“Low Cost” Pore Expanded SBA-15 Functionalized with Amine Groups Applied to CO2 Adsorption
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Siliceous Porous Structures
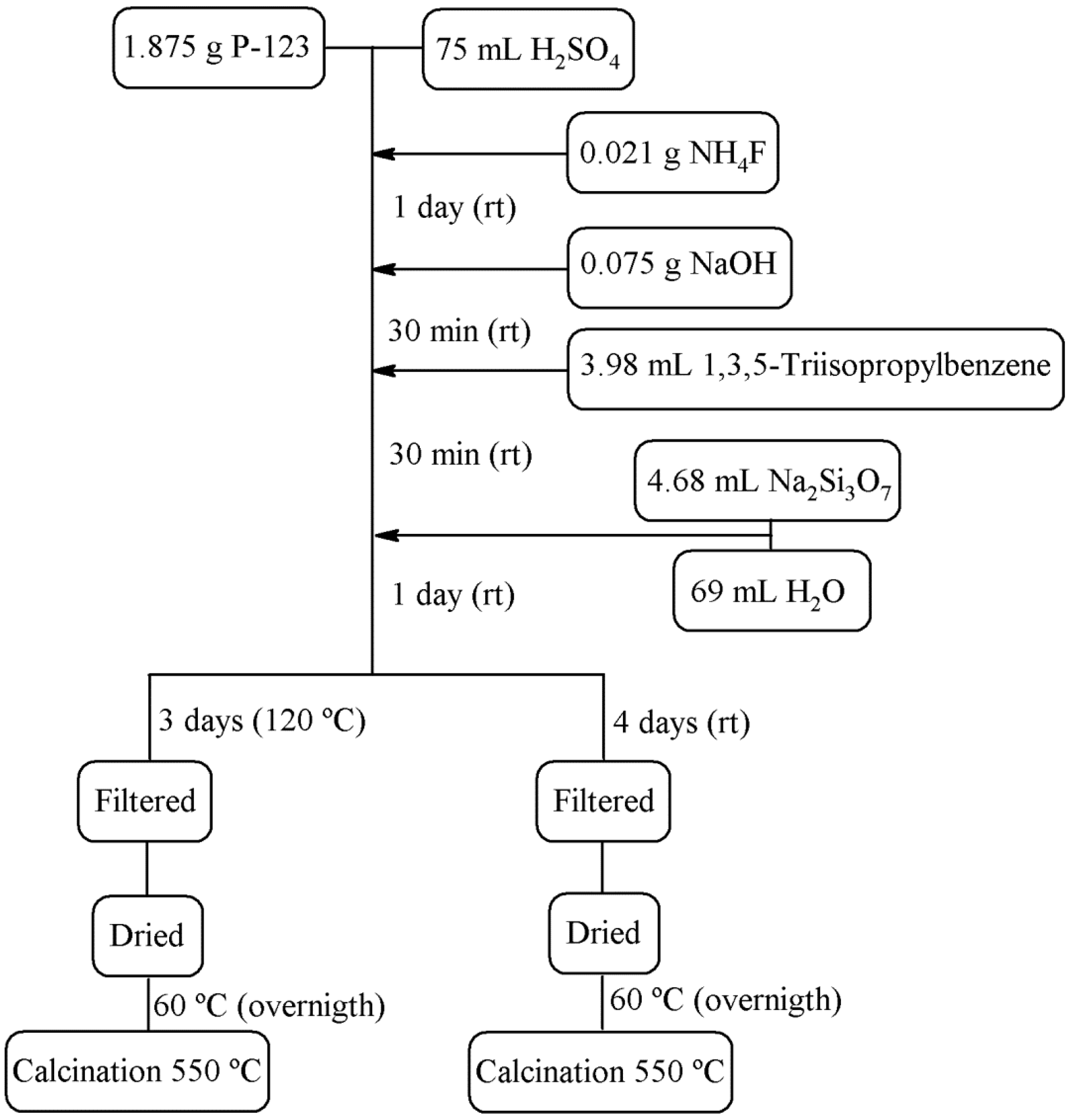
| Sample | Description | P123/SiO2/H2SO4/NaOH/NH4F/TIPB/H2O |
|---|---|---|
| RT | Room temperature SBA15 | 1/189/93/5.8/0/0/4447 |
| RT-F | Room temperature porous silica modified with fluoride | 1/189/93/5.8/1.7/0/4447 |
| HT | Hydrothermal SBA15 | 1/189/93/5.8/0/0/4447 |
| HT-F | Hydrothermal porous silica modified with fluoride | 1/189/93/5.8/1.7/0/4447 |
| TiPB | Hydrothermal SBA-15 modified with pore expander (TiPB) | 1/189/93/5.8/0/49/4447 |
| TiPB-F | Hydrothermal porous silica modified with pore expander (TIPB) | 1/189/93/5.8/1.7/49/4447 |
2.3. Characterization Techniques
2.4. CO2 Adsorption Tests
3. Results and Discussion
3.1. Characterization
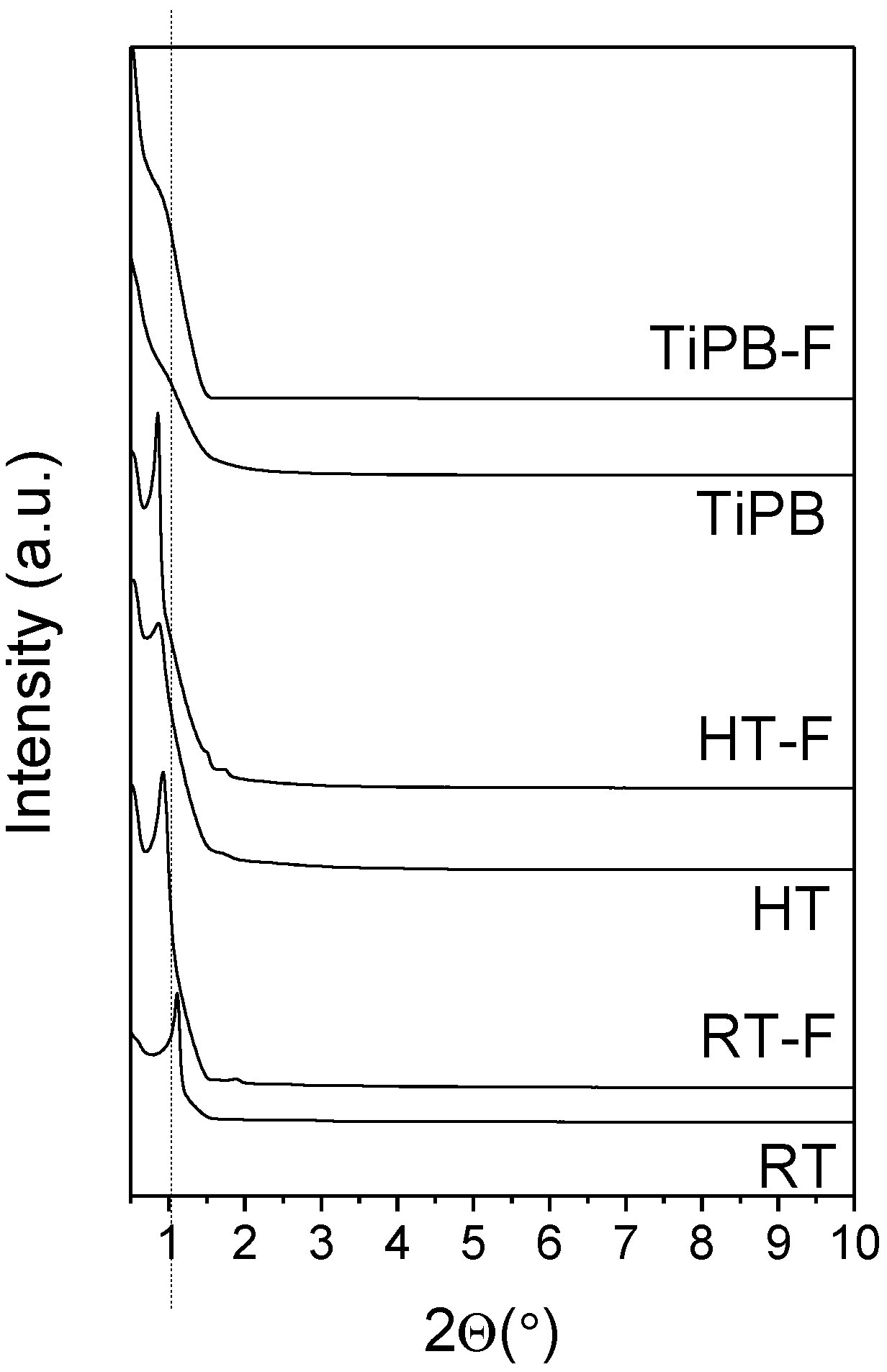
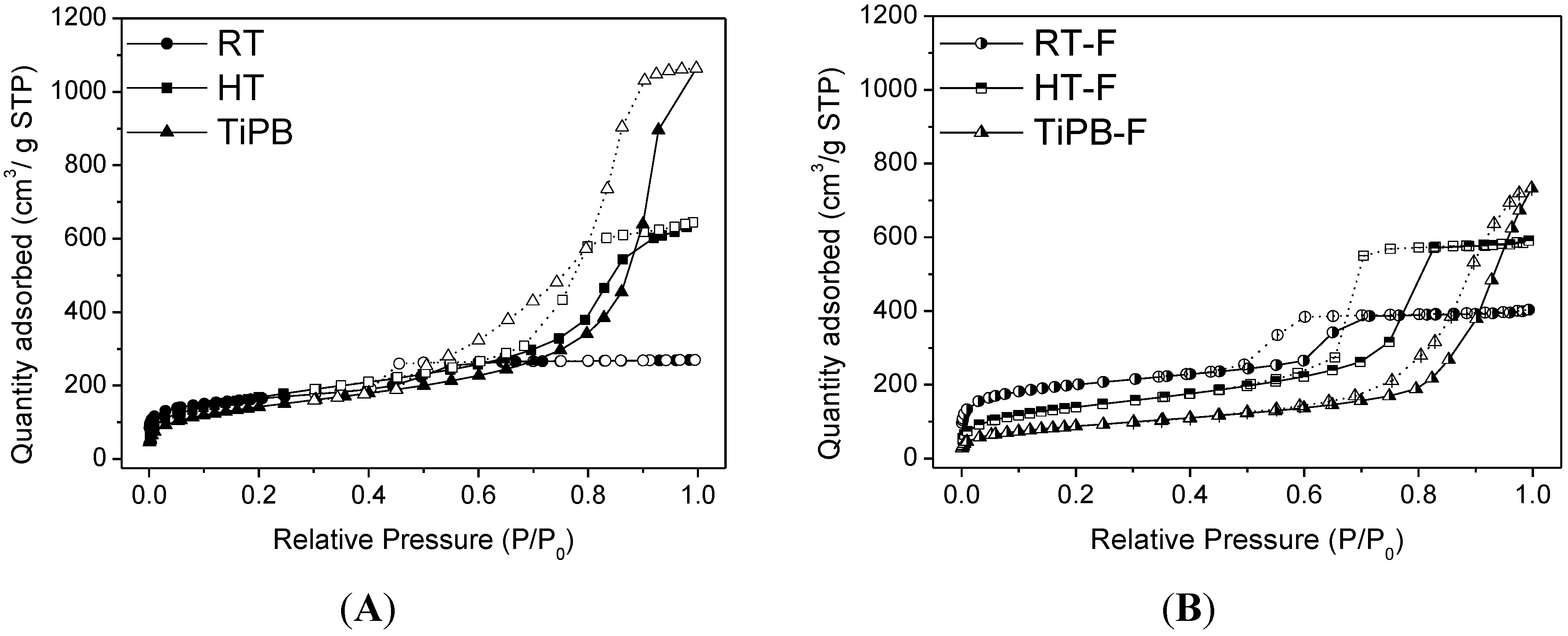
| Sample | SBET (m2/g) | Smp t.plot (m2/g) | VP (cm3/g) | Vmp t-plot (cm3/g) | DP (nm) |
|---|---|---|---|---|---|
| RT | 571 | 256 | 0.42 | 0.12 | 3.6 |
| RT-F | 699 | 385 | 0.62 | 0.17 | 4.6 |
| HT | 613 | 148 | 0.99 | 0.06 | 7.5 |
| HT-F | 508 | 74 | 0.91 | 0.03 | 6.3 |
| TiPB | 519 | 74 | 1.64 | 0.02 | 7.9 |
| TiPB-F | 321 | 75 | 1.13 | 0.02 | 13.2 |
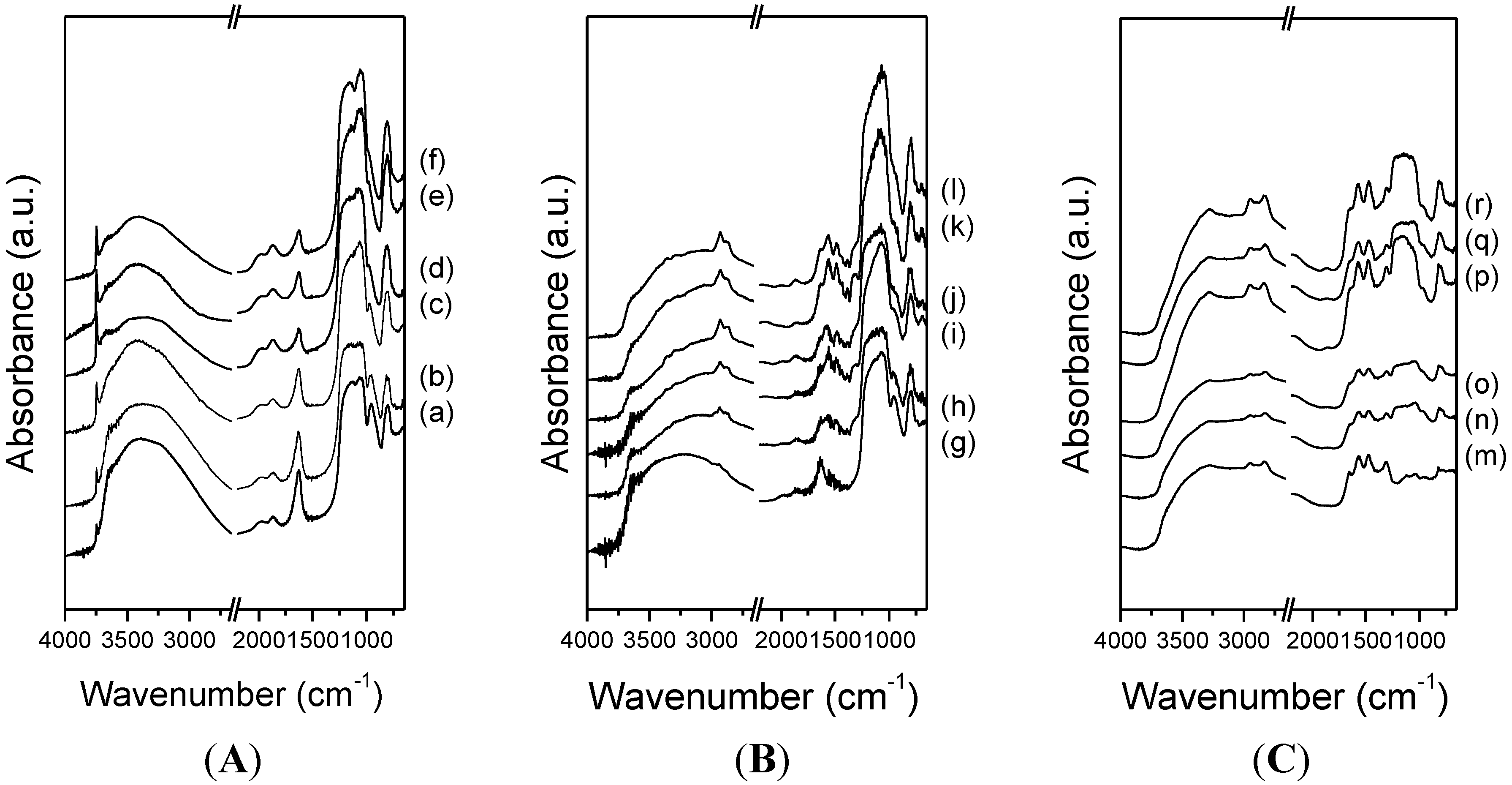
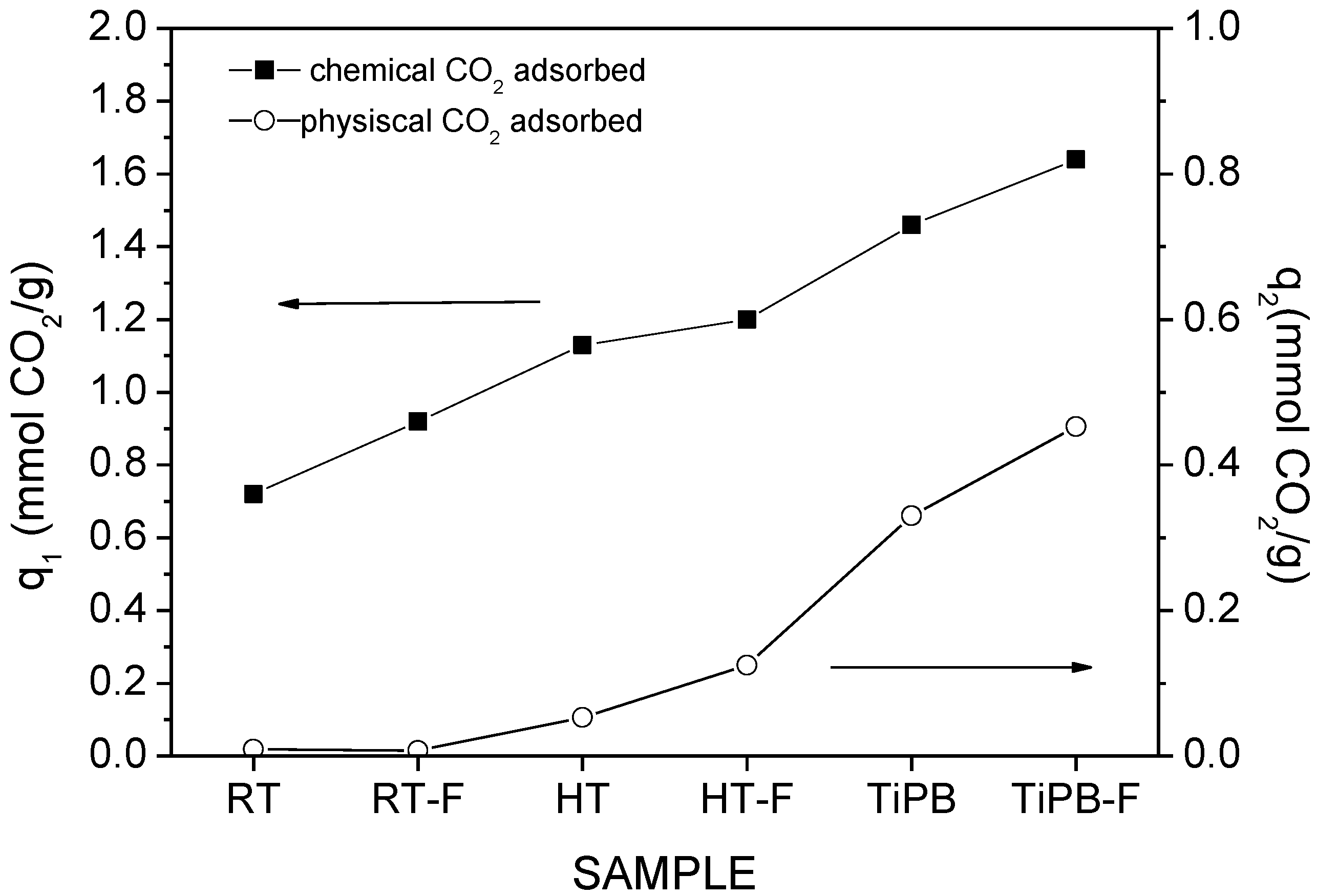
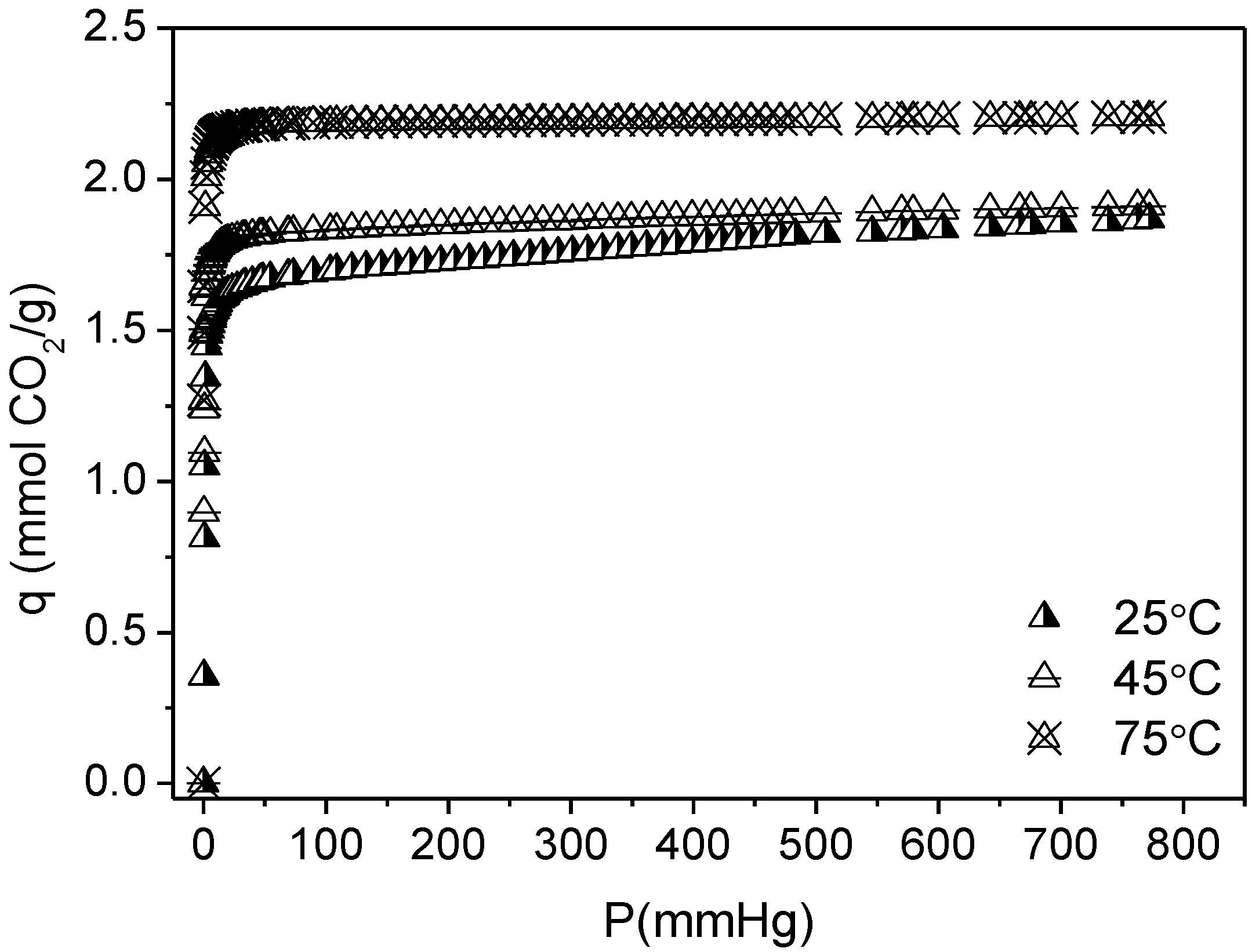
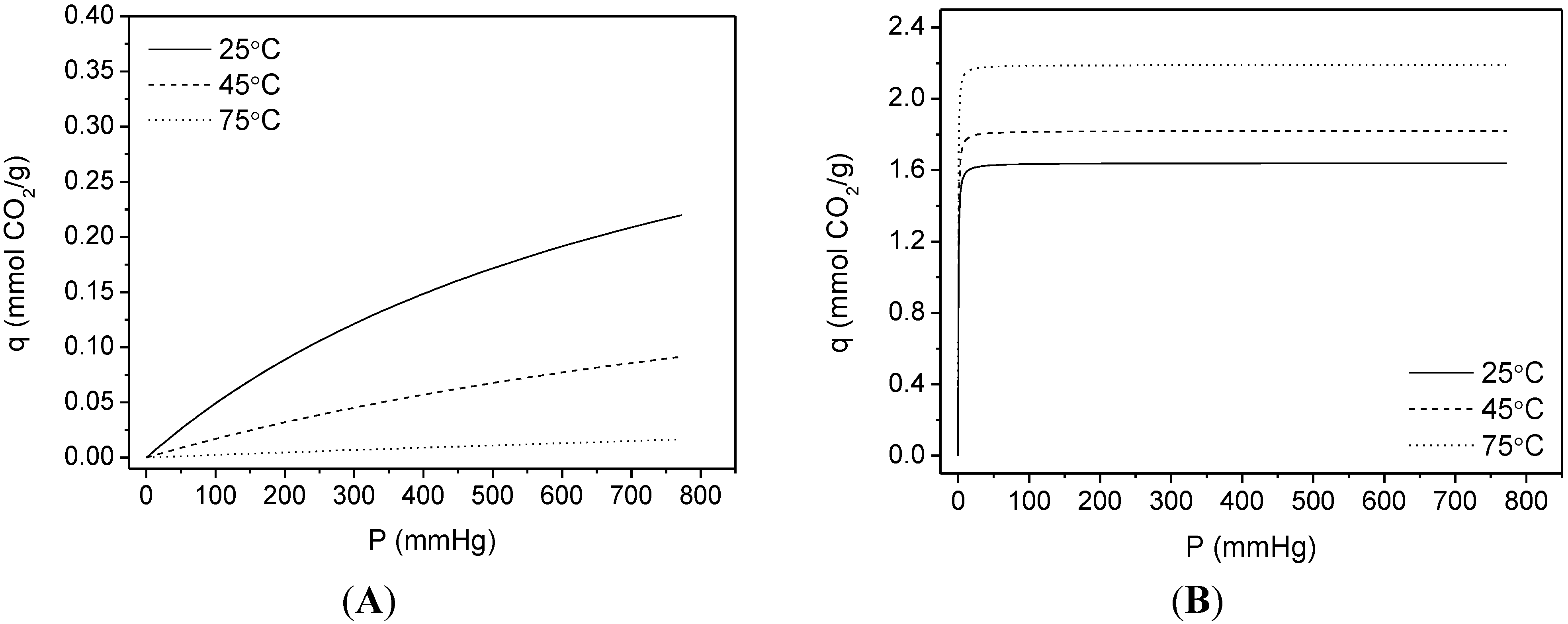
3.2. CO2 Adsorption
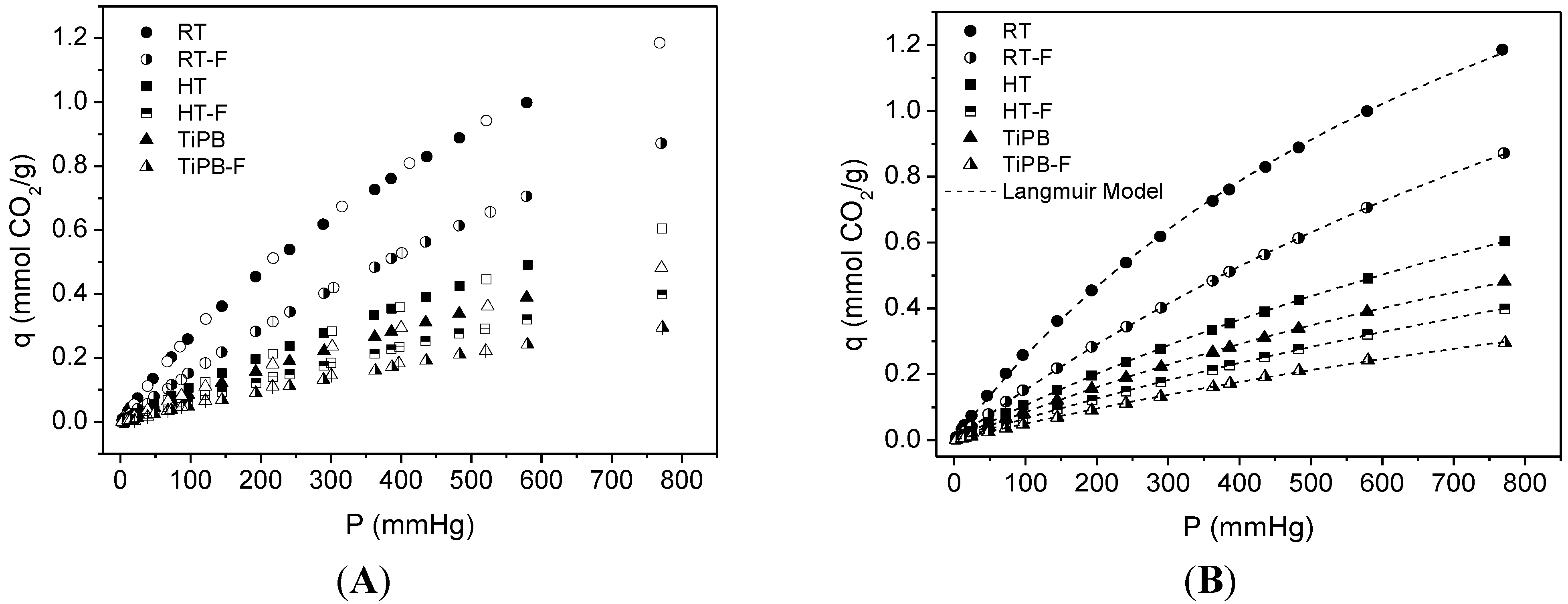
| Sample | Langmuir Model | q (25 °C, 1 bar) | |
|---|---|---|---|
| q (mmol CO2/g) | K (mmHg−1) | ||
| RT | 2.53 ± 0.05 | 0.0011 ± 3.4 × 10−4 | 1.19 |
| RT-F | 2.88 ± 0.04 | 5.61 × 10−4 ± 1.03 × 10−5 | 0.87 |
| HT | 1.99 ± 0.03 | 5.59 × 10−4 ± 1.23 × 10−5 | 0.61 |
| HT-F | 1.69 ± 0.04 | 4.08 × 10−4 ± 1.06 × 10−5 | 0.39 |
| TiPB | 1.58 ± 0.03 | 5.66 × 10−4 ± 1.59 × 10−5 | 0.48 |
| TiPB-F | 1.16 ± 0.06 | 4.47 × 10−4 ± 2.73 × 10−5 | 0.29 |
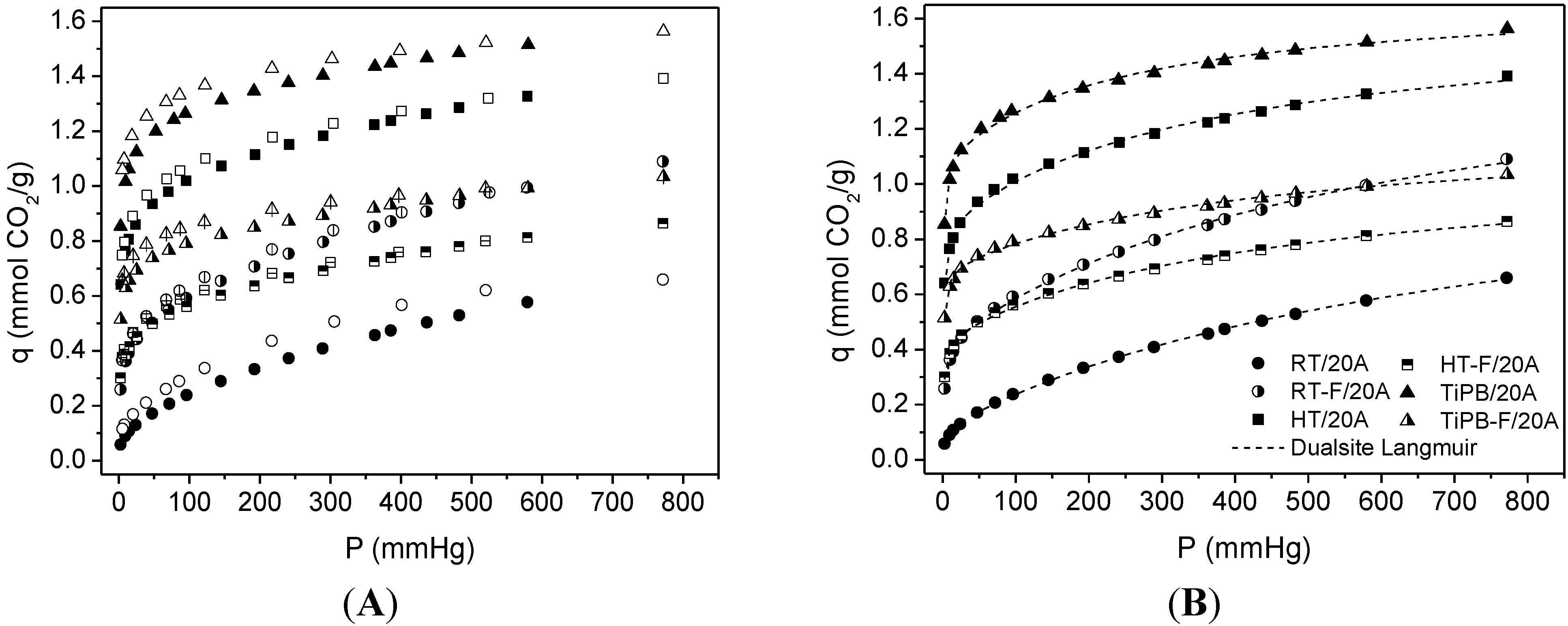
| Sample | SBET | %N | %APTES | Dualsite Langmuir model | q* | |||
|---|---|---|---|---|---|---|---|---|
| q1 | K1 | q2 | K2 | |||||
| RT | 22 | 1.37 | 144 | 0.12 ± 0.01 | 0.247 ± 0.045 | 1.07 ± 0.03 | 1.31 × 10−3 ± 9.95 × 10−4 | 0.66 |
| RT-F | 102 | 2.85 | 65 | 0.43 ± 0.01 | 0.538 ± 0.071 | 1.17 ± 0.06 | 1.65 × 10−3 ± 1.82 × 10−4 | 1.09 |
| HT | 245 | 3.36 | 32 | 0.85 ± 0.01 | 0.984 ± 0.111 | 0.77 ± 0.04 | 2.77 × 10−3 ± 3.89 × 10−4 | 1.39 |
| HT-F | 260 | 2.41 | 21 | 0.44 ± 0.01 | 0.727 ± 0.079 | 0.66 ± 0.03 | 2.25 × 10−3 ± 2.61 × 10−4 | 0.86 |
| TiPB | 135 | 3.89 | 67 | 1.09 ± 0.02 | 1.449 ± 0.148 | 0.59 ± 0.02 | 4.16 × 10−3 ± 6.22 × 10−4 | 1.56 |
| TiPB-F | 198 | 2.70 | 32 | 0.69 ± 0.01 | 1.107 ± 0.099 | 0.52 ± 0.03 | 2.30 × 10−3 ± 3.55 × 10−4 | 1.03 |
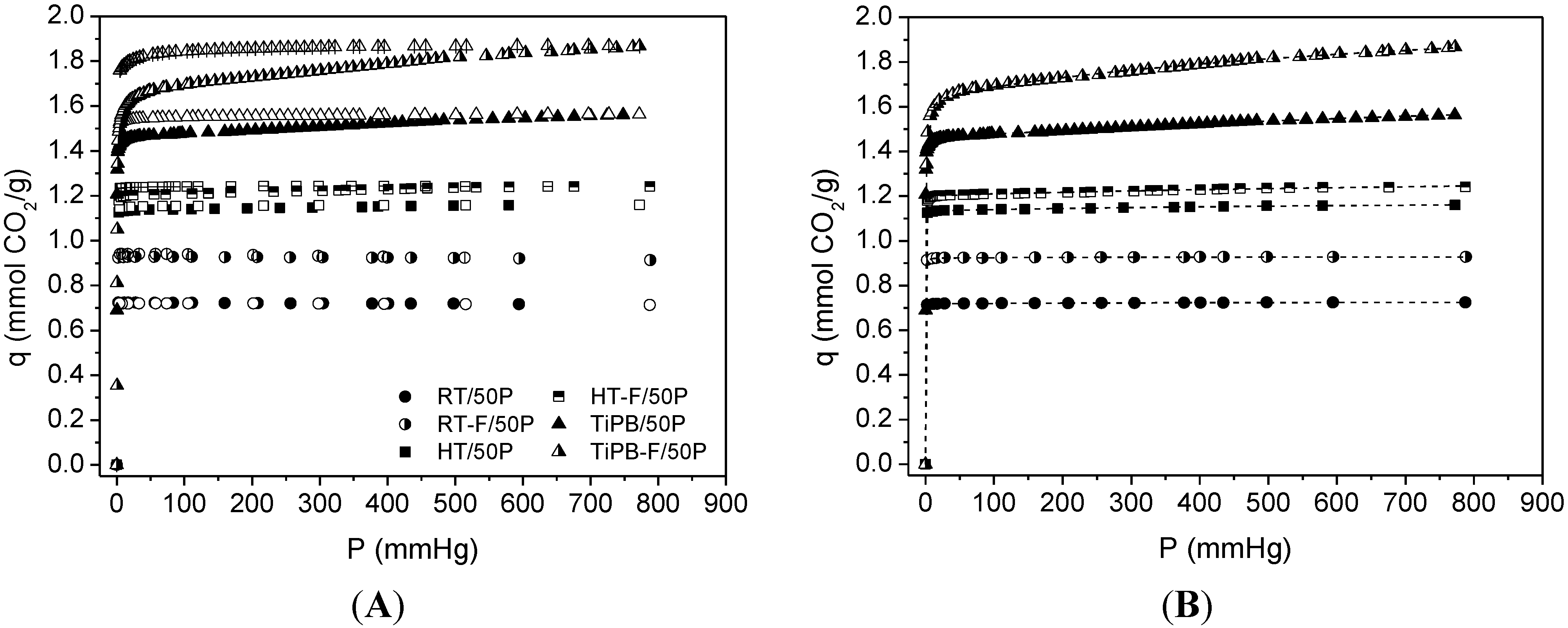
| Sample | SBET | %N | Dualsite Langmuir model | q* | |||
|---|---|---|---|---|---|---|---|
| q1 | K1 | q2 | K2 | ||||
| RT | 40 | 15.81 | 0.72 ± 0.01 | 56.051 ± 5.095 | 9.21 × 10−3 ± 1.13 × 10−3 | 2.31 × 10−3 ± 7.40 × 10−4 | 0.72 |
| RT-F | 81 | 15.07 | 0.92 ± 0.01 | 35.113 ± 2.874 | 7.29 × 10−3 ± 1.91 × 10−3 | 2.33 × 10−3 ± 1.58 × 10−3 | 0.93 |
| HT | 103 | 15.23 | 1.13 ± 0.01 | 42.840 ± 5.312 | 0.05 ± 0.01 | 1.32 × 10−3 ± 2.64 × 10−4 | 1.16 |
| HT-F | 101 | 14.18 | 1.20 ± 0.01 | 19.234 ± 1.299 | 0.12 ± 0.02 | 6.53 × 10−4 ± 1.51 × 10−4 | 1.24 |
| TiPB | 146 | 13.62 | 1.46 ± 0.01 | 11.783 ± 0.028 | 0.33 ± 0.03 | 5.95 × 10−4 ± 7.19 × 10−5 | 1.56 |
| TiPB-F | 171 | 14.63 | 1.64 ± 0.01 | 2.795 ± 0.069 | 0.45 ± 0.03 | 1.12 × 10−4 ± 1.35 × 10−5 | 1.87 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change: The Scientific Basis, Intergovernmental Panel on Climate Change; The Press Syndicate of the University of Cambridge: Cambridge, UK, 2001. [Google Scholar]
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, S.J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Song, C.-F.; Kitamura, Y.; Li, S.-H. Evaluation of Stirling cooler system for cryogenic CO2 capture. Appl. Energ. 2012, 98, 491–501. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.R. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Favre, E. Membrane processes and postcombustion carbon dioxide capture: Challenges and prospects. Chem. Eng. J. 2011, 171, 782–793. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications. A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Oh, T.H. Carbon capture and storage potential in coal-fired plant in Malaysia: A review. Renew. Sustain. Energy Rev. 2010, 14, 2697–2709. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, R.; Webley, P.A. Alkali and alkaline-earth cation exchanged chabazite zeolites for adsorption based CO2 capture. Micropor. Mesopor. Mater. 2008, 111, 478–487. [Google Scholar] [CrossRef]
- Walton, K.S.; Abney, M.B.; LeVan, M.D. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Micropor. Mesopor. Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Adsorption equilibrium of methane, carbon dioxide and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Bai, B.C.; Cho, S.; Yu, H.-R.; Yi, K.-B.; Kim, K.-D.; Lee, Y.-S. Effects of aminated carbon molecular sieves on breakthrough curve behavior in CO2/CH4 separation. J. Ind. Eng. Chem. 2013, 13, 776–783. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Yao, K.-X.; Yang, Y.; Zhang, Q.; Han, Y. Novel porous carbon materials with ultrahigh nitrogen contents for selective CO2 capture. J. Mater. Chem. 2012, 22, 19726–19731. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J. Colloid Interf. Sci. 2011, 353, 549–556. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal−Organic Frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. CO2 adsorption on branched polyethyleneimine-impregnated mesoporous silica SBA-15. Appl. Surf. Sci. 2010, 256, 5323–5328. [Google Scholar] [CrossRef]
- Khatri, R.A.; Chuang, S.S.C.; Soong, Y.; Gray, M. Thermal and chemical stability of regenerable solid amine sorbent for CO2 capture. Energy Fuels 2006, 20, 1516–1520. [Google Scholar] [CrossRef]
- Yue, M.B.; Chun, Y.; Cao, Y.; Dong, X.; Zhu, J.H. CO2 capture by as-prepared SBA15 with an occluded organic template. Adv. Funct. Mater. 2006, 16, 1717–1722. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption characteristics of carbón dioxide on organically functionalized SBA-15. Micropor. Mesopor. Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
- Zelenak, V.; Badanicova, M.; Malanova, D.; Cejka, J.; Zukai, A.; Murafa, N.; Goerigk, G. Amine-modified ordered mesoporous silica. Effect of pore size on carbon dioxide capture. Chem. Eng. J. 2008, 144, 336–342. [Google Scholar] [CrossRef]
- Srikanth, C.S.; Chuang, S.S.C. Infrared study of strongly and weakly adsorbed CO2 on fresh and oxidatively degraded amine sorbents. J. Phys. Chem. C 2013, 117, 9196–9205. [Google Scholar] [CrossRef]
- Tumuluri, U.; Isenberg, M.; Tan, C.-S.; Chuang, S.C. In situ infrared study of the effect of amine density on the nature of adsorbed CO2 on amine-functionalized solid sorbents. Langmuir 2014, 30, 7405–7413. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa-García, E.; Ortigosa Moya, E.M.; Cecilia, J.A.; Cavalcante, C.L., Jr.; Jiménez-Jiménez, J.; Azevedo, D.C.S.; Rodríguez-Castellón, E. CO2 adsorption on amine modified mesoporous silicas: Effect of the progressive disorder of the honeycomb arrangement. Micropor. Mesopor. Mater. 2015, 209, 172–183. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Santos, S.M.L.; Cavalcante, C.L., Jr.; Jiménez-Jiménez, J.; Azevedo, D.C.S.; Rodríguez-Castellón, E. CO2 adsorption on APTES functionalized mesocellular foams obtained from mesoporosu silicas. Micropor. Mesopor. Mater. 2014, 187, 125–134. [Google Scholar] [CrossRef]
- Harlick, P.J.E.; Sayari, A. Applications of pore-expanded mesoporous silica. Triamine grafted material with exceptional CO2 dynamic and equilibrium adsorption performance. Ind. Eng. Chem. Res. 2007, 46, 446–458. [Google Scholar] [CrossRef]
- Mello, M.R.; Phanon, D.; Silveira, G.Q.; Llewellyn, P.L.; Ronconi, C.M. Amine-modified MCM-41 mesoporous silica for carbon dioxide capture. Micropor. Mesopor. Mater. 2011, 143, 174–179. [Google Scholar] [CrossRef]
- Builes, S.; Vega, L.F. Understanding CO2 capture in amine-functionalized MCM-41 by molecular simulation. J. Phys. Chem. C 2012, 116, 3017–3024. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Yang, Y.; Sayari, A. Effect of the pore length on CO2 adsorption over amine-modified mesoporous silicas. Energy Fuels 2011, 25, 4206–4216. [Google Scholar] [CrossRef]
- Feng, X.; Hu, G.; Hu, X.; Xie, G.; Xie, Y.; Lu, J.; Luo, M. Tetraethylenepentamine-modified siliceious mesocellular foam (MCF) CO2 capture. Ind. Eng. Chem. Res. 2013, 52, 4221–4228. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Pikus, S.; Jaroniec, M. Short-time synthesis of SBA-15 using various silica sources. J. Colloid Interf. Sci. 2005, 287, 717–720. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Ma, D.; Bao, X.; Klein-Hoffmann, A.; Weinberg, G.; Su, D.; Schlögl, R. Unusual mesoporous SBA-15 with parallel channels running along the short axis. J. Am. Chem. Soc. 2004, 126, 7440–7441. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Stucky, G.D. Synthesis of highly ordered mesoporous silica materials using sodium silicate and amphiphilic block copolymers. Chem. Commun. 2000, 13, 1159–1160. [Google Scholar] [CrossRef]
- Kassab, H.; Maksoud, M.; Aguado, S.; Pera-Titus, M.; Albela, B.; Bonneviot, L. Polyethylenimine covalently grafted on mesostructured porous silica for CO2 capture. RSC Adv. 2012, 2, 2508–2516. [Google Scholar] [CrossRef]
- Gómez-Cazalilla, M.; Mérida-Robles, J.M.; Gurbani, A.; Rodríguez-Castellón, E.; Jiménez-López, A. Characterization and acidic properties of Al-SBA-15 materials prepared by post-synthesis alumination of a low-cost ordered mesoporous silica. J. Solid State Chem. 2007, 180, 1130–1140. [Google Scholar] [CrossRef]
- De Boer, J.H.; Lippens, B.C.; Linsen, J.C.P.; Broekhoff, J.C.P.; van den Heuvel, A.; Osinga, T.J. The t-curve of multimolecular N2-adsorption. J. Colloid Interf. Sci. 1966, 21, 405–414. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer synthesis of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Winkel, P.; Lukens, W.W., Jr.; Zhao, D.; Yang, P.; Chmelka, B.F.; Stucky, G.D. Mesocellular siliceous foams with uniformly sized cells and windows. J. Am. Chem. Soc. 1999, 121, 254–255. [Google Scholar] [CrossRef]
- Lettow, J.S.; Han, Y.J.; Schmidt-Winkel, P.; Yang, P.; Zhao, D.; Stucky, G.D.; Ying, J.Y. Hexagonal to mesocellular foam phase transition in polymer-templated mesoporous silicas. Langmuir 2000, 16, 8291–8295. [Google Scholar] [CrossRef]
- Miyazawa, K.; Inagaki, S. Control of the microporosity within the pore walls of ordered mesoporous silica SBA-15. Chem. Commun. 2000, 2121–2122. [Google Scholar] [CrossRef]
- Imperor-Clerc, M.; Davidson, P.; Davidson, A. Existence of a microporous corona around the mesopores of silica-based SBA-15 materials templated by triblock copolymers. J. Am. Chem. Soc. 2000, 122, 11925–11933. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; di Renzo, F.; Fajula, F. True microporosity and surface area of mesoporous SBA-15 silicas as a function of synthesis temperature. Langmuir 2001, 17, 8328–8335. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of micro- and mesoporosity in SBA-15 materials from adsorption data by the NLDFT method. J. Phys. Chem. B 2001, 105, 6817–6823. [Google Scholar] [CrossRef]
- Luan, Z.; Fournier, J.A.; Wooten, J.B.; Miser, D.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-modified mesoporous SBA-15 silica molecular sieves. Microp. Mesop. Mater. 2005, 83, 150–158. [Google Scholar] [CrossRef]
- Tanev, P.T.; Vlaev, L.T. An attempt at a more precise evaluation of the approach to mesopore size distribution calculations depending on the degree of pore blocking. J. Colloid Interface Sci. 1993, 160, 110–116. [Google Scholar] [CrossRef]
- Shen, S.C.; Kawi, S. Understanding of the effect of Al substitution on the hydrothermal stability of MCM-41. J. Phys. Chem. B 1999, 103, 8870–8876. [Google Scholar] [CrossRef]
- Kosslick, H.; Landmesser, H.; Fricke, R. Acidity of substituted MCM-41-type mesoporous silicates probed by ammonia. J. Chem. Soc., Faraday Trans. 1997, 93, 1849–1854. [Google Scholar] [CrossRef]
- Wang, X.; Schwartz, V.; Clark, J.C.; Ma, X.; Overbury, S.H.; Xu, X.; Song, C. Infrared study of CO2 sorption over “Molecular Basket” sorbent consisting of polyethylenimine-modified mesoporous molecular sieve. J. Phys. Chem. C 2009, 113, 7260–7268. [Google Scholar] [CrossRef]
- Wang, X.G.; Lin, K.S.K.; Chen, J.C.C.; Cheng, S. Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials. J. Phys. Chem. B 2005, 109, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ruan, J.F.; Li, Y.S.; Terasaki, O.; Che, S.A. Synthesis and characterization of the amphoteric amino acid bifunctional mesoporous silica. Chem. Mater. 2007, 19, 2860–2867. [Google Scholar] [CrossRef]
- Heydary-Gorji, A.; Belmabkhout, Y.; Sayari, A. Polyethylenimine-impregnated mesoporous silica: Effect of amine loading and surface alkyl chains on CO2 adsorption. Langmuir 2011, 27, 12411–12416. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa-García, E.; Cecilia, J.A.; Cavalcante, C.L., Jr.; Jiménez-Jiménez, J.; Azevedo, D.C.S.; Rodríguez-Castellón, E. Adsorción de CO2 y separación CO2/CH4 en SBA-15 de poro expandido modificada con Ti y funcionalizada con APTES. Mater. Adsorc. Catál. 2014, 7, 30–47. (In Spanish) [Google Scholar]
- Chang, F.Y.; Chao, K.J.; Cheng, H.H. Adsorption of CO2 onto amine-grafted mesoporous Silicas. Sep. Pur. Technol. 2009, 70, 87–95. [Google Scholar] [CrossRef]
- Shenderovich, L.G.; Buntkwosky, G.; Schreiber, A.; Gedat, E.; Sharif, S.; Albrecht, J.; Golubev, N.S. Pyridine-15N a mobile NMR sensor for surface acidity and surface defects of mesoporous silica. J. Phys. Chem. B 2003, 107, 11924–11939. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J. Phys. Chem. C 2011, 115, 21264–21272. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of amine-containing CO2 adsorbents: Dramatic effect of water vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef] [PubMed]
- Knofel, C.; Martin, C.; Hornebecq, V.; Llewellyn, P.L. Study of carbon dioxide adsorption on mesoporous aminopropylsilane-functionalized silica and Titania combining microcalorimetry and in situ infrared spectroscopy. J. Phys. Chem. C 2009, 113, 21726–21734. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Bermúdez, J.M.; Dominguez, P.H.; Arenillas, A.; Cot, J.; Weber, J.; Luque, R. CO2 separation and capture properties of porous carbonaceous materials from leather residues. Materials 2013, 6, 4641–4653. [Google Scholar] [CrossRef]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Pennline, H.; Baltrus, J.P.; Stevens, R.W. Improved immobilized carbon dioxide capture sorbents. Fuel Process Technol. 2005, 86, 1449–1455. [Google Scholar] [CrossRef]
- Xu, X.C.; Song, C.S.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Micropor. Mesopor. Mater. 2003, 62, 29–45. [Google Scholar] [CrossRef]
- Sun, N.; Tang, Z.; Wei, W.; Snape, C.E.; Sun, Y. Solid adsorbents for low temperature CO2 capture with low energy penalties leading to more effective integrated solutions for power generation and industrial processes. Front. Energy Res. 2015. [Google Scholar] [CrossRef]
- Wang, X.X.; Song, C.S. Temperature-programmed desorption of CO2 from polyethylenimine-loaded SBA-15 as molecular basket sorbents. Catal. Today 2012, 194, 44–52. [Google Scholar] [CrossRef]
- Chen, Z.H.; Deng, S.B.; Wei, H.R.; Wang, B.; Huang, J.; Yu, G. Polyethylenimine-impregnated resin for high CO2 adsorption: An efficient adsorbent for CO2 capture from simulated flue gas and ambient air. ACS Appl. Mater. Inter. 2013, 5, 6937–6945. [Google Scholar] [CrossRef]
- Yang, R.T. Gas Separation by Adsorption Process; Imperial College Press: London, UK, 1997; Volume 1. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilarrasa-García, E.; Cecilia, J.A.; Moya, E.M.O.; Cavalcante, C.L., Jr.; Azevedo, D.C.S.; Rodríguez-Castellón, E. “Low Cost” Pore Expanded SBA-15 Functionalized with Amine Groups Applied to CO2 Adsorption. Materials 2015, 8, 2495-2513. https://doi.org/10.3390/ma8052495
Vilarrasa-García E, Cecilia JA, Moya EMO, Cavalcante CL Jr., Azevedo DCS, Rodríguez-Castellón E. “Low Cost” Pore Expanded SBA-15 Functionalized with Amine Groups Applied to CO2 Adsorption. Materials. 2015; 8(5):2495-2513. https://doi.org/10.3390/ma8052495
Chicago/Turabian StyleVilarrasa-García, Enrique, Juan Antonio Cecilia, Elisa Maria Ortigosa Moya, Celio Loureiro Cavalcante, Jr., Diana Cristina Silva Azevedo, and Enrique Rodríguez-Castellón. 2015. "“Low Cost” Pore Expanded SBA-15 Functionalized with Amine Groups Applied to CO2 Adsorption" Materials 8, no. 5: 2495-2513. https://doi.org/10.3390/ma8052495
APA StyleVilarrasa-García, E., Cecilia, J. A., Moya, E. M. O., Cavalcante, C. L., Jr., Azevedo, D. C. S., & Rodríguez-Castellón, E. (2015). “Low Cost” Pore Expanded SBA-15 Functionalized with Amine Groups Applied to CO2 Adsorption. Materials, 8(5), 2495-2513. https://doi.org/10.3390/ma8052495










