Photocatalytic, Morphological and Structural Properties of the TiO2-SiO2-Ag Porous Structures Based System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Performance of the Obtained Nanocomposites

2.2. Characterization of the Photocatalysts
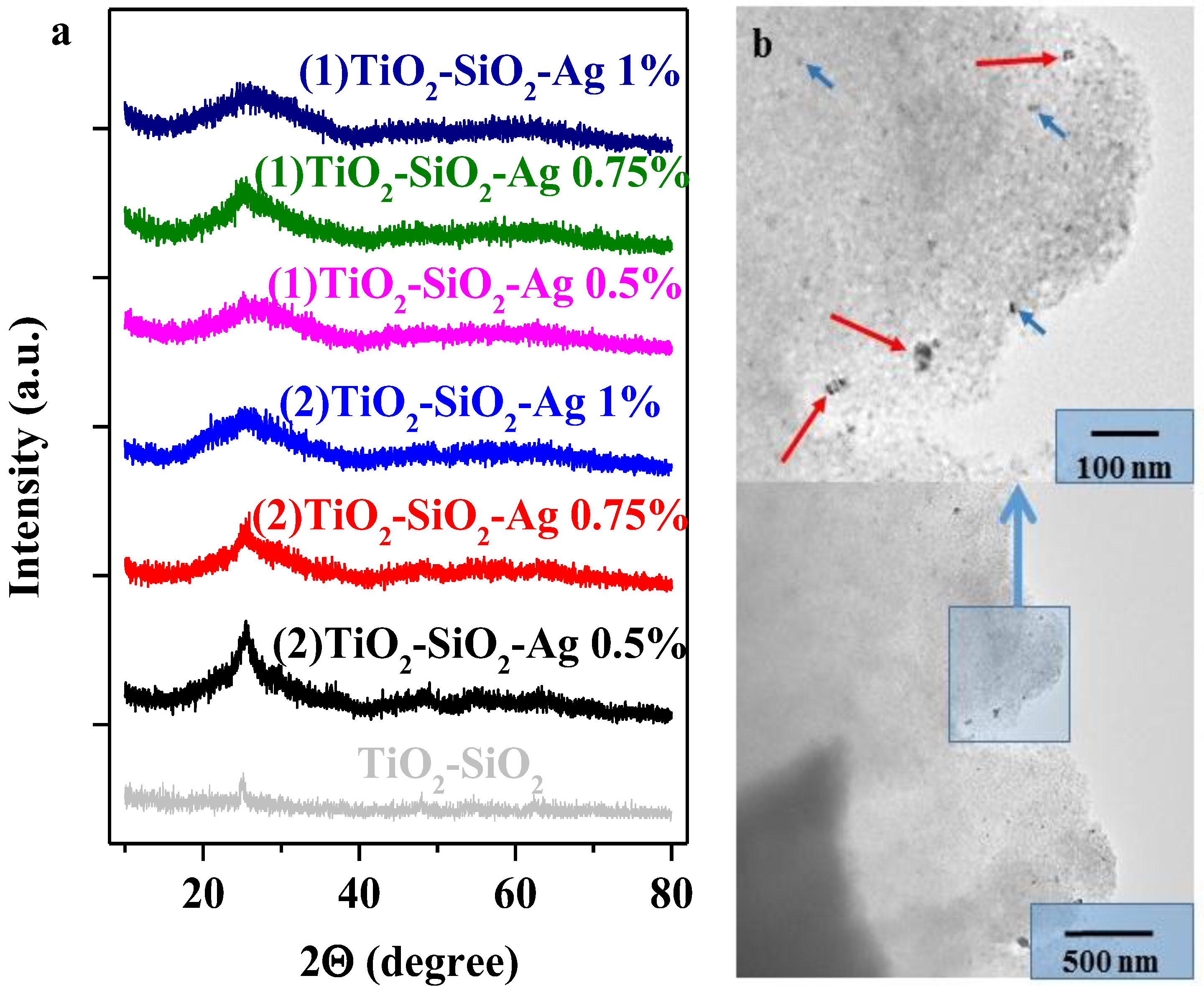
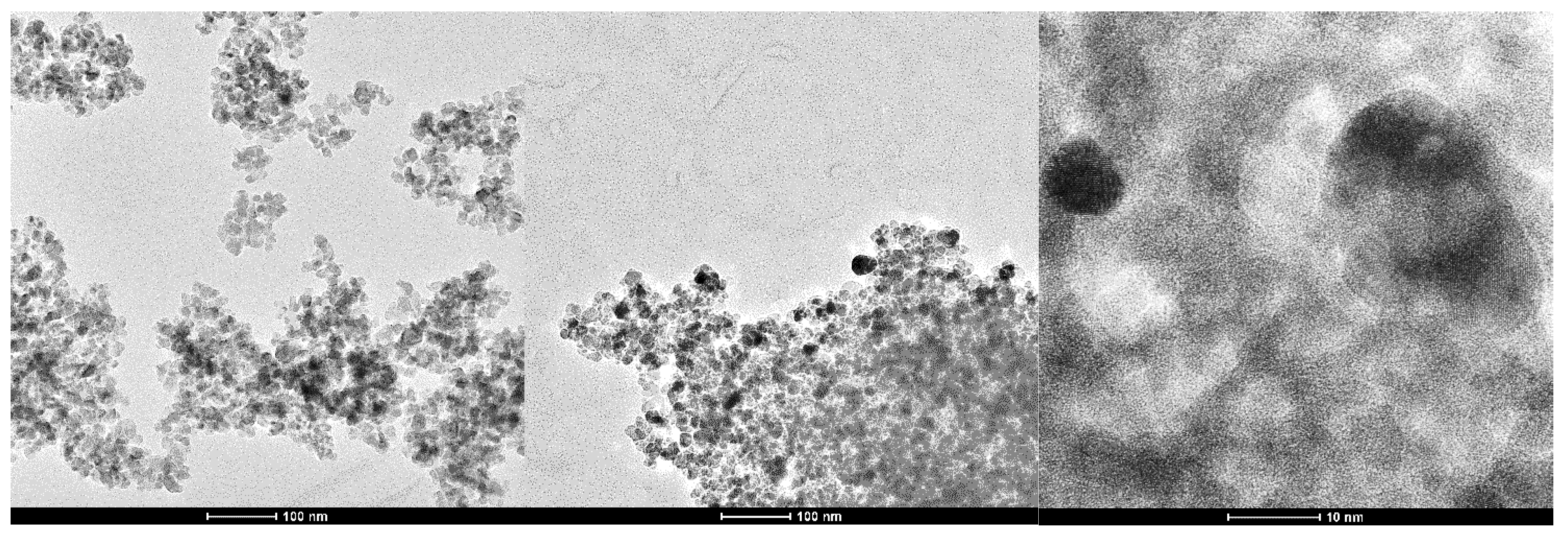
2.2.1. Morpho-Structural Characterization of the Composites (XRD, TEM)
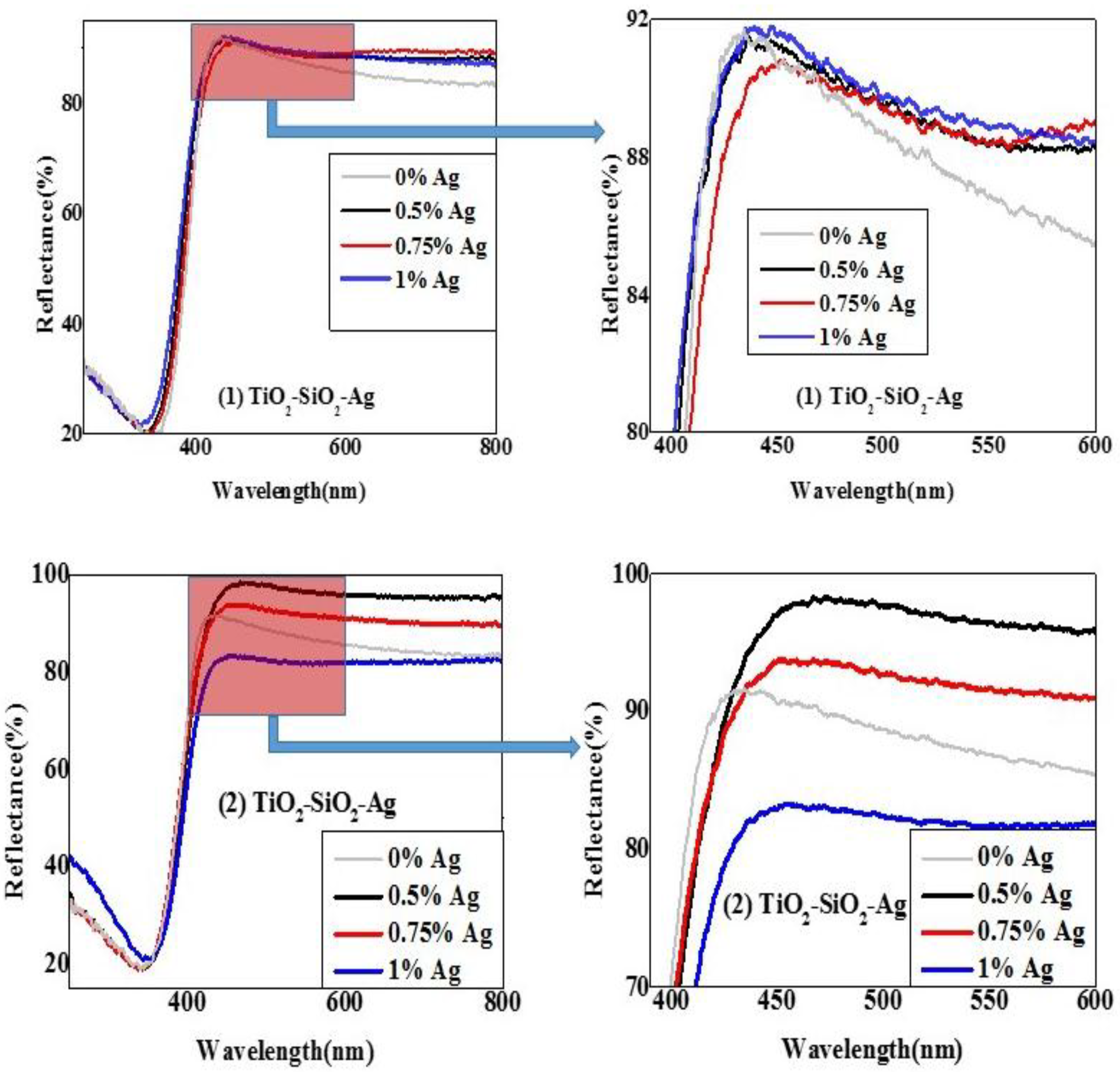
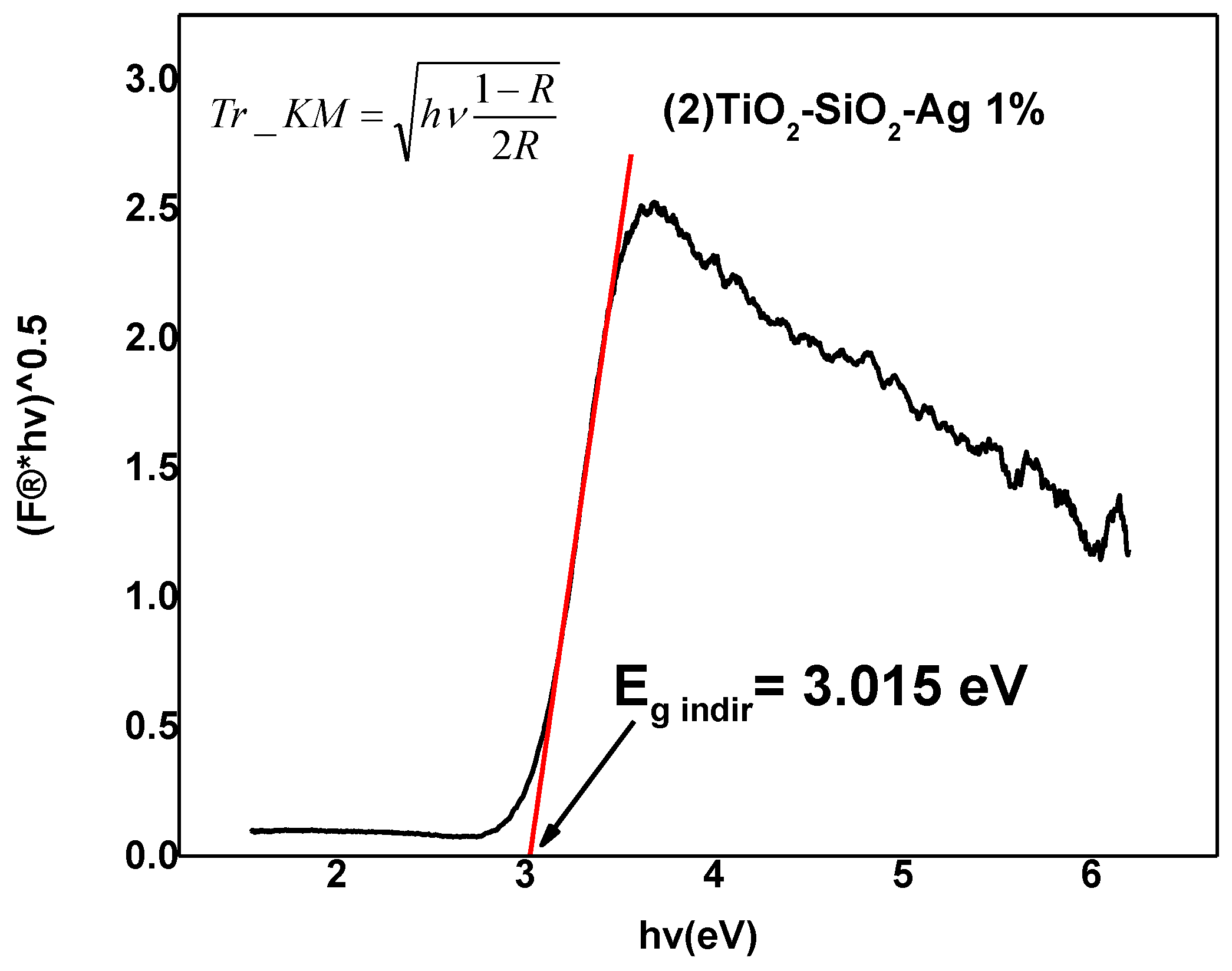
2.2.2. Diffuse Reflectance Spectroscopy (DRS)
| Sample | kapp × 10−3 (min−1) | Egdir | Egindir | Crystallites mean size [25] |
|---|---|---|---|---|
| TiO2-SiO2 | - | 3.30 | 2.99 | 3 |
| (1) TiO2-SiO2-Ag 0.5% | 2.07 | 3.35 | 3.08 | 2 |
| (1) TiO2-SiO2-Ag 0.75% | 2.37 | 3.27 | 2.88 | 3 |
| (1) TiO2-SiO2-Ag 1% | 1.65 | 3.38 | 3.09 | 2 |
| (2) TiO2-SiO2-Ag 0.5% | 12.37 | 3.23 | 2.97 | 4 |
| (2) TiO2-SiO2-Ag 0.75% | 13.07 | 3.28 | 3.01 | 5 |
| (2) TiO2-SiO2-Ag 1% | 12.73 | 3.3 | 3.015 | 4 |
| P25-Aeroxide | 11.89 | 3.45 | 3.25 | - |
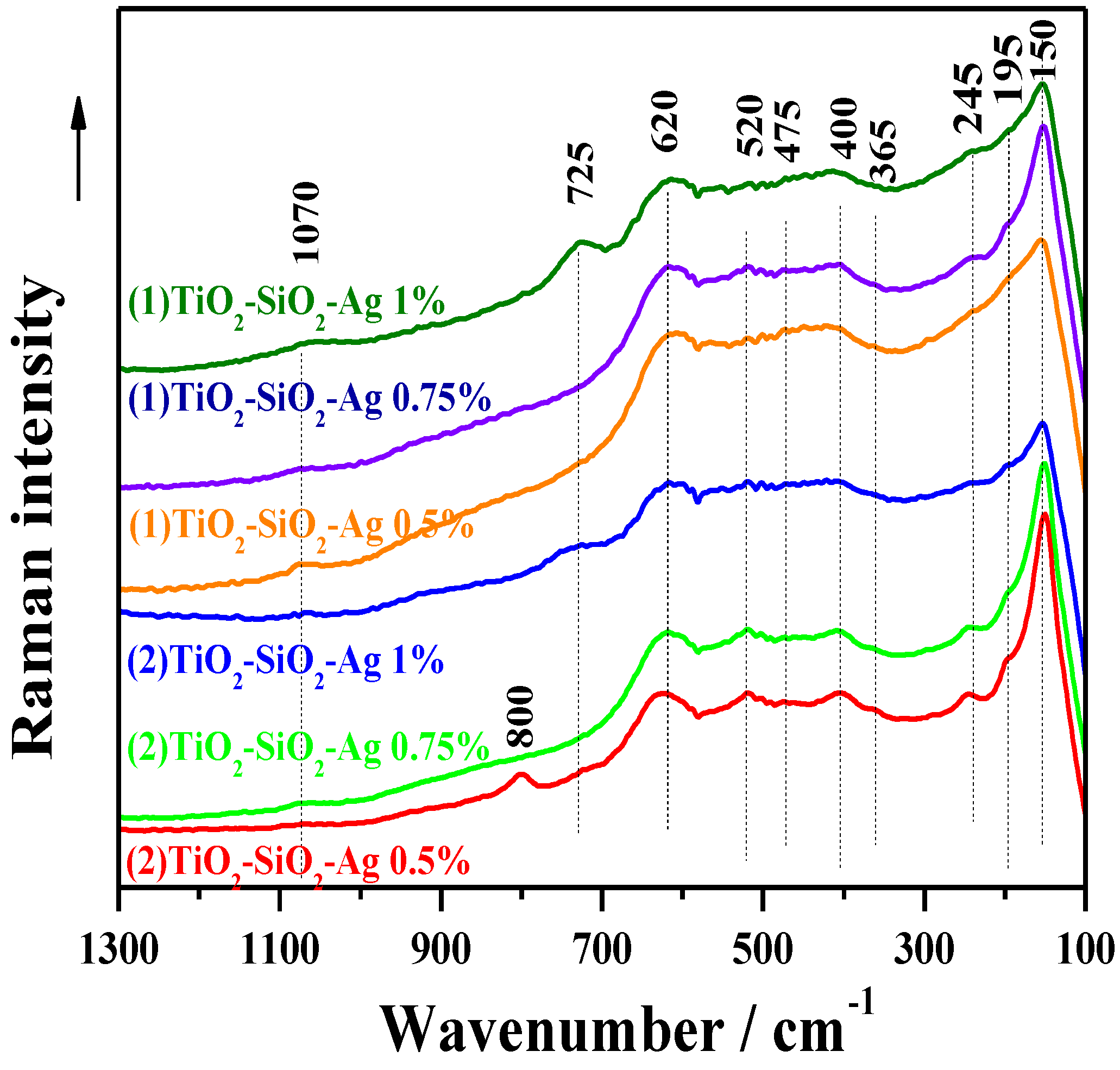
2.2.3. Raman Spectroscopy
2.2.4. X-ray Photoelectron Spectroscopy (XPS)
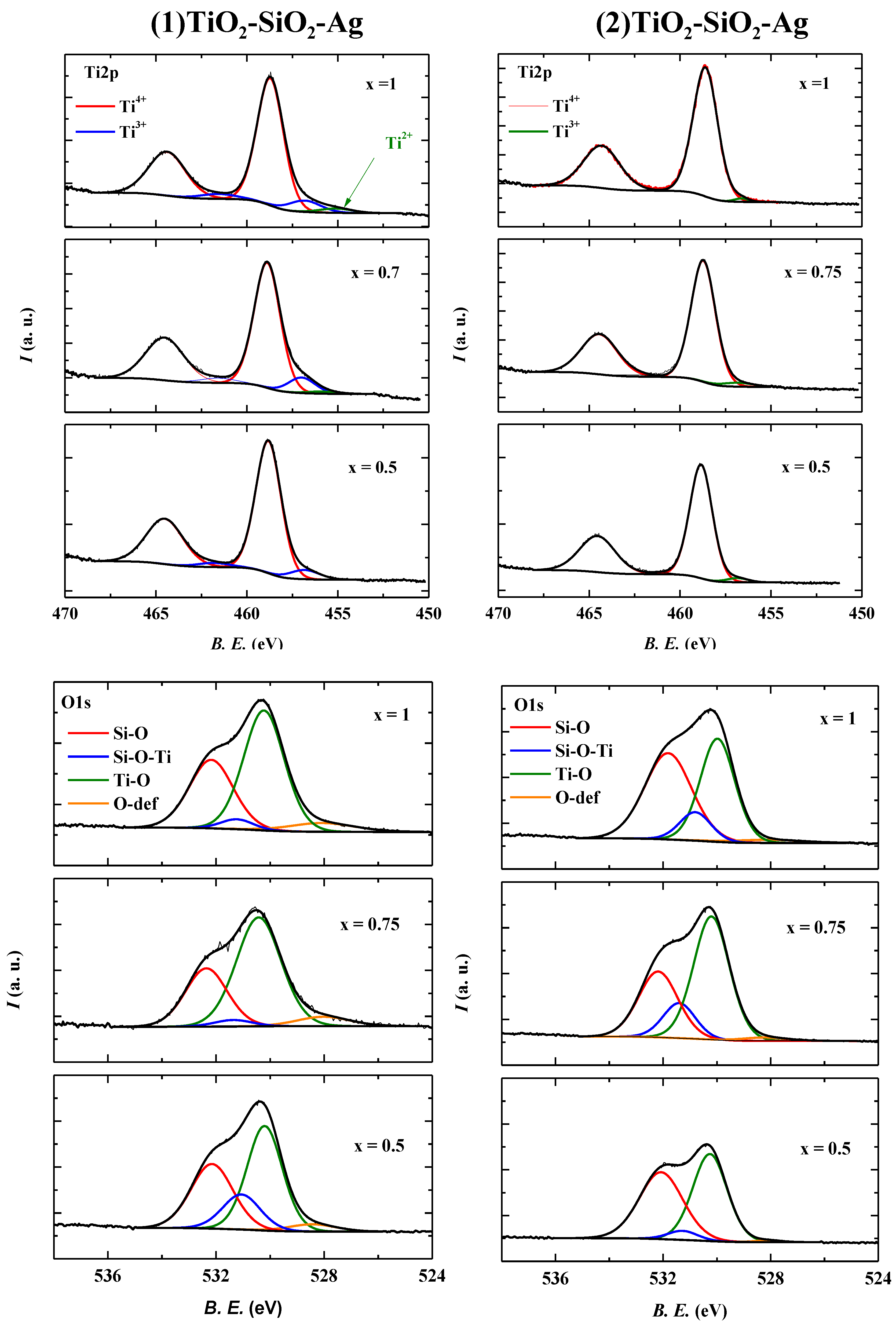
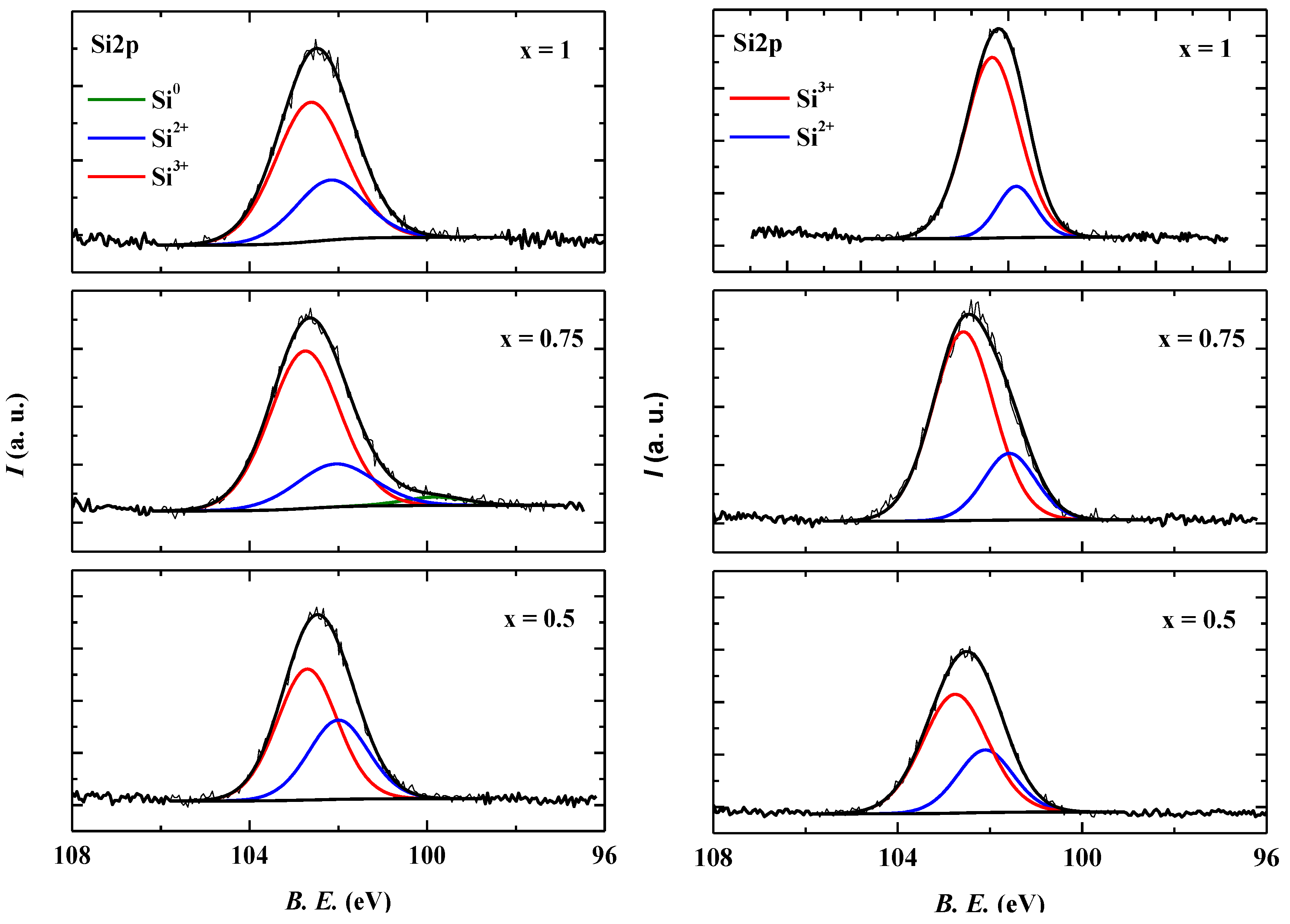
2.2.5. The Activity Governing Structural Elements of the TiO2-SiO2-Ag Ternary Composite Materials
3. Experimental Section
3.1. Materials
3.2. The Synthesis of the TiO2-SiO2-Ag-Based Composites
- Synthesis pathway, by in situ impregnation of TiO2-SiO2 aerogels with the Ag precursor (AgNO3 dissolved in EtOH) followed by chemical reduction;
- Mixing the gel precursors for TiO2-SiO2-based composites with AgNO3.
3.3. Characterization Methods and Instrumentation
4. Conclusions
Acknowledgments
Author Contributions
Appendix
Conflicts of Interest
References
- Chhor, K. Comparative studies of phenol and salicylic acid photocatalytic degradation: Influence of adsorbed oxygen. Mater. Chem. Phys. 2004, 86, 123–131. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; Navío, J.A. Photocatalytic behaviour of sulphated TiO2 for phenol degradation. Appl. Catal. B Environ. 2003, 45, 39–50. [Google Scholar] [CrossRef]
- Bandala, E. Solar photoreactors comparison based on oxalic acid photocatalytic degradation. Sol. Energy 2004, 77, 503–512. [Google Scholar] [CrossRef]
- McMurray, T. Intrinsic kinetics of photocatalytic oxidation of formic and oxalic acid on immobilised TiO2 films. Appl. Catal. A Gen. 2004, 262, 105–110. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hidalgo, M.C.; Navío, J.A.; Araña, J.; Doña-Rodríguez, J.M. Correlation study between photo-degradation and surface adsorption properties of phenol and methyl orange on TiO2 vs platinum-supported TiO2. Appl. Catal. B Environ. 2014, 150–151, 107–115. [Google Scholar] [CrossRef]
- Brown, L.; Anderson, A.; Carroll, M. Fabrication of titania and titania–silica aerogels using rapid supercritical extraction. J. Sol-Gel Sci. Technol. 2012, 62, 404–413. [Google Scholar] [CrossRef]
- Ayers, M.R.; Hunt, A.J. Titanium oxide aerogels prepared from titanium metal and hydrogen peroxide. Mater. Lett. 1998, 34, 290–293. [Google Scholar] [CrossRef]
- Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Norén, K.; Canton, S.E.; et al. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity “from degradation intermediates to catalysts’ structural peculiarities” Part II: Aerogel based composites—fine details by spectroscopic means. Appl. Catal. B Environ. 2014, 148–149, 589–600. [Google Scholar] [CrossRef]
- Baia, M.; Danciu, V.; Cosoveanu, V.; Baia, L. Porous nanoarchitectures based on TiO2 aerogels and Au particles as potential SERS sensor for monitoring of water quality. Vib. Spectrosc. 2008, 48, 206–209. [Google Scholar] [CrossRef]
- D’Elia, D.; Beauger, C.; Hochepied, J.-F.; Rigacci, A.; Berger, M.-H.; Keller, N.; Keller-Spitzer, V.; Suzuki, Y.; Valmalette, J.-C.; Benabdesselam, M.; et al. Impact of three different TiO2 morphologies on hydrogen evolution by methanol assisted water splitting: Nanoparticles, nanotubes and aerogels. Int. J. Hydrog. Energy 2011, 36, 14360–14373. [Google Scholar] [CrossRef]
- Pietron, J.; Rolison, D. Improving the efficiency of titania aerogel-based photovoltaic electrodes by electrochemically grafting isopropyl moieties on the titania surface. J. Non-Cryst. Solids 2004, 350, 107–112. [Google Scholar] [CrossRef]
- Popa, M.; Macovei, D.; Indrea, E.; Mercioniu, I.; Popescu, I.C.; Danciu, V. Synthesis and structural characteristics of nitrogen doped TiO2 aerogels. Microporous Mesoporous Mater. 2010, 132, 80–86. [Google Scholar] [CrossRef]
- Kesmez, Ö.; Erdem Çamurlu, H.; Burunkaya, E.; Arpaç, E. Sol–gel preparation and characterization of anti-reflective and self-cleaning SiO2–TiO2 double-layer nanometric films. Sol. Energy Mater. Sol. Cells 2009, 93, 1833–1839. [Google Scholar]
- Zhang, M.; Shi, L.; Yuan, S.; Zhao, Y.; Fang, J. Synthesis and photocatalytic properties of highly stable and neutral TiO2/SiO2 hydrosol. J. Colloid Interface Sci. 2009, 330, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar]
- Al-Ahmed, A. Metal doped TiO2 photocatalysts for CO2 photoreduction. Mater. Sci. Forum 2013, 757, 243–256. [Google Scholar] [CrossRef]
- Primo, A.; Corma, A.; Garcia, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef] [PubMed]
- Sreethawong, T.; Yoshikawa, S. Impact of photochemically deposited monometallic Pt and bimetallic Pt-Au nanoparticles on photocatalytic dye-sensitized H2 production activity of mesoporous-assembled TiO2-SiO2 mixed oxide nanocrystal. Chem. Eng. J. 2012, 197, 272–282. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Rodriguezreinoso, F.; Ruizmartinez, J.; Sepulvedaescribano, A.; Yus, M. Platinum nanoparticles supported on titania as an efficient hydrogen-transfer catalyst. J. Catal. 2008, 260, 113–118. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, H.; Kim, N.D.; Yi, J. Characterization of photocatalytic performance of silver deposited TiO2 nanorods. Electrochem. Commun. 2009, 11, 363–366. [Google Scholar] [CrossRef]
- Chou, C.S.; Yang, R.Y.; Yeh, C.K.; Lin, Y.J. Preparation of TiO2/nano-metal composite particles and their applications in dye-sensitized solar cells. Powder Technol. 2009, 194, 95–105. [Google Scholar] [CrossRef]
- Goei, R.; Lim, T.-T. Ag-decorated TiO2 photocatalytic membrane with hierarchical architecture: Photocatalytic and anti-bacterial activities. Water Res. 2014, 59, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, Z.; Balázs, N.; Alapi, T.; Wittmann, G.; Sipos, P.; Dombi, A.; Mogyorósi, K. Synthesis, structure and photocatalytic properties of Fe(III)-doped TiO2 prepared from TiCl3. Appl. Catal. B Environ. 2008, 81, 27–37. [Google Scholar] [CrossRef]
- Ambrus, Z.; Mogyorósi, K.; Szalai, Á.; Alapi, T.; Demeter, K.; Dombi, A.; Sipos, P. Low temperature synthesis, characterization and substrate-dependent photocatalytic activity of nanocrystalline TiO2 with tailor-made rutile to anatase ratio. Appl. Catal. A Gen. 2008, 340, 153–161. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Baia, L.; Peter, A.; Cosoveanu, V.; Indrea, E.; Baia, M.; Popp, J.; Danciu, V. Synthesis and nanostructural characterization of TiO2 aerogels for photovoltaic devices. Thin Solid Films 2006, 511, 512–516. [Google Scholar] [CrossRef]
- Iancu, V.; Baia, L.; Tarcea, N.; Popp, J.; Baia, M. Towards TiO2Ag porous nanocomposites based SERS sensors for chemical pollutant detection. J. Mol. Struct. 2014, 1073. [Google Scholar] [CrossRef]
- Rusu, M.; Baia, M.; Pap, Z.; Danciu, V.; Baia, L. Structural investigations of TiO2-WO3-Au porous composites. J. Mol. Struct. 2014, 1073, 150–156. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chan, C.K.; Porter, J.F.; Guo, W. Micro-raman spectroscopic characterization of nanosized TiO2 powders prepared by vapor hydrolysis. J. Mater. Res. 1998, 13, 2602–2609. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, Q.F.; Chou, K.C. Structure of CaO-B2O3-SiO2-TiO2 glasses: A raman spectral study. ISIJ Int. 2011, 51, 1021–1027. [Google Scholar] [CrossRef]
- Zheng, K.; Liao, J.L.; Wang, X.D.; Zhang, Z.T. Raman spectroscopic study of the structural properties of CaO-MgO-SiO2-TiO2 slags. J. Non-Cryst. Solids 2013, 376, 209–215. [Google Scholar] [CrossRef]
- Marycz, K.; Krzak-Ros, J.; Donesz-Sikorska, A.; Smieszek, A. The morphology, proliferation rate, and population doubling time factor of adipose-derived mesenchymal stem cells cultured on to non-aqueous SiO2, TiO2, and hybrid sol-gel-derived oxide coatings. J. Biomed. Mater. Res. Part A 2014, 102, 4017–4026. [Google Scholar] [CrossRef]
- Pap, Z.; Danciu, V.; Cegléd, Z.; Kukovecz, Á.; Oszkó, A.; Dombi, A.; Mogyorósi, K. The influence of rapid heat treatment in still air on the photocatalytic activity of titania photocatalysts for phenol and monuron degradation. Appl Catal. B Environ. 2011, 101, 461–470. [Google Scholar] [CrossRef]
- Mogyorósi, K.; Balázs, N.; Srankó, D.F.; Tombácz, E.; Dékány, I.; Oszkó, A.; Sipos, P.; Dombi, A. The effect of particle shape on the activity of nanocrystalline TiO2 photocatalysts in phenol decomposition. Part 3: The importance of surface quality. Appl. Catal. B Environ. 2010, 96, 577–585. [Google Scholar] [CrossRef]
- Pap, Z.; Karácsonyi, É.; Cegléd, Z.; Dombi, A.; Danciu, V.; Popescu, I.C.; Baia, L.; Oszkó, A.; Mogyorósi, K. Dynamic changes on the surface during the calcination of rapid heat treated TiO2 photocatalysts. Appl. Catal. B Environ. 2012, 111–112, 595–604. [Google Scholar] [CrossRef]
- Pap, Z.; Radu, A.; Hidi, I.J.; Melinte, G.; Diamandescu, L.; Popescu, T.; Baia, L.; Danciu, V.; Baia, M. Behavior of gold nanoparticles in a titania aerogel matrix: Photocatalytic activity assessment and structure investigations. Chin. J. Catal. 2013, 34, 734–740. [Google Scholar] [CrossRef]
- Mayer, J.T.; Diebold, U.; Madey, T.E.; Garfunkel, E. Titanium and reduced titania overlayers on titanium dioxide(110). J. Electron. Spectrosc. Relat. Phenom. 1995, 73, 1–11. [Google Scholar] [CrossRef]
- Kovács, G.; Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Pap, Z. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity, “from degradation intermediates to catalysts’ structural peculiarities”, Part I: Aeroxide P25 based composites. Appl. Catal. B Environ. 2014, 147, 508–517. [Google Scholar] [CrossRef]
- Abad, J.; Gonzalez, C.; de Andres, P.L.; Roman, E. Characterization of thin silicon overlayers on rutile TiO2(110) − (1 × 1). Phys. Rev. B 2010, 82. [Google Scholar] [CrossRef]
- Zhang, F.X.; Guan, N.J.; Li, Y.Z.; Zhang, X.; Chen, J.X.; Zeng, H.S. Control of morphology of silver clusters coated on titanium dioxide during photocatalysis. Langmuir 2003, 19, 8230–8234. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, X.; Zhang, Y. XPS study of Al-Alkyl effect on Ziegler-Natta catalytic system. Surf. Interface Anal. 2012, 44, 924–926. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-ray Powder Diffractometry; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, G.; Pap, Z.; Coteț, C.; Coșoveanu, V.; Baia, L.; Danciu, V. Photocatalytic, Morphological and Structural Properties of the TiO2-SiO2-Ag Porous Structures Based System. Materials 2015, 8, 1059-1073. https://doi.org/10.3390/ma8031059
Kovács G, Pap Z, Coteț C, Coșoveanu V, Baia L, Danciu V. Photocatalytic, Morphological and Structural Properties of the TiO2-SiO2-Ag Porous Structures Based System. Materials. 2015; 8(3):1059-1073. https://doi.org/10.3390/ma8031059
Chicago/Turabian StyleKovács, Gábor, Zsolt Pap, Cosmin Coteț, Veronica Coșoveanu, Lucian Baia, and Virginia Danciu. 2015. "Photocatalytic, Morphological and Structural Properties of the TiO2-SiO2-Ag Porous Structures Based System" Materials 8, no. 3: 1059-1073. https://doi.org/10.3390/ma8031059
APA StyleKovács, G., Pap, Z., Coteț, C., Coșoveanu, V., Baia, L., & Danciu, V. (2015). Photocatalytic, Morphological and Structural Properties of the TiO2-SiO2-Ag Porous Structures Based System. Materials, 8(3), 1059-1073. https://doi.org/10.3390/ma8031059