Effects of Combined Surface and In‐Depth Absorption on Ignition of PMMA
Abstract
:1. Introduction
2. Theoretical Analysis
3. Numerical Simulation
3.1. Model
3.2. Simulation Parameters
4. Results and Discussion
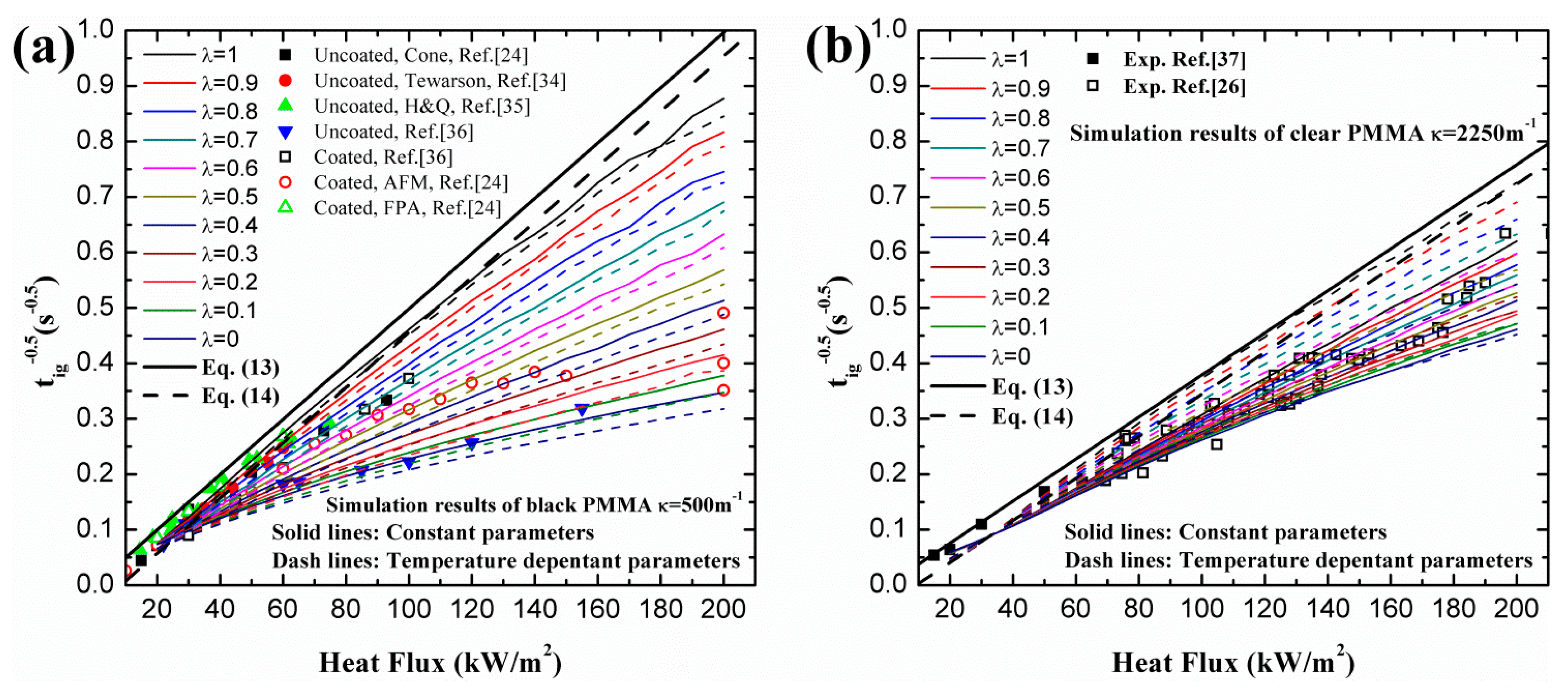
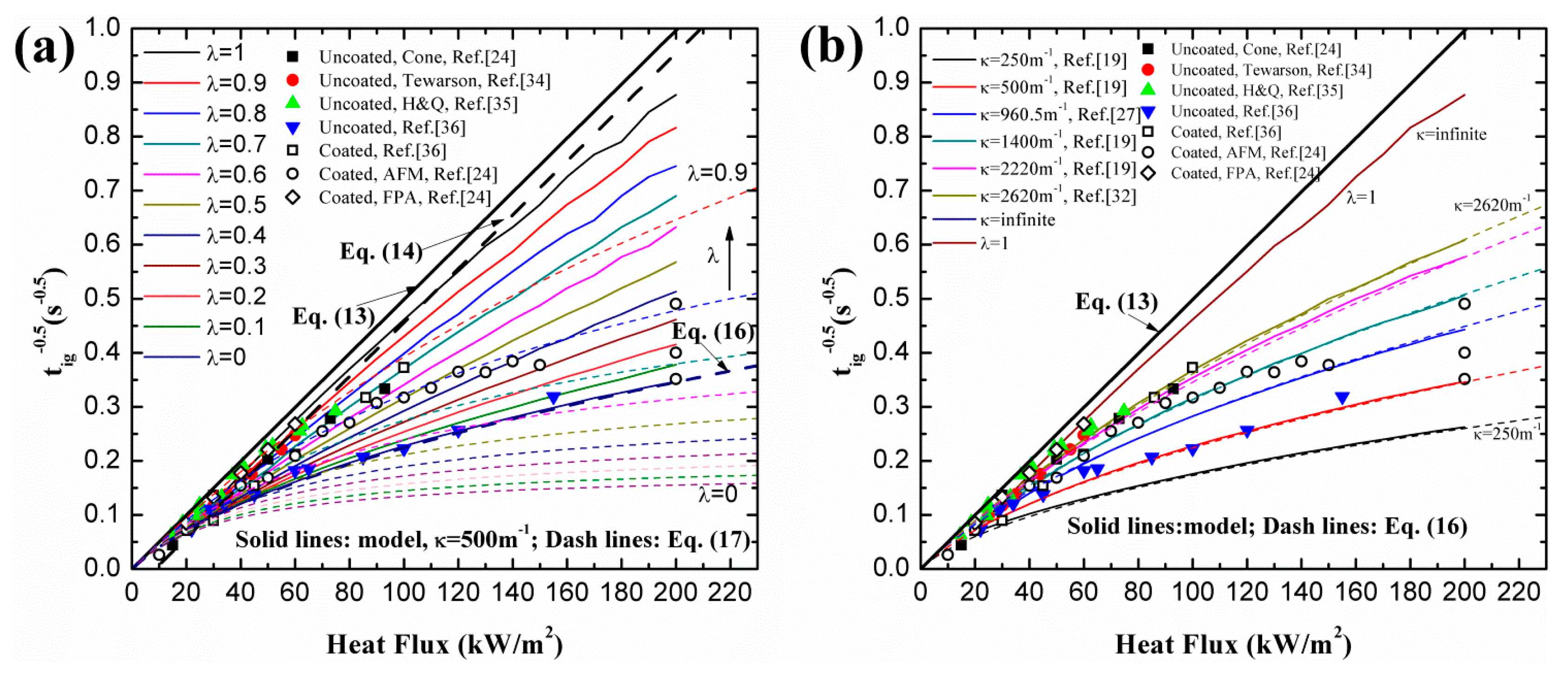
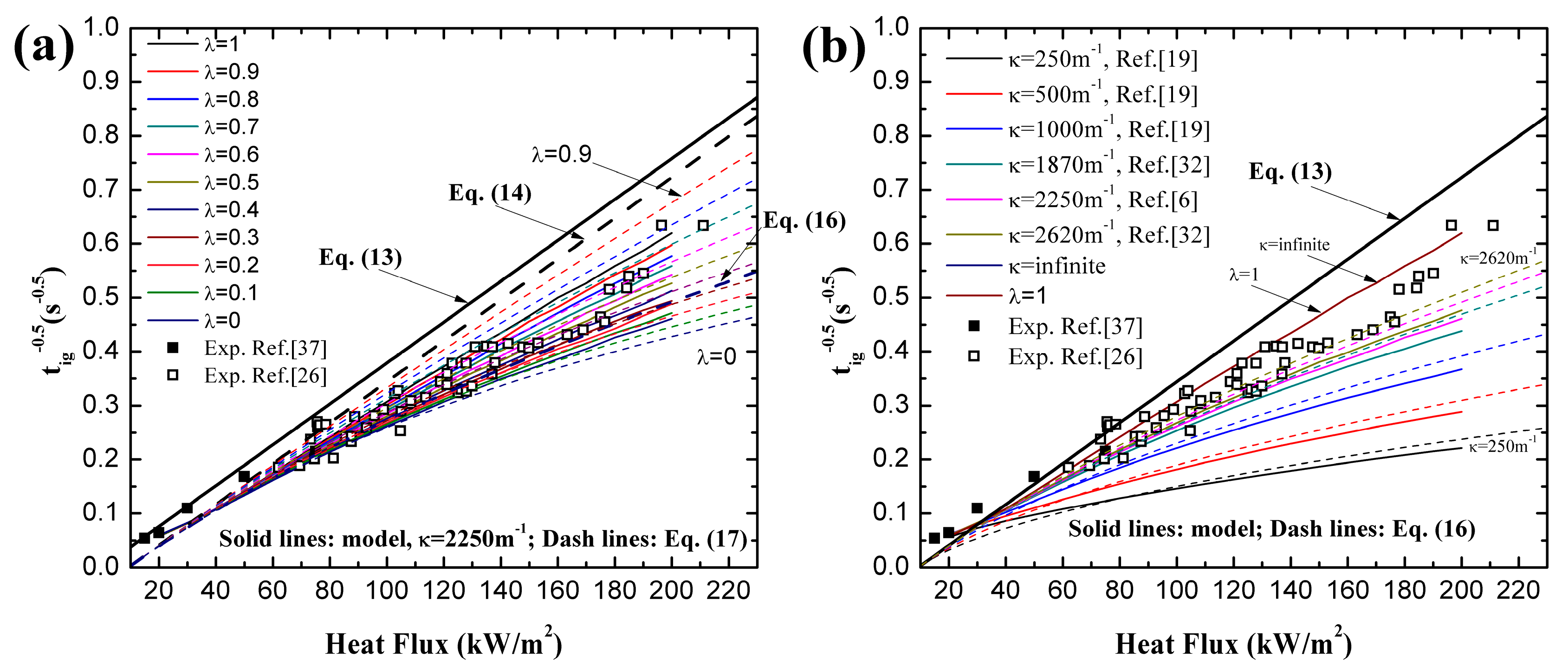
4.1. Ignition Time of PMMA
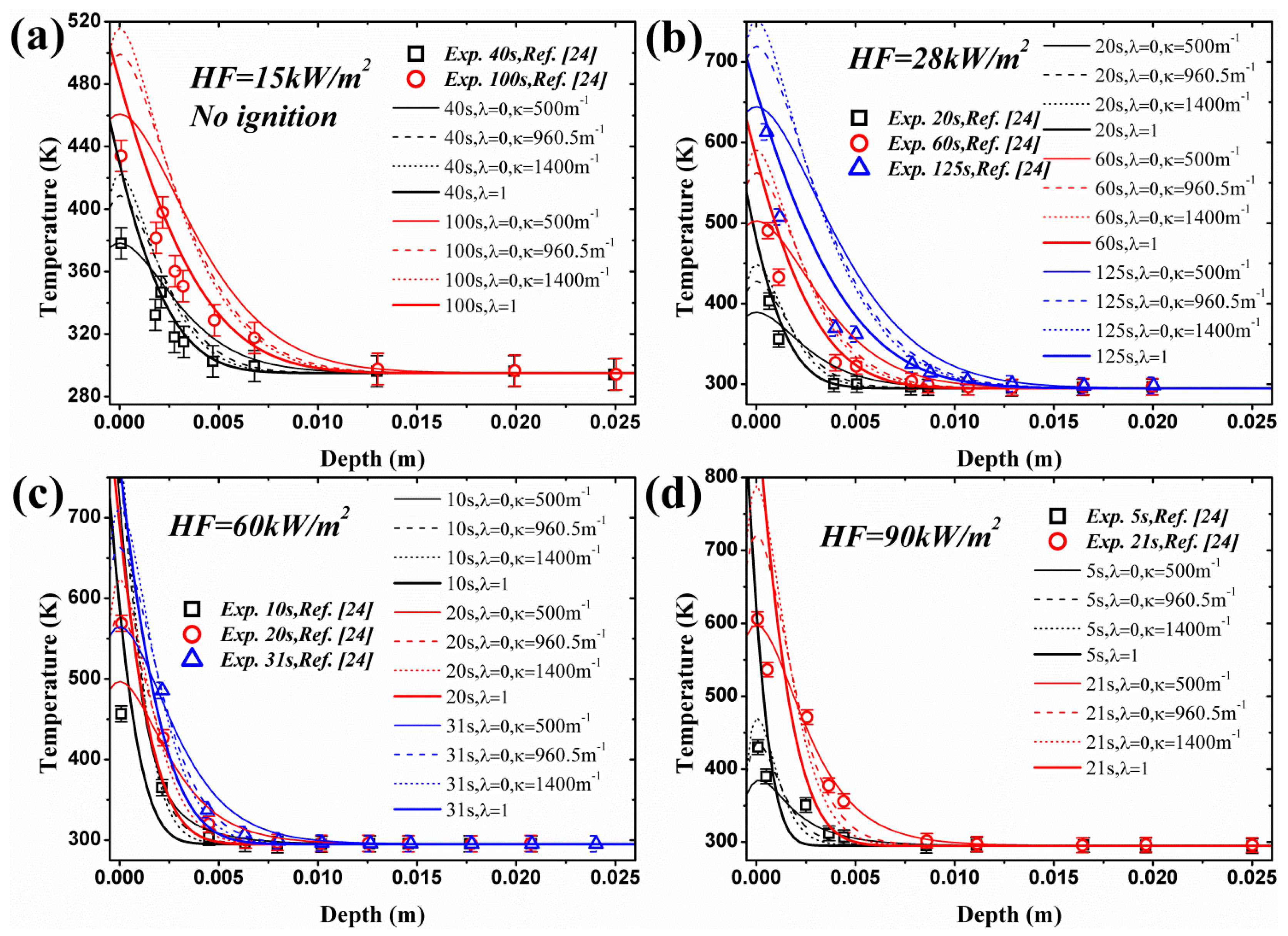
4.2. Transient Temperature Distribution in a Solid
4.3. Optimization of λ
5. Conclusions
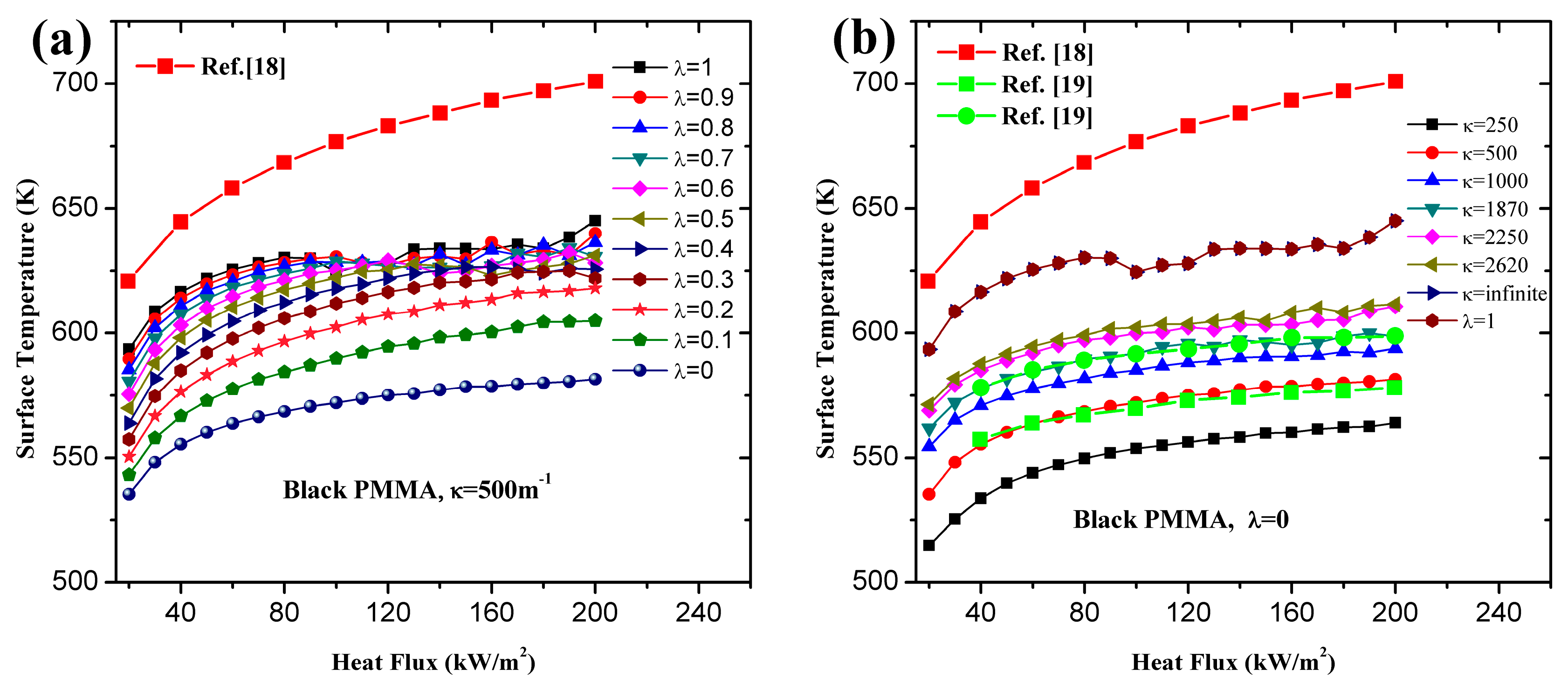
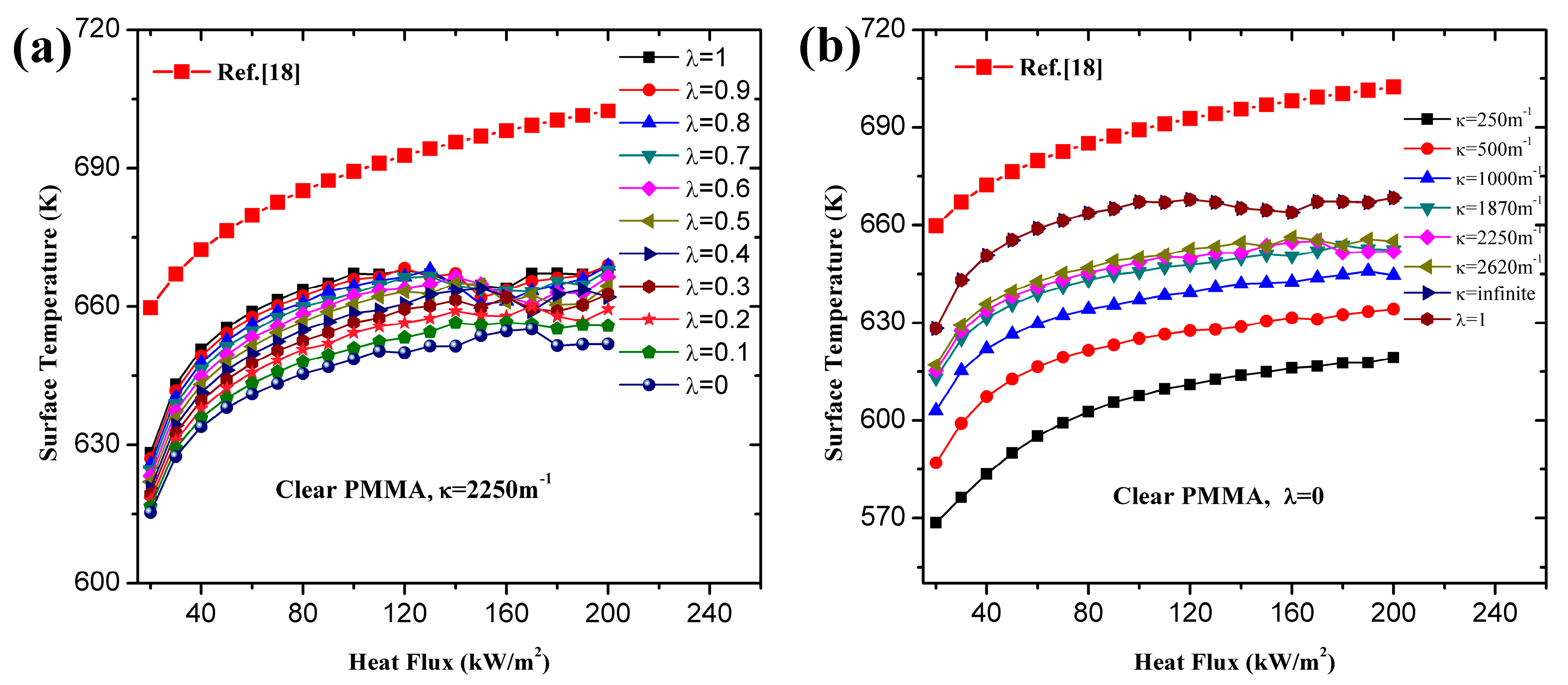
- Utilization of constant or variable thermal parameters has little effect on ignition time for a wide range of heat fluxes, which agrees with the conclusion of Bal [19]. For both black and clear PMMA, linearity of increases with increasing surface absorptivity, λ. In-depth absorption contributes to the curve, especially under high heat flux. For pure in-depth absorption, the analytical model results fit the numerical ones well with different radiative extinction coefficient values.
- For surface absorption, surface temperature is the maximum value in a solid, while for in-depth absorption, the peak exists below the surface and the surface temperature cannot be used as the ignition criterion. Both analytical and numerical models overestimate the temperature in the heat penetration layer and underestimate the temperature beyond this region for surface absorption. The opposite is true for in-depth absorption. Affected by this overestimation, the predicted ignition time is much lower than the measured value in tests due to the fact that the critical temperature is used as the ignition criterion in the analytical model. However, the agreement between numerical and experimental results is much better when the critical mass flux is used. The surface temperature increases with increasing heat flux, and the increasing rate declines as the heat flux gets larger. Furthermore, larger λ and κ, contributing to the opacity of material, also lead to higher surface temperature.
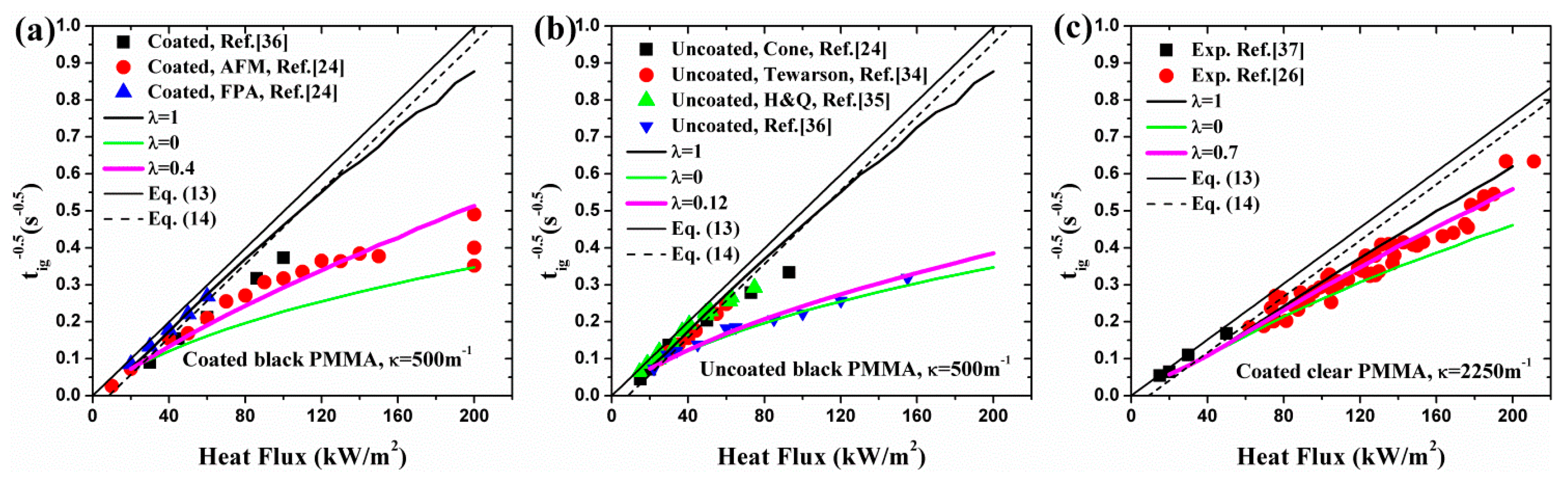
- The best combination of these two absorption modes is explored for black and clear PMMA, including coated and uncoated conditions, by ignition time, mass loss rate and transient temperature in material. The optimized λ was found to be 0.40 and 0.12 for coated and uncoated black PMMA, leading to an enhancement of surface absorptivity by 0.28. While for clear PMMA, the optimized λ was computed to be 0.70 and 0.17 for coated and uncoated ones, respectively, resulting in an increase of 0.53 in the painted black layer. Moreover, the optimized λ decreases with increasing incident heat flux based on the analytical and numerical calculations.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stoliarov, S.I.; Walters, R.N. Determination of the heats of gasification of polymers using differential scanning calorimetry. Polym. Degrad. Stab. 2008, 93, 422–427. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Crowley, S.; Lyon, R.E.; Linteris, G.T. Prediction of the burning rates of non-charring polymers. Combust. Flame 2009, 156, 1068–1083. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Crowley, S.; Walters, R.N.; Lyon, R.E. Prediction of the burning rates of charring polymers. Combust. Flame 2010, 157, 2024–2034. [Google Scholar] [CrossRef]
- Li, J.; Stoliarov, S.I. Measurement of kinetics and thermodynamics of the thermal degradation for non-charring polymers. Combust. Flame 2013, 160, 1287–1297. [Google Scholar] [CrossRef]
- Li, J.; Stoliarov, S.I. Measurement of kinetics and thermodynamics of the thermal degradation for charring polymers. Polym. Degrad. Stab. 2014, 106, 2–15. [Google Scholar] [CrossRef]
- Li, J.; Gong, J.; Stoliarov, S.I. Gasification experiments for pyrolysis model parameterization and validation. Int. J. Heat Mass Transf. 2014, 77, 738–744. [Google Scholar] [CrossRef]
- Li, J.; Gong, J.; Stoliarov, S.I. Development of pyrolysis models for charring polymers. Polym. Degrad. Stab. 2015, 115, 138–152. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Leventon, I.T.; Lyon, R.E. Two-dimensional model of burning for pyrolyzable solids. Fire Mater. 2014, 38, 391–408. [Google Scholar] [CrossRef]
- McGrattan, K.; Hostikka, S.; Floyd, J.; Baum, H.; Rehm, R.; Mell, W.; McDermott, R. Fire dynamics simulator (version 6) technical reference guide, National Institute of Standards and Technology 2013. Available online: https://pages.nist.gov/fds-smv/manuals.html (accessed on 4 October 2016).
- Stagges, J.E.J. A simple model of polymer pyrolysis including transport of volatiles. Fire Saf. J. 2000, 34, 69–80. [Google Scholar] [CrossRef]
- Butler, K.M. A mixed layer pyrolysis model for polypropylene. Fire Saf. Sci. 2000, 6, 313–324. [Google Scholar] [CrossRef]
- Kempel, F.; Schartel, B.; Linteris, G.T.; Stoliarov, S.I.; Lyon, R.E.; Walters, R.N. Prediction of the mass loss rate of polymer materials: Impact of residue formation. Combust. Flame 2012, 159, 2974–2984. [Google Scholar] [CrossRef]
- Walters, R.N.; Safronava, N.; Lyon, R.E. A microscale combustion calorimeter study of gas phase combustion of polymers. Combust. Flame 2015, 162, 855–863. [Google Scholar] [CrossRef]
- Dai, J.; Delichatsios, M.A.; Yang, L.; Zhang, J. Piloted ignition and extinction for solid fuels. Proc. Combust. Inst. 2013, 34, 2487–2495. [Google Scholar] [CrossRef]
- Vermesi, I.; Roenner, N.; Pironi, P.; Hadden, R.M.; Rein, G. Pyrolysis and ignition of a polymer by transient irradiation. Combust. Flame 2016, 163, 31–41. [Google Scholar] [CrossRef]
- Torero, J.L. Flaming Ignition of Solids Fuels. In SFPE Hand Book of Fire Protection Engineering, 4th ed.; National Fire Protection Association: Quincy, MA, USA, 2008; pp. 260–277. [Google Scholar]
- Cordova, J.L.; Walther, D.C.; Torero, J.L.; Fernandez-Pello, C. Oxidizer flow effects on the flammability of solid combustibles. Combust. Sci. Technol. 2001, 164, 253–278. [Google Scholar] [CrossRef]
- Lautenberger, C.; Fernandez-Pello, C. Approximate analytical solutions for the transient mass loss rate and piloted ignition time of a radiatively heated solid in the high heat flux limit. Fire Saf. Sci. 2005, 8, 445–456. [Google Scholar] [CrossRef]
- Bal, N.; Rein, G. Numerical investigation of the ignition delay time of a translucent solid at high radiant heat fluxes. Combust. Flame 2011, 158, 1109–1116. [Google Scholar] [CrossRef]
- Babrauskas, V. Ignition Handbook; Fire Science Publishers: Issaquah, WA, USA, 2003; pp. 234–251. [Google Scholar]
- Reszka, P.; Borowiec, P.; Steinhaus, T.; Torero, J.L. A methodology for the estimation of ignition delay times in forest fire modelling. Combust. Flame 2012, 159, 3652–3657. [Google Scholar] [CrossRef]
- Lautenberger, C.; Fernandez-Pello, C. Generalized pyrolysis model for combustible solids. Fire Saf. J. 2009, 44, 819–839. [Google Scholar] [CrossRef]
- Beaulieu, P.A.; Dembsey, N.A. Flammability characteristics at applied heat flux levels up to 200 kW/m2. Fire Mater. 2008, 32, 61–86. [Google Scholar] [CrossRef]
- Beaulieu, P.A. Flammability Characteristics at Heat Flux Levels up to 200 kW/m2 and the Effect of Oxygen on Flame Heat Flux. Ph.D. Dissertation, Worcester Polytechnic Institute, Worcester, MA, USA, 2005. [Google Scholar]
- Kashiwagi, T. Experimental observation of radiative ignition mechanisms. Combust. Flame 1979, 34, 231–244. [Google Scholar] [CrossRef]
- Jiang, F.; De Ris, J.L.; Khan, M.M. Absorption of thermal energy in PMMA by in-depth radiation. Fire Saf. J. 2009, 44, 106–112. [Google Scholar] [CrossRef]
- Staggs, J. The effects of gas-phase and in-depth radiation absorption on ignition and steady burning rate of PMMA. Combust. Flame 2014, 161, 3229–3236. [Google Scholar] [CrossRef]
- Delichatsios, M.A.; Zhang, J. An alternative way for the ignition times for solids with radiation absorption in-depth by simple asymptotic solutions. Fire Mater. 2012, 36, 41–47. [Google Scholar] [CrossRef]
- Gong, J.; Chen, Y.; Jiang, J.; Yang, L.; Li, J. A numerical study of thermal degradation of polymers: Surface and in-depth absorption. Appl. Therm. Eng. 2016, 106, 1366–1379. [Google Scholar] [CrossRef]
- Steinhaus, T. Evaluation of the Thermophysical Properties of Poly(Methyl Methacrylate): A Reference Material for the Development of a Flammability Test for Micro-Gravity Environments. Master’s Thesis, University of Maryland, College Park, MD, USA, 1999. [Google Scholar]
- Linteris, G.; Zammarano, M.; Wilthan, B.; Hanssen, L. Absorption and reflection of infrared radiation by polymers in fire-like environments. Fire Mater. 2012, 36, 537–553. [Google Scholar] [CrossRef]
- Deepak, D.; Drysdale, D. Flammability of solids: An apparatus to measure the critical mass flux at the firepoint. Fire Saf. J. 1983, 5, 167–169. [Google Scholar] [CrossRef]
- Tewarson, A.; Ogden, S. Fire behavior of polymethylmethacrylate. Combust. Flame 1992, 89, 237–259. [Google Scholar] [CrossRef]
- Hopkins, D.; Quintiere, J. Material fire properties and predictions for thermoplastics. Fire Saf. J. 1996, 26, 241–268. [Google Scholar] [CrossRef]
- Delichatsios, M.A.; Saito, K. Upward fire spread: Key flammability properties, similarity solution and flammability indices. Fire Saf. Sci. 1991, 3, 217–226. [Google Scholar] [CrossRef]
- Safronava, N.; Lyon, R.E.; Crowley, S.; Stoliarov, S.I. Effect of moisture on ignition time of polymers. Fire Technol. 2013, 51, 78–93. [Google Scholar] [CrossRef]


| Parameters | Black PMMA | Clear PMMA | ||
|---|---|---|---|---|
| Values | Ref. | Values | Ref. | |
| Density, ρ(kg/m3) | 1187.8 | [19] | 1200 | [27] |
| [30] | [29] | |||
| Pre-exponential factor, As (1/s) | 5 × 108 | [19] | 8.6 × 1012 | [6] |
| Activation energy, Es (J/mol) | 1.25 × 105 | [19] | 1.88 × 105 | [6] |
| Heat of vaporization, ∆Hv (J/g) | 900 | [23] | 846 | [6] |
| Absorption coefficient, κ (1/m) | 500 | [19] | 1000 | [27] |
| 960 | [26] | 1870 | [31] | |
| 1400 | [19] | 2250 | [6] | |
| Specific heat, Cs (J/(g·K)) | 1.665 | [19] | 1.7 | [27] |
| [30] | 0.6 + 0.00367Ts | [6] | ||
| Thermal conductivity, k (J/(s·m·K) | 0.21 | [19] | 0.2 | [27] |
| [30] | [6] | |||
| Reflectivity, r (-) | 0 | [19] | 0.05 | [6] |
| Critical mass flux at ignition, (g/m2·s) | 2.42 | [19] | 2.5 | [9] |
| 4.5 | [32] | |||
| Convection coefficient, h (J/sm2·K) | 10 | [19] | 5 | [6] |
| Equation(15) | [29] | 25 | [27] | |
| Equation(15) | [29] | |||
| Ambient temperature, T∞, (K) | 300 | * | 300 | * |
| (kW/m2) | tig(s) | ||||
| Experiential data Ref. [23] | Equation (15), λ = 0 κ = 500 m−1 | Equation (15), λ = 0 κ = 960.5 m−1 | Equation (15), λ = 0 κ = 1400 m−1 | Equation (14), λ = 1 | |
| 28 | 125 | 247.35 | 172.16 | 149.59 | 105.84 |
| 60 | 31 | 87.17 | 45.28 | 34.10 | 15.19 |
| 90 | 21 | 62.45 | 28.03 | 19.41 | 6.07 |
| kW/m2) | Experiential data Ref. [23] | Equation (16), λ = 0 κ = 500 m−1 | Equation (16), λ = 0 κ = 960.5 m−1 | Equation (16), λ = 0 κ = 1400 m−1 | Equation (14), λ = 1 |
| 28 | 125 | 202.36 | 158.83 | 143.08 | 105.84 |
| 60 | 31 | 48.28 | 33.64 | 28.31 | 15.19 |
| 90 | 21 | 25.94 | 17.18 | 14 | 6.07 |
| HF (kW/m2) | 15 | 28 | 60 | 90 | Average |
|---|---|---|---|---|---|
| Analytical | 0.77 | 0.63 | 0.47 | 0.23 | 0.53 |
| Numerical | 0.54 | 0.49 | 0.38 | 0.21 | 0.41 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Chen, Y.; Li, J.; Jiang, J.; Wang, Z.; Wang, J. Effects of Combined Surface and In‐Depth Absorption on Ignition of PMMA. Materials 2016, 9, 820. https://doi.org/10.3390/ma9100820
Gong J, Chen Y, Li J, Jiang J, Wang Z, Wang J. Effects of Combined Surface and In‐Depth Absorption on Ignition of PMMA. Materials. 2016; 9(10):820. https://doi.org/10.3390/ma9100820
Chicago/Turabian StyleGong, Junhui, Yixuan Chen, Jing Li, Juncheng Jiang, Zhirong Wang, and Jinghong Wang. 2016. "Effects of Combined Surface and In‐Depth Absorption on Ignition of PMMA" Materials 9, no. 10: 820. https://doi.org/10.3390/ma9100820
APA StyleGong, J., Chen, Y., Li, J., Jiang, J., Wang, Z., & Wang, J. (2016). Effects of Combined Surface and In‐Depth Absorption on Ignition of PMMA. Materials, 9(10), 820. https://doi.org/10.3390/ma9100820





