Quantitative Evaluation of Contamination on Dental Zirconia Ceramic by Silicone Disclosing Agents after Different Cleaning Procedures
Abstract
:1. Introduction
| Material | Company | Composition | Batch No. |
|---|---|---|---|
| IPS e.max ZirCad | Ivoclar Vivadent | ZrO2: 87%–95%, Y2O3: 4%–6%, HfO2: 1%–5%, Al2O3: 0%–0.5% | L48411 |
| GC Fit Checker white—Base | GC Corporation | Dimethyl methylhydrogenpolysiloxane: 65% CaCO3: 35% | 1302221 |
| GC Fit Checker white—Catalyst | GC Corporation | Petrolatum: 55%, Ethylsilicate: 40%, fatty acid salt: 5% | 1302221 |
| GC Fit Checker II Base | GC Corporation | SiO2: 40%–60%, Polydimethyl siloxane: 38%–42% | 1305151 |
| GC Fit Checker II Catalyst | GC Corporation | SiO2: 42%–45%, Vinyldimethylpolysiloxane: 50%–60% | 1305151 |
| Ivoclean | Ivoclar Vivadent | ZrO2: 10%–15%, H2O: 65%–80%, PEG: 8%–10%, NaOH: <1%, Pigments, Additives: 4%–5% | P66483 |
2. Results and Discussion
2.1. Results
2.2. Discussion
| Test Group | Zr | C | Si |
|---|---|---|---|
| REF a | 0.7723 ± 0.0207 | 0.2033 ± 0.0216 | 0.0234 ± 0.0057 |
| REF b | 0.7100 ± 0.0141 | 0.2683 ± 0.0147 | 0.0201 ± 0.0037 |
| REF c | 0.7350 ± 0.0152 | 0.2383 ± 0.0147 | 0.0243 ± 0.0024 |
| FC1 a | 0.5517 ± 0.0488 | 0.3900 ± 0.0522 | 0.0571 ± 0.0134 |
| FC1 b | 0.6633 ± 0.0163 | 0.2883 ± 0.0194 | 0.0514 ± 0.0071 |
| FC1 c | 0.6783 ± 0.0184 | 0.2800 ± 0.0141 | 0.0410 ± 0.0043 |
| FC2 a | 0.5117 ± 0.1277 | 0.4050 ± 0.1161 | 0.0826 ± 0.0334 |
| FC2 b | 0.6683 ± 0.0240 | 0.2867 ± 0.0216 | 0.0455 ± 0.0162 |
| FC2 c | 0.6367 ± 0.0513 | 0.3217 ± 0.0471 | 0.0411 ± 0.0099 |
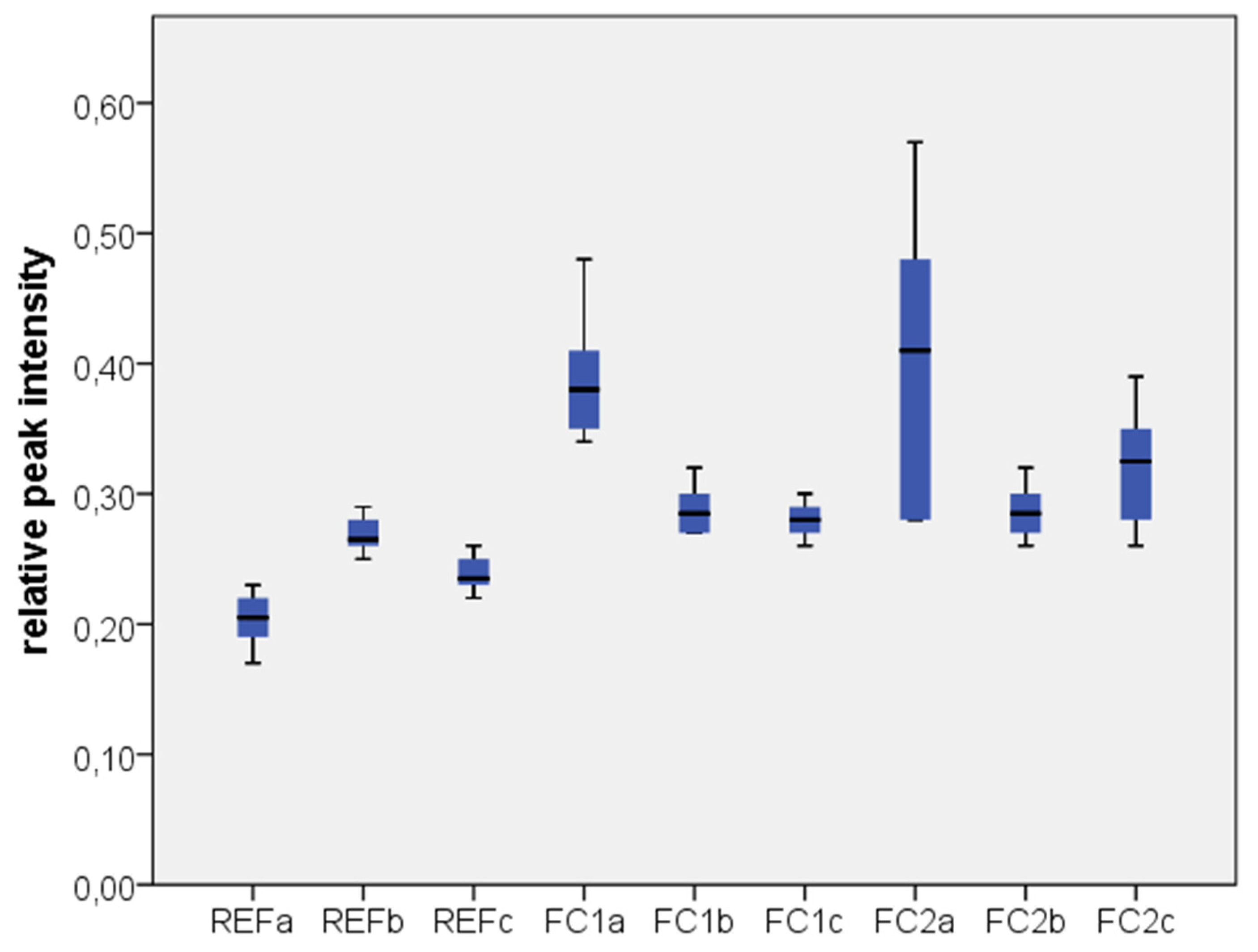
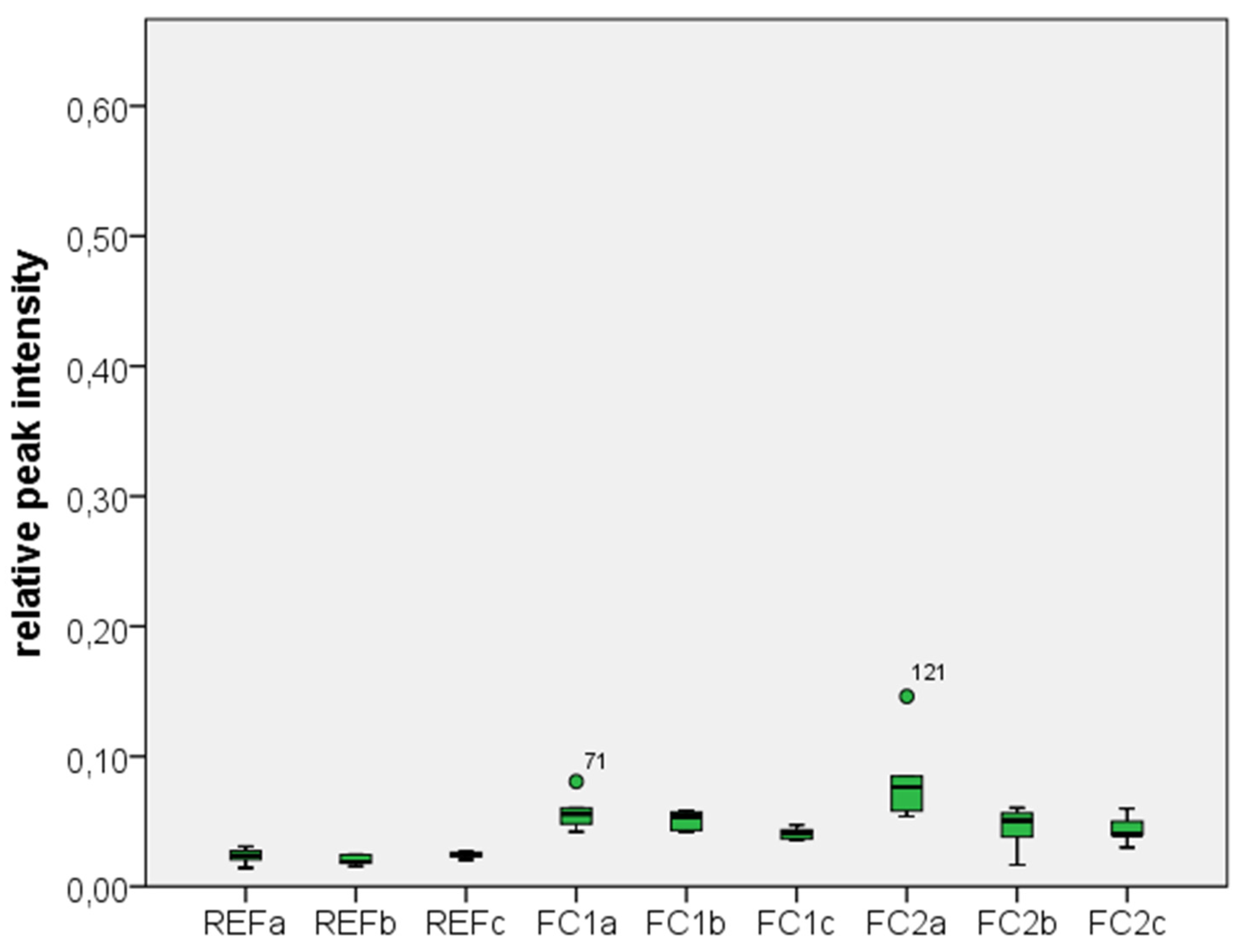
3. Experimental Section
3.1. Specimen Preparation
3.2. Cleaning Procedures
3.3. XPS Analysis
4. Conclusions
- There is no difference between the two investigated silicone disclosing agents concerning the cleanability, with respect to the investigated cleaning processes.
- Additional ultrasonic cleaning in 99% isopropanol will lead to a significant reduction of contamination.
- Alternative cleaning processes need to be developed in order to achieve a better removal of contaminants in order to achieve optimal bonding conditions.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blatz, M.B.; Sadan, A.; Kern, M. Resin-Ceramic Bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sasse, M.; Kern, M. CAD/CAM single retainer zirconia-ceramic resin-bonded fixed dental prostheses: Clinical outcome after 5 years. Int. J. Comput. Dent. 2013, 16, 109–118. [Google Scholar] [PubMed]
- Sasse, M.; Kern, M. Survival of anterior cantilevered all-ceramic resin-bonded fixed dental protheses made from zirconia ceramic. J. Dent. 2014, 42, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Wegner, S.M. Bonding to zirconia ceramic: Adhesion methods and their durability. Dent. Mater. 1998, 14, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.M.; Gerdes, W.; Kern, M. Effect of different artificial aging conditions on ceramic/composite bond strength. Int. J. Prosthodont. 2002, 15, 267–272. [Google Scholar] [PubMed]
- Kern, M. Resin bonding to oxide ceramics for dental restorations. J. Adhes. Sci. Technol. 2009, 23, 1097–1111. [Google Scholar] [CrossRef]
- Blatz, M.B.; Phark, J.H.; Ozer, F.; Mante, F.K.; Saleh, N.; Bergler, M.; Sadan, A. In vitro comparative bond strength of contemporary self-adhesive resin cements to zirconium oxide ceramic with and without air-particle abrasion. Clin. Oral. Investig. 2010, 14, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kern, M. Letter to the editor: Bond strength of luting cements to zirconium oxide ceramics. Int. J. Prosthodont. 2000, 13, 350. [Google Scholar] [PubMed]
- Wegner, S.M.; Kern, M. Long-term resin bond strength to zirconia ceramic. J. Adhes. Dent. 2000, 2, 139–145. [Google Scholar] [PubMed]
- Yang, B.; Scharnberg, M.; Wolfart, S.; Quaas, A.C.; Ludwig, K.; Adelung, R.; Kern, M. Influence of contamination on bonding to zirconia ceramic. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2007, 81, 283–290. [Google Scholar] [CrossRef]
- Sheth, J.; Jensen, M.; Tolliver, D. Effect of surface treatment on etched porcelain bond strength to enamel. Dent. Mater. 1988, 4, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.A. Improved seating of ceramic inlays with a silicone fit-checking medium. J. Prosthet. Dent. 1991, 65, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Szep, S.; Schmid, C.; Weigl, P.; Hahn, L.; Heidemann, D. Effect of the silicone disclosing procedure on the shear bond strength of composite cements to ceramic restorations. J. Prosthet. Dent. 2003, 89, 60–65. [Google Scholar] [CrossRef]
- Yang, B.; Wolfart, S.; Scharnberg, M.; Ludwig, K.; Adelung, R.; Kern, M. Influence of contamination on zirconia ceramic bonding. J. Dent. Res. 2007, 86, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.; Kern, M. Effect of cleaning methods after reduced-pressure air abrasion on bonding to zirconia ceramic. J. Adhes. Dent. 2011, 13, 561–567. [Google Scholar] [PubMed]
- Sato, H.; Yamada, K.; Pezzotti, G.; Nawa, M.; Ban, S. Mechanical properties of dental zirconia ceramics changed with sandblasting and heat treatment. Dent. Mater. J. 2008, 27, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Degrange, M. Shear bond strengths of self-adhesive luting resins fixing dentine to different restorative materials. J. Biomater. Sci. 2010, 21, 593–608. [Google Scholar] [CrossRef]
- Han, G.J.; Chung, S.N.; Chun, B.H.; Kim, C.K.; Oh, K.H.; Cho, B.H. Effect of the applied power of atmospheric pressure plasma on the adhesion of composite resin to dental ceramic. J. Adhes. Dent. 2013, 14, 461–469. [Google Scholar]
- Valverde, G.B.; Coelho, P.G.; Janal, M.N.; Lorenzoni, F.C.; Carvalho, R.M.; Thompson, V.P.; Weltemann, K.D.; Silva, N.R. Surface characterisation and bonding of Y-TZP following non-thermal plasma treatment. J. Dent. 2013, 41, 51–59. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wille, S.; Van Broeck, V.; Strunskus, T.; Faupel, F.; Kern, M. Quantitative Evaluation of Contamination on Dental Zirconia Ceramic by Silicone Disclosing Agents after Different Cleaning Procedures. Materials 2015, 8, 2650-2657. https://doi.org/10.3390/ma8052650
Wille S, Van Broeck V, Strunskus T, Faupel F, Kern M. Quantitative Evaluation of Contamination on Dental Zirconia Ceramic by Silicone Disclosing Agents after Different Cleaning Procedures. Materials. 2015; 8(5):2650-2657. https://doi.org/10.3390/ma8052650
Chicago/Turabian StyleWille, Sebastian, Vincent Van Broeck, Thomas Strunskus, Franz Faupel, and Matthias Kern. 2015. "Quantitative Evaluation of Contamination on Dental Zirconia Ceramic by Silicone Disclosing Agents after Different Cleaning Procedures" Materials 8, no. 5: 2650-2657. https://doi.org/10.3390/ma8052650
APA StyleWille, S., Van Broeck, V., Strunskus, T., Faupel, F., & Kern, M. (2015). Quantitative Evaluation of Contamination on Dental Zirconia Ceramic by Silicone Disclosing Agents after Different Cleaning Procedures. Materials, 8(5), 2650-2657. https://doi.org/10.3390/ma8052650









