Dihydrogen Phosphate Stabilized Ruthenium(0) Nanoparticles: Efficient Nanocatalyst for The Hydrolysis of Ammonia-Borane at Room Temperature
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Water Soluble Dihydrogen Phosphate Stabilized Ruthenium(0) Nanoparticles
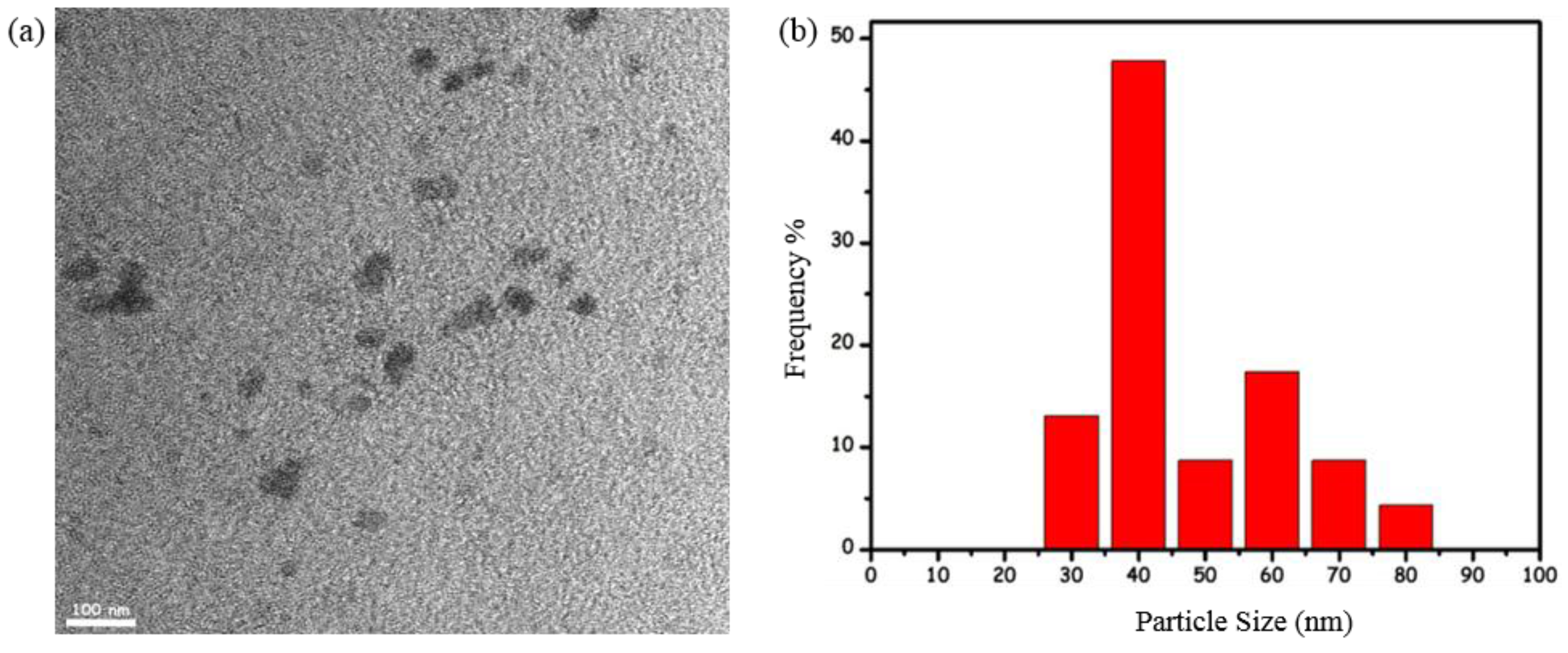
2.3. Transmission Electron Microscopy Analyses
2.4. Testing the Catalytic Activity of Ruthenium(0) Nanoparticles in the Hydrolysis of Ammonia-Borane
2.5. Determination of Activation Energy for Ruthenium(0) Nanoparticles Catalyzed Hydrolysis of Ammonia-Borane
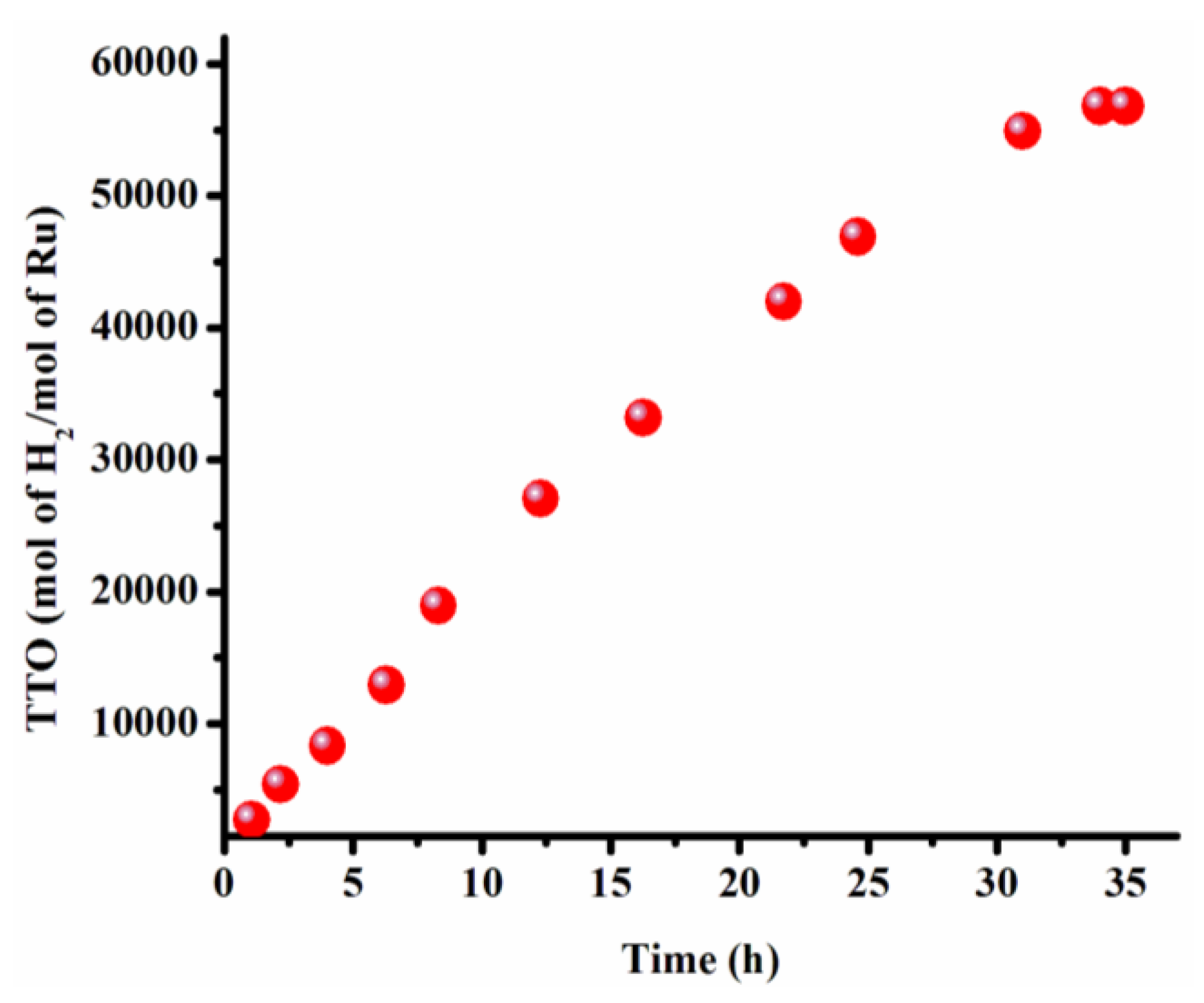
2.6. Determination of Catalytic Lifetime of Ruthenium(0) Nanoparticles in the Hydrolysis of Ammonia-Borane
2.7. Recyclability of Ruthenium(0) Nanoparticles in the Hydrolysis of Ammonia-Borane
3. Results and Discussion
3.1. Catalytic Activity of Water-Soluble Dihydrogen Phosphate-Stabilized Ruthenium(0) Nanoparticles in the Hydrolysis of Ammonia-Borane
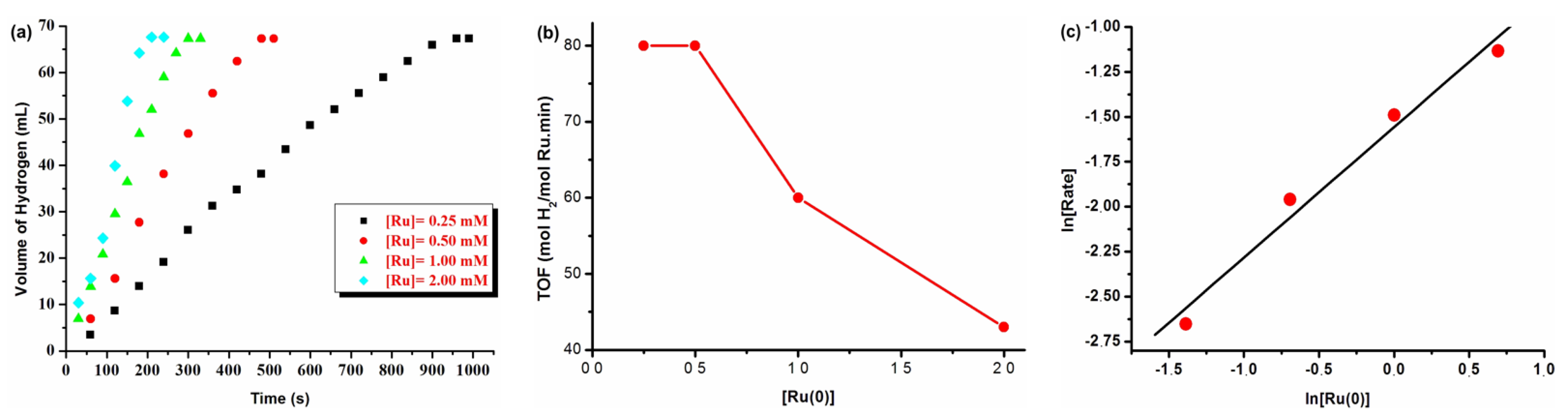
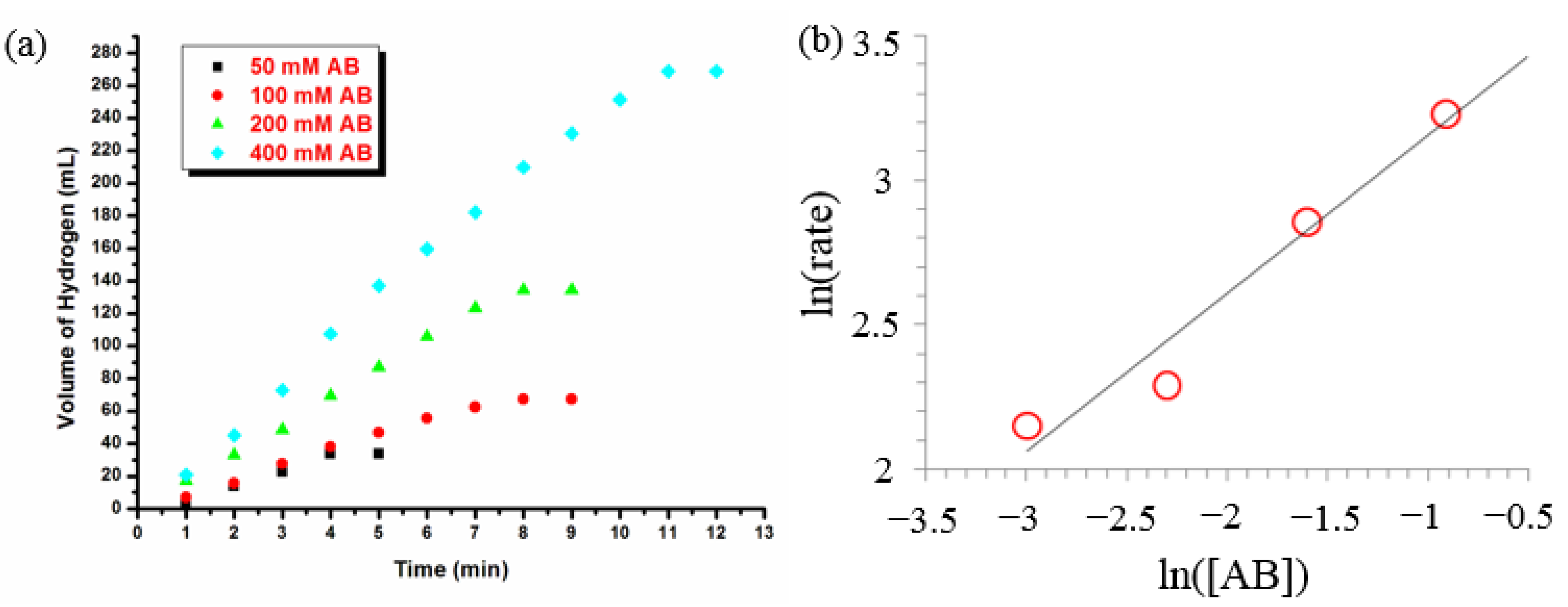
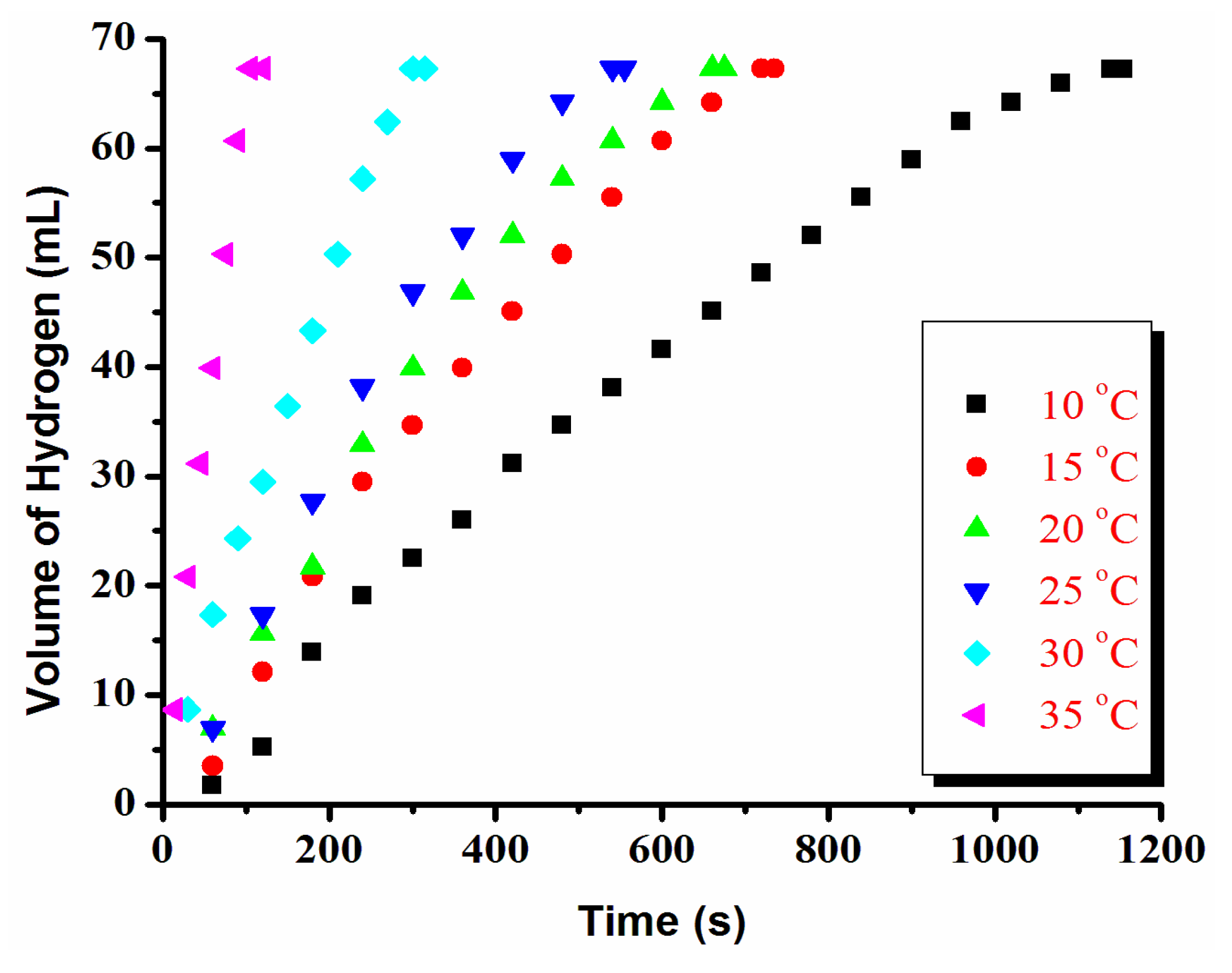
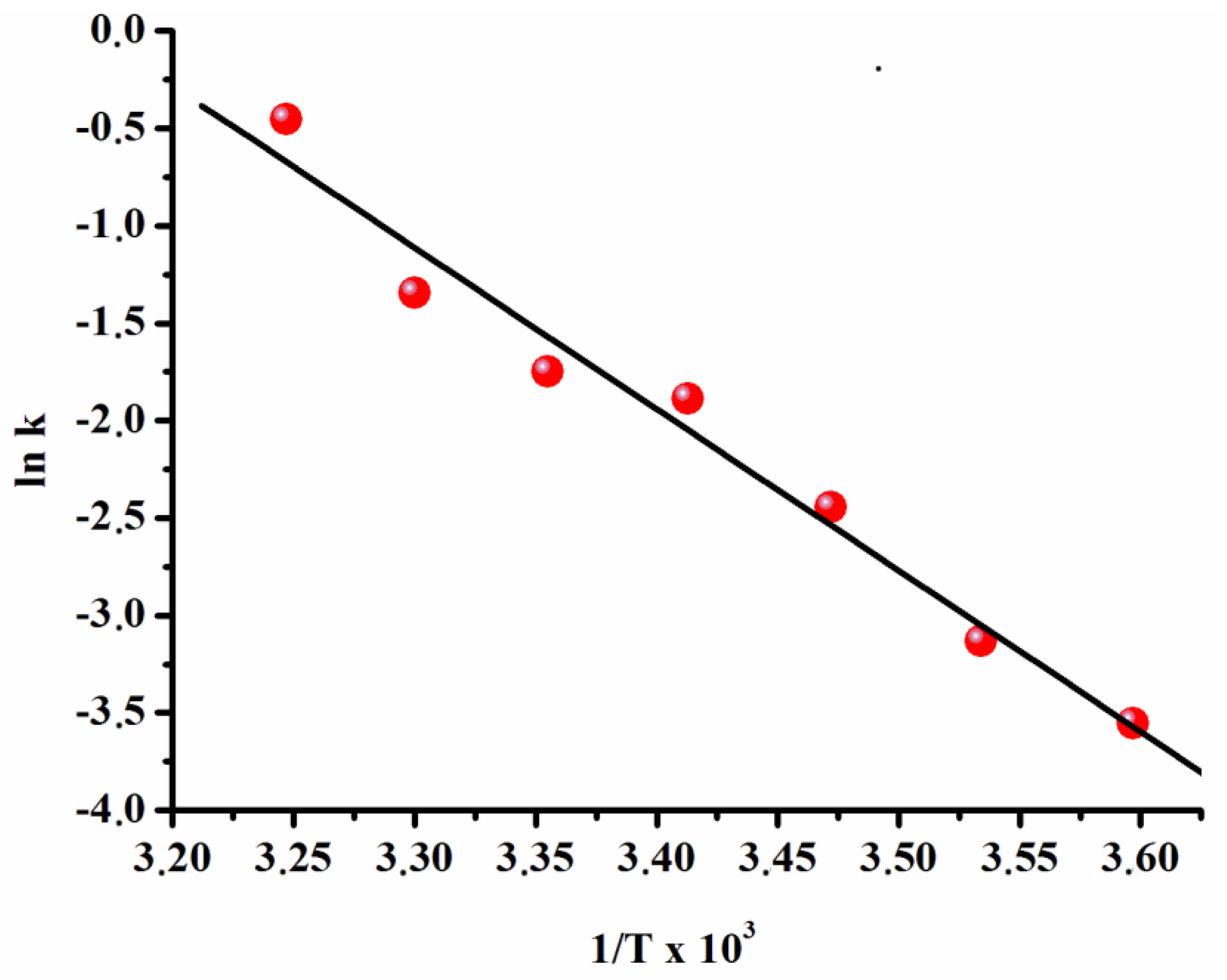
3.2. Recyclability and Catalytic Lifetime of Ruthenium(0) Nanoparticles in the Hydrolysis of Ammonia-Borane
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sperling, D.; DeLuchi, M.A. Transportation energy futures. Annu. Rev. Energy 1989, 14, 375–424. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
- Metin, Ö.; Şahin, Ş.; Özkar, S. Water-soluble poly(4-styrene sulfonic acid-co-maleic acid) stabilized ruthenium(0) and palladium(0) nanoclusters as highly active catalyst in hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hyd. Energy 2009, 34, 6304–6313. [Google Scholar] [CrossRef]
- Wu, B.H.; Zheng, N.F. Surface and interface control of noble metal nano crystals for catalytic and electrocatalytic applications. Nano Today 2013, 8, 168–197. [Google Scholar] [CrossRef]
- Guo, S.J.; Wang, E.K. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ruthenium(0) nanoparticles supported on multiwalled carbon nanotube as highly active catalyst for hydrogen generation from ammonia borane. ACS Appl. Mater. Interfaces 2012, 4, 6302–6310. [Google Scholar] [CrossRef] [PubMed]
- Basic Research Needs Catalysis for Energy, Report from the US Department of Energy, Basic Energy Sciences Workshop Report. Available online: http://www.sc.doe.gov/bes/reports/list.html (accessed on 6 August 2007).
- Marder, T. Will we soon be fueling our automobiles with ammonia-borane? Angew. Chem. Int. Ed. 2007, 46, 8116–8118. [Google Scholar] [CrossRef] [PubMed]
- Satyapala, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. department of energy’s national hydrogen storage project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef]
- Zahmakıran, M. Preparation and characterization LTA-type zeolite framework dispersed ruthenium nanoparticles and their catalytic application in the hydrolytic dehydrogenation of ammonia-borane for efficient hydrogen generation. Mater. Sci. Eng. B 2012, 177, 606–613. [Google Scholar] [CrossRef]
- Wolf, G.; Baumann, J.; Baitalow, F.; Hoffmann, F.P. Calorimetric process monitoring of thermal decomposition of B-N-H compounds. Thermochim. Acta 2000, 343, 19–25. [Google Scholar] [CrossRef]
- Gutowska, A.; Li, L.; Shin, Y.; Wang, C.M.; Li, X.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew. Chem. Int. Ed. 2005, 44, 3578–3582. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, M.E.; Bradley, M.G.; Butterick, R.; Kusari, U.; Sneddon, L.G. Amineborane-based chemical hydrogen storage: Enhanced ammonia borane dehydrogenation in ionic liquids. J. Am. Chem. Soc. 2006, 128, 7748–7749. [Google Scholar] [CrossRef] [PubMed]
- Stephens, F.H.; Pons, V.; Baker, R.T. Ammonia-borane: The hydrogen source par excellence? Dalton Transactions 2007, 25, 2613–2626. [Google Scholar] [CrossRef] [PubMed]
- Proceedings of the 2002 U.S. DOE Hydrogen Program Review. Available online: http://www.eere.energy.gov/hydrogenandfuelcells/pdfs/32405b15.pdf (accessed on 17 April 2015).
- Chandra, M.; Xu, Q. Dissociation and hydrolysis of ammoniaborane with solid acids and carbon dioxide: An efficient hydrogen generation system. J. Power Sources 2006, 159, 855–860. [Google Scholar] [CrossRef]
- Xu, Q.; Chandra, M. Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia-borane at room temperature. J. Power Sources 2006, 163, 364–370. [Google Scholar] [CrossRef]
- Xu, Q.; Chandra, M. A portable hydrogen generation system: Catalytic hydrolysis of ammonia-borane. J. Alloys Compd. 2007, 446, 729–732. [Google Scholar] [CrossRef]
- Graham, T.W.; Tsang, C.W.; Chen, X.; Guo, R.; Jia, W.; Lu, S.M.; Sui-Sen, C.; Ewart, C.B.; Lough, A.; Amoroso, D.; et al. Catalytic solvolysis of ammonia borane. Angew. Chem. Int. Ed. 2010, 49, 8708–8711. [Google Scholar] [CrossRef] [PubMed]
- Fortman, G.C.; Slawin, A.M.Z.; Nolan, S.P. Highly active iridium(III)–NHC system for the catalytic B-N bond activation and subsequent solvolysis of ammonia-borane. Organometallics 2011, 30, 5487–5492. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.; Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef] [PubMed]
- Zahmakıran, M.; Durap, F.; Özkar, S. Zeolite confined copper(0) nanoclusters as cost-effective and reusable catalyst in hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2010, 35, 187–197. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A high performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Clark, T.J.; Whittell, G.R.; Manners, I. Highly efficient colloidal cobalt- and rhodium-catalyzed hydrolysis of H3N-BH3 in air. Inorg. Chem. 2007, 46, 7522–7527. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Ma, H.; Li, Y.; Chen, J. Ni1-xPtx (x = 0–0.12) hollow spheres as catalysts for hydrogen generation from ammonia borane. Inorg. Chem. 2007, 46, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Xu, Q. Room temperature hydrogen generation from aqueous ammonia-borane using noble metal nanoclusters as highly active catalysts. J. Power Sources 2007, 168, 135–142. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Gagare, P.D. Preparation of ammonia borane in high yield and purity, methanolysis, and regeneration. Inorg. Chem. 2007, 46, 7810–7817. [Google Scholar] [CrossRef] [PubMed]
- Metin, Ö.; Kayhan, E.; Özkar, S.; Schneider, J.J. Palladium nanoparticles supported on chemically derived graphene: An efficient and reusable catalyst for the dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2012, 37, 8161–8169. [Google Scholar]
- Yan, J.M.; Zang, X.B.; Han, S.; Shioyama, H.; Xu, Q. Iron nanoparticle catalyzed hydrolytic dehydrogenation of ammonia-borane for chemical hydrogen storage. Angew. Chem. Int. Ed. 2008, 47, 2287–2289. [Google Scholar] [CrossRef] [PubMed]
- Kalindindi, S.B.; Indirani, M.; Jagirdar, B.R. First row transition metal ion-assisted ammonia-borane hydrolysis for hydrogen generation. Inorg. Chem. 2008, 47, 7424–7429. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, T.; Yan, J.M.; Zhang, X.B.; Shioyama, H.; Kuriyama, N.; Xu, Q. Preparation and catalysis of poly(N-vinyl-2-pyrrolidone) (PVP) stabilized nickel catalyst for hydrolytic dehydrogeneration of ammonia borane. Int. J. Hydrogen Energy 2009, 34, 3816–3822. [Google Scholar] [CrossRef]
- Yao, C.F.; Zhuang, L.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Hydrogen release from hydrolysis of borazane on Pt- and Ni-based alloy catalysts. Int. J. Hydrogen Energy 2008, 33, 2462–2467. [Google Scholar] [CrossRef]
- Umegaki, T.; Yan, J.M.; Zhang, X.B.; Shioyama, H.; Kuriyama, N.; Xu, Q. Hollow Ni-SiO2 nanosphere-catalyzed hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. J. Power Sources 2009, 191, 209–216. [Google Scholar] [CrossRef]
- Durap, F.; Zahmakıran, M.; Özkar, S. Water soluble laurate stabilized ruthenium(0) nanoclusters catalyst for hydrogen generation from the hydrolysis of ammonia-borane: High activity and long life time. Int. J. Hydrogen Energy 2009, 34, 7223–7230. [Google Scholar] [CrossRef]
- Durap, F.; Zahmakıran, M.; Özkar, S. Water soluble laurate stabilized rhodium(0) nanoclusters catalyst: Unprecedented catalytic lifetime in the hydrolytic dehydrogenation of ammonia-borane. Appl. Catal. A. General 2009, 369, 53–59. [Google Scholar] [CrossRef]
- Zahmakıran, M.; Ayvalı, T.; Akbayrak, S.; Çalışkan, S.; Çelik, D.; Özkar, S. Zeolite framework stabilized nickel(0) nanoparticles: Active and long-lived catalyst for hydrogen generation from the hydrolysis of ammonia-borane and sodiumborohydride. Catalysis Today 2011, 170, 76–84. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Hydrogen generation from the hydrolysis of ammonia-borane using intrazeolite cobalt(0) nanoclusters catalyst. Int. J. Hydrogen Energy 2010, 35, 3341–3346. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Zeolite confined palladium(0) nanoclusters as effective and reusable catalyst for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2010, 35, 1305–1312. [Google Scholar] [CrossRef]
- Zahmakıran, M.; Özkar, S. Zeolite framework stabilized rhodium(0) nanoclusters catalyst for the hydrolysis of ammonia-borane in air: Outstanding catalytic activity, reusability and life time. Appl. Catal. B 2009, 89, 104–110. [Google Scholar] [CrossRef]
- Pool, R. Clusters: Strange morsels of matter: when metals or semiconductor are shrunk down to clumps only 10 or 100 atoms in size, they become a “totally new class of materials” with potentially valuable applications. Science 1990, 248, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, S.; Zahmakıran, M.; Durap, F.; Özkar, S. Hydrogen liberation from the hydrolytic dehydrogenation of dimethylamine-borane at room temperature by using a novel ruthenium nanocatalyst. Dalton Trans. 2012, 41, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Özkar, S.; Finke, R.G. Transition-metal nanocluster stabilization fundamental studies: Hydrogenphosphate as a simple, effective, readily available, robust and previously unappreciated stabilizer for well-formed, isolable, and redissolvable Ir(0) and other transition-metal nanoclusters. Langmuir 2003, 19, 6247–6260. [Google Scholar]
- Özkar, S.; Finke, R.G. Molecular insights for how preferred oxoanions bind to and stabilize transition-metal nanoclusters: A tridentate, C3 symmetry, lattice size-matching binding model. Coord. Chem. Rev. 2004, 248, 135–146. [Google Scholar]
- Metin, Ö.; Özkar, S. Water soluble nickel(0) and cobalt(0) nanoclusters stabilized by poly(4-styrenesulfonic acid-co-maleic acid): Highly active, durable and cost effective catalysts in hydrogen generation from the hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2011, 36, 1424–1432. [Google Scholar]
- Zahmakiran, M.; Özkar, S. Metal nanoparticles in liquid phase catalysis; from recent advances to future goals. Nanoscale 2011, 3, 3462–3481. [Google Scholar] [CrossRef] [PubMed]
- Can, H.; Metin, Ö. A facile synthesis of nearly monodisperse ruthenium nanoparticles and their catalysis in the hydrolytic dehydrogenation of ammonia borane for chemical hydrogen store. Appl. Cat. B Environ. 2012, 125, 304–310. [Google Scholar] [CrossRef]
- Liang, H.; Chen, G.; Desinan, S.; Rosei, R.; Rosei, F.; Ma, D. In situ facile synthesis of ruthenium nanocluster catalyst supported on carbon black for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2012, 37, 17921–17927. [Google Scholar] [CrossRef]
- Basu, S.; Brockman, A.; Gagore, P.; Zheng, Y.; Ramachandran, P.V.; Delgass, W.N. Chemical kinetics of Ru-catalyzed ammonia borane hydrolysis. J. Power Sources 2009, 188, 238–243. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durap, F.; Caliskan, S.; Özkar, S.; Karakas, K.; Zahmakiran, M. Dihydrogen Phosphate Stabilized Ruthenium(0) Nanoparticles: Efficient Nanocatalyst for The Hydrolysis of Ammonia-Borane at Room Temperature. Materials 2015, 8, 4226-4238. https://doi.org/10.3390/ma8074226
Durap F, Caliskan S, Özkar S, Karakas K, Zahmakiran M. Dihydrogen Phosphate Stabilized Ruthenium(0) Nanoparticles: Efficient Nanocatalyst for The Hydrolysis of Ammonia-Borane at Room Temperature. Materials. 2015; 8(7):4226-4238. https://doi.org/10.3390/ma8074226
Chicago/Turabian StyleDurap, Feyyaz, Salim Caliskan, Saim Özkar, Kadir Karakas, and Mehmet Zahmakiran. 2015. "Dihydrogen Phosphate Stabilized Ruthenium(0) Nanoparticles: Efficient Nanocatalyst for The Hydrolysis of Ammonia-Borane at Room Temperature" Materials 8, no. 7: 4226-4238. https://doi.org/10.3390/ma8074226
APA StyleDurap, F., Caliskan, S., Özkar, S., Karakas, K., & Zahmakiran, M. (2015). Dihydrogen Phosphate Stabilized Ruthenium(0) Nanoparticles: Efficient Nanocatalyst for The Hydrolysis of Ammonia-Borane at Room Temperature. Materials, 8(7), 4226-4238. https://doi.org/10.3390/ma8074226




