Preparation and Characterization of Surface Photocatalytic Activity with NiO/TiO2 Nanocomposite Structure
Abstract
:1. Introduction
2. Material Preparation and Experimental Procedure
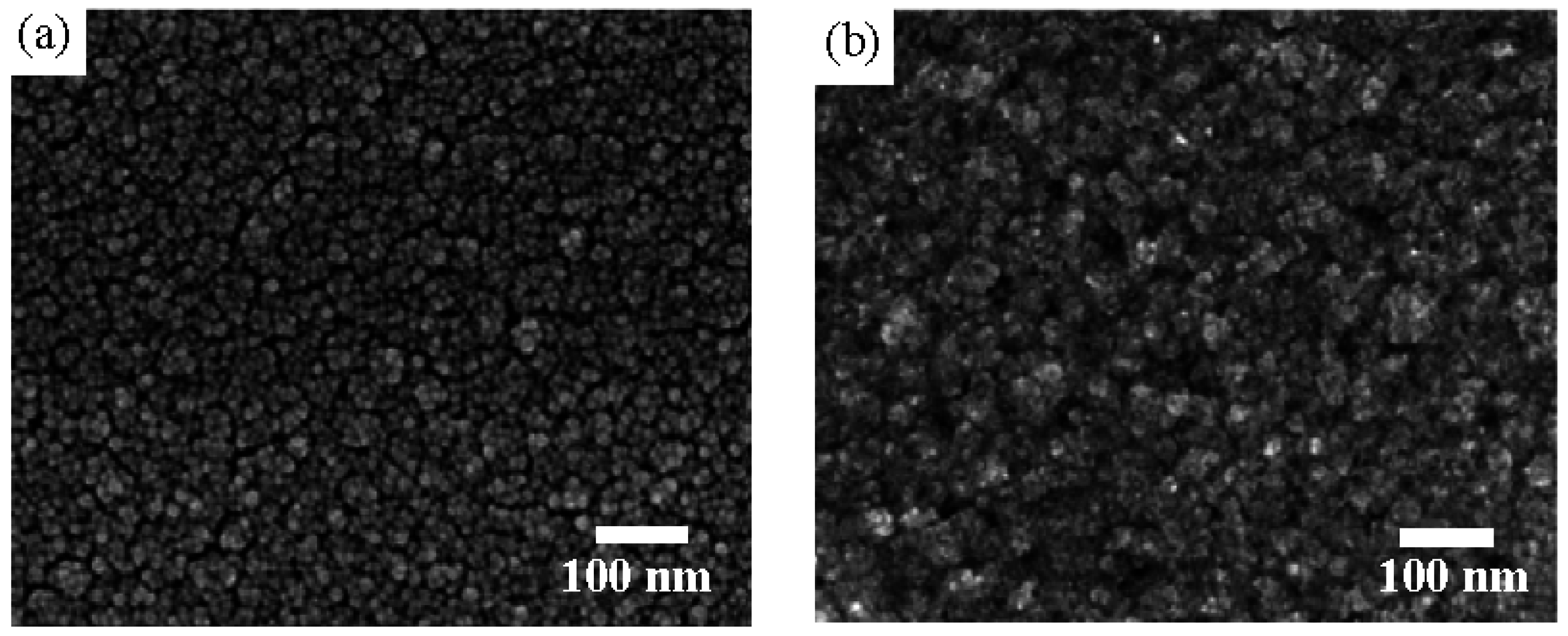
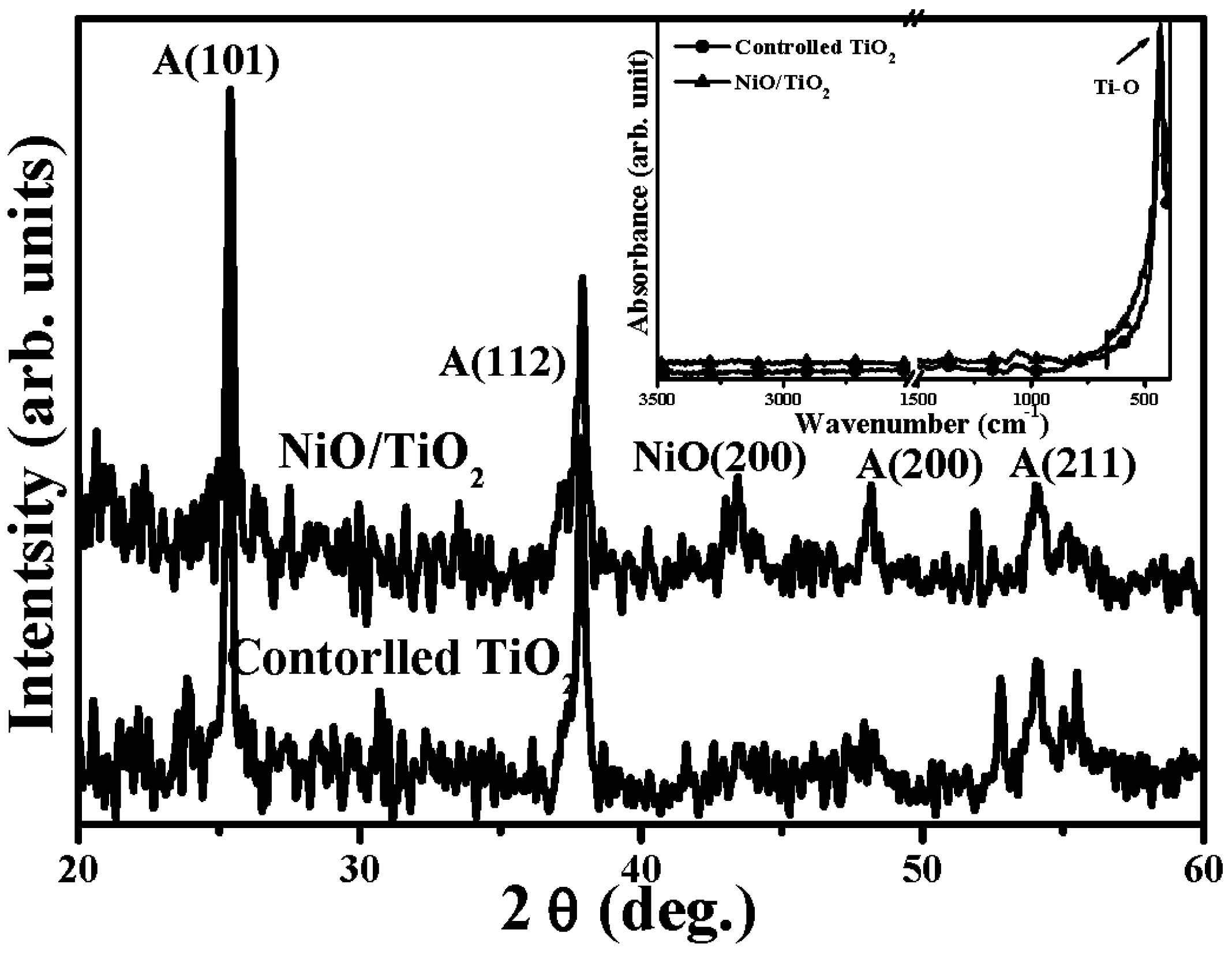
3. Results and Discussion
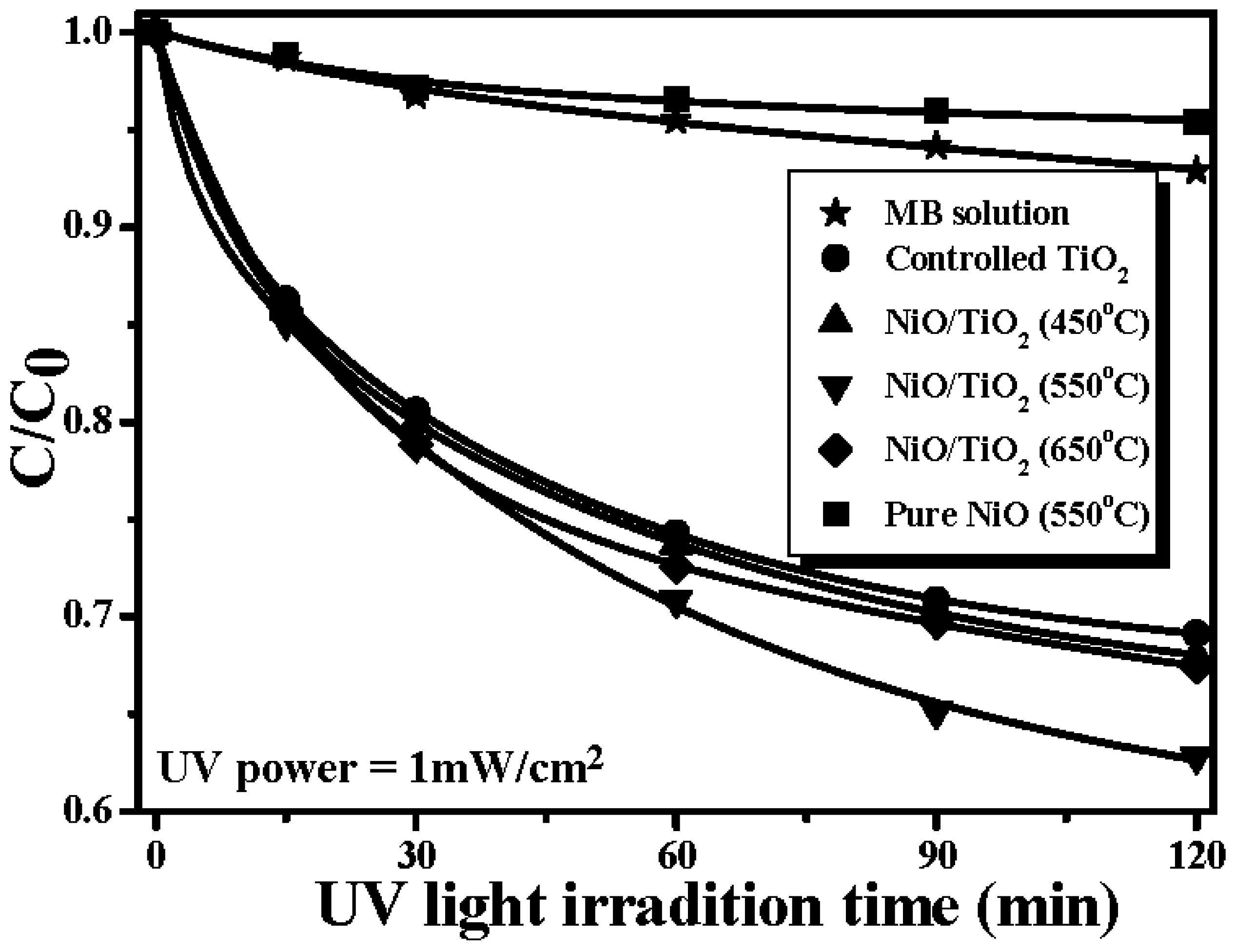
| Values | Controlled TiO2 | NiO/TiO2 (450 °C) | NiO/TiO2 (550 °C) | NiO/TiO2 (650 °C) |
|---|---|---|---|---|
| k (min−1) | 0.0232 | 0.0236 | 0.0304 | 0.0251 |
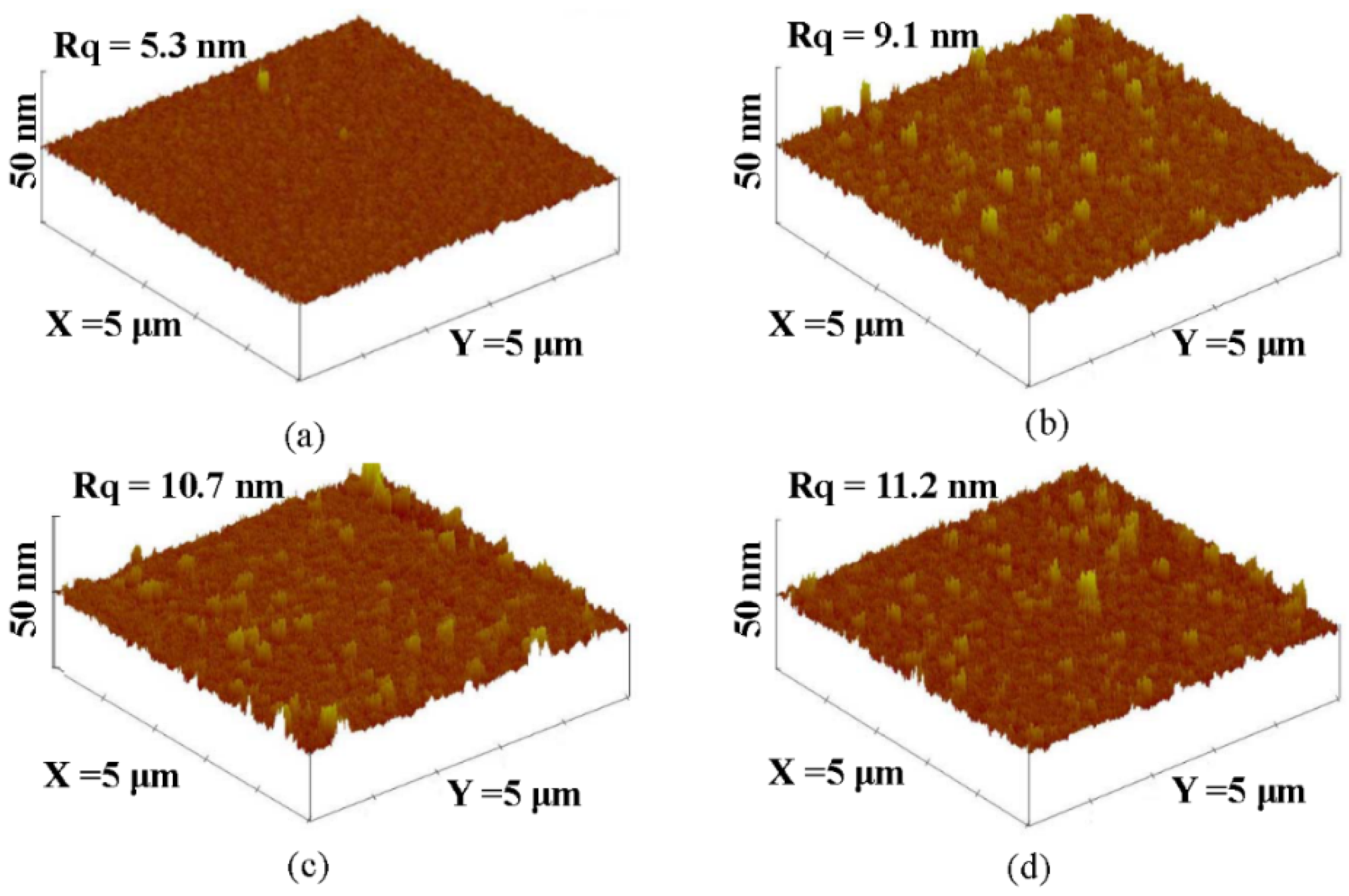
| Rq (nm) | 5.3 | 9.1 | 10.7 | 11.2 |
| Samples | Concentration (cm−3) | Mobility (cm2·V−1·s−1) | Resistivity (Ω·cm) |
|---|---|---|---|
| As-deposited Ni | -8.23 × 1023 | 4.74 | 1.64 × 10−6 |
| NiO (450 °C) | N/A | N/A | N/A |
| NiO (550 °C) | 2.60 × 1018 | 21.6 | 1.11 × 10−1 |
| NiO (650 °C) | 1.29 × 1014 | 4.30 | 1.13 × 104 |
| Controlled TiO2 | -3.55 × 1013 | 40.8 | 4.32 × 103 |

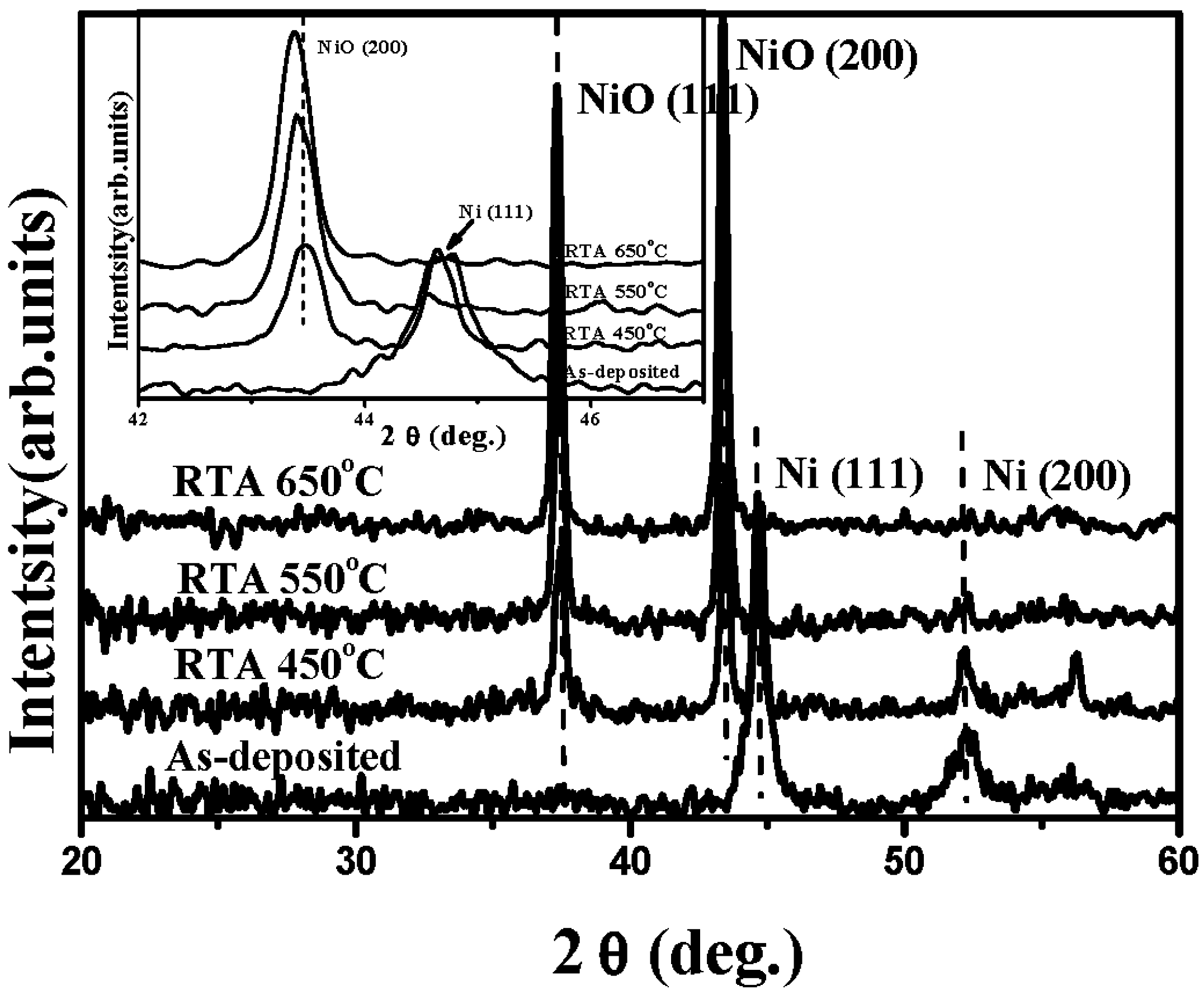

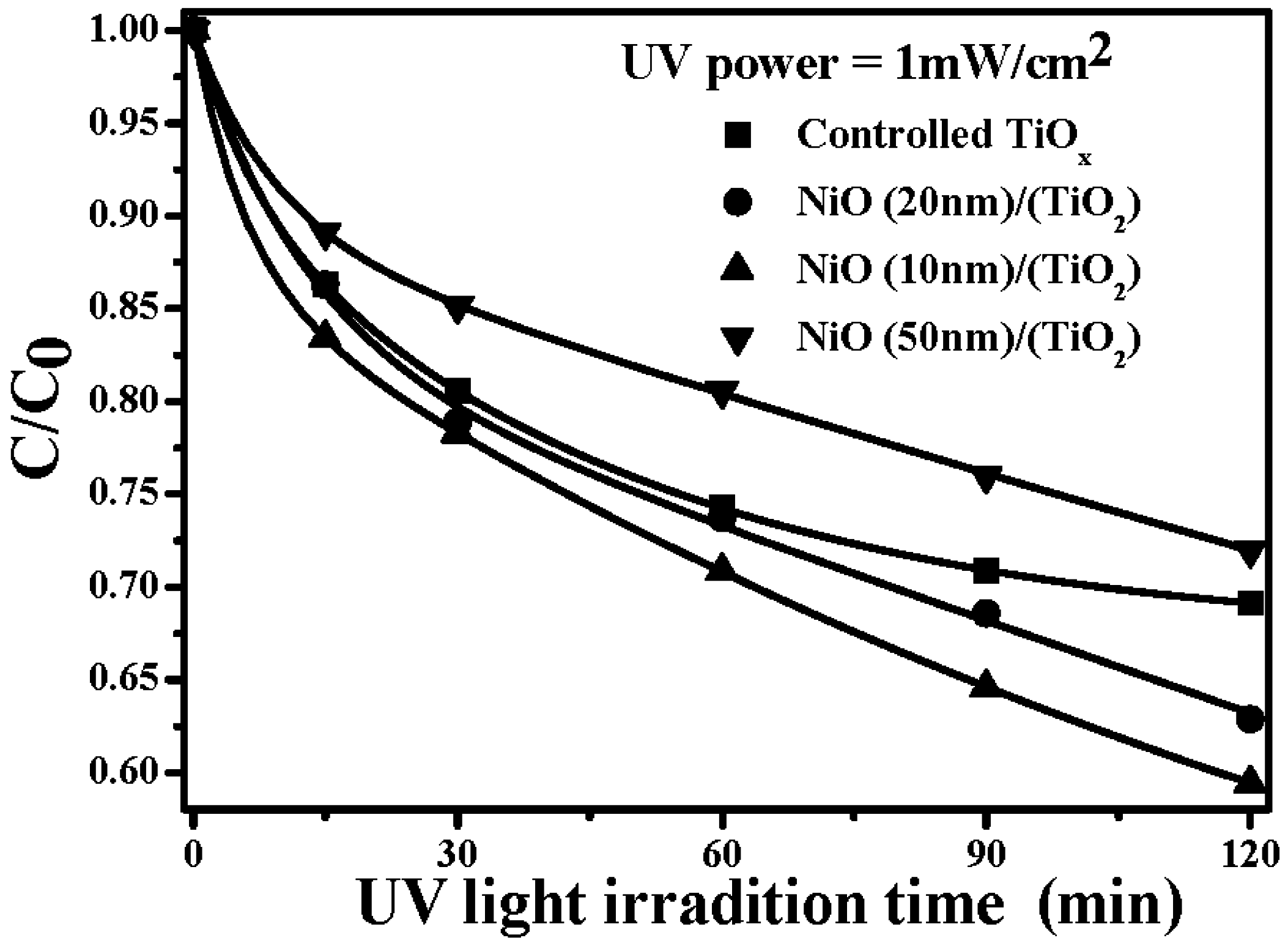
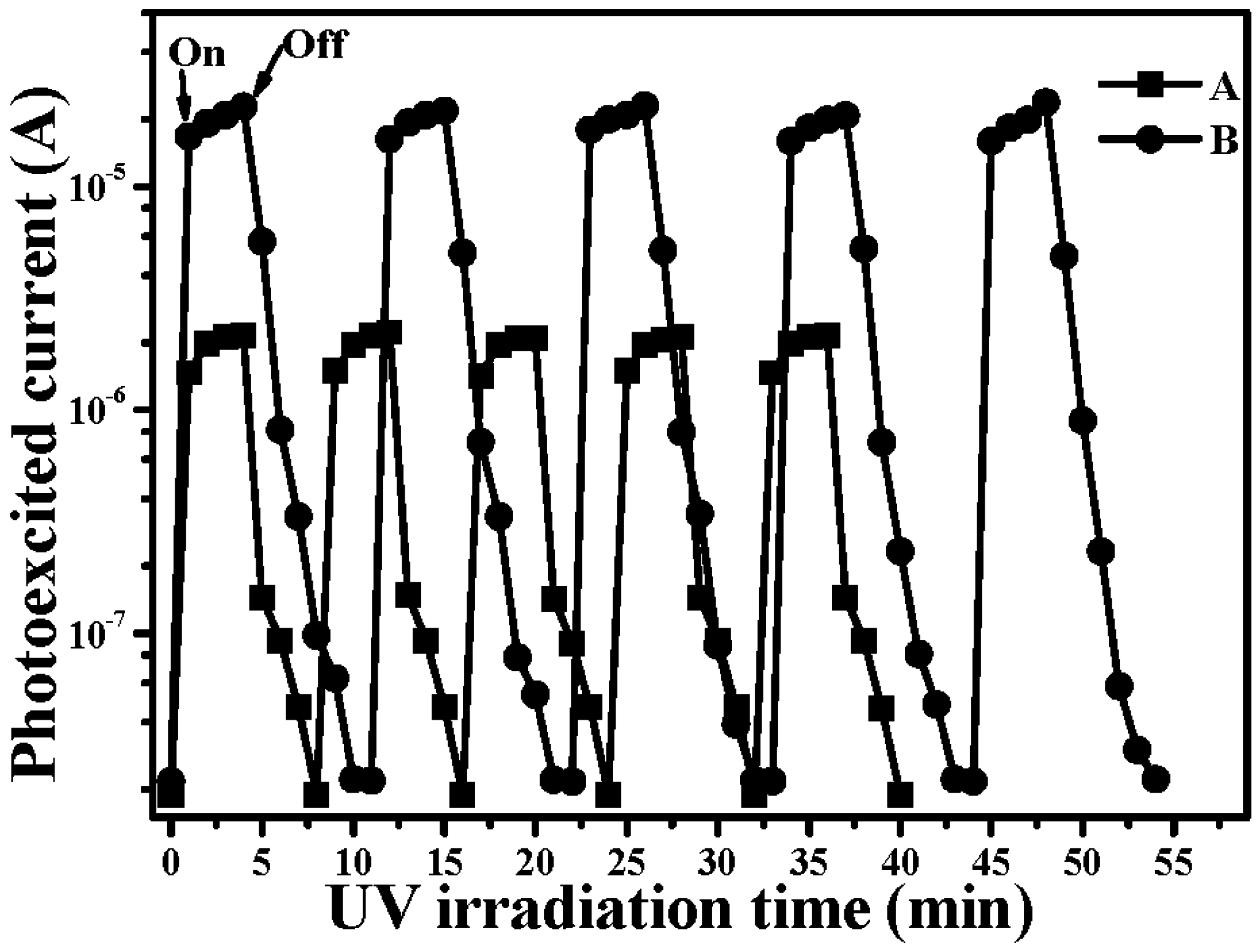
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, K.M.; Abd Hamid, S.B. Simple response surface methodology: Investigation on advance photocatalytic oxidation of 4-chlorophenoxyacetic acid using UV-active ZnO photocatalyst. Materials 2015, 8, 339–354. [Google Scholar] [CrossRef]
- Kumar, R.G.; Umar, A. Zinc oxide nanomaterials for photocatalytic degradation of methyl orange: A review. Nanosci. Nanotechnol. Lett. 2014, 6, 631–650. [Google Scholar] [CrossRef]
- Kmetyko, A.; Mogyorosi, K.; Gerse, V.; Konya, Z.; Pusztai, P.; Dombi, A.; Hernadi, K. Photocatalytic H2 production using Pt-TiO2 in the presence of oxalic acid: Influence of the noble metal size and the carrier gas flow rate. Materials 2014, 7, 7022–7038. [Google Scholar] [CrossRef]
- Bayal, N.; Jeevanandam, P. Sol-gel synthesis of SnO2-MgO nanoparticles and their photocatalytic activity towards methylene blue degradation. Mater. Res. Bull. 2013, 48, 3790–3799. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olvier, C.; Servant, L.; Toupance, T.; Li, S.; Klein, A.; Jaegermann, W. Improved photocatalytic activity in RuO2-ZnO nanoparticulate heterostructures due to inhomogeneous space charge effects. Phys. Chem. Chem. Phys. 2015, 17, 5090–5102. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.; Lin, T.W.; Huang, B.W.; Juang, F.S.; Lei, P.H.; Hu, C.Z. Light-extraction enhancement in GaN-based light-emitting diodes using grade-refractive-index amorphous titanium oxide films with porous structures. Appl. Phys. Lett. 2009, 94, 143502. [Google Scholar] [CrossRef]
- Necula, B.S.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In vitro antibacterial activity of porous TiO2-Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomater. 2009, 5, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.L.; Wang, X.M.; Ai, X.L.; Huang, J.G.; Wang, Q.; Wu, T. Sputtered TiOx thin film as compact layer for solid-state dye sensitized solar cells. Ceram. Int. 2014, 40, 15941–15949. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Deng, K.; Chen, X.; Zou, Z. Low Temperature Synthesis and Photocatalytic Activity of Rutile TiO2 Nanorod Superstructures. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chiang, B.S.; Chang, S.; Liu, D.S. Determination of photocatalytic activity in amorphous and crystalline titanium oxide films prepared using plasma-enhanced chemical vapor deposition. Appl. Surf. Sci. 2011, 257, 1893–1897. [Google Scholar] [CrossRef]
- Wu, Z.B.; Dong, F.; Zhao, W.R.; Wang, H.Q.; Liu, Y.; Guan, B.H. The fabrication and characterization of novel carbon doped TiO2 nanotubes, nanowires and nanorods with high visible light photocatalytic activity. Nanotechnology 2009, 20, 235701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, C.X.; Zhang, M.; Yang, J.J.; Zhang, Z.J. Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl. Catal. B Environ. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Lin, X.X.; Rong, F.; Ji, X.; Fu, D.G. Visible light photocatalytic activity and Photoelectrochemical property of Fe-doped TiO2 hollow spheres by sol-gel method. J. Sol-Gel Sci. Technol. 2011, 59, 283–289. [Google Scholar] [CrossRef]
- Ma, C.; Dong, W.; Fang, L.; Zheng, F.G.; Shen, M.R.; Wang, Z.L. Synthesis of TiO2/Pt/TiO2 multilayer films via radio frequency magnetron sputtering and their enhanced photocatalytic activity. Thin Solid Films 2012, 520, 5727–5732. [Google Scholar] [CrossRef]
- Sun, T.; Liu, E.Z.; Fan, J.; Hu, X.Y.; Wu, F.; Hou, W.Q.; Yang, Y.H.; Kang, L.M. High photocatalytic activity of hydrogen production from water over Fe doped and Ag deposited anatase TiO2 catalyst synthesized by solvothermal method. Chem. Engineer. J. 2013, 228, 896–906. [Google Scholar] [CrossRef]
- Shih, P.C.; Huang, C.H.; Chen, T.H.; Lai, L.W.; Lu, Y.S.; Liu, D.S. Enhancement on photocatalytic activity of an amorphous titanium oxide film with nano-textured surface by selective-fluorination etching process. Mater. Res. Bull. 2014, 52, 177–182. [Google Scholar] [CrossRef]
- Van Overschelde, O.; Guisbiers, G.; Hamadi, F.; Hemberg, A.; Snyders, R.; Wautelet, M. Alternative to classic annealing treatments for fractally patterned TiO2 thin films. J. Appl. Phys. 2008, 104, 103106. [Google Scholar] [CrossRef]
- Yang, S.G.; Quan, X.; Li, X.Y.; Liu, Y.Z.; Chen, S.; Chen, G.H. Preparation, characterization and photoelectrocatalytic properties of nanocrystalline Fe2O3/TiO2, ZnO/TiO2, and Fe2O3/ZnO/TiO2 composite film electrodes towards pentachlorophenol degradation. Phys. Chem. Chem. Phys. 2004, 6, 659–664. [Google Scholar]
- Zhang, J.; Zhu, H.; Zheng, S.; Pan, F.; Wang, T. TiO2 film/Cu2O microgrid heterojunction with photocatalytic activity under solar light irradiation. Appl. Mater. Inter. 2009, 1, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zeng, Y.P.; Jiang, D. The improved photocatalytic properties of p-type NiO loaded porous TiO2 sheets prepared via freeze tape-casting. Solid State Sci. 2010, 12, 138–143. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Li, C.; Wang, W.; Wang, T. Synthesis, characterization and photocatalytic activity of TiO2 film/Bi2O3 microgrid heterojunction. J. Mater. Sci. Technol. 2011, 27, 59–63. [Google Scholar] [CrossRef]
- Yang, G.; Yang, B.; Xiao, T.; Yan, Z. One-step solvothermal synthesis of hierarchically porous nanostructured CdS/TiO2 heterojunction with higher visible light photocatalytic activity. Appl. Surf. Sci. 2013, 283, 402–410. [Google Scholar] [CrossRef]
- Uddin, Md.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Müller, M.M.; Kleebe, H.J.; Rachut, K.; Ziegler, J.; Klein, A.; Jaegermann, W. Preparation of RuO2/TiO2 mesoporous heterostructures and rationalization of their enhanced photocatalytic properties by band alignment investigations. J. Phys. Chem. C 2013, 117, 22098–22110. [Google Scholar] [CrossRef]
- Kokane, S.B.; Sartale, S.D.; Betty, C.A.; Sasikala, R. Pd-TiO2-SrIn2O4 heterojunction photocatalyst: Enhanced photocatalytic activity for hydrogen. RSC Adv. 2014, 4, 55539–55547. [Google Scholar] [CrossRef]
- Uddin, Md.T.; Babot, O.; Thomas, L.; Oliver, C.; Redaelli, M.; D’Arienzo, M.; Morazzoni, F.; Jaegermann, W.; Rockstroh, N.; Junge, H.; Toupance, T. New insights into the photocatalytic properties of RuO2/TiO2 mesoporous heterostructures for hydrogen production and organic pollutant photodecomposition. J. Phys. Chem. C 2015, 119, 7006–7015. [Google Scholar] [CrossRef]
- Ai, L.; Fang, G.; Yuan, L.; Liu, N.; Wang, M.; Li, C.; Zhang, Q.; Li, J.; Zhao, X. Influence of substrate temperature on electrical and optical properties of p-type semitransparent conductive nickel oxide thin films deposited by radio frequency sputtering. Appl. Surf. Sci. 2008, 254, 2401–2405. [Google Scholar] [CrossRef]
- Chen, C.J.; Liao, C.H.; Hsu, K.C.; Wu, Y.T.; Wu, C.S. P-N junction mechanism on improved NiO/TiO2 photocatalyst. Catal. Commun. 2011, 12, 1307–1310. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hong, S.C.; Hwang, F.T.; Lai, L.W.; Lin, T.W.; Liu, D.S. Effect of nickel oxide seed layers on annealed-amorphous titanium oxide thin films prepared using plasma-enhanced chemical vapor deposition. Thin Solid Films 2011, 520, 320–327. [Google Scholar] [CrossRef]
- Li, H.; Zhao, G.; Han, G.; Song, B. Hydrophilicity and photocatalysis of Ti1−xVxO2 films prepared by sol-gel method. Surf. Coat. Technol. 2007, 201, 7615–7618. [Google Scholar] [CrossRef]
- Vlachos, D.; Kamaratos, M.; Foulias, S.D.; Argirusis, C.; Borchardt, G. Adsorption of oxygen on a nickel covered SrTiO3(100) surface studied by means of Auger electron spectroscopy and work function measurements. J. Phys. Condens. Mater. 2005, 17, 635–642. [Google Scholar] [CrossRef]
- Gayán, P.; Dueso, C.; Abad, A.; Adanez, J.; de Diego, L.F.; Garćia-Labiano, F. NiO/Al2O3 oxygen carriers for chemical-looping combustion prepared by impregnation and deposition-precipitation methods. Fuel 2009, 88, 1016–1023. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Hou, Y.; Huang, J.; Wu, L.; Fu, X. The effect of postnitridation annealing on the surface property and photocatalytic performance of N-doped TiO2 under visible light irradiation. J. Catal. 2008, 255, 59–67. [Google Scholar] [CrossRef]
- Que, W.; Sun, Z.; Zhou, Y.; Lam, Y.L.; Chan, Y.C.; Kam, C.H. Optical and mechanical properties of TiO2/SiO2/organically modified silane composite films prepared by sol-gel processing. Thin Solid Films 2000, 359, 177–183. [Google Scholar] [CrossRef]
- Yang, J.L.; Lai, Y.S.; Chen, J.S. Effect of heat treatment on the properties of non-stoichiometric p-type nickel oxide films deposited by reactive sputtering. Thin Solid Films 2005, 488, 242–246. [Google Scholar] [CrossRef]
- Sasi, B.; Gopchandran, K.G. Nanostructured mesoporous nickel oxide thin films. Nanotechology 2007, 18, 115613. [Google Scholar] [CrossRef]
- Kroeze, J.E.; Savenije, T.J. The application of a low-bandgap conjugated oligomer for the sensitization of SnO2 and TiO2. Thin Solid Films 2004, 451, 54–59. [Google Scholar] [CrossRef]
- Lany, S.; Osorio-Guillen, J.; Zunger, A. Origins of the doping asymmetry in oxides: Hole doping in NiO versus electron doping in ZnO. Phys. Rev. B 2007, 75, 241203. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wu, Y.C.; Lai, B.Y. Collection Efficiency enhancement of injected electrons in dye-sensitized solar cells with a Ti interfacial layer and TiCl4 treatment. Int. J. Electrochem. Sci. 2012, 7, 9478–9487. [Google Scholar]
- Abbasi, M.A.; Ibupoto, Z.H.; Hussain, M.; Nur, O.; Wilander, M. The fabrication of white light-emitting diodes using the n-ZnO/NiO/p-GaN heterojunction with enhanced luminescence. Nanoscale Res. Lett. 2013, 8, 320. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Z.; Chen, T.-H.; Lai, L.-W.; Li, P.-Y.; Liu, H.-W.; Hong, Y.-Y.; Liu, D.-S. Preparation and Characterization of Surface Photocatalytic Activity with NiO/TiO2 Nanocomposite Structure. Materials 2015, 8, 4273-4286. https://doi.org/10.3390/ma8074273
Chen J-Z, Chen T-H, Lai L-W, Li P-Y, Liu H-W, Hong Y-Y, Liu D-S. Preparation and Characterization of Surface Photocatalytic Activity with NiO/TiO2 Nanocomposite Structure. Materials. 2015; 8(7):4273-4286. https://doi.org/10.3390/ma8074273
Chicago/Turabian StyleChen, Jian-Zhi, Tai-Hong Chen, Li-Wen Lai, Pei-Yu Li, Hua-Wen Liu, Yi-You Hong, and Day-Shan Liu. 2015. "Preparation and Characterization of Surface Photocatalytic Activity with NiO/TiO2 Nanocomposite Structure" Materials 8, no. 7: 4273-4286. https://doi.org/10.3390/ma8074273
APA StyleChen, J.-Z., Chen, T.-H., Lai, L.-W., Li, P.-Y., Liu, H.-W., Hong, Y.-Y., & Liu, D.-S. (2015). Preparation and Characterization of Surface Photocatalytic Activity with NiO/TiO2 Nanocomposite Structure. Materials, 8(7), 4273-4286. https://doi.org/10.3390/ma8074273





