Heteroepitaxy of Cerium Oxide Thin Films on Cu(111)
Abstract
:1. Applied and Model Catalysis over Ceria
2. Highly Controlled Ceria Model Catalysts
2.1. Continuous CeO2(111) Films on the Cu(111) Substrate
CeO2(111) || Cu(111); CeO2[0 1] || Cu[0 1]
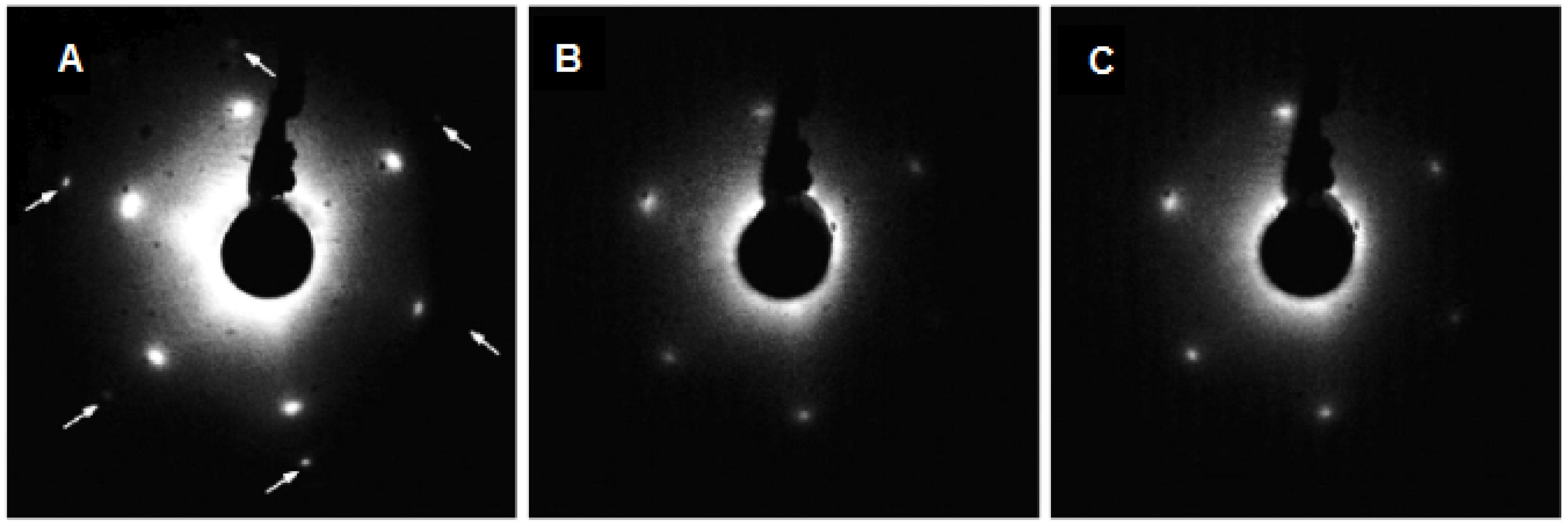
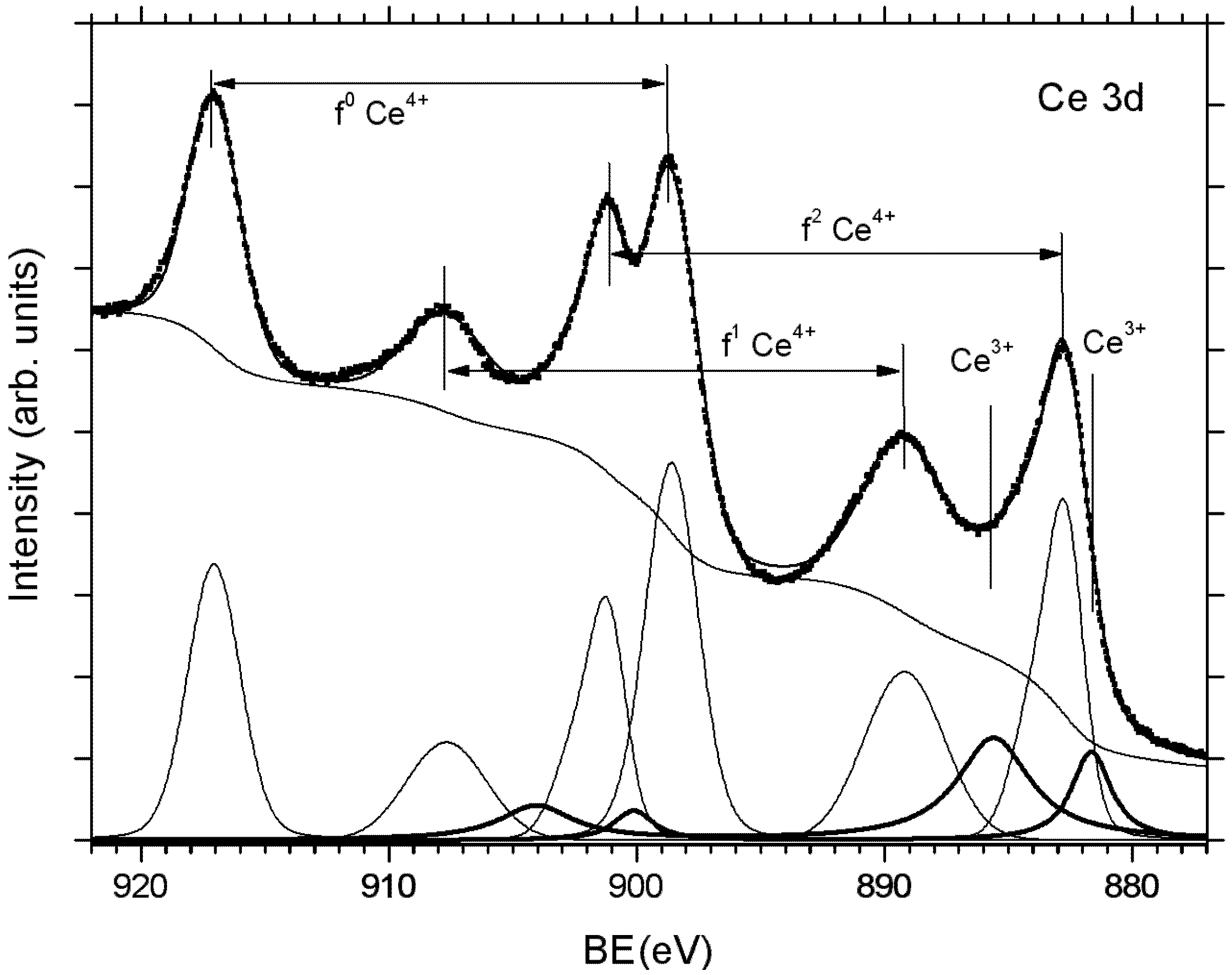
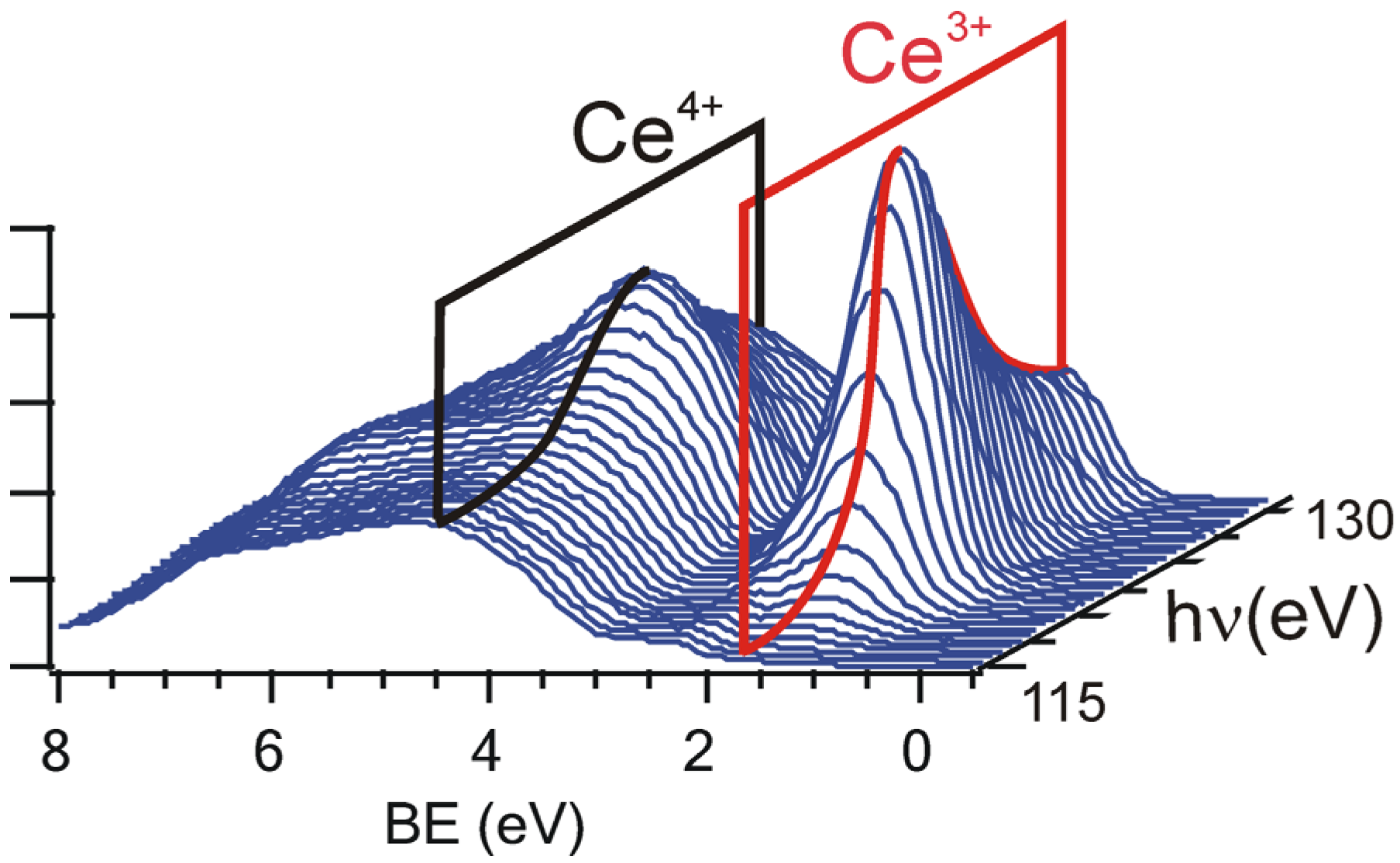
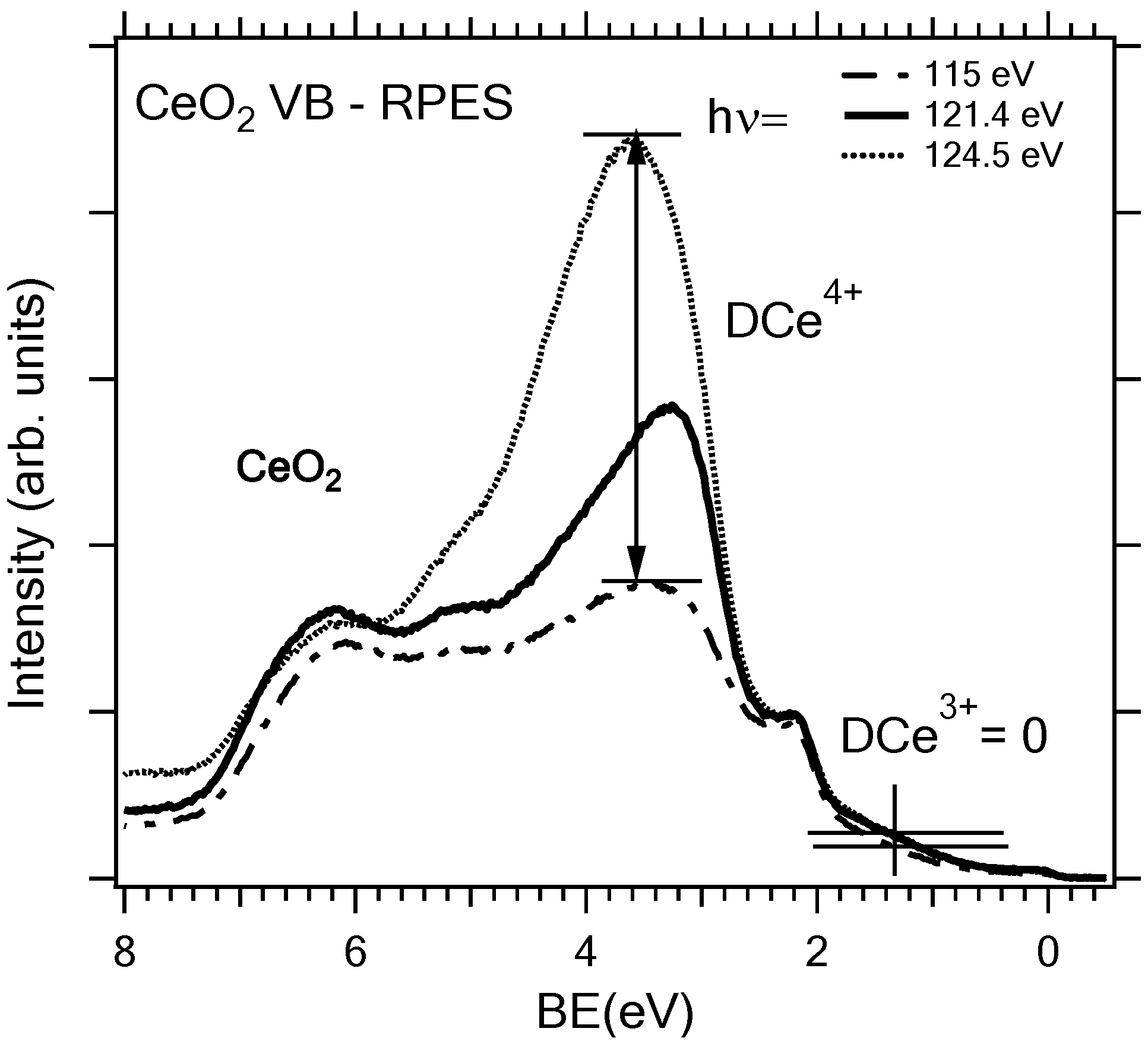
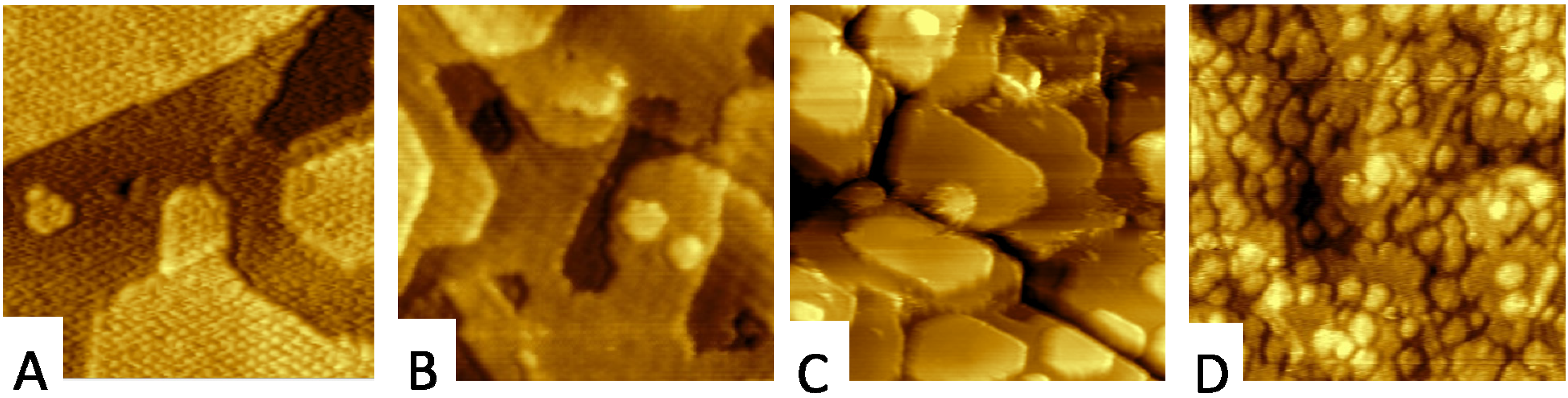
2.2. Adjusting the Morphology of CeO2(111) Nanostructured Thin Films on Cu(111)
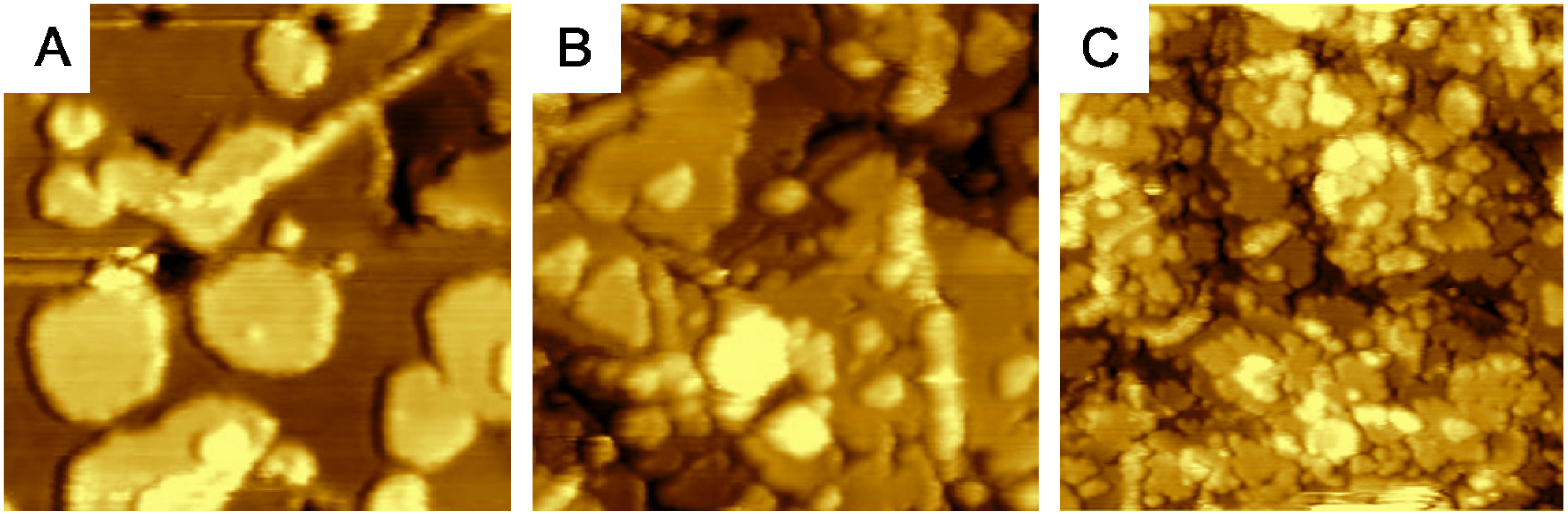
2.3. Adjusting the Stoichiometry of CeO2(111) Nanostructured Thin Films on Cu(111)
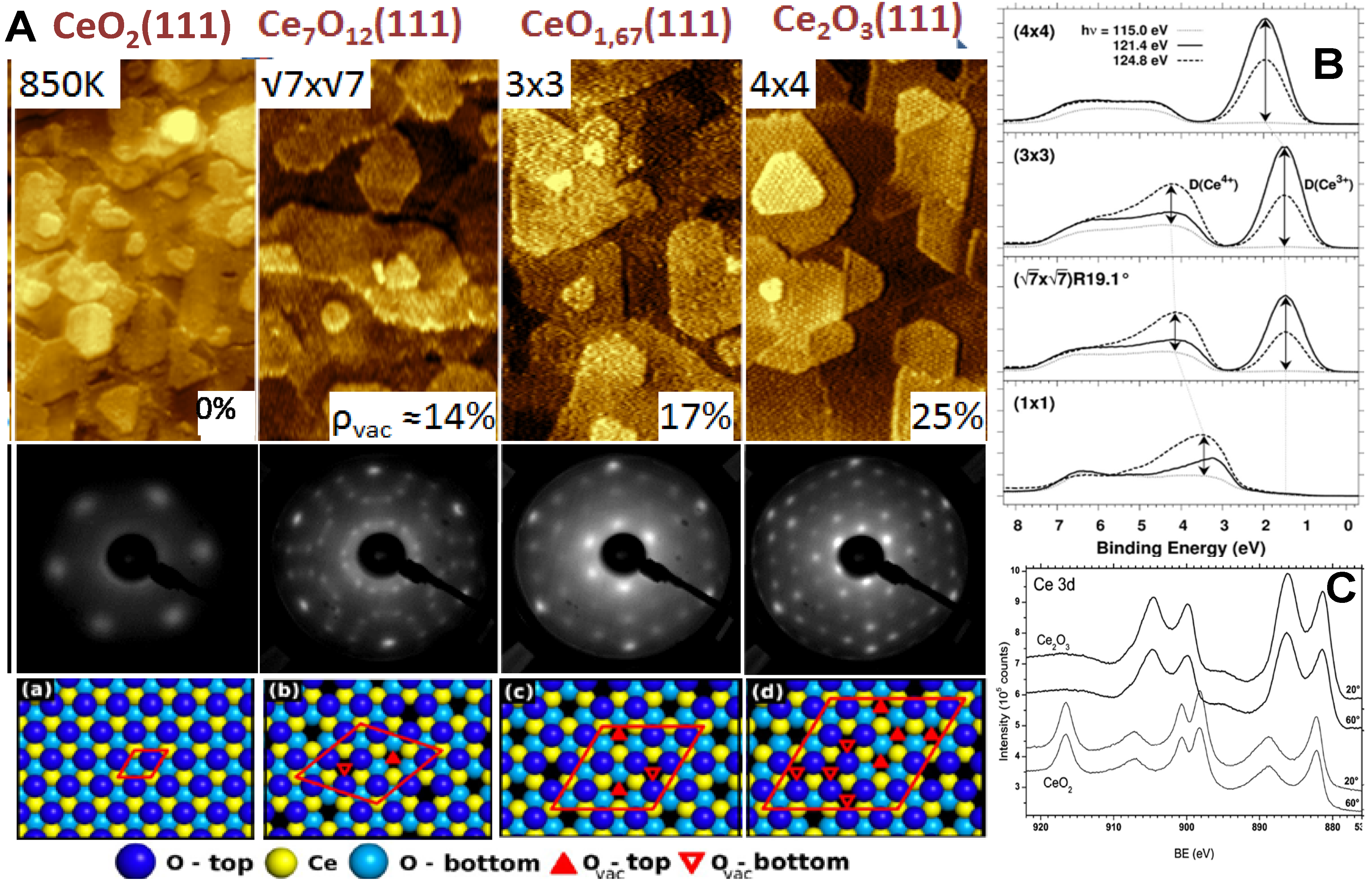
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trovarelli, A.; Fornasiero, P. Catalysis by Ceria and Related Materials, 2nd ed.; World Scientific: Singapore, 2013. [Google Scholar]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef]
- Gandhi, H.S.; Graham, G.W.; McCabe, R.W. Automotive exhaust catalysis. J. Catal. 2003, 216, 433–442. [Google Scholar] [CrossRef]
- Hatanaka, M.; Takahashi, N.; Takahashi, N.; Tanabe, T.; Nagai, Y.; Suda, A.; Shinjoh, H. Reversible changes in the Pt oxidation state and nanostructure on a ceria-based supported Pt. J. Catal. 2009, 266, 182–190. [Google Scholar] [CrossRef]
- Thomas, J.M. Heterogeneous catalysis: Enigmas, illusions, challenges, realities, and emergent strategies of design. J. Chem. Phys. 2008, 128. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Matolín, V. Method for Preparing Oxidation Catalyst and Catalyst Prepared by the Oxidation. U.S. Patent 8435921 B2, 7 May 2013. [Google Scholar]
- Fiala, R.; Vaclavu, M.; Rednyk, A.; Khalakhan, I.; Vorokhta, M.; Lavkova, J.; Potin, V.; Matolinova, I.; Matolin, V. Pt-CeOx thin film catalysts for PEMFC. Catal. Today 2015, 240, 236–241. [Google Scholar] [CrossRef]
- Matolín, V.; Matolínová, I.; Václavů, M.; Khalakhan, I.; Vorokhta, M.; Fiala, R.; Pis, I.; Sofer, Z.; Poltierová-Vejpravová, J.; Mori, T.; et al. Platinum-doped CeO2 thin film catalysts prepared by magnetron sputtering. Langmuir 2010, 26, 12824–12831. [Google Scholar] [CrossRef] [PubMed]
- Bruix, A.; Lykhach, Y.; Matolínová, I.; Neitzel, A.; Skála, T.; Tsud, N.; Vorokhta, M.; Stetsovych, V.; Ševčíková, K.; Mysliveček, J.; et al. Maximum noble-metal efficiency in catalytic materials: Atomically dispersed surface platinum. Angew. Chem. Int. Ed. Engl. 2014, 53, 10525–10530. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.S.; Madras, G.; Patil, K.C. Noble metal ionic catalysts. Acc. Chem. Res. 2009, 42, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Freund, H.J.; Bäumer, M.; Libuda, J.; Risse, T.; Rupprechter, G.; Shaikhutdinov, S. Preparation and characterization of model catalysts: From ultrahigh vacuum to in situ conditions at the atomic dimension. J. Catal. 2003, 216, 223–235. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Vang, R.T.; Besenbacher, F. From atom-resolved scanning tunneling microscopy (STM) studies to the design of new catalysts. Catal. Today 2006, 111, 34–43. [Google Scholar] [CrossRef]
- Ertl, G. Reactions at surfaces: From atoms to complexity (nobel lecture). Angew. Chem. Int. Ed. Engl. 2008, 47, 3524–3535. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U.; Li, S.-C.; Schmid, M. Oxide surface science. Ann. Rev. Phys. Chem. 2010, 61, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Stacchiola, D. Catalysis and the nature of mixed-metal oxides at the nanometer level: Special properties of MOx/TiO2(110) {M = V, W, Ce} surfaces. Phys. Chem. Chem. Phys. 2010, 12, 9557–9565. [Google Scholar] [CrossRef] [PubMed]
- Surnev, S.; Fortunelli, A.; Netzer, F.P. Structure-property relationship and chemical aspects of oxide-metal hybrid nanostructures. Chem. Rev. 2013, 113, 4314–4372. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, F.; Stetsovych, O.; Steger, M.; Cherradi, E.; Matolínová, I.; Tsud, N.; Škoda, M.; Skála, T.; Mysliveček, J.; Matolín, V. Adjusting morphology and surface reduction of CeO2(111) thin films on Cu(111). J. Phys. Chem. C 2011, 115, 7496–7503. [Google Scholar] [CrossRef]
- Duchoň, T.; Dvořák, F.; Aulická, M.; Stetsovych, V.; Vorokhta, M.; Mazur, D.; Veltruská, K.; Skála, T.; Mysliveček, J.; Matolínová, I.; et al. Ordered phases of reduced ceria as epitaxial films on Cu(111). J. Phys. Chem. C 2014, 118, 357–365. [Google Scholar] [CrossRef]
- Matolín, V.; Matolínová, I.; Dvořák, F.; Johánek, V.; Mysliveček, J.; Prince, K.C.; Skála, T.; Stetsovych, O.; Tsud, N.; Václavů, M.; et al. Water interaction with CeO2(111)/Cu(111) model catalyst surface. Catal. Today 2012, 181, 124–132. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.W. Correlations between surface science models and “real-world” catalysts. J. Phys. Chem. 1996, 100, 13090–13102. [Google Scholar] [CrossRef]
- Kuhlenbeck, H.; Shaikhutdinov, S.; Freund, H. Well-ordered transition metal oxide layers in model catalysis-a series of case studies. Chem. Rev. 2013, 113, 3986–4034. [Google Scholar] [CrossRef] [PubMed]
- Mullins, D.R.; Radulovic, P.V.; Overbury, S.H. Ordered cerium oxide thin films grown on Ru(0001) and Ni(111). Surf. Sci. 1999, 429, 186–198. [Google Scholar] [CrossRef]
- Lu, J.-L.; Gao, H.-J.; Shaikhutdinov, S.; Freund, H.-J. Morphology and defect structure of the CeO2(111) films grown on Ru(0001) as studied by scanning tunneling microscopy. Surf. Sci. 2006, 600, 5004–5010. [Google Scholar] [CrossRef]
- Kaemena, B.; Senanayake, S.D.; Meyer, A.; Sadowski, J.T.; Falta, J.; Flege, J.I. Growth and Morphology of Ceria on Ruthenium (0001). J. Phys. Chem. C 2013, 117, 221–232. [Google Scholar] [CrossRef]
- Jerratsch, J.-F.; Shao, X.; Nilius, N.; Freund, H.-J.; Popa, C.; Ganduglia-Pirovano, M.V.; Burow, A.M.; Sauer, J. Electron localization in defective ceria films: A study with scanning-tunneling microscopy and density-functional theory. Phys. Rev. Lett. 2011, 106. [Google Scholar] [CrossRef]
- Nilius, N.; Kozlov, S.M.; Jerratsch, J.-F.; Baron, M.; Shao, X.; Viñes, F.; Shaikhutdinov, S.; Neyman, K.M.; Freund, H.-J. Formation of one-dimensional electronic states along the step edges of CeO2(111). ACS Nano 2012, 6, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.M.; Viñes, F.; Nilius, N.; Shaikhutdinov, S.; Neyman, K.M. Absolute surface step energies: Accurate theoretical methods applied to ceria nanoislands. J. Phys. Chem. Lett. 2012, 3, 1956–1961. [Google Scholar] [CrossRef]
- Berner, U.; Schierbaum, K.; Jones, G.; Wincott, P.; Haq, S.; Thornton, G. Ultrathin ordered CeO2 overlayers on Pt(111): Interaction with NO2, NO, H2O and CO. Surf. Sci. 2000, 467, 201–213. [Google Scholar] [CrossRef]
- Grinter, D.C.; Ithnin, R.; Pang, C.L.; Thornton, G. Defect structure of ultrathin ceria films on Pt(111): Atomic views from scanning tunnelling microscopy. J. Phys. Chem. C 2010, 114, 17036–17041. [Google Scholar] [CrossRef]
- Luches, P.; Pagliuca, F.; Valeri, S. Morphology, stoichiometry, and interface structure of CeO2 ultrathin films on Pt(111). J. Phys. Chem. C 2011, 115, 10718–10726. [Google Scholar] [CrossRef]
- Luches, P.; Pagliuca, F.; Valeri, S. Structural and morphological modifications in thermally reduced cerium oxide ultrathin epitaxial films on Pt(111). Phys. Chem. Chem. Phys. 2014, 16, 18848–18857. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Ma, S.; Liu, P.; Hrbek, J.; Evans, J.; Pérez, M. Activity of CeOx and TiOx nanoparticles grown on Au(111) in the water-gas shift reaction. Science 2007, 318, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Šutara, F.; Cabala, M.; Sedláček, L.; Skála, T.; Škoda, M.; Matolín, V.; Prince, K.C.; Cháb, V. Epitaxial growth of continuous CeO2(111) ultra-thin films on Cu(111). Thin Solid Films 2008, 516, 6120–6124. [Google Scholar] [CrossRef]
- Staudt, T.; Lykhach, Y.; Hammer, L.; Schneider, M.A.; Matolín, V.; Libuda, J. A route to continuous ultra-thin cerium oxide films on Cu(111). Surf. Sci. 2009, 603, 3382–3388. [Google Scholar] [CrossRef]
- Szabová, L.; Camellone, M.F.; Huang, M.; Matolín, V.; Fabris, S. Thermodynamic, electronic and structural properties of Cu/CeO2 surfaces and interfaces from first-principles DFT+U calculations. J. Chem. Phys. 2010, 133. [Google Scholar] [CrossRef] [PubMed]
- Szabová, L.; Skála, T.; Matolínová, I.; Fabris, S.; Farnesi Camellone, M.; Matolín, V. Copper-ceria interaction: A combined photoemission and DFT study. Appl. Surf. Sci. 2013, 267, 12–16. [Google Scholar] [CrossRef]
- Szabová, L.; Stetsovych, O.; Dvořák, F.; Farnesi Camellone, M.; Fabris, S.; Mysliveček, J.; Matolín, V. Distinct physicochemical properties of the first ceria monolayer on Cu(111). J. Phys. Chem. C 2012, 116, 6677–6684. [Google Scholar] [CrossRef]
- Stetsovych, O.; Dvořák, F.; Szabová, L.; Fabris, S.; Mysliveček, J.; Matolín, V. Nanometer-range strain distribution in layered incommensurate systems. Phys. Rev. Lett. 2012, 109. [Google Scholar] [CrossRef]
- Skorodumova, N.; Simak, S.; Lundqvist, B.; Abrikosov, I.; Johansson, B. Quantum origin of the oxygen storage capability of ceria. Phys. Rev. Lett. 2002, 89. [Google Scholar] [CrossRef]
- Matolín, V.; Matolínová, I.; Sedláček, L.; Prince, K.C.; Skála, T. A resonant photoemission applied to cerium oxide based nanocrystals. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Matolín, V.; Cabala, M.; Cháb, V.; Matolínová, I.; Prince, K.C.; Škoda, M.; Šutara, F.; Skála, T.; Veltruská, K. A resonant photoelectron spectroscopy study of Sn(Ox) doped CeO2 catalysts. Surf. Interface Anal. 2008, 40, 225–230. [Google Scholar] [CrossRef]
- Matsumoto, M.; Soda, K.; Ichikawa, K.; Tanaka, S.; Taguchi, Y.; Jouda, K.; Aita, O.; Tezuka, Y.; Shin, S. Resonant photoemission study of CeO2. Phys. Rev. B 1994, 50, 11340–11346. [Google Scholar] [CrossRef]
- Skála, T.; Šutara, F.; Prince, K.C.; Matolín, V. Cerium oxide stoichiometry alteration via Sn deposition: Influence of temperature. J. Electron Spectrosc. Relat. Phenome. 2009, 169, 20–25. [Google Scholar] [CrossRef]
- Kucherenko, Y.; Molodtsov, S.; Heber, M.; Laubschat, C. 4f-derived electronic structure at the surface and in the bulk of α-Ce metal. Phys. Rev. B 2002, 66. [Google Scholar] [CrossRef]
- Hüfner, S. Photoelectron Spectroscopy, Principles and Applications; Springer-Verlag: Berlin, Germany, 2003. [Google Scholar]
- Happel, M.; Mysliveček, J.; Johánek, V.; Dvořák, F.; Stetsovych, O.; Lykhach, Y.; Matolín, V.; Libuda, J. Adsorption sites, metal-support interactions, and oxygen spillover identified by vibrational spectroscopy of adsorbed CO: A model study on Pt/ceria catalysts. J. Catal. 2012, 289, 118–126. [Google Scholar] [CrossRef]
- Stetsovych, V.; Pagliuca, F.; Dvořák, F.; Duchoň, T.; Vorokhta, M.; Aulická, M.; Lachnitt, J.; Schernich, S.; Matolínová, I.; Veltruská, K.; et al. Epitaxial cubic Ce2O3 films via Ce-CeO2 interfacial reaction. J. Phys. Chem. Lett. 2013, 4, 866–871. [Google Scholar] [PubMed]
- Weststrate, C.J.; Westerström, R.; Lundgren, E.; Mikkelsen, A.; Andersen, J.N.; Resta, A. Influence of oxygen vacancies on the properties of ceria-supported gold. J. Phys. Chem. C 2009, 113, 724–728. [Google Scholar] [CrossRef]
- Baron, M.; Bondarchuk, O.; Stacchiola, D.; Shaikhutdinov, S.; Freund, H.-J. Interaction of Gold with Cerium Oxide Supports: CeO2(111) Thin Films vs CeOx Nanoparticles. J. Phys. Chem. C 2009, 113, 6042–6049. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Zhou, J.; Baddorf, A.P.; Mullins, D.R. The reaction of carbon monoxide with palladium supported on cerium oxide thin films. Surf. Sci. 2007, 601, 3215–3223. [Google Scholar]
- Lu, J.-L.; Gao, H.-J.; Shaikhutdinov, S.; Freund, H.-J. Gold supported on well-ordered ceria films: Nucleation, growth and morphology in CO oxidation reaction. Catal. Lett. 2007, 114, 8–16. [Google Scholar] [CrossRef]
- Duchoň, T.; Dvořák, F.; Aulická, M.; Stetsovych, V.; Vorokhta, M.; Mazur, D.; Veltruská, K.; Skála, T.; Mysliveček, J.; Matolínová, I.; et al. Comment on “Ordered phases of reduced ceria as epitaxial films on Cu(111)”. J. Phys. Chem. C 2014, 118, 5058–5059. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mysliveček, J.; Matolín, V.; Matolínová, I. Heteroepitaxy of Cerium Oxide Thin Films on Cu(111). Materials 2015, 8, 6346-6359. https://doi.org/10.3390/ma8095307
Mysliveček J, Matolín V, Matolínová I. Heteroepitaxy of Cerium Oxide Thin Films on Cu(111). Materials. 2015; 8(9):6346-6359. https://doi.org/10.3390/ma8095307
Chicago/Turabian StyleMysliveček, Josef, Vladimir Matolín, and Iva Matolínová. 2015. "Heteroepitaxy of Cerium Oxide Thin Films on Cu(111)" Materials 8, no. 9: 6346-6359. https://doi.org/10.3390/ma8095307
APA StyleMysliveček, J., Matolín, V., & Matolínová, I. (2015). Heteroepitaxy of Cerium Oxide Thin Films on Cu(111). Materials, 8(9), 6346-6359. https://doi.org/10.3390/ma8095307





