A Natural Bacterium-Produced Membrane-Bound Nanocarrier for Drug Combination Therapy
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
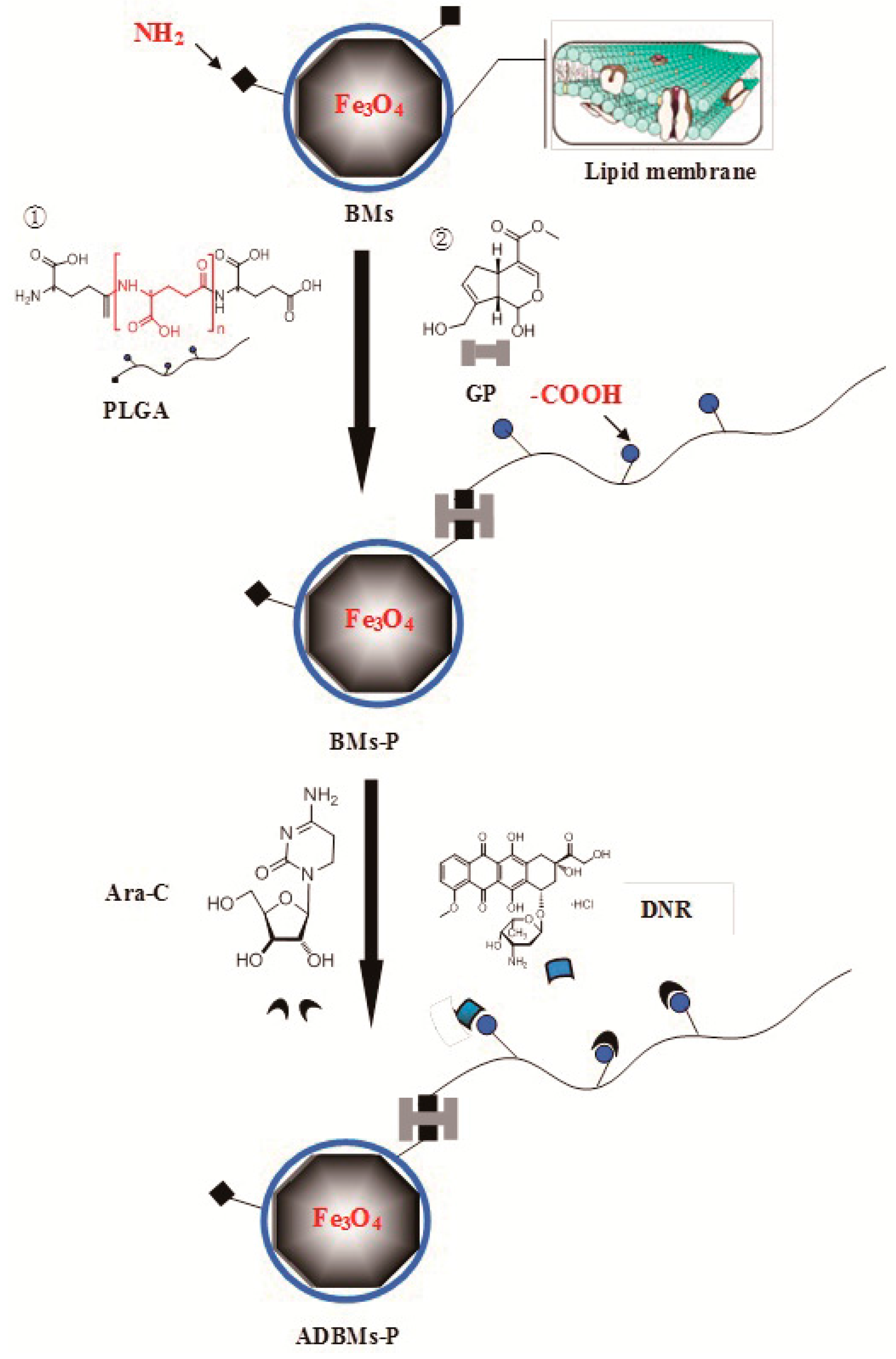
2.2. Preparation of the ADBMs-Ps
2.3. Characterization of the ADBMs-Ps
2.4. The Drug-Loading and Encapsulation Efficiency of ADBMs-Ps
2.5. In Vitro Release Studies
2.6. Cytotoxicity Assay
3. Results and Discussion
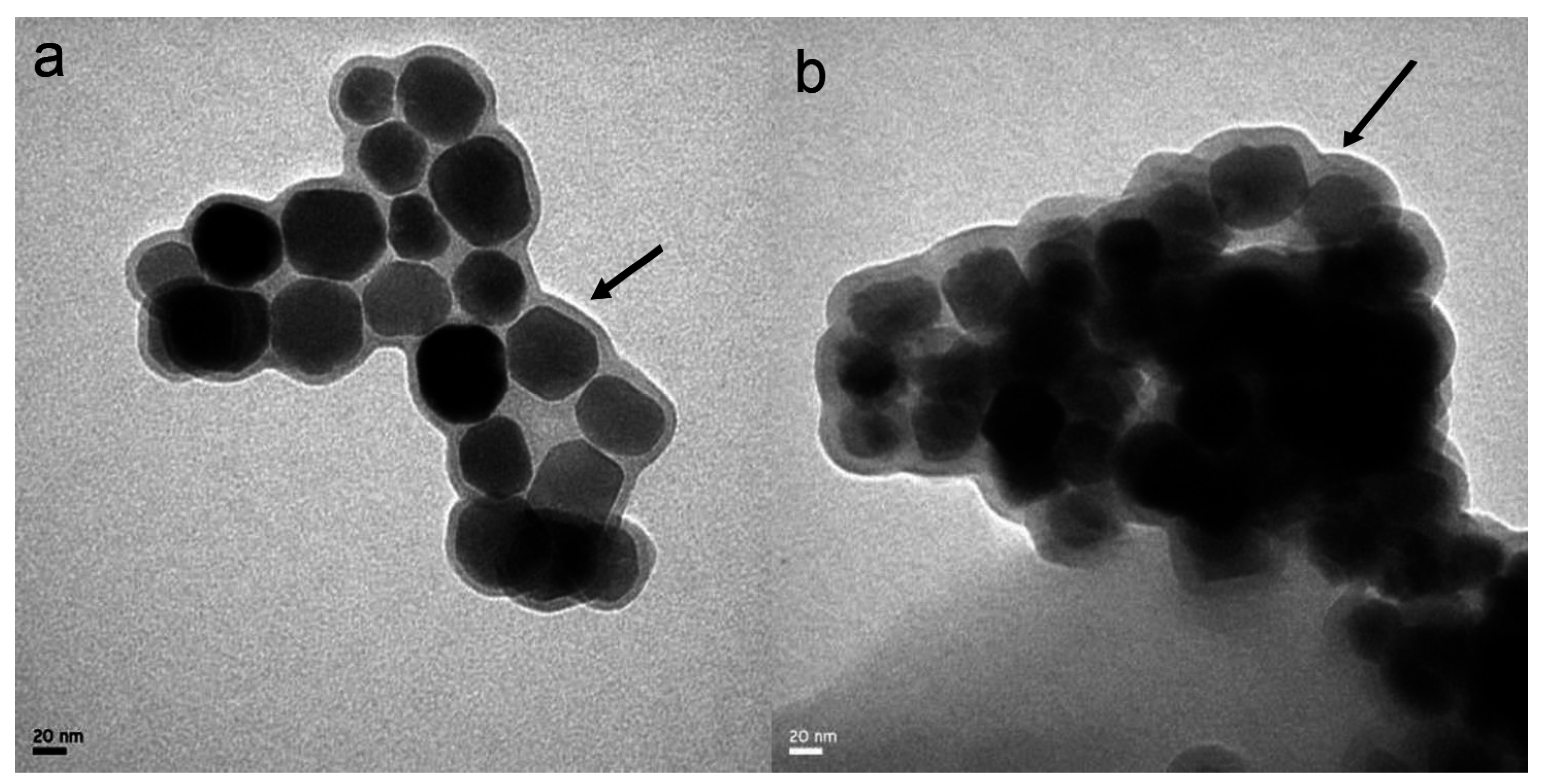
3.1. Characterization of the ADBMs-Ps
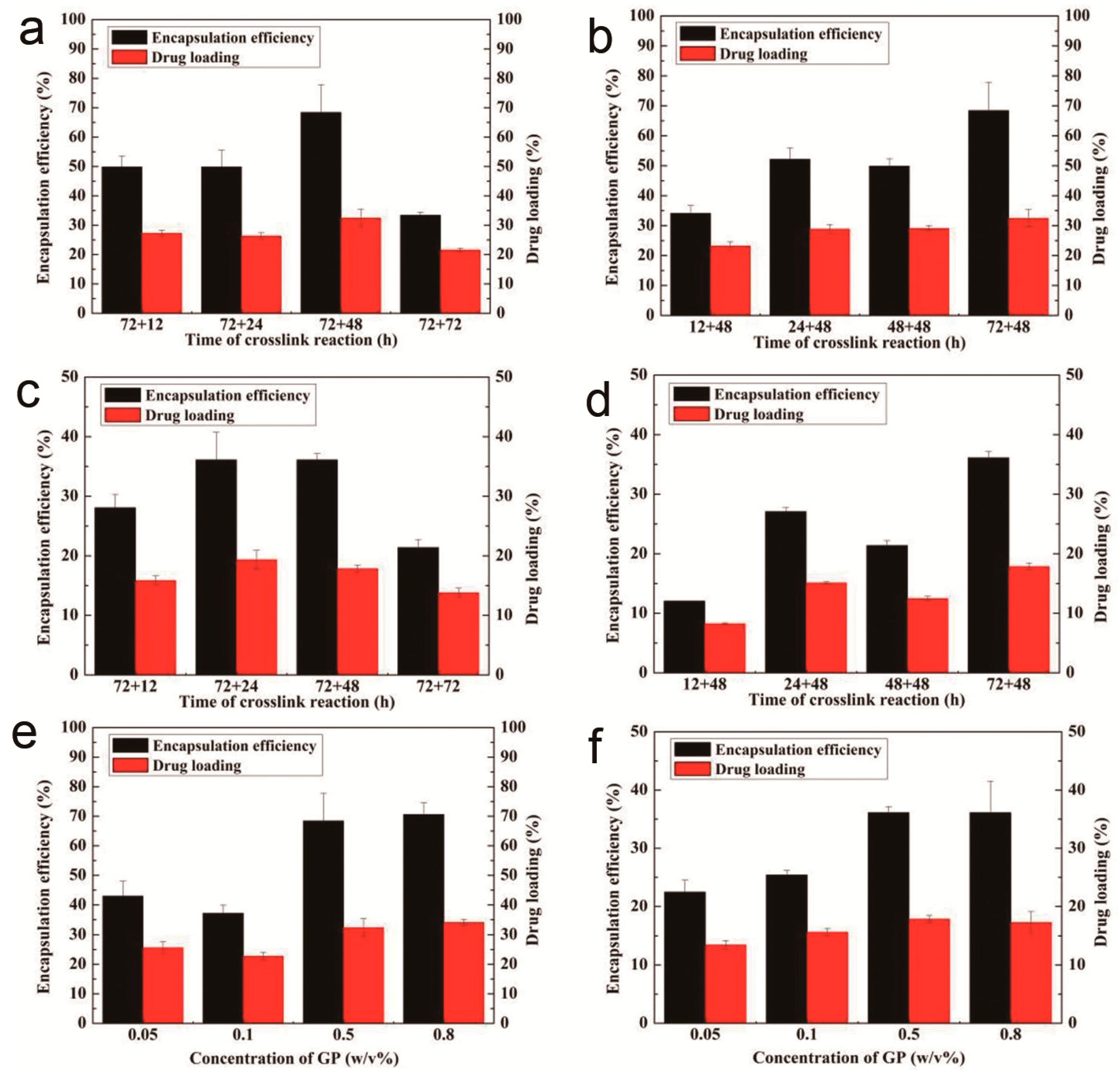
3.2. The Drug-Loading and Encapsulation Efficiency of ADBMs-Ps
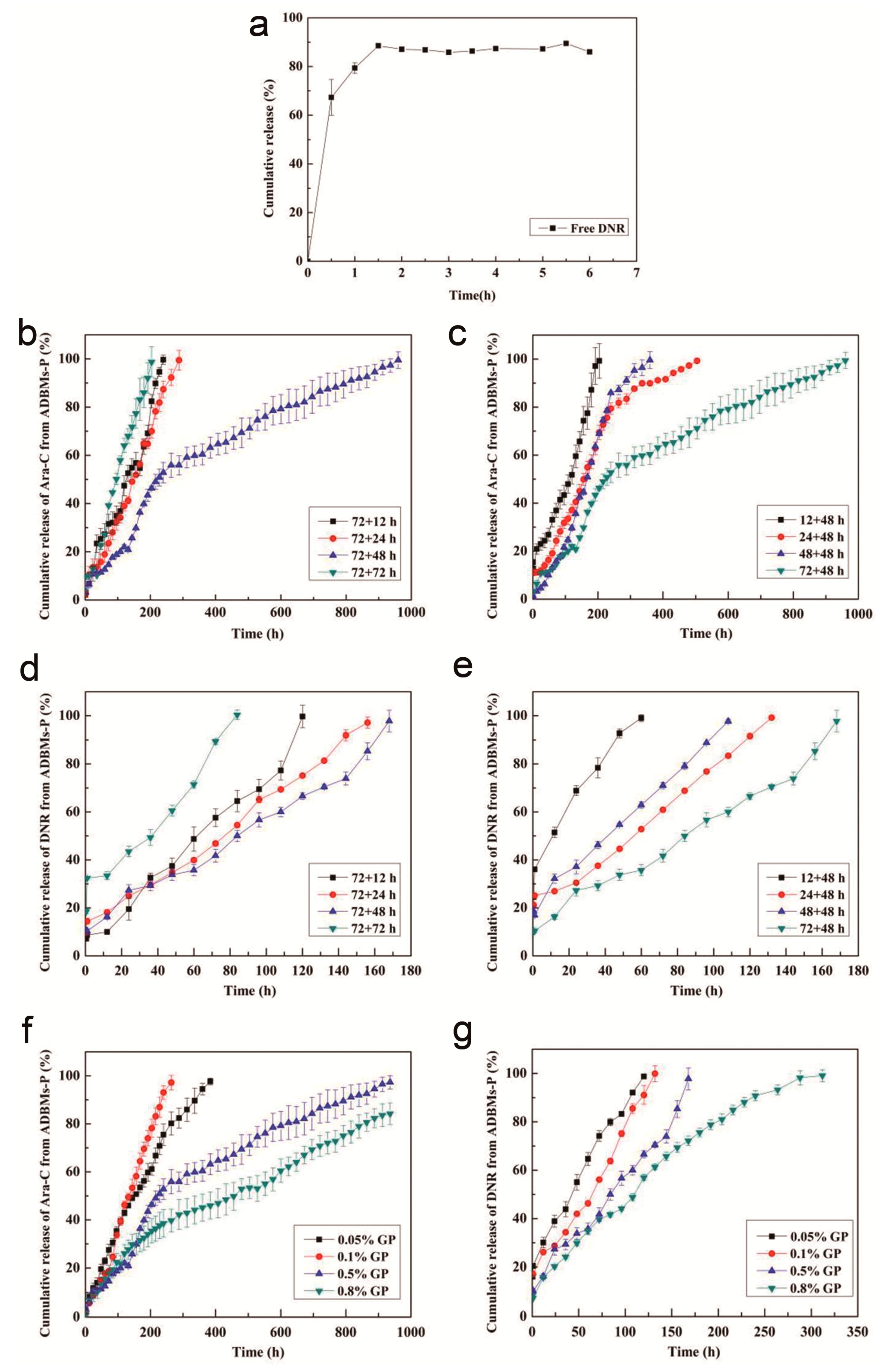
3.3. In Vitro Release Studies
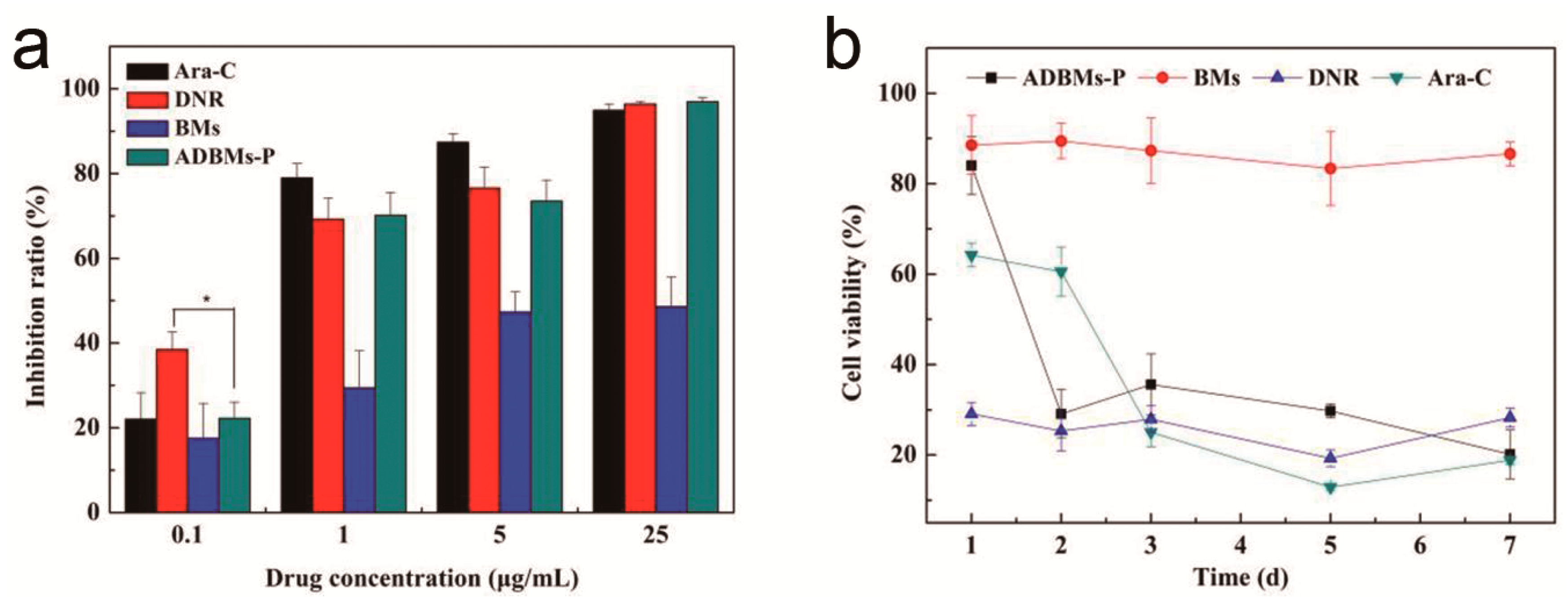
3.4. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Y.C.; Bathula, S.R.; Li, J.; Huang, L. Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. J. Biol. Chem. 2010, 285, 22639–22650. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Girija, A.R.; Nagaoka, Y.; Iwai, S.; Suzuki, M.; Kizhikkilot, V.; Yoshida, Y.; Maekawa, T.; Nair, S.D. Curcumin and 5-Fluorouracil-loaded, folate- and transferrin-decorated polymeric magnetic nanoformulation: A synergistic cancer therapeutic approach, accelerated by magnetic hyperthermia. Int. J. Nanomed. 2014, 9, 437–459. [Google Scholar]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, C.M.; Dong, Q.Q.; Zhang, L.; Zhang, X.; Ma, Z.Y.; Han, Q.R. Preparation and characterization of magnetic Fe3O4-chitosan nanoparticles loaded with isoniazid. J. Magn. Magn. Mater. 2015, 381, 120–126. [Google Scholar] [CrossRef]
- Sagar, V.; Pilakka-Kanthikeel, S.; Atluri, V.S.R.; Ding, H.; Arias, A.Y.; Jayant, R.D.; Kaushik, A.; Nair, M. Therapeutical neurotargeting via magnetic nanocarrier: Implications to opiate-induced neuropathogenesis and neuroAIDS. J. Biomed. Nanotechnol. 2015, 11, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.J.; Jiang, W.; Xu, S.S.; Tian, R.B. Synthesis and characterization of recyclable clusters of magnetic nanoparticles as doxorubicin carriers for cancer therapy. Appl. Surf. Sci. 2014, 321, 43–49. [Google Scholar] [CrossRef]
- Alphandéry, E.; Faure, S.; Seksek, O.; Guyot, F.; Chebbi, I. Chains of magnetosomes extracted from AMB-1 magnetotactic bacteria for application in alternative magnetic field cancer therapy. ACS Nano 2011, 5, 6279–6296. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.M.; Galloway, J.M.; Rawlings, A.E.; Bramble, J.P.; Staniland, S.S. Taking a hard line with biotemplating: Cobalt-doped magnetite magnetic nanoparticle arrays. Nanoscale 2015, 7, 7340–7351. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Guyot, F.; Chebbi, I. Preparation of chains of magnetosomes, isolated from Magnetospirillum magneticum strain AMB-1 magnetotactic bacteria, yielding efficient treatment of tumors using magnetic hyperthermia. Int. J. Pharm. 2012, 434, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yue, X.; Zhang, S.; Chen, P.; Xu, Z.; Li, Y.; Li, H. Biocompatibility evaluation of magnetosomes formed by Acidithiobacillus ferrooxidans. Mater. Sci. Eng. C 2012, 32, 1802–1807. [Google Scholar] [CrossRef]
- Prozorov, T.; Bazylinski, D.A.; Mallapragada, S.K.; Prozorov, R. Novel magnetic nanomaterials inspired by magnetotactic bacteria: Topical review. Mater. Sci. Eng. R Rep. 2013, 74, 133–172. [Google Scholar] [CrossRef]
- Stanley, S. Biological nanoparticles and their influence on organisms. Curr. Opin. Biotech. 2014, 28, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.J.; Liu, Y.G.; Wang, S.B.; Xie, M.B.; Wu, S.J.; Chen, A.Z.; Wu, W.G. Construction of a novel magnetic targeting anti-tumor drug delivery system: Cytosine arabinoside-loaded bacterial magnetosome. Materials 2013, 6, 3755–3763. [Google Scholar] [CrossRef]
- Borg, S.; Rothenstein, D.; Bill, J.; Schüler, D. Generation of multishell magnetic hybrid nanoparticles by encapsulation of genetically engineered and fluorescent bacterial magnetosomes with ZnO and SiO2. Small 2015, 11, 4209–4217. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, B.; Jin, H.L.; Jiang, W.; Tian, J.S.; Guan, F.; Li, Y. Bacterial magnetic particles (BMPs)-PEI as a novel and efficient non-viral gene delivery system. J. Gene Med. 2007, 9, 679–690. [Google Scholar]
- Tang, Y.S.; Wang, D.; Zhou, C.; Ma, W.; Zhang, Y.Q.; Liu, B.; Zhang, S. Bacterial magnetic particles as a novel and efficient gene vaccine delivery system. Gene Ther. 2012, 19, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Dai, Q.L.; Wang, S.B.; Deng, Q.J.; Wu, W.G.; Chen, A.Z. Preparation and in vitro antitumor effects of cytosine arabinoside-loaded genipin-poly-L-glutamic acid-modified bacterial magnetosomes. Int. J. Nanomed. 2015, 10, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, J.; Zhang, X.; Li, Y.; Zheng, L.M. Bacterial magnetic nanoparticles as drug carriers. J. Mater. Chem. 2008, 18, 5993–5997. [Google Scholar] [CrossRef]
- Sun, J.B.; Duan, J.H.; Dai, S.L.; Ren, J.; Guo, L.; Jiang, W.; Li, Y. Preparation and anti-tumor efficiency evaluation of doxorubicin-loaded bacterial magnetosomes: Magnetic nanoparticles as drug carriers isolated from magnetospirillum gryphiswaldense. Biotechnol. Bioeng. 2008, 101, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, J.; Zheng, L.M. Efficient conjugation doxorubicin to bacterial magnetic nanoparticles via a triplex hands coupling reagent. J. Nanosci. Nanotechnol. 2010, 10, 6514–6519. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.B.; Li, Y.; Liang, X.J.; Wang, P.C. Bacterial magnetosome: A novel biogenetic magnetic targeted drug carrier with potential multifunctions. J. Nanomater. 2011, 2011, 469031. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, J.; Zheng, L.M. Control generating of bacterial magnetic nanoparticle-doxorubicin conjugates by poly-L-glutamic acid surface modification. Nanotechnology 2011, 22, 175102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Xie, M.B.; Wang, S.B.; Zheng, Q.Y.; Chen, A.Z.; Deng, Q.J. Facile fabrication of high performances MTX nanocomposites with natural biomembrane bacterial nanoparticles using GP. Mater. Lett. 2013, 100, 248–251. [Google Scholar] [CrossRef]
- Sun, J.B.; Duan, J.H.; Dai, S.L.; Ren, J.; Zhang, Y.D.; Tian, J.S.; Li, Y. In vitro and in vivo antitumor effects of doxorubicin loaded with bacterial magnetosomes (DBMs) on H22 cells: The magnetic bio-nanoparticles as drug carriers. Cancer Lett. 2007, 258, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wei, J.; Jianbo, S.; Guili, W.; Feng, G.; Ying, L. Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett. Appl. Microbiol. 2007, 45, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tang, T.; Duan, J.; Xu, P.; Wang, Z.; Zhang, Y.; Wu, L.; Li, Y. Biocompatibility of bacterial magnetosomes: Acute toxicity, immunotoxicity and cytotoxicity. Nanotoxicology 2010, 4, 271–283. [Google Scholar] [CrossRef] [PubMed]
| Type | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| BMs | 42.0 ± 8.6 | –28.8 ± 7.6 |
| ADBMs-P | 65.5 ± 8.9 | –42.0 ± 6.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, R.; Liu, Y.; Dai, Q.; Wang, S.; Deng, Q.; Zhou, X. A Natural Bacterium-Produced Membrane-Bound Nanocarrier for Drug Combination Therapy. Materials 2016, 9, 889. https://doi.org/10.3390/ma9110889
Long R, Liu Y, Dai Q, Wang S, Deng Q, Zhou X. A Natural Bacterium-Produced Membrane-Bound Nanocarrier for Drug Combination Therapy. Materials. 2016; 9(11):889. https://doi.org/10.3390/ma9110889
Chicago/Turabian StyleLong, Ruimin, Yuangang Liu, Qinglei Dai, Shibin Wang, Qiongjia Deng, and Xia Zhou. 2016. "A Natural Bacterium-Produced Membrane-Bound Nanocarrier for Drug Combination Therapy" Materials 9, no. 11: 889. https://doi.org/10.3390/ma9110889
APA StyleLong, R., Liu, Y., Dai, Q., Wang, S., Deng, Q., & Zhou, X. (2016). A Natural Bacterium-Produced Membrane-Bound Nanocarrier for Drug Combination Therapy. Materials, 9(11), 889. https://doi.org/10.3390/ma9110889






