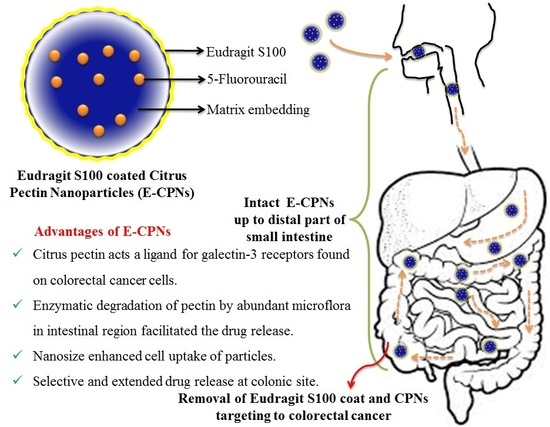
Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Citrus Pectin Nanoparticles (CPNs)
2.2. Coating of CPNs
2.3. Characterization of CPNs and E-CPNs
2.3.1. Particle Size
2.3.2. Zeta Potential
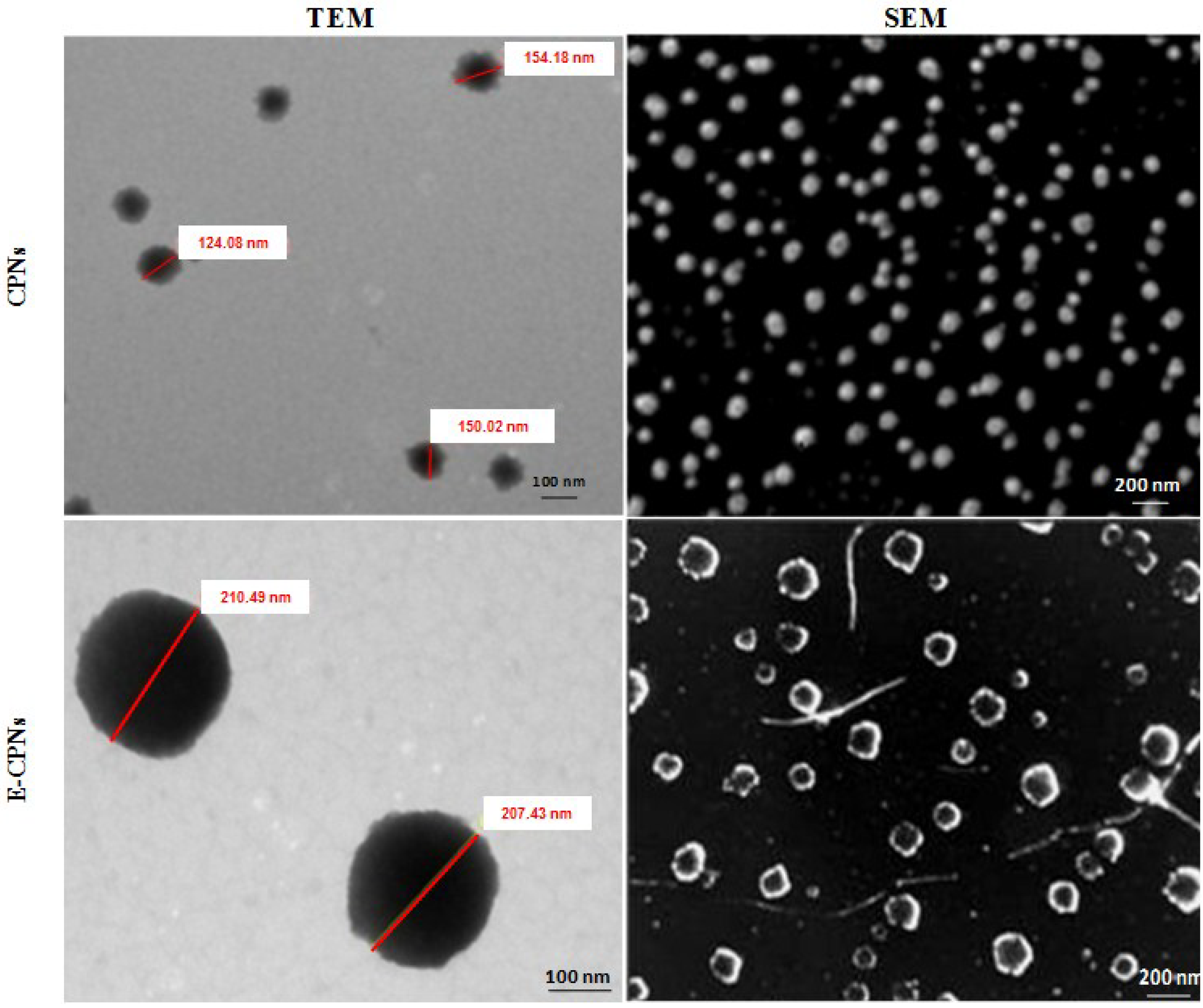
2.3.3. Particle Shape and Surface Morphology
Transmission Electron Microscopy
Scanning Electron Microscopy
2.3.4. Entrapment Efficiency
| Formulation code | Particle size (nm) | PDI | Zeta potential (mV) | % Entrapment efficiency | % Drug loading |
|---|---|---|---|---|---|
| CPNs | 174.65 ± 5.32 | 0.095 | −18.4 ± 0.4 | 38.75 ± 0.74 | 21.45 ± 0.87 |
| E-CPNs | 218.12 ± 10.25 | 0.117 | −27.5 ± 0.8 | 35.15 ± 0.52 | 20.84 ± 0.75 |
2.4. In vitro Release Study
2.4.1. In Simulated Gastric Fluids of Different pH Conditions
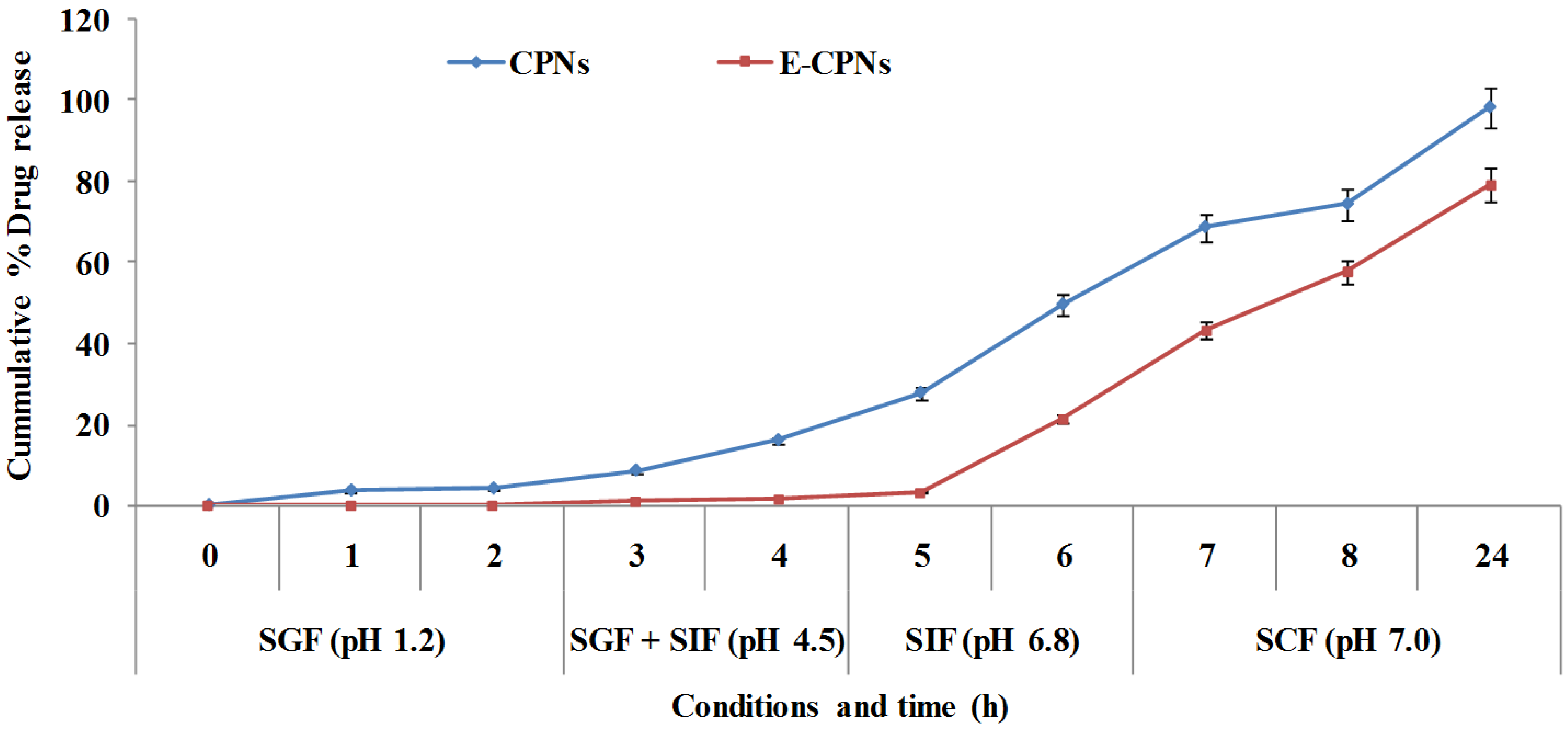
2.4.2. In vitro Drug Release Study in the Presence and Absence of Rat Caecal Content
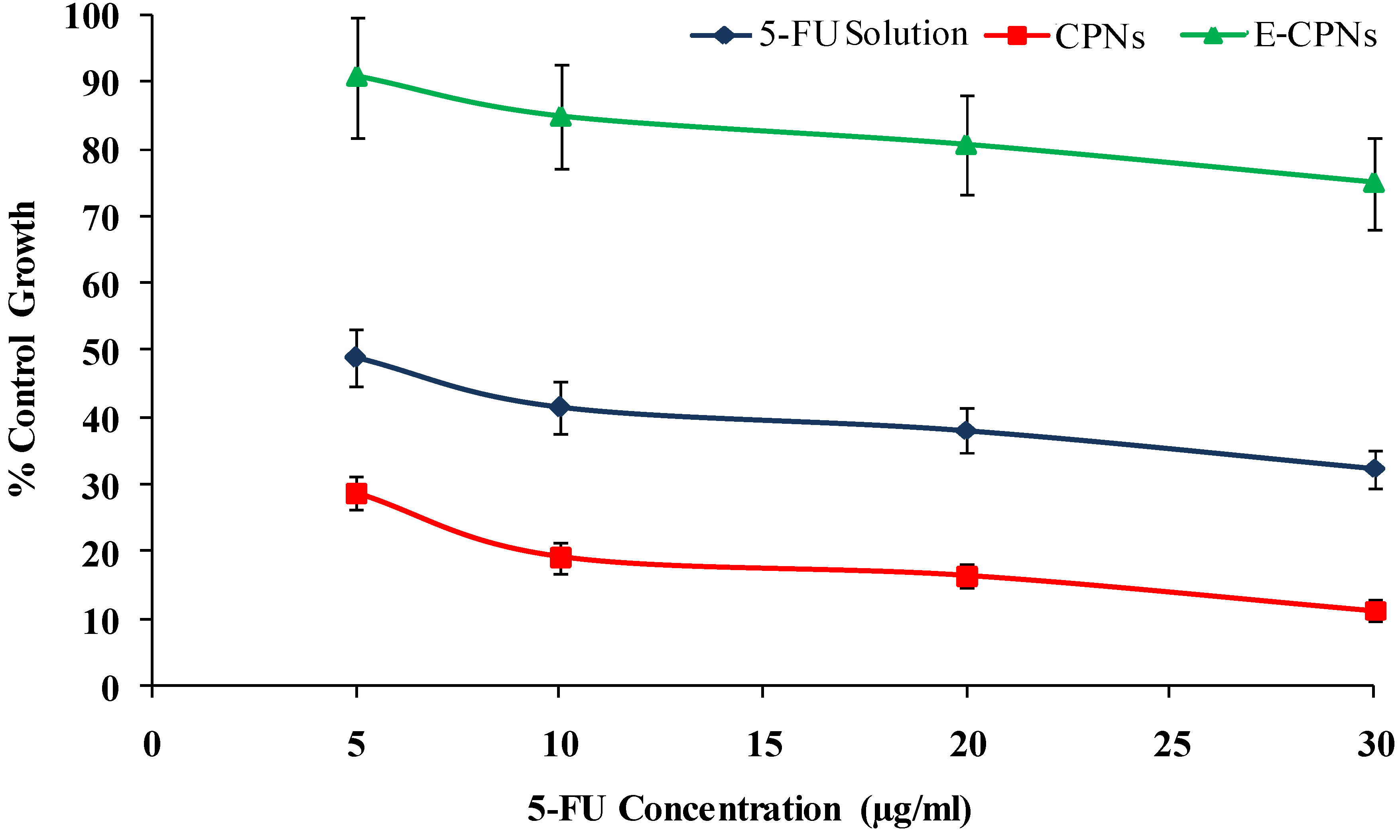
2.5. In vitro Cytotoxicity Studies

Endpoint Measurement
2.6. In vivo Studies
2.6.1. Experimental Protocol Approval
2.6.2. Blood Profile and GI Organ Distribution Study

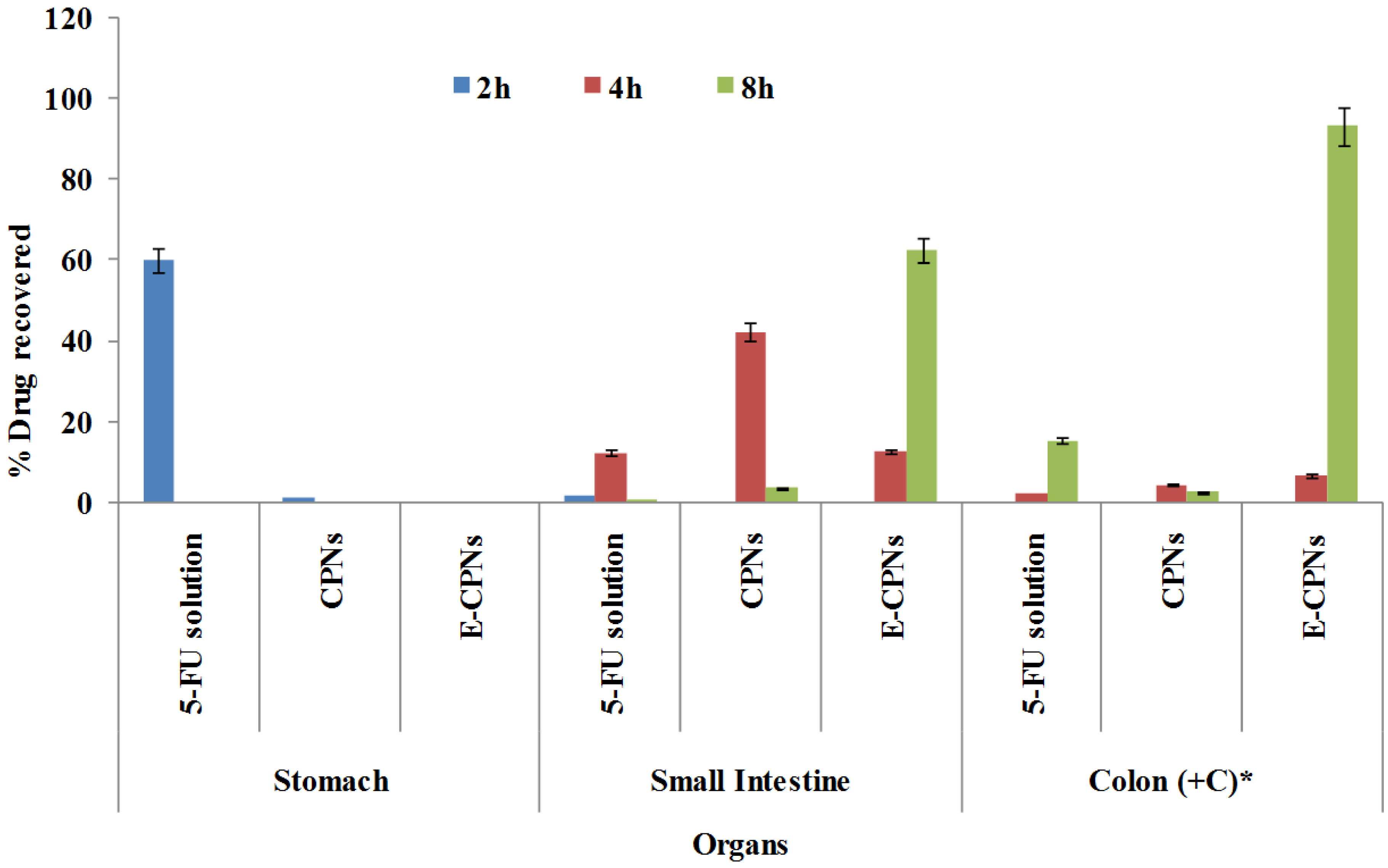
3. Statistical Analysis
4. Results and Discussion
| Formulation | LC50 (μg/mL) | TGI (μg/mL) | GI50 (μg/mL) |
|---|---|---|---|
| 5-FU solution | 56.7 ± 4.2 | 33.7 ± 2.4 | 9.3 ± 0.7 |
| CPNs | 36.4 ± 3.2 | 25.8 ± 1.8 | 6.5 ± 0.4 |
| E-CPNs | 94.2 ± 3.8 | 76.4 ± 2.2 | 18.5 ± 0.5 |
5. Conclusions
Supplementary Information
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chaurasia, M.; Chourasia, M.K.; Jain, N.K.; Jain, A.; Soni, V.; Gupta, Y.; Jain, S.K. Cross-linked guar gum microspheres: A viable approach for improved delivery of anticancer drugs for the treatment of colorectal cancer. AAPS PharmSciTech 2006, 7, E143–E151. [Google Scholar] [CrossRef]
- Dangi, R.; Hurkat, P.; Jain, A.; Shilpi, S.; Jain, A.; Gulbake, A.; Jain, S.K. Targeting liver cancer via ASGP receptor using 5-FU-loaded surface-modified PLGA nanoparticles. J. Microencapsul. 2014, 31, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, X.; Ding, Y.; Ge, H.; Yuan, Y.; Yang, C. Synthesis and characterization of chitosan–poly (acrylic acid) nanoparticles. Biomaterials 2002, 23, 3193–3201. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Jiang, M.; Peng, H.; Chen, D.; Hong, Y. Ph-dependent self-assembly: Micellization and micelle–hollow-sphere transition of cellulose-based copolymers. Angew. Chem. Int. Ed. 2003, 42, 1516–1519. [Google Scholar] [CrossRef]
- Tang, M.; Dou, H.; Sun, K. One-step synthesis of dextran-based stable nanoparticles assisted by self-assembly. Polymer 2006, 47, 728–734. [Google Scholar] [CrossRef]
- BeMiller, J.N. An introduction to pectins: Structure and properties. In Chemistry and Function of Pectins; ACS Symposium Series-American Chemical Society: Washington, DC, USA, 1986; Volume 310, pp. 2–12. [Google Scholar]
- Sriamornsak, P.; Sungthongjeen, S.; Puttipipatkhachorn, S. Use of pectin as a carrier for intragastric floating drug delivery: Carbonate salt contained beads. Carbohydr. Polym. 2007, 67, 436–445. [Google Scholar] [CrossRef]
- Mennini, N.; Furlanetto, S.; Maestrelli, F.; Pinzauti, S.; Mura, P. Response surface methodology in the optimization of chitosan–Ca pectinate bead formulations. Eur. J. Pharm. Sci. 2008, 35, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mishra, R.K.; Banthia, A.K. Development of pectin based hydrogel membranes for biomedical applications. Int. J. Plast. Technol. 2010, 14, 213–223. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Cambier, P.; Cutsem, P. Drug encapsulation in pectin hydrogel beads—A systematic study of simulated digestion media. Int. J. Pharm. Pharm. Sci. 2011, 3, 292–299. [Google Scholar]
- Birch, N.P.; Schiffman, J.D. Characterization of self-assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A new horizon in modifcations of chitosan: Syntheses and applications. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 91–181. [Google Scholar] [PubMed]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polymers 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Kohn, R. Ion binding on polyuronates-alginate and pectin. Pure Appl. Chem. 1975, 42, 371–397. [Google Scholar] [CrossRef]
- Niwa, K.; Takaya, T.; Morimoto, T.; Takada, K. Preparation and evaluation of a time-controlled release capsule made of ethylcellulose for colon delivery of drugs. J. Drug Target. 1995, 3, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.K.; Jain, S.K. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharm. Sci. 2003, 6, 33–66. [Google Scholar] [PubMed]
- Leclere, L.; van Cutsem, P.; Michiels, C. Anti-cancer activities of ph-or heat-modified pectin. Front. Pharmacol. 2013, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Cao, H.; Zhang, X.-C.; Zhou, F.-Z.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Hybrid nanospheres and vesicles based on pectin as drug carriers. Langmuir 2009, 25, 11720–11726. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, F.; Cirri, M.; Corti, G.; Mennini, N.; Mura, P. Development of enteric-coated calcium pectinate microspheres intended for colonic drug delivery. Eur. J. Pharm. Biopharm. 2008, 69, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, M.; Chourasia, M.; Jain, N.K.; Jain, A.; Soni, V.; Gupta, Y.; Jain, S. Methotrexate bearing calcium pectinate microspheres: A platform to achieve colon-specific drug release. Curr. Drug Deliv. 2008, 5, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Souder, J.; Ellenbogen, W. Control of d-amphetamine sulphate sustained release capsule. Drug Stand. 1985, 26, 77–79. [Google Scholar]
- Jain, A.; Jain, S.K.; Ganesh, N.; Barve, J.; Beg, A.M. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine 2010, 6, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Van den Mooter, G.; Samyn, C.; Kinget, R. The relation between swelling properties and enzymatic degradation of azo polymers designed for colon-specific drug delivery. Pharm. Res. 1994, 11, 1737–1741. [Google Scholar]
- Momin, M.; Pundarikakshudu, K. In vitro studies on guar gum based formulation for the colon targeted delivery of sennosides. J. Pharm. Pharm. Sci. 2004, 7, 325–331. [Google Scholar] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Akamanchi, K.G. Oleic acid based heterolipid synthesis, characterization and application in self-microemulsifying drug delivery system. Int. J. Pharm. 2012, 425, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Y.; Chen, G.; Wei, P.; Ping, Q. Plga nanoparticles for the oral delivery of 5-fluorouracil using high pressure homogenization-emulsification as the preparation method and in vitro/in vivo studies. Drug Dev. Ind. Pharm. 2008, 34, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Alsarra, I.A.; Yassin, A.E.; Abdel-Hamid, M.; Alanazi, F.K.; Aljuffali, I.A. Direct UPLC-MS-MS validated method for the quantification of 5-aminolevulinic acid: Application to in vitro assessment of colonic-targeted oral tablets. J. Chromatogr. Sci. 2011, 49, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Sharma, V.K.; Jain, A.; Soni, V. Nano-aggregates: Emerging delivery tools for tumor therapy. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Licht, T.R.; Hansen, M.; Bergström, A.; Poulsen, M.; Krath, B.N.; Markowski, J.; Dragsted, L.O.; Wilcks, A. Effects of apples and specific apple components on the cecal environment of conventional rats: Role of apple pectin. BMC Microbiol. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Gulati, M.; Singh, S.K.; Shivapooja, A. Facts, fallacies and future of dissolution testing of polysaccharide based colon-specific drug delivery. J. Controll. Release 2014, 178, 55–62. [Google Scholar] [CrossRef]
- Paharia, A.; Yadav, A.K.; Rai, G.; Jain, S.K.; Pancholi, S.S.; Agrawal, G.P. Eudragit-coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS PharmSciTech 2007, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.K.; Sahu, S. Development of a novel probe sonication assisted enhanced loading of 5-FU in SPION encapsulated pectin nanocarriers for magnetic targeted drug delivery system. Eur. J. Pharm. Biopharm. 2012, 82, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Voigt, W. Sulforhodamine B assay and chemosensitivity. In Chemosensitivity; Springer: Berlin/Heidelberg, Germany, 2005; pp. 39–48. [Google Scholar]
- Bergman, M.; Djaldetti, M.; Salman, H.; Bessler, H. Effect of citrus pectin on malignant cell proliferation. Biomed. Pharmacother. 2010, 64, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Olano-Martin, E.; Rimbach, G.H.; Gibson, G.R.; Rastall, R.A. Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res. 2002, 23, 341–346. [Google Scholar]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-W.; Giri, N.; Lee, C.H. Ph-sensitive eudragit nanoparticles for mucosal drug delivery. Int. J. Pharm. 2011, 403, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Jain, A.; Gupta, Y.; Ahirwar, M. Design and development of hydrogel beads for targeted drug delivery to the colon. AAPS PharmSciTech 2007, 8, E56. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Jain, A.; Khare, P.; Agrawal, R.K.; Jain, S.K. Metronidazole loaded pectin microspheres for colon targeting. J. Pharm. Sci. 2009, 98, 4229–4236. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Du, Q.; Cao, D.-Y.; Xiang, B.; Fang, F. Pectin/ethylcellulose as film coatings for colon-specific drug delivery: Preparation and in vitro evaluation using 5-fluorouracil pellets. PDA J. Pharm. Sci. Technol./PDA 2007, 61, 121–130. [Google Scholar]
- Bose, A.; Elyagoby, A.; Wong, T.W. Oral 5-fluorouracil colon-specific delivery through in vivo pellet coating for colon cancer and aberrant crypt foci treatment. Int. J. Pharm. 2014, 468, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Zinutti, C.; Barberi-Heyob, M.; Hoffman, M.; Maincent, P. In-vivo evaluation of sustained release microspheres of 5-FU in rabbits. Int. J. Pharm. 1998, 166, 231–234. [Google Scholar] [CrossRef]
- Haupt, S.M.; Rubinstein, A. The colon as a possible target for orally administered peptide and protein drugs. Crit. Rev. Ther. Drug Carrier Syst. 2002, 19, 499–551. [Google Scholar] [CrossRef] [PubMed]
- Hoffart, V.; Lamprecht, A.; Maincent, P.; Lecompte, T.; Vigneron, C.; Ubrich, N. Oral bioavailability of a low molecular weight heparin using a polymeric delivery system. J. Controll. Release 2006, 113, 38–42. [Google Scholar] [CrossRef]
- Gupta, E.; Vyas, V.; Ahmed, F.; Sinko, P.; Cook, T.; Rubin, E. Pharmacokinetics of orally administered camptothecins. Ann. N. Y. Acad. Sci. 2000, 922, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, A.; Chao, P.; Hu, P.; Stein, S.; Sinko, P.J. Pharmacokinetic and pharmacodynamic evaluation of a novel in situ forming poly(ethylene glycol)-based hydrogel for the controlled delivery of the camptothecins. J. Controll. Release 2006, 112, 333–342. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil. Materials 2015, 8, 832-849. https://doi.org/10.3390/ma8030832
Subudhi MB, Jain A, Jain A, Hurkat P, Shilpi S, Gulbake A, Jain SK. Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil. Materials. 2015; 8(3):832-849. https://doi.org/10.3390/ma8030832
Chicago/Turabian StyleSubudhi, M. Biswaranjan, Ankit Jain, Ashish Jain, Pooja Hurkat, Satish Shilpi, Arvind Gulbake, and Sanjay K. Jain. 2015. "Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil" Materials 8, no. 3: 832-849. https://doi.org/10.3390/ma8030832
APA StyleSubudhi, M. B., Jain, A., Jain, A., Hurkat, P., Shilpi, S., Gulbake, A., & Jain, S. K. (2015). Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil. Materials, 8(3), 832-849. https://doi.org/10.3390/ma8030832