8 ± 1.3 | 18.1 ± 1.5 | |
| A1BS | UTS (MPa) | 198.4 ± 7.5 | 265.7 ± 8.3 | 272.5 ± 9.1 |
| YS (MPa) | 106.9 ± 4.5 | 124.2 ± 6.5 | 135.2 ± 6.5 | |
| El (%) | 5.6 ± 0.36 | 18.1 ± 1.3 | 23.7 ± 1.5 |
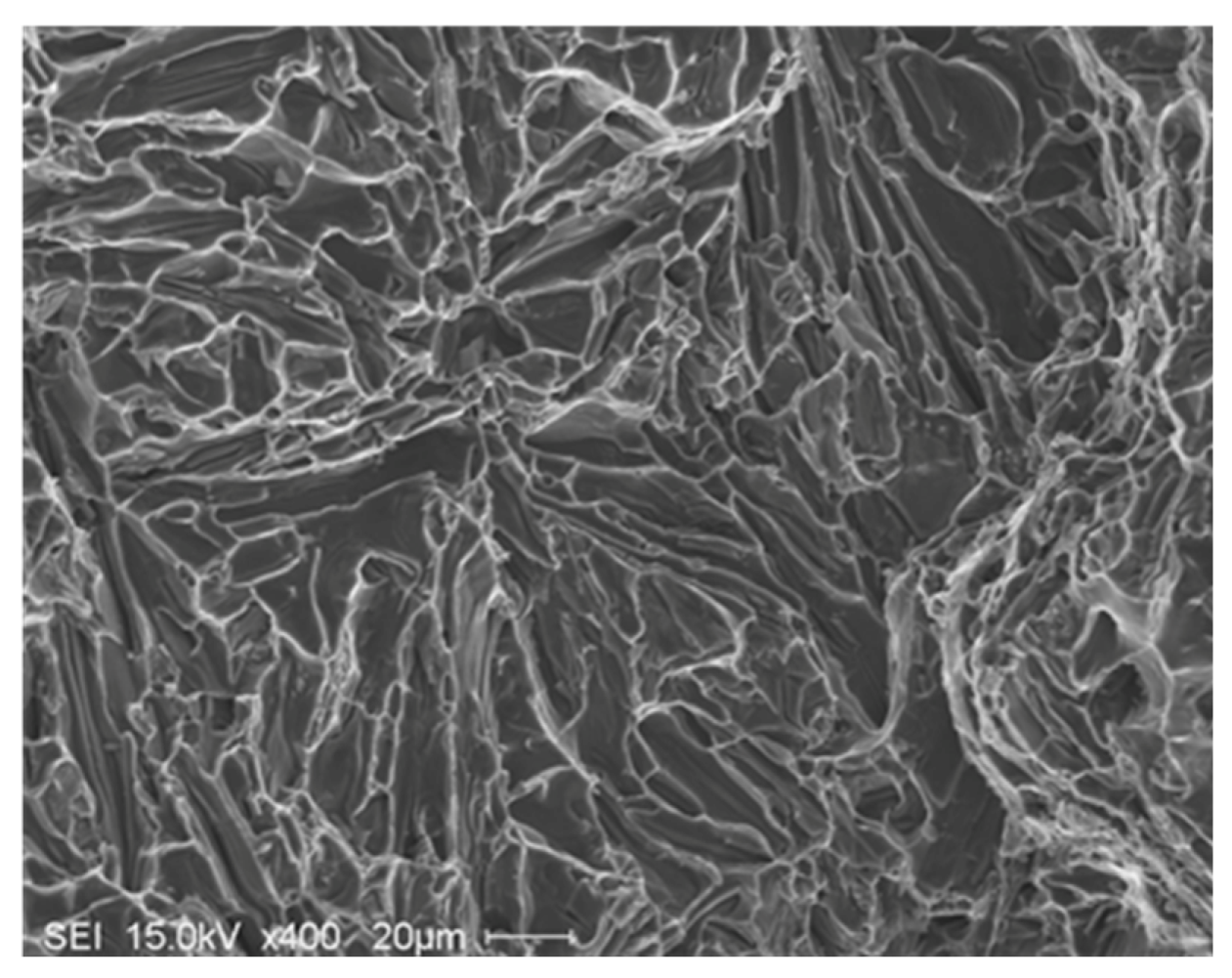
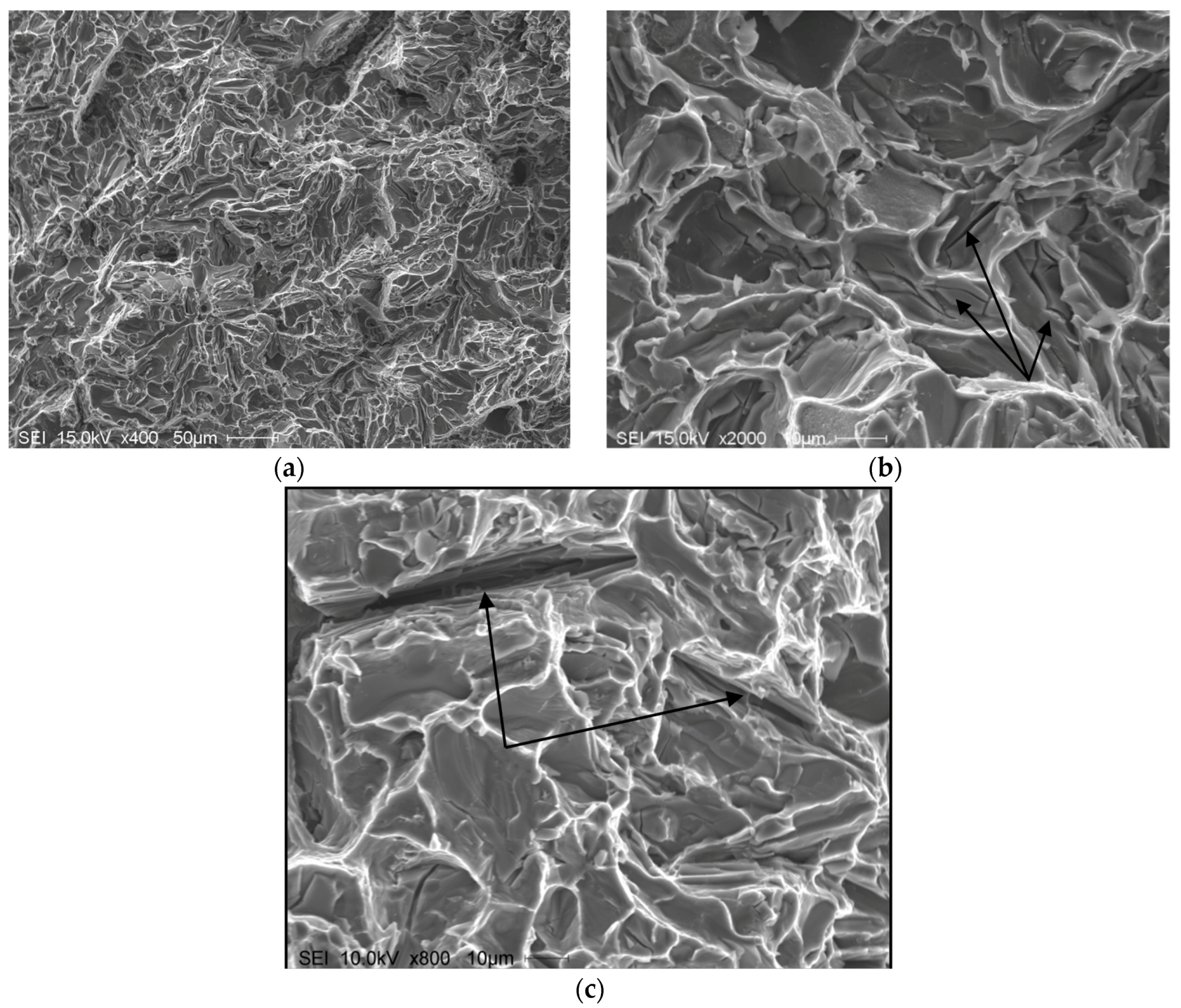
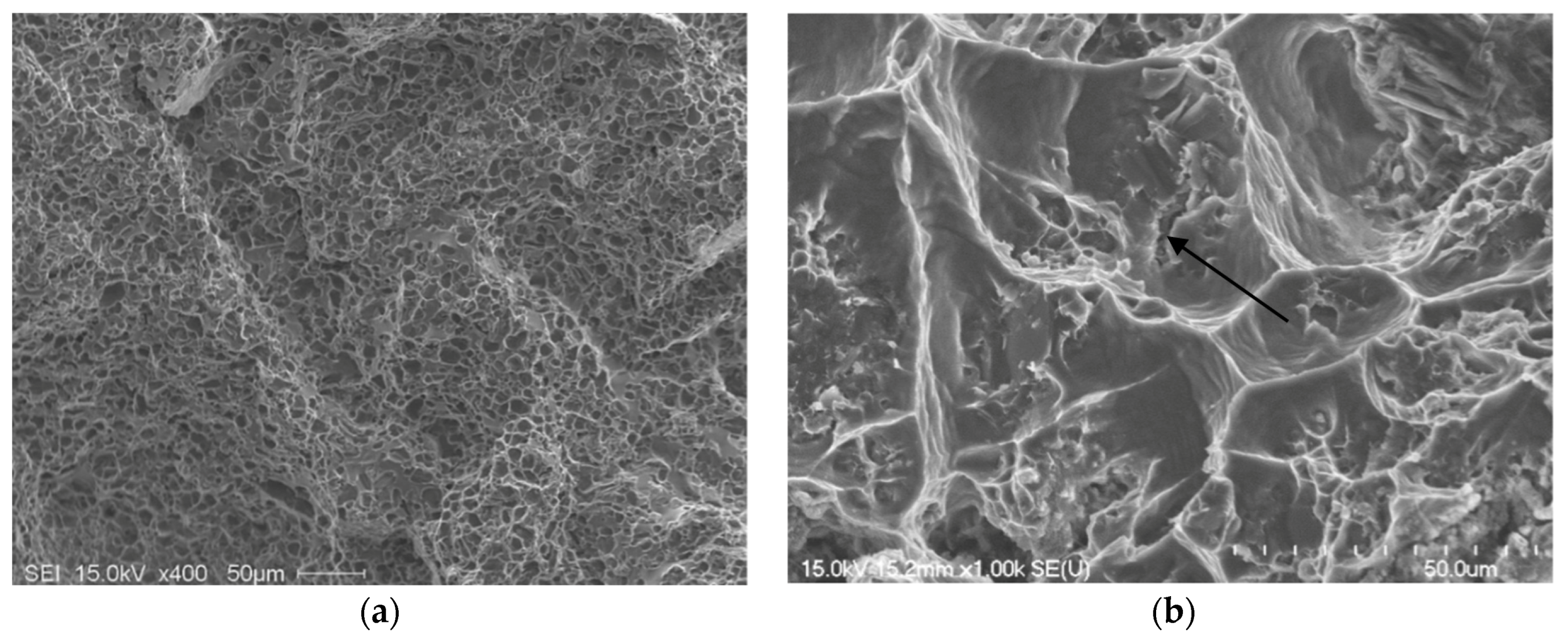

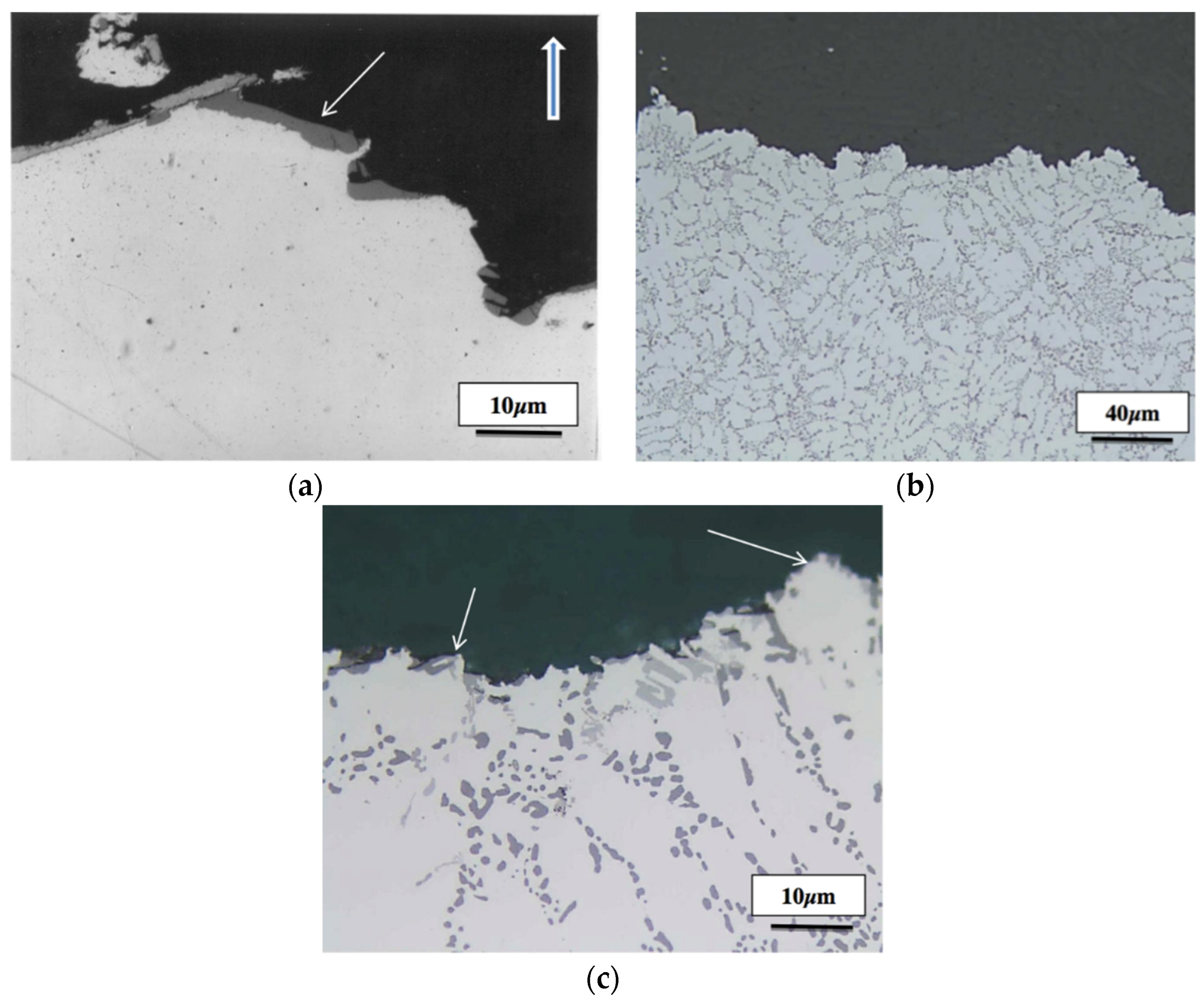
5. Conclusions
- In non-modified alloys, the average values of the Si parameters are more or less equal to standard deviations. Chemical modification (specially with addition of 150 ppm Sr) found to reduce this difference to a great extent.
- Beryllium causes partial modification of the eutectic Si particles, similar to that reported for Mg addition.
- The addition of 0.8% Mg reduced the eutectic temperature by ~10 °C, i.e., 1.3 °C per 0.1% of Mg addition. Interactions between Fe-Be and Sr-Mg may reduce the modification efficiency of Be and Sr.
- The presence of long, acicular Si particles in the matrix accelerates crack propagation, leading to poor ductility. Additionally, the possibility of the existence the planar bifilm cracks in the Si particles may accelerate fracture.
- Depending on the solidification rate, full modification of the eutectic Si may be achieved by the addition of 150–200 ppm of Sr. Exceeding this concentration leads to the precipitation of Sr in the form of primary Al4SrSi2 particles which have a negative influence on the alloy tensile properties. The Sr amount may be reduced through reducing the oxide films.
- Exceeding the proper solution temperature would lead to incipient melting of the aluminum matrix with a change in the eutectic Si particles from spherical to tetrahedral form.
- In low iron (less than 0.1 wt%) Al-Si-Mg alloys, the mechanical properties in the as-cast and heat-treated conditions are mainly controlled by the eutectic Si particle charactersitics. Increasing the iron content, and hence the volume fraction of Fe-based intermetallics, leads to a complex failure mode.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liao, C.; Chen, J.; Pan, C. Microstructure of Al4Sr phase in Al-Sr master alloy and its effect on modification properties. Procedia Eng. 2012, 27, 805–814. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Aluminum and Aluminum Alloys; ASM International, the Materials Information Society: Materials Park, OH, USA, 1994; pp. 1–30. [Google Scholar]
- Sokolowski, J.H.; Djurdjevic, M.B.; Kierkus, C.A.; Northwood, D.O. Improvement of 319 aluminum alloy casting durability by high temperature solution treatment. J. Mater. Process. Technol. 2001, 109, 174–180. [Google Scholar] [CrossRef]
- Sarada, B.N.; Srinivasamurthy, P.L. Swetha, microstructural characteristics of Sr And Na modified Al-Mg-Si alloy. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 3975–3938. [Google Scholar]
- Shivkumar, S.; Keller, C.; Trazzera, M.; Apelian, D. Precipitation Hardening in A356 Alloys. In Proceedings of the International Symposium on Production, Refining, Fabrication and Recycling of Light Metals, Hamilton, ON, Canada, 26–30 August 1990; pp. 264–278.
- Cáceres, C.H.; Davidson, C.J.; Griffiths, J.R.; Wang, Q.G. The effect of Mg on the microstructure and mechanical behavior of Al-Si-Mg casting alloys. Mater. Metall. Trans. A 1999, 30, 1999–2611. [Google Scholar] [CrossRef]
- Hatch, J.E. Aluminum: Properties and Physical Metallurgy; American Society for Metals: Metals Park, OH, USA, 1984. [Google Scholar]
- Murali, S.; Raman, K.S.; Murthy, K.S.S. The formation of β-phase and Be-Fe phases in Al-7Si-0.3Mg alloy containing Be. Mater. Sci. Eng. A 1995, 190, 165–172. [Google Scholar] [CrossRef]
- Kammer, C. Aluminum Handbook, Vol. 1: Fundamentalsand Materials; Aluminium-Verlag Marketing and Kommunication: Dusseldorf, Germany, 1999. [Google Scholar]
- Han, Y.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Optimizing the tensile properties of Al-Si-Cu-Mg 319-type alloys: Role of solution heat treatment. Mater. Des. 2014, 58, 426–438. [Google Scholar] [CrossRef]
- Samuel, E.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Intermetallic phases in Al-Si based cast alloys: New perspective. Int. J. Cast Metals Res. 2014, 27, 107–114. [Google Scholar] [CrossRef]
- Apelian, D.; Shivkumar, S.; Sigworth, G. Fundamental aspects of heat treatment of cast Al-Si-Mg alloys. AFS Trans. 1989, 97, 727–742. [Google Scholar]
- Roy, E.L. Castings, 9th ed.; ASM International: Materials Park, OH, USA, 1992; Volume 15, pp. 743–769. [Google Scholar]
- Samuel, F.H.; Liu, H.; Samuel, A.M. Effect of melt cleanliness on the properties of an Al-10 wt% Si-10 Vol% SIC(P) composite. Metall. Trans. A Phys. Metall. Mater. Sci. 1993, 24, 1631–1645. [Google Scholar] [CrossRef]
- Tiryakioğlu, M.; Campbell, J.; Nyahumwa, C. Fracture surface facets and fatigue life potential of castings. Metall. Mater. Trans. B 2011, 42, 1098–1103. [Google Scholar] [CrossRef]
- Campbell, J. An overview of the effects of bifilms on the structure and properties of cast alloys. Metall. Mater. Trans. B 2006, 37, 857–863. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, H.-C.; Oh, K.H.; Dahl, A.K. Effect of strontium and phosphorus on eutectic Al-Si nucleation and formation of β-Al5FeSi in hypoeutectic Al-Si foundry alloys. Metall. Mater. Trans. A 2008, 39, 2435–2448. [Google Scholar] [CrossRef]
- Ibrahim, M.F. Microstructural characterization of beryllium treated Al-Si alloys. Adv. Mater. Sci. Eng. 2015, 2015. [Google Scholar] [CrossRef]
- Standard Specification for Aluminum-Alloy Permanent Mold Castings; ASTM B108/B108M; ASTM International (ASTM): Englewood, NJ, USA, May 2015.
- Nafisi, S.; Ghomashchi, R.; Vali, H. Eutectic nucleation in hypoeutectic Al-Si alloys. Mater. Charact. 2008, 59, 1466–1473. [Google Scholar] [CrossRef]
- Ma, Z.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Effect of Fe content on the fracture behaviour of Al-Si-Cu cast alloys. Mater. Des. 2014, 57, 366–373. [Google Scholar] [CrossRef]
- Taylor, J.A.; StJohn, D.H.; Zheng, L.H.; Edwards, G.A.; Barresi, J.; Couper, M.J. Solution treatment effects in Al-Si-Mg casting alloys: Part 1—Intermetallic phases. Alum. Trans. 2001, 45, 95–110. [Google Scholar]
- Dasgupta, R.; Brown, C.G.; Mark, S. Analysis of overmodified 356 aluminum alloy. AFS Trans. 1988, 96, 297–310. [Google Scholar]
- Mohamed, A.M.A.; Samuel, F.H. A review on the heat treatment of Al-Si-Cu/Mg casting alloys. In Heat Treatment: Conventional and Novel Applications; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Elsharkawi, E.A.; Samuel, E.; Samuel, A.M.; Samuel, F.H. Effects of Mg, Fe, Be additions and solution heat treatment on the pi-AlMgFeSi iron intermetallic phase in Al-7Si-Mg alloys. J. Mater. Sci. 2010, 45, 1528–1539. [Google Scholar] [CrossRef]
- Ibrahim, M.F. Effects of Be, Sr, Fe and Mg Interactions on the Microstructure and Mechanical Properties of Aluminum Based Aeronautical Alloys. Ph.D. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 9 April 2015. [Google Scholar]
- Wang, Q.G. Microstructural effects on the tensile and fracture behavior of aluminum casting alloys A356/357. Metall. Mater. Trans. A 2003, 34, 2887–2899. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.F.; Elgallad, E.M.; Valtierra, S.; Doty, H.W.; Samuel, F.H. Metallurgical Parameters Controlling the Eutectic Silicon Charateristics in Be-Treated Al-Si-Mg Alloys. Materials 2016, 9, 78. https://doi.org/10.3390/ma9020078
Ibrahim MF, Elgallad EM, Valtierra S, Doty HW, Samuel FH. Metallurgical Parameters Controlling the Eutectic Silicon Charateristics in Be-Treated Al-Si-Mg Alloys. Materials. 2016; 9(2):78. https://doi.org/10.3390/ma9020078
Chicago/Turabian StyleIbrahim, Mohamed F., Emad M. Elgallad, Salvador Valtierra, Herbert W. Doty, and Fawzy H. Samuel. 2016. "Metallurgical Parameters Controlling the Eutectic Silicon Charateristics in Be-Treated Al-Si-Mg Alloys" Materials 9, no. 2: 78. https://doi.org/10.3390/ma9020078
APA StyleIbrahim, M. F., Elgallad, E. M., Valtierra, S., Doty, H. W., & Samuel, F. H. (2016). Metallurgical Parameters Controlling the Eutectic Silicon Charateristics in Be-Treated Al-Si-Mg Alloys. Materials, 9(2), 78. https://doi.org/10.3390/ma9020078





