Mechanical Strength Improvements of Carbon Nanotube Threads through Epoxy Cross-Linking
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
- MLCA—mass of liquid curing agent;
- M—molecular weight of LCA;
- G—epoxide number of glycidyl amine epoxy;
- Hn—number of active hydrogen.
2.2. Characterization
3. Results and Discussion
3.1. Mechanical Properties and Chemical Reaction Mechanism
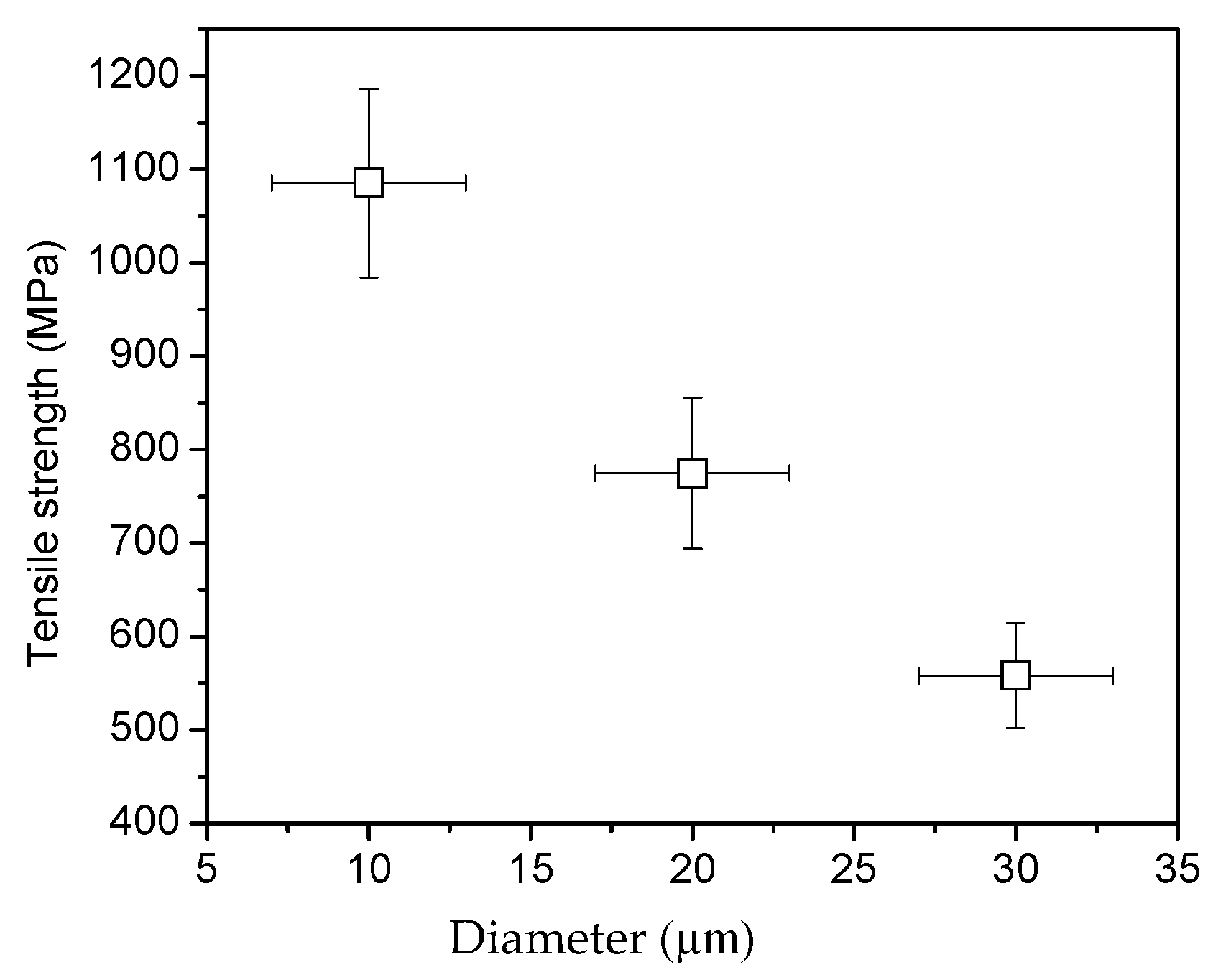

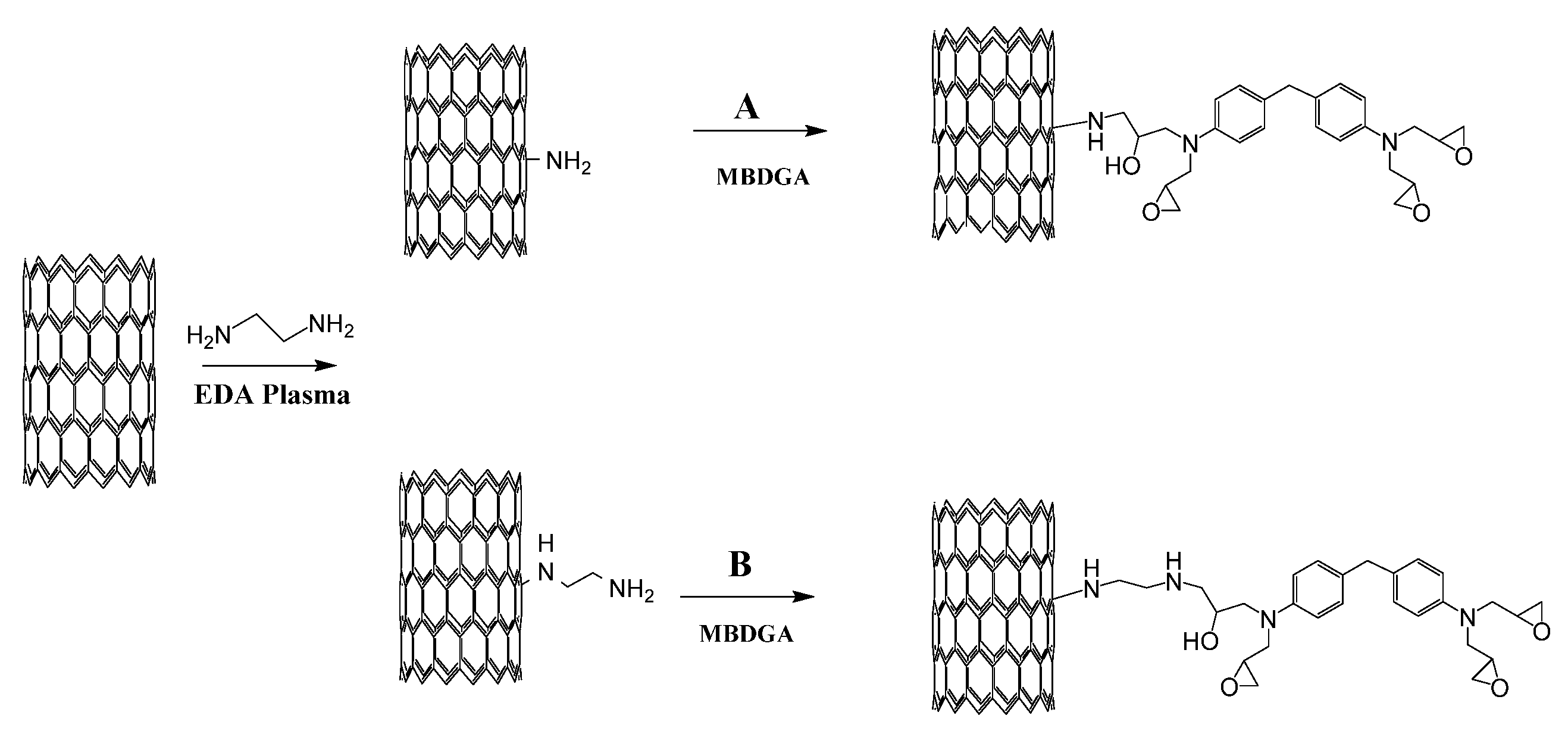
3.2. The Effects of Defects and Functional Groups
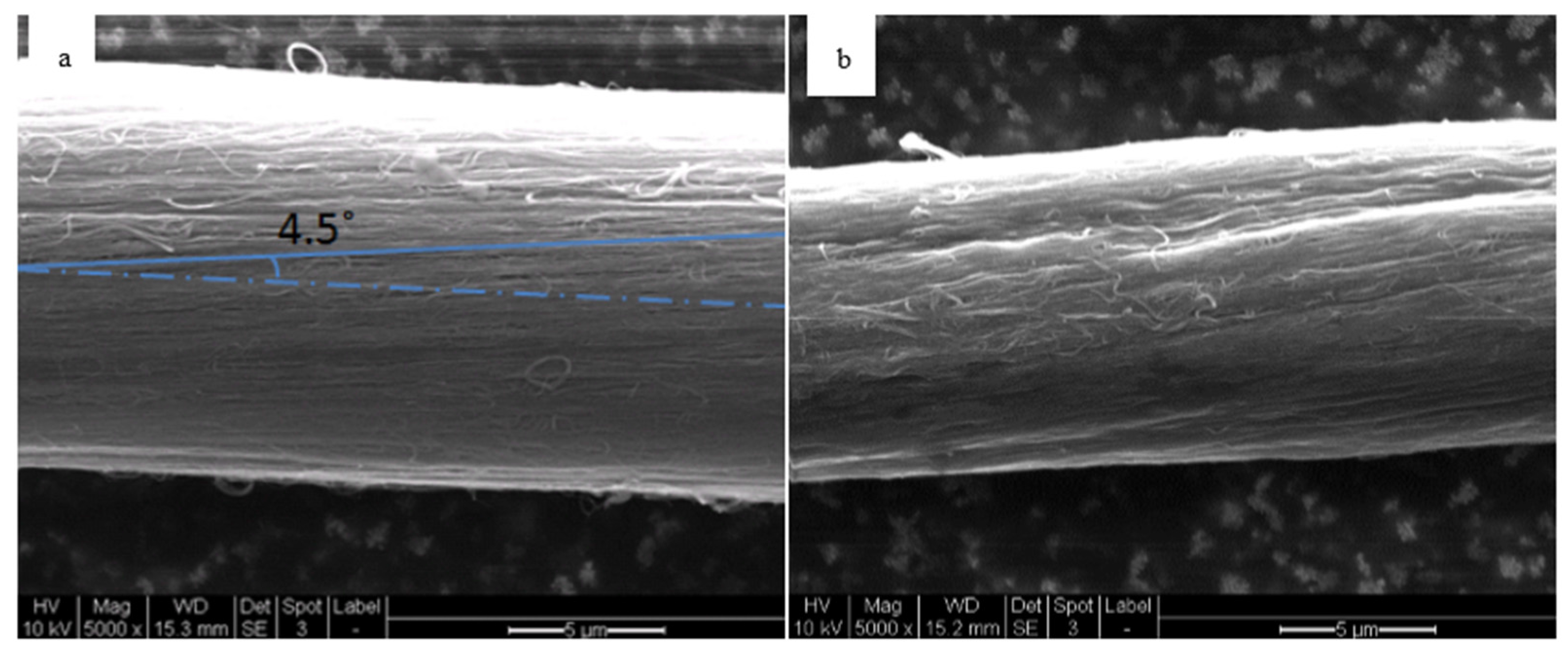
3.2.1. SEM Analysis
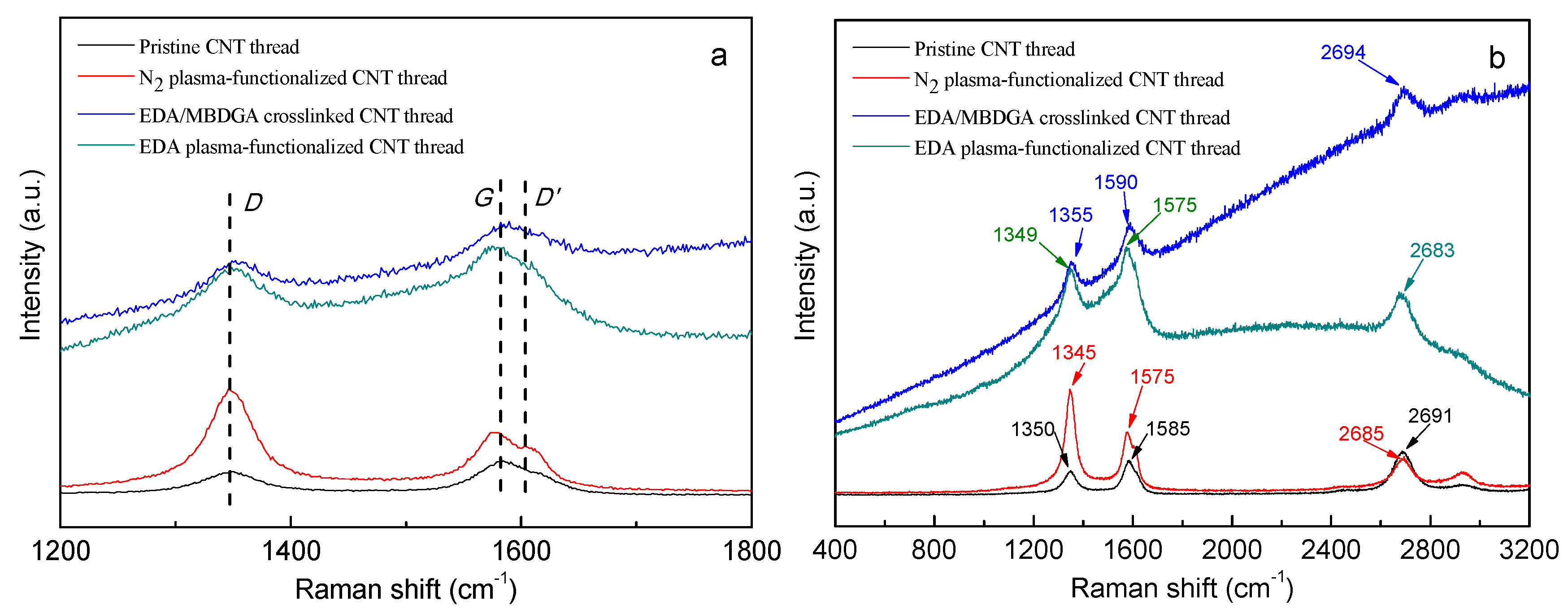
3.2.2. Raman Analysis
3.2.3. XPS Analysis
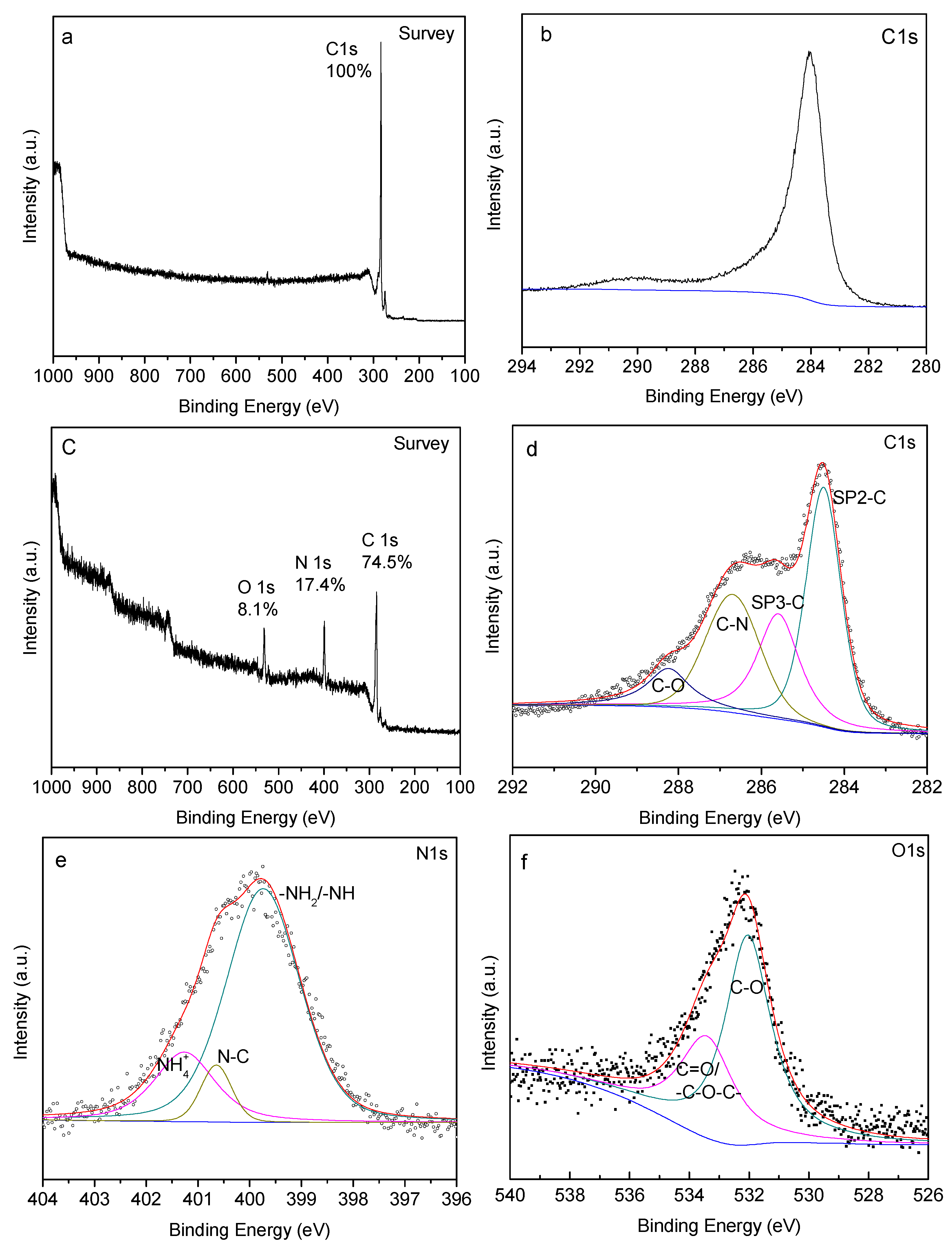
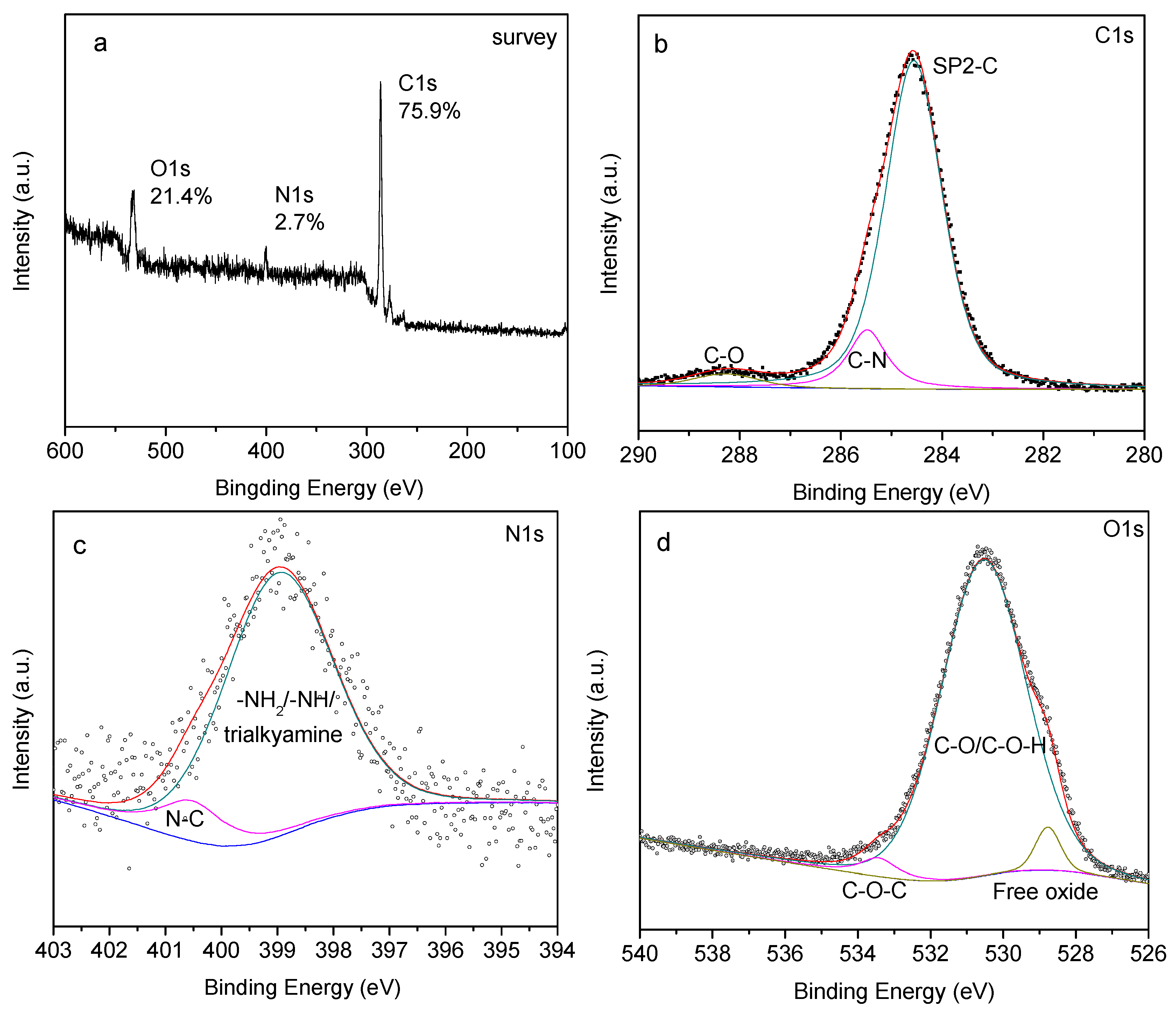
3.2.4. Thermogravimetric Analysis

| Sample | Conductivity (S·cm−1) | Standard Deviation (S·cm−1) |
|---|---|---|
| Pristine CNT thread | 3984.26 | 110.67 |
| EDA/MBDGA cross-linked CNT thread | 2123.40 | 86.16 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dalton, A.B.; Collins, S.; Muñoz, E.; Razal, J.M.; Ebron, V.H.; Ferraris, J.P.; Coleman, J.N.; Kim, B.G.; Baughman, R.H. Super-tough carbon-nanotube fibers. Nature 2003, 423, 703. [Google Scholar] [CrossRef] [PubMed]
- Demczyk, B.G.; Wang, Y.M.; Cumings, J.; Hetman, M.; Han, W.; Zettl, A.; Ritchie, R.O. Direct mechanical measurement of the tensile strength and elastic modulus of multiwalled carbon nanotubes. Mater. Sci. Eng. A 2002, 334, 173–178. [Google Scholar] [CrossRef]
- Yu, M.F.; Siles, B.S.; Arepalli, S.; Ruoff, R.S. Tensile loading of ropes of single wall carbon nanotubes and their mechanical properties. Phys. Rev. Lett. 2000, 84, 5552–5555. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Vilatela, A.C.; Koziol, K.K.K. Performance of carbon nanotube wires in extreme conditions. Carbon 2013, 62, 438–446. [Google Scholar] [CrossRef]
- McEuen, P.L.; Fuhrer, M.S.; Park, H. Single-walled carbon nanotube electronics. IEEE Trans. Nanotechnol. 2002, 1, 78–85. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Tu, Y.; Li, Y.; Coulter, J.Y.; Zheng, L.; Zhao, Y.; Jia, Q.; Peterson, D.E.; Zhu, Y. Strong carbon-nanotube fibers spun from long carbon-nanotube arrays. Small 2007, 3, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Schulte, K. Influence of different carbon nanotubes on the mechanical properties of epoxy matrix composites—A comparative study. Compos. Sci. Technol. 2005, 65, 2300–2313. [Google Scholar] [CrossRef]
- Bahr, J.L.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.T.; Li, Y.; Schmidt, H.K.; Tour, J.M. Selective redox chemistry with metallic swnt. 2008; unpublished. [Google Scholar]
- Cai, J.Y.; Min, J.; McDonnell, J.; Church, J.S.; Easton, C.D.; Humphries, W.; Lucas, S.; Woodhead, A.L. An improved method for functionalisation of carbon nanotube spun yarns with aryldiazonium compounds. Carbon 2012, 50, 4655–4662. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Banerjee, S.; Wong, S.S. Rational sidewall functionalization and purification of single-walled carbon nanotubes by solution-phase ozonolysis. J. Phys. Chem. B 2002, 106, 12144–12151. [Google Scholar] [CrossRef]
- Ritts, A.C.; Yu, Q.; Li, H.; Lombardo, S.J.; Han, X.; Xia, Z.; Lian, J. Plasma treated multi-walled carbon nanotubes (MWCNTs) for epoxy nanocomposites. Polymers 2011, 3, 2142–2155. [Google Scholar] [CrossRef]
- Gao, Y.; He, P.; Lian, J.; Wang, L.; Qian, D.; Zhao, J.; Wang, W.; Schulz, M.J.; Zhang, J.; Zhou, X.; et al. Improving the mechanical properties of polycarbonate nanocomposites with plasma-modified carbon nanofibers. J. Macromol. Sci. 2006, 45, 671–679. [Google Scholar] [CrossRef]
- Shi, D.; Lian, J.; He, P.; Wang, L.M.; van Ooij, W.J.; Schulz, M.J.; Liu, Y.; Mast, D. Plasma deposition of ultrathin polymer film on carbon nanotubes. Appl. Phys. Lett. 2002, 81, 5216–5218. [Google Scholar] [CrossRef]
- Yu, Q.; Kim, Y.J.; Ma, H. Plasma treatment of diamond nanoparticles for dispersion improvement in water. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Avila-Orta, C.A.; Cruz-Delgado, V.J.; Neira-Velazquez, M.G.; Hernandez-Hernandez, E.; Mendez-Padilla, M.G.; Medellın-Rodrıguez, F.J. Surface modification of carbon nanotubes with ethylene glycol plasma. Carbon 2009, 47, 1916–1921. [Google Scholar] [CrossRef]
- Naseh, M.V.; Khodadadi, A.A.; Mortazavi, Y.; Pourfayaz, F.; Alizadeh, O.; Maghrebi, M. Fast and clean functionalization of carbon nanotubes by dielectric barrier discharge plasma in air compared to acid treatment. Carbon 2010, 48, 1369–1379. [Google Scholar] [CrossRef]
- Wei, H.; Wei, Y.; Wu, Y.; Liu, L.; Fan, S.; Jiang, K. High-strength composite yarns derived from oxygen plasma modified super-aligned carbon nanotube arrays. Nano Res. 2013, 6, 208–215. [Google Scholar] [CrossRef]
- Chen, X.; Wudl, F.; Mal, A.K.; Shen, H.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Rocks, J.; Rintoul, L.; Vohwinkel, F.; George, G. The kinetics and mechanism of cure of an amino-glycidyl epoxy resin by a co-anhydride as studied by FT-Raman spectroscopy. Polymer 2004, 45, 6799–6811. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Park, B.; Sa, J.H.; Jung, A.; Kim, T.; Park, J.; Hwang, W.; Lee, K.H. Improving the tensile strength of carbon nanotube yarn via one-step double [2+1] cycloadditions. Korean J. Chem. Eng. 2015, 33. [Google Scholar] [CrossRef]
- Min, J.; Cai, J.Y.; Sridhar, M.; Easton, C.D.; Gengenbach, T.R.; McDonnell, J.; Humphries, W.; Lucas, S. High performance carbon nanotube spun yarns from a crosslinked network. Carbon 2013, 52, 520–527. [Google Scholar] [CrossRef]
- Kumar, R.; Rao, C.N.R. Assemblies of single-walled carbon nanotubes generated by covalent cross-linking with organic linkers. J. Mater. Chem. A 2015, 3, 6747–6750. [Google Scholar] [CrossRef]
- Cheng, F.; Adronov, A. Suzuki coupling reactions for the surface functionalization of single-walled carbon nanotubes. Chem. Mater. 2006, 18, 5389–5391. [Google Scholar] [CrossRef]
- Miller, S.G.; Williams, T.S.; Baker, J.S.; Solá, F.; Lebron-Colon, M.; McCorkle, L.S.; Wilmoth, N.G.; Gaier, J.; Chen, M.; Meador, M.A. Increased tensile strength of carbon nanotube yarns and sheets through chemical modification and electron beam irradiation. ACS Appl. Mater. Interfaces 2014, 6, 6120–6126. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.D.; Hudson, J.L.; Fan, H.; Booker, R.; Simpson, L.J.; O′Neill, K.J.; Parilla, P.A.; Heben, M.J.; Pasquali, M.; Kittrell, C.; et al. Nanoengineered carbon scaffolds for hydrogen storage. J. Am. Chem. Soc. 2008, 131, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.T.; Miller, P.; Haase, M.; Kienzle, N.; Zhang, L.; Schulz, M.; Shanov, V. Carbon nanotube assembly at near-industrial natural-fiber spinning rates. Carbon 2015, 86, 350–357. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Chakrabarti, S.; Schulz, M.J.; Shanov, V. Spinning yarn from long carbon nanotube arrays. J. Mater. Res. 2011, 26, 645–651. [Google Scholar] [CrossRef]
- Alvarez, N.T.; Li, F.; Pint, C.L.; Mayo, J.T.; Fisher, E.Z.; Tour, J.M.; Colvin, V.L.; Hauge, R.H. Uniform large diameter carbon nanotubes in vertical arrays from premade near-monodisperse nanoparticles. Chem. Mater. 2011, 23, 3466–3475. [Google Scholar] [CrossRef]
- Alvarez, N.T.; Orbaek, A.; Barron, A.R.; Tour, J.M.; Hauge, R.H. Dendrimer-assisted self-assembled monolayer of iron nanoparticles for vertical array carbon nanotube growth. ACS Appl. Mater. Interfaces 2010, 2, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Miao, M. Yarn spun from carbon nanotube forests: Production, structure, properties and applications. Particuology 2013, 11, 378–393. [Google Scholar] [CrossRef]
- Gao, G.; Jin, Y.Z.; Kong, H.; Whitby, R.L.D.; Acquah, S.F.A.; Chen, G.Y.; Qian, H.; Hartschuh, A.; Silva, S.R.P.; Henley, S.; et al. Polyurea-functionalized multiwalled carbon nanotubes: Synthesis, morphology, and Raman spectroscopy. J. Phys. Chem. B 2005, 109, 11925–11932. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Seong, D.G.; Kang, T.J.; Youn, J.R. Effects of surface modification on rheological and mechanical properties of CNT/epoxy composites. Carbon 2006, 44, 1898–1905. [Google Scholar] [CrossRef]
- Maldonado, S.; Morin, S.; Stevenson, K.J. Structure, composition, and chemical reactivity of carbon nanotubes by selective nitrogen doping. Carbon 2006, 44, 1429–1437. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, B.S.; Chen, S.; Shao-Horn, Y.; Hammond, P.T. Layer-by-layer assembly of all carbon nanotube ultrathin films for electrochemical applications. J. Am. Chem. Soc. 2009, 131, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution XPS and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Kundu, S.; Xia, W.; Busser, W.; Becker, M.; Schmidt, D.A.; Havenith, M.; Muhler, M. The formation of nitrogen-containing functional groups on carbon nanotube surfaces: A quantitative XPS and TPD study. Phys. Chem. Chem. Phys. 2010, 12, 4351–4359. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Huang, W.; Wu, L.; Hu, Y.; Ye, M. Thermo-physical properties of epoxy nanocomposites reinforced with amino-functionalized multi-walled carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1331–1336. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Alvarez, N.T.; Miller, P.; Malik, R.; Haase, M.R.; Schulz, M.; Shanov, V.; Zhu, X. Mechanical Strength Improvements of Carbon Nanotube Threads through Epoxy Cross-Linking. Materials 2016, 9, 68. https://doi.org/10.3390/ma9020068
Yu Q, Alvarez NT, Miller P, Malik R, Haase MR, Schulz M, Shanov V, Zhu X. Mechanical Strength Improvements of Carbon Nanotube Threads through Epoxy Cross-Linking. Materials. 2016; 9(2):68. https://doi.org/10.3390/ma9020068
Chicago/Turabian StyleYu, Qingyue, Noe T. Alvarez, Peter Miller, Rachit Malik, Mark R. Haase, Mark Schulz, Vesselin Shanov, and Xinbao Zhu. 2016. "Mechanical Strength Improvements of Carbon Nanotube Threads through Epoxy Cross-Linking" Materials 9, no. 2: 68. https://doi.org/10.3390/ma9020068
APA StyleYu, Q., Alvarez, N. T., Miller, P., Malik, R., Haase, M. R., Schulz, M., Shanov, V., & Zhu, X. (2016). Mechanical Strength Improvements of Carbon Nanotube Threads through Epoxy Cross-Linking. Materials, 9(2), 68. https://doi.org/10.3390/ma9020068







