Poly(vinyl alcohol) Nanocomposites Reinforced with Bamboo Charcoal Nanoparticles: Mineralization Behavior and Characterization
Abstract
:1. Introduction
2. Results and Discussion
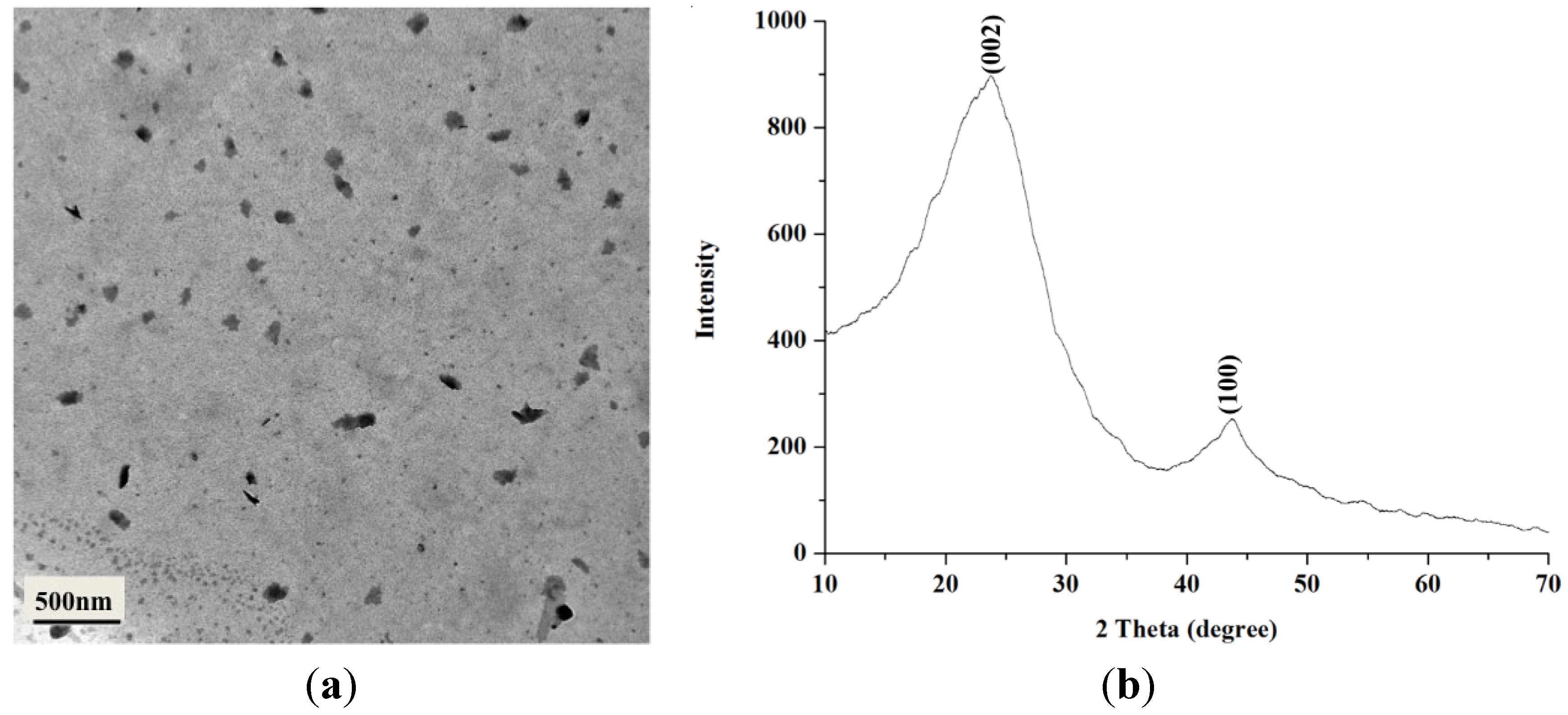
2.1. Characterization of BCNPs
2.2. Characterization of PVA/BCNP Nanocomposite Membranes
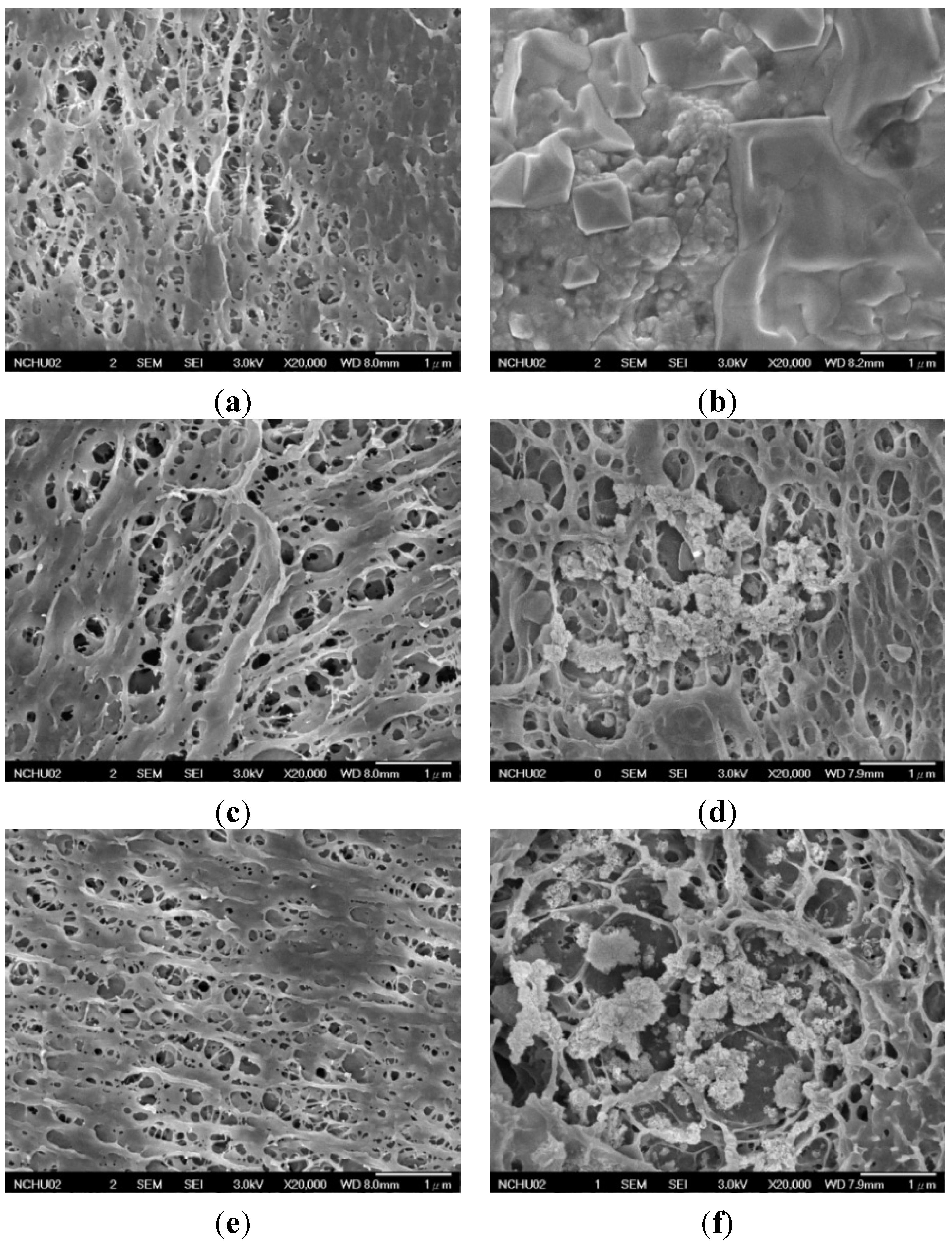
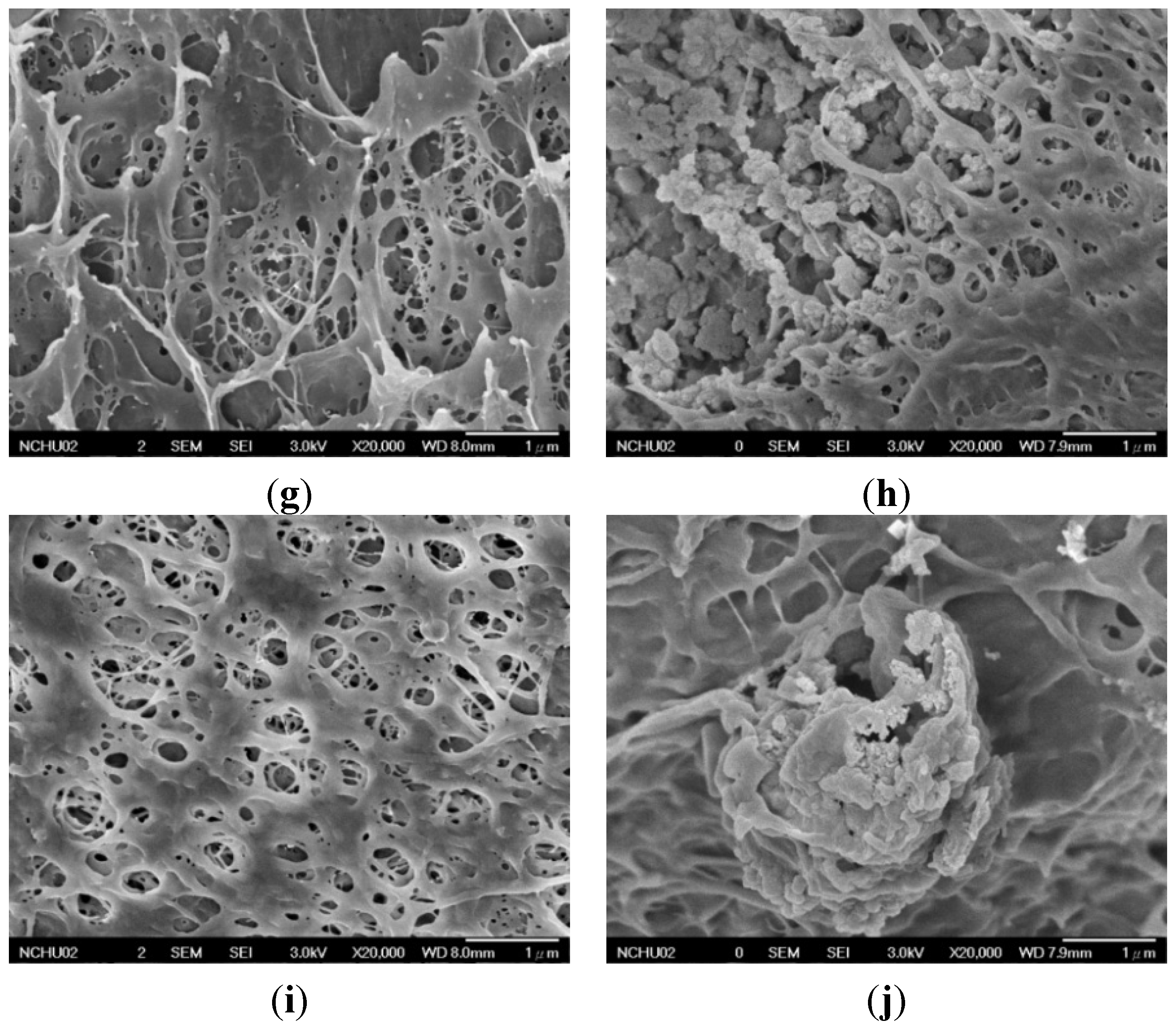
2.2.1. Surface Morphology of PVA and PVA/BCNP Nanocomposite Membranes
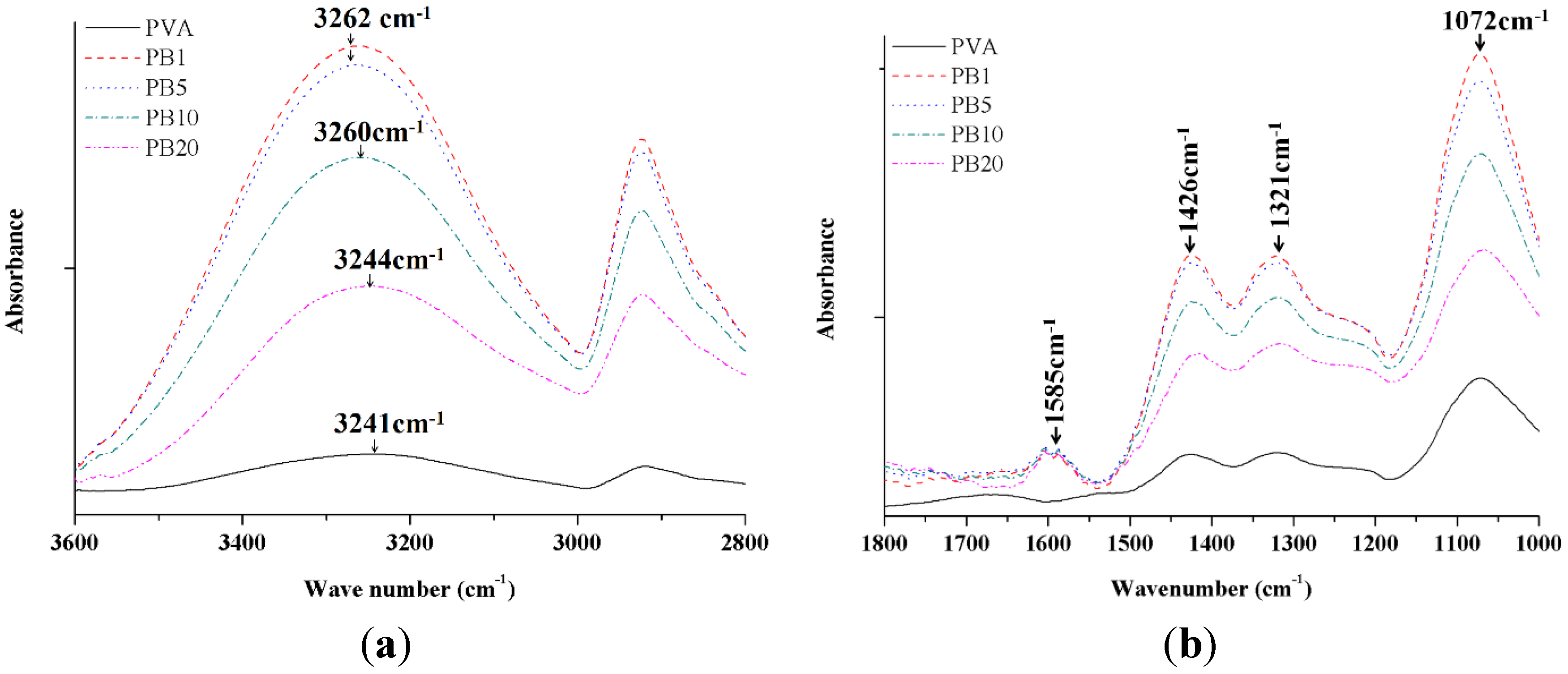
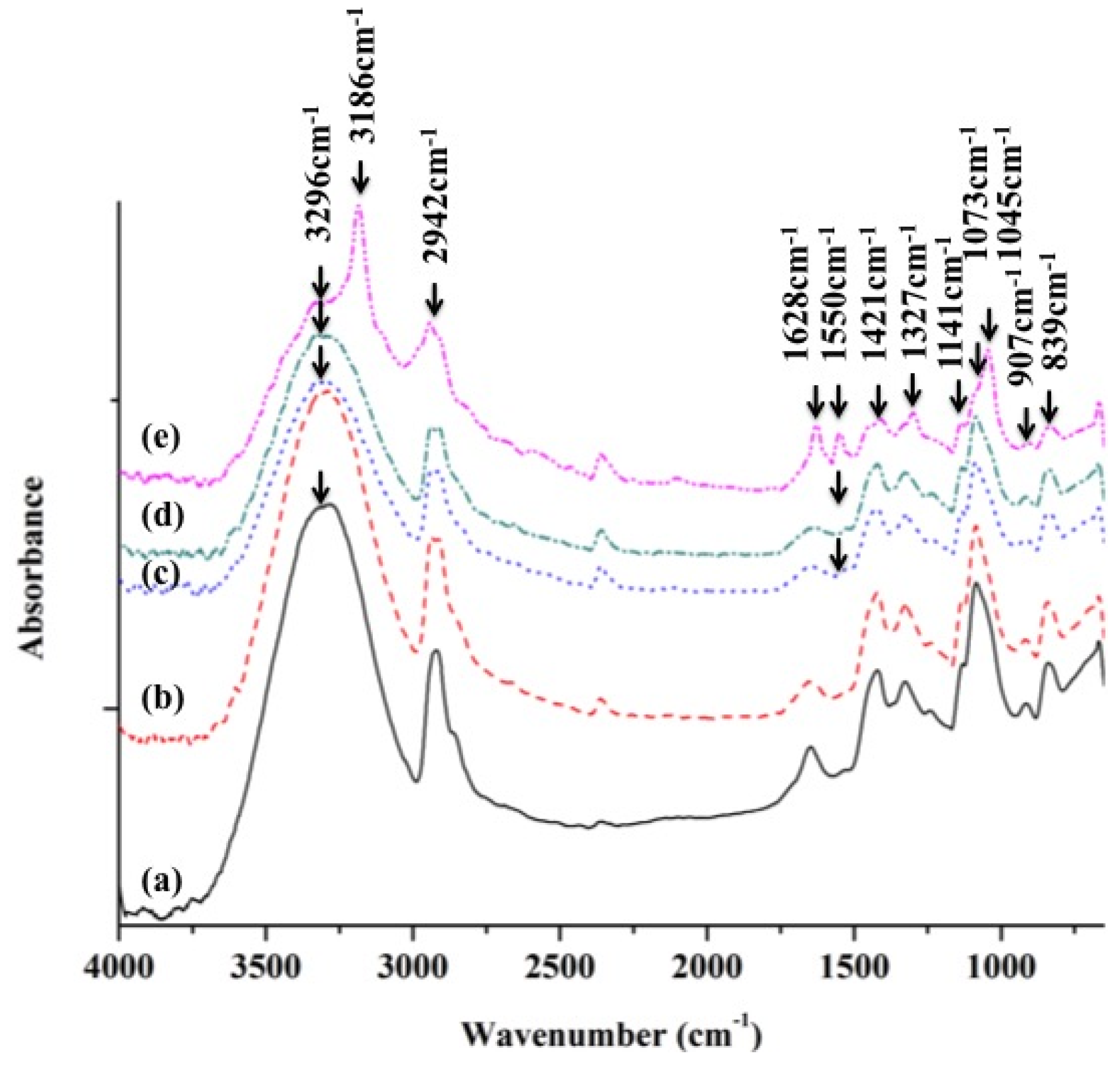
2.2.2. ATR-FTIR Analysis of PVA and PVA/BCNP Nanocomposites
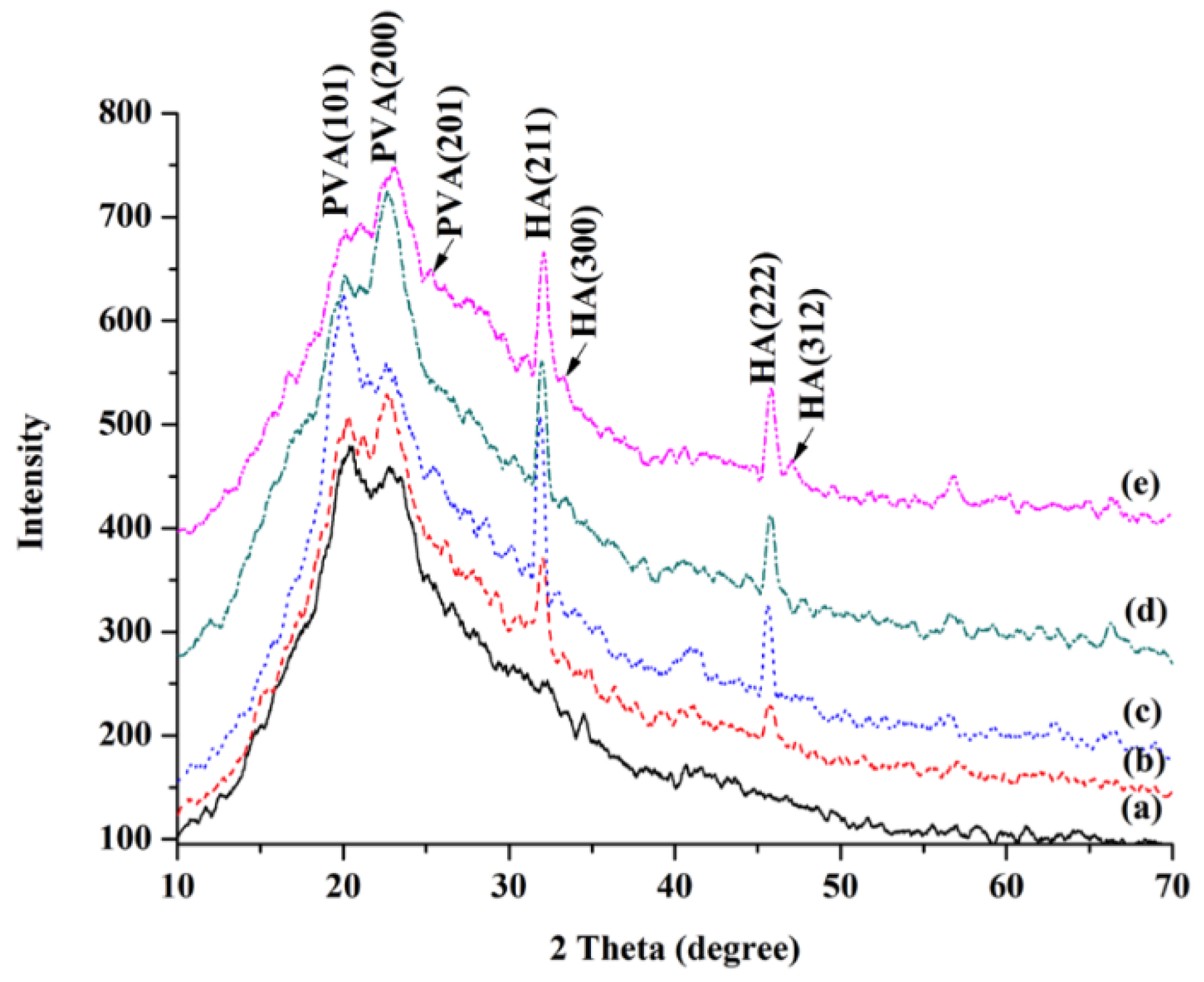
2.2.3. XRD Analysis of PVA and PVA/BCNP Nanocomposites
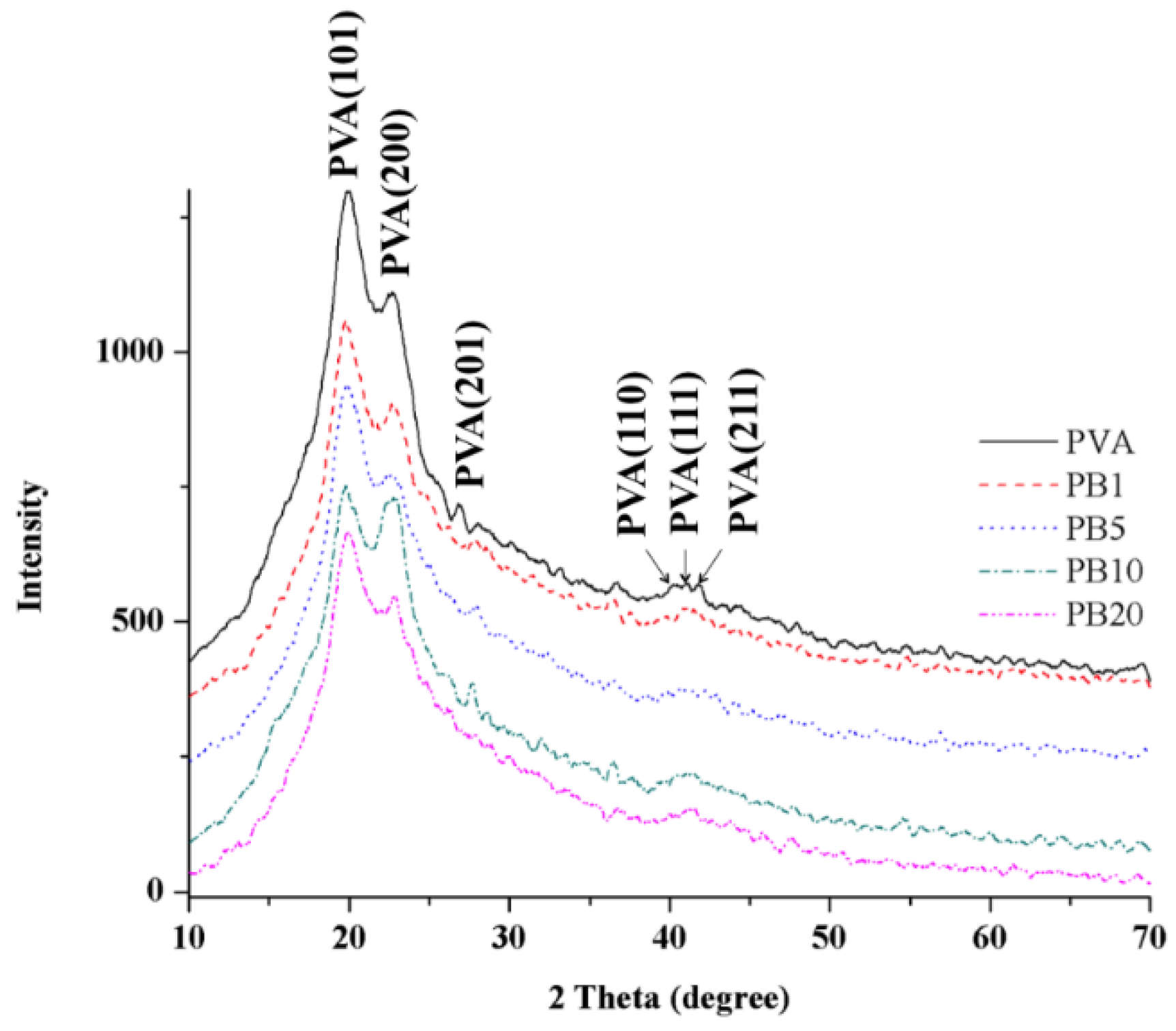
2.2.4. Thermal Properties of PVA and PVA/BCNP Nanocomposite Membranes
| Sample | Pyrolytic Temperature (°C) | Tc (°C) | Tm (°C) | ∆Hc (J/g) | Crystallinity Xc (%) | |
|---|---|---|---|---|---|---|
| Tonset | Tp | |||||
| PVA | 122.9 | 303.7 | 186.28 | 220.99 | 3.641 | 2.6 |
| PB1 | 130.1 | 304.3 | 198.93 | 224.35 | 58.57 | 42.7 |
| PB5 | 139.8 | 311.5 | 192.78 | 221.99 | 13.09 | 9.9 |
| PB10 | 138.4 | 318.0 | 196.76 | 224.84 | 13.46 | 10.8 |
| PB20 | 121.8 | 338.5 | 190.61 | 221.54 | 4.986 | 4.5 |
2.2.5. Mechanical Properties of PVA and PVA/BCNP Nanocomposites
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PVA | 13.8 ± 0.9 | 305.0 ± 61.1 |
| PB1 | 19.0 ± 0.9 * | 369.0 ± 25.4 |
| PB5 | 12.9 ± 1.8 | 336.9 ± 46.3 |
| PB10 | 15.8 ± 1.3 | 305.0 ± 96.1 |
| PB20 | 14.9 ± 1.8 | 303.4 ± 63.9 |
2.2.6. Swelling Properties of PVA and PVA/BCNP Nanocomposite Hydrogels
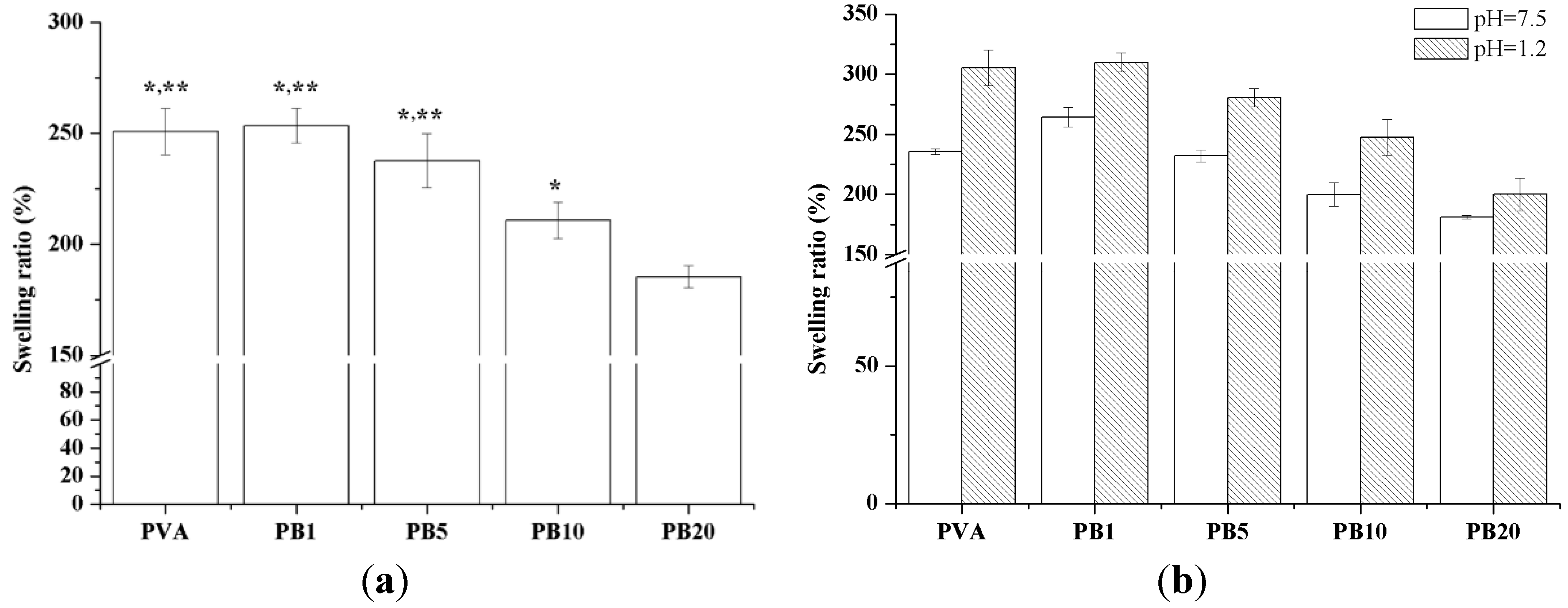
2.2.7. Free Radical Scavenging Effects of Nanocomposites
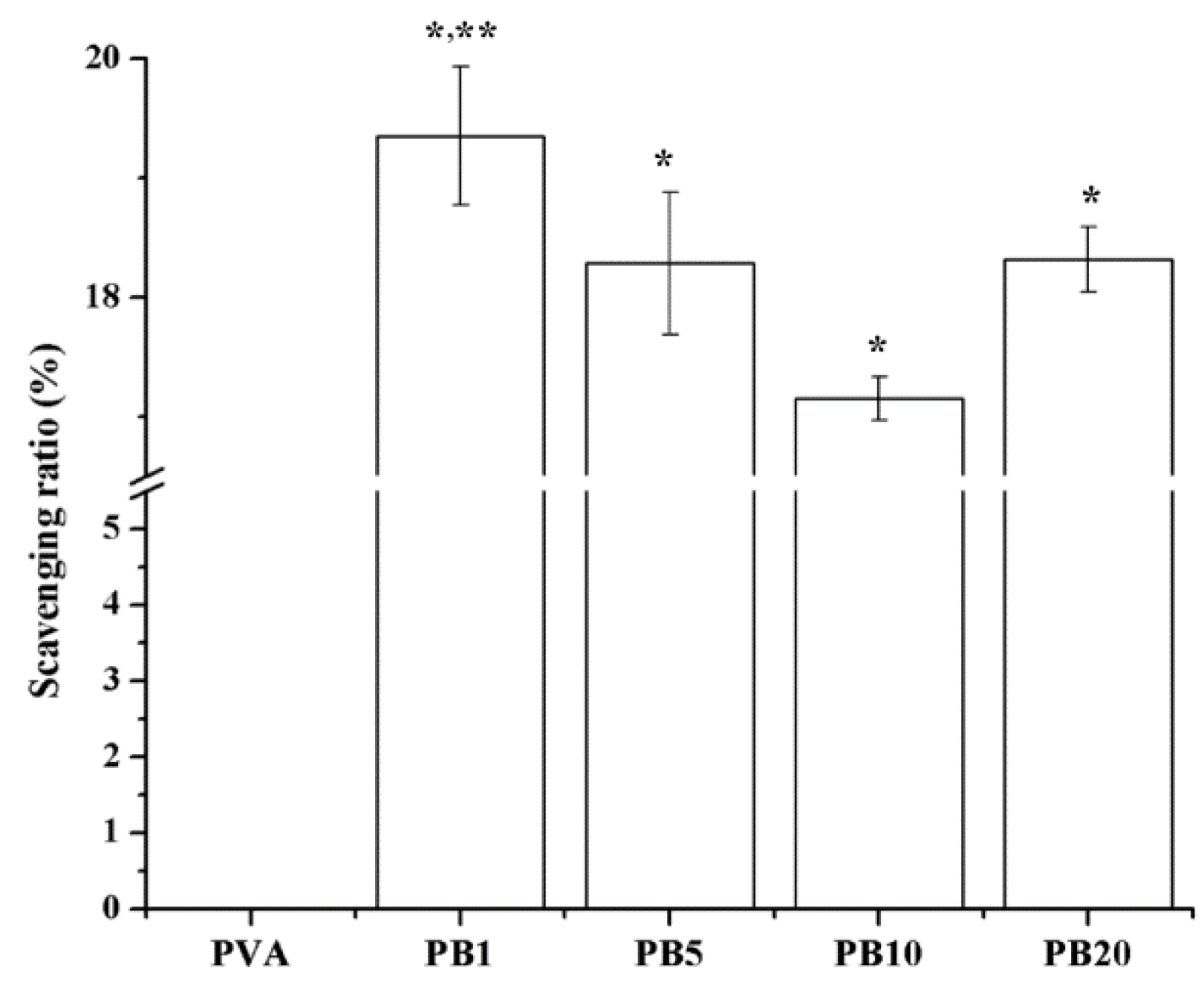
2.2.8. Far Infrared Ray Emission Measurement of PVA/BCNP Nanocomposites
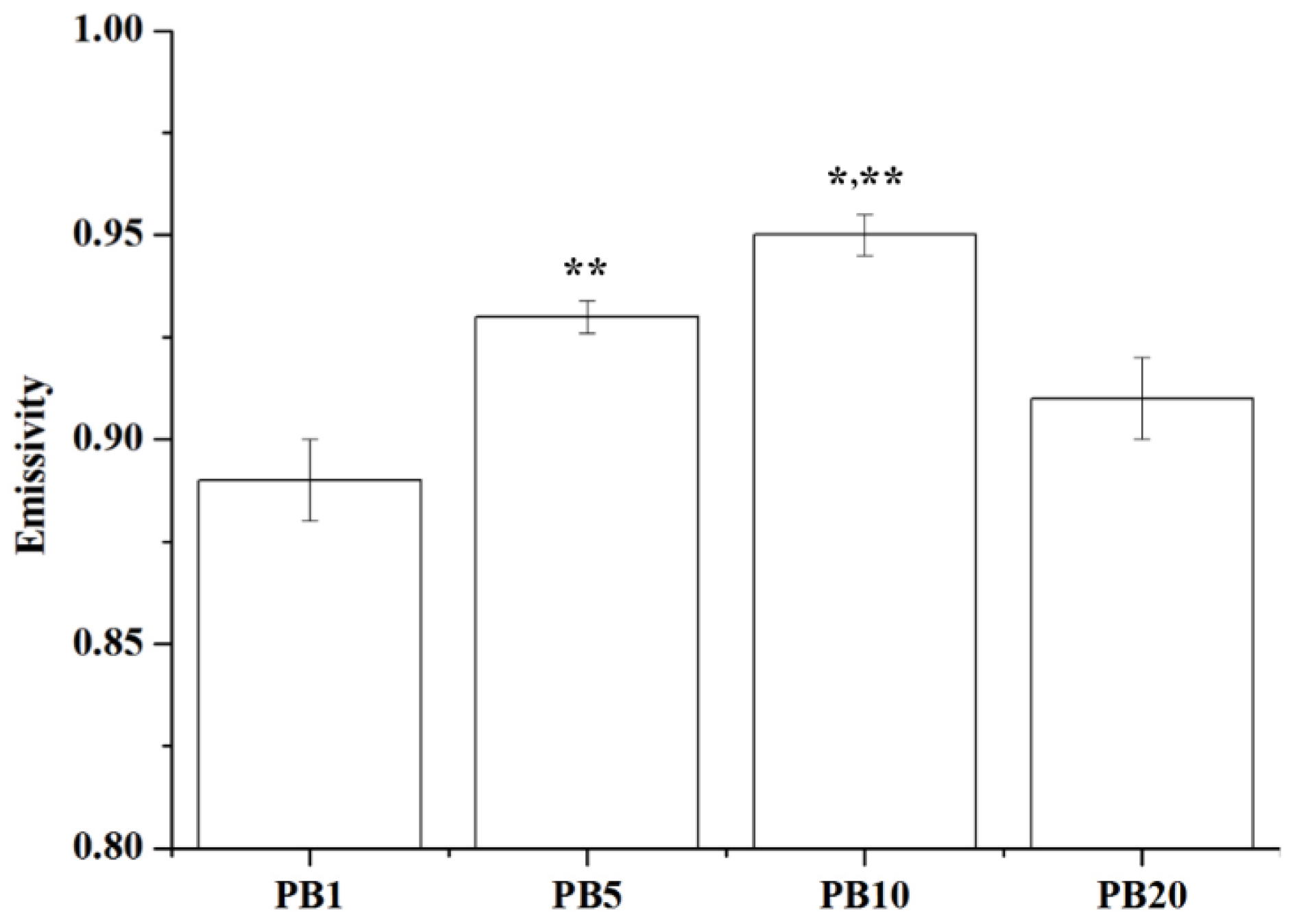
2.3. Biocompatibility of PVA and PVA/BCNP Nanocomposites
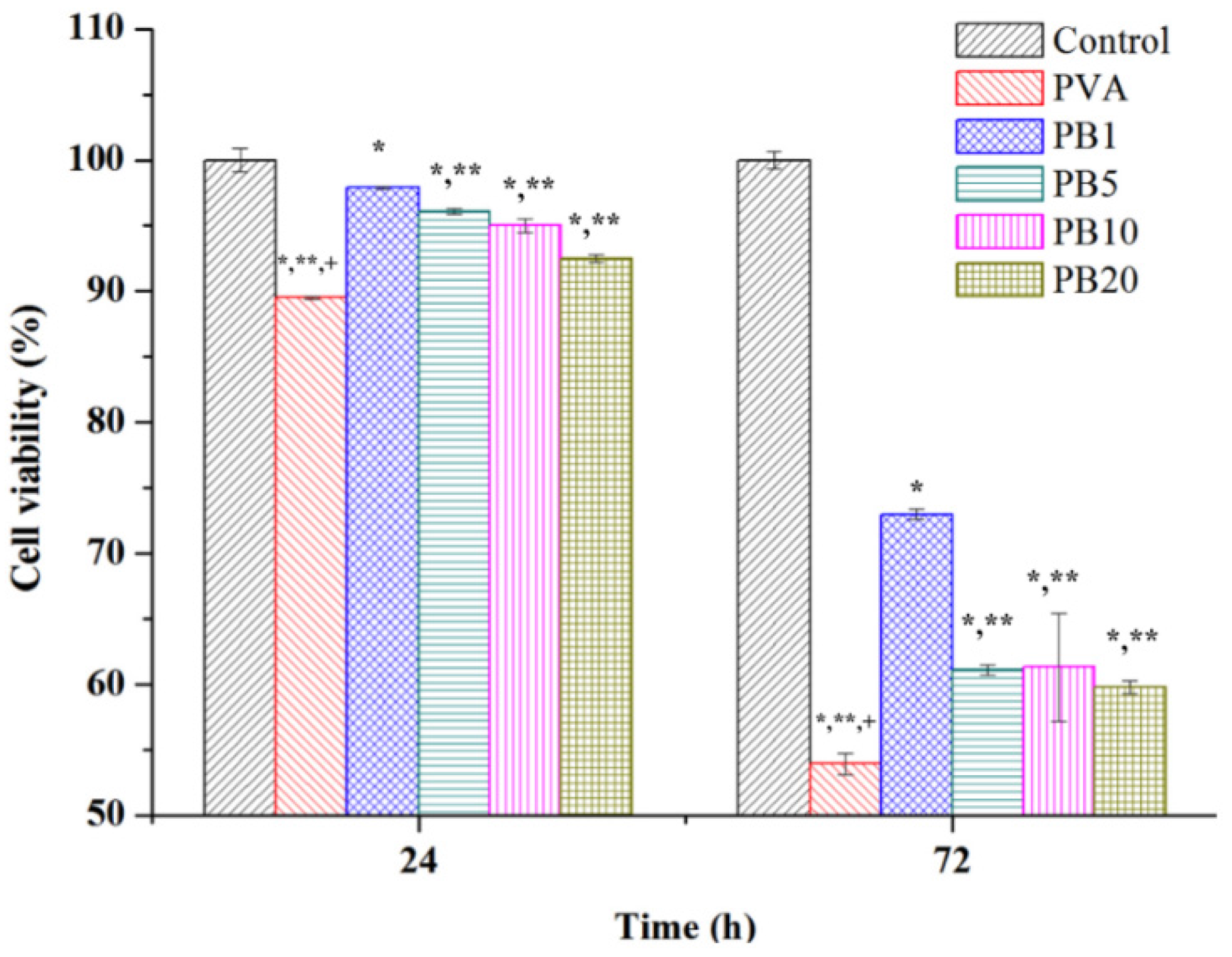
2.4. Biomineralization of PVA and PVA/BCNP Nanocomposite Membranes
3. Materials and Methods
3.1. Preparation of BCNPs
3.2. Preparation of PVA and PVA/BCNP Nanocomposite Membranes
3.3. Characterization of the Nanocomposites
3.3.1. Chemical Analysis
3.3.2. X-ray Diffraction
3.3.3. Surface Morphology
3.3.4. Mechanical Properties of PVA and PVA/BCNP Composite Membranes
3.3.5. Swelling Behaviors of PVA and PVA/BCNP Nanocomposite Membranes in Water and at various pH
3.3.6. Free Radical Scavenging Effects of the PVA and PVA/BCNP Nanocomposites
3.3.7. Far Infrared Ray Emission Test of the PVA and PVA/BCNP Nanocomposites
3.4. Biocompatibility of the PVA and PVA/BCNP Nanocomposites
3.5. Biomineralization of PVA/BCNP Nanocomposites
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Supplementary Materials
Conflicts of Interest
References
- Burillo, G.; Ogawa, T. Formation of hydrogel of polyacrylamide in solution by gamma irradiation. Die Makromol. Chem. Rapid Commun. 1980, 1, 545–550. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Matsumura, K.; Hayami, T.; Hyon, S.H.; Tsutsumi, S. Control of proliferation and differentiation of osteoblasts on apatite-coated poly(vinyl alcohol) hydrogel as an artificial articular cartilage material. J. Biomed. Mater. Res. Part A 2010, 92A, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Costa, H.S. Nanostructured poly(vinyl alcohol)/bioactive glass and poly(vinyl alcohol)/chitosan/bioactive glass hybrid scaffolds for biomedical applications. Chem. Eng. J. 2008, 137, 72–83. [Google Scholar] [CrossRef]
- Ismail, H.; Freakley, P.K.; Sheng, E. The effect of carbon black particle size on multifunctional additive-carbon black interaction. Eur. Polym. J. 1995, 31, 1049–1056. [Google Scholar] [CrossRef]
- Hsu, S.H.; Tang, C.M.; Tseng, H.J. Gold nanoparticles induce surface morphological transformation in polyurethane and affect the cellular response. Biomacromolecules 2007, 9, 241–248. [Google Scholar]
- Hsu, S.H.; Tang, C.M.; Tseng, H.J. Biostability and biocompatibility of poly(ester urethane)–gold nanocomposites. Acta Biomater. 2008, 4, 1797–1808. [Google Scholar]
- Chen, A.P.; Lin, C.M.; Lin, C.W.; Hsieh, C.T.; Lou, C.W.; Young, Y.H.; Lin, J.H. Electromagnetic shielding effectiveness and manufacture technique of functional bamboo charcoal/metal composite woven. Adv. Mater. Res. 2010, 123–125, 967–970. [Google Scholar] [CrossRef]
- Ge, Z.D.; Hu, Y.Q. Tribological behavior of friction materials containing bamboo charcoal under high-pressure and high-speed. Lubr. Eng. 2011, 36. [Google Scholar] [CrossRef]
- Zhao, R.S.; Liu, Y.L.; Zhou, J.B.; Chen, X.F.; Wang, X. Bamboo charcoal as a novel solid-phase microextraction coating material for enrichment and determination of eleven phthalate esters in environmental water samples. Anal. Bioanal. Chem. 2013, 405, 4993–4996. [Google Scholar]
- Zhao, R.S.; Hu, C.; Zhou, J.B.; Yuan, J.P.; Wang, S.S.; Wang, X. Preconcentration and sensitive determination of hexabromocyclododecane diastereomers in environmental water samples using solid phase extraction with bamboo charcoal cartridge prior to rapid resolution liquid chromatography–electrospray tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 1189–1195. [Google Scholar]
- Hsieh, M.F.; Shyu, C.L.; Huang, S.H.; Wang, W.C.; Chen, W.C. Application of bamboo charcoal particles in blood purification: Cytotoxicity and absorption capability assessments. J. Med. Biolog. Eng. 2007, 27, 47–51. [Google Scholar]
- Hsieh, M.F.; Wen, H.W.; Shyu, C.L.; Chen, S.H.; Li, W.T.; Wang, W.C.; Chen, W.C. Synthesis, in vitro macrophage response and detoxification of bamboo charcoal beads for purifying blood. J. Biomed. Mater. Res. Part A 2010, 94, 1133–1140. [Google Scholar]
- Kowuwon, W.; Laupattarakasem, W.; Saengnipanthkul, S.; Mahaisavariya, B.; Therapongpakdee, S. Charcoal bamboo as a bone substitute: An animal study. J. Med. Assoc. Thail. Chotmaihet Thangphaet 1994, 77, 496–500. [Google Scholar]
- Hamada, Y.; Teraoka, F.; Matsumoto, T.; Madachi, A.; Toki, F.; Uda, E.; Hase, R.; Takahashi, J.; Matsuura, N. Effects of far infrared ray on hela cells and wi-38 cells. Int. Congr. Ser. 2003, 1255, 339–341. [Google Scholar]
- Teraoka, F.; Hamada, Y.; Takahashi, J. Bamboo charcoal inhibits growth of hela cells in vitro. Dent. Mater. J. 2004, 23, 633–637. [Google Scholar]
- Li, S.H.; Liu, Q.; De Wijn, J.R.; Zhou, B.L.; De Groot, K. In vitro calcium phosphate formation on a natural composite material, bamboo. Biomaterials 1997, 18, 389–395. [Google Scholar]
- Li, S.H.; Liu, Q.; De Wijn, J.; Zhou, B.L.; De Groot, K. Calcium phosphate formation induced on silica in bamboo. J. Mater. Sci. Mater. Med. 1997, 8, 427–433. [Google Scholar]
- Bai, H.; Li, C.; Wang, X.; Shi, G. A pH-sensitive graphene oxide composite hydrogel. Chem. Commun. 2010, 46, 2376–2378. [Google Scholar]
- Oberlin, A.; Rousseaux, F. Graphitation partielle de quelques carbones durs. Etude en microscopie et microdiffraction electroniques. J. Appl. Crystallogr. 1968, 1, 218–226. [Google Scholar]
- Singjai, P.; Wongjamras, A.; Yu, L.D.; Tunkasiri, T. Production and characterization of beaded nanofibers from current heating of charcoal. Chem. Phys. Let. 2002, 366, 51–55. [Google Scholar]
- Zou, L.; Huang, B.; Huang, Y.; Huang, Q.; Wang, C.A. An investigation of heterogeneity of the degree of graphitization in carbon–carbon composites. Mater. Chem. Phys. 2003, 82, 654–662. [Google Scholar]
- Nie, L.; Chen, D.; Suo, J.; Zou, P.; Feng, S.; Yang, Q.; Yang, S.; Ye, S. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. Colloids Surf. B Biointerfaces 2012, 100, 169–176. [Google Scholar]
- Guo, Y.; Rockstraw, D.A. Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation. Bioresour. Technol. 2007, 98, 1513–1521. [Google Scholar]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. X-ray diffraction analysis of poly(vinyl alcohol) hydrogels, obtained by freezing and thawing techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar]
- Peppas, N.A.; Hansen, P.J. Crystallization kinetics of poly(vinyl alcohol). J. Appl. Polym. Sci. 1982, 27, 4787–4797. [Google Scholar]
- Li, J.; Suo, J.; Zou, P.; Jia, L.; Wang, S. Structure, corrosion behavior and mechanical property of a novel poly(vinyl alcohol) composite in simulated body fluid. J. Biomater. Sci. Polym. Ed. 2010, 21, 863–876. [Google Scholar]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. drug deliv. Rev. 2012, 64, 49–60. [Google Scholar]
- Hsu, S.H.; Tseng, H.J.; Lin, Y.C. The biocompatibility and antibacterial properties of waterborne polyurethane-silver nanocomposites. Biomaterials 2010, 31, 6796–6808. [Google Scholar]
- Lin, C.A.; An, T.C.; Hsu, Y.H. Study on the far infrared ray emission property and adsorption performance of bamboo charcoal/polyvinyl alcohol fiber. Polym. Plast. Technol. Eng. 2007, 46, 1073–1078. [Google Scholar]
- Toyokawa, H.; Matsui, Y.; Uhara, J.; Tsuchiya, H.; Teshima, S.; Nakanishi, H.; Kwon, A.H.; Azuma, Y.; Nagaoka, T.; Ogawa, T.; Kamiyama, Y. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp. Biol. Med. 2003, 228, 724–729. [Google Scholar]
- Saeed, A.M.; Hassan, R.A.; Thajeel, K.M. Synthesis of calcium hydroxyapatite powder from hen’s eggshell. Iraqi J. Phys. 2011, 9, 24–28. [Google Scholar]
- Adhikari, B.B.; Kanemitsu, M.; Kawakita, H.; Ohto, K. Synthesis and application of a highly efficient polyvinylcalix [4] arene tetraacetic acid resin for adsorptive removal of lead from aqueous solutions. Chem. Eng. J. 2011, 172, 341–353. [Google Scholar]
- Patterson, A.L. The Scherrer formula for x-ray particle size determination. Phys. Rev. 1939, 56. [Google Scholar] [CrossRef]
- United States Pharmacopoeia. Simulated gastric fluid and simulated intestinal fluid, TS. In the National Formulary; The United States Pharmacopeial Convention Inc.: Rockville, MD, USA, 1995; Volume 23, p. 2053. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Deng, Y.; Zhao, K.; Zhang, X.F.; Hu, P.; Chen, G.Q. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials 2002, 23, 4049–4056. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-M.; Tian, Y.-H.; Hsu, S.-H. Poly(vinyl alcohol) Nanocomposites Reinforced with Bamboo Charcoal Nanoparticles: Mineralization Behavior and Characterization. Materials 2015, 8, 4895-4911. https://doi.org/10.3390/ma8084895
Tang C-M, Tian Y-H, Hsu S-H. Poly(vinyl alcohol) Nanocomposites Reinforced with Bamboo Charcoal Nanoparticles: Mineralization Behavior and Characterization. Materials. 2015; 8(8):4895-4911. https://doi.org/10.3390/ma8084895
Chicago/Turabian StyleTang, Cheng-Ming, Yi-Hung Tian, and Shan-Hui Hsu. 2015. "Poly(vinyl alcohol) Nanocomposites Reinforced with Bamboo Charcoal Nanoparticles: Mineralization Behavior and Characterization" Materials 8, no. 8: 4895-4911. https://doi.org/10.3390/ma8084895
APA StyleTang, C.-M., Tian, Y.-H., & Hsu, S.-H. (2015). Poly(vinyl alcohol) Nanocomposites Reinforced with Bamboo Charcoal Nanoparticles: Mineralization Behavior and Characterization. Materials, 8(8), 4895-4911. https://doi.org/10.3390/ma8084895







