Gelatin Tight-Coated Poly(lactide-co-glycolide) Scaffold Incorporating rhBMP-2 for Bone Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
2.1. Scaffold Characterizations
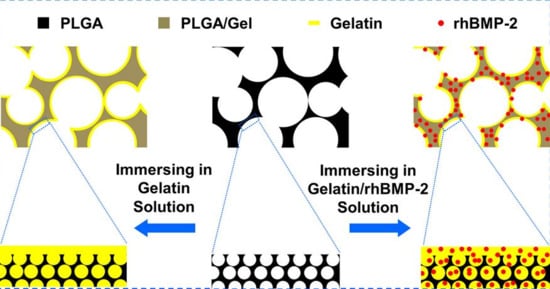
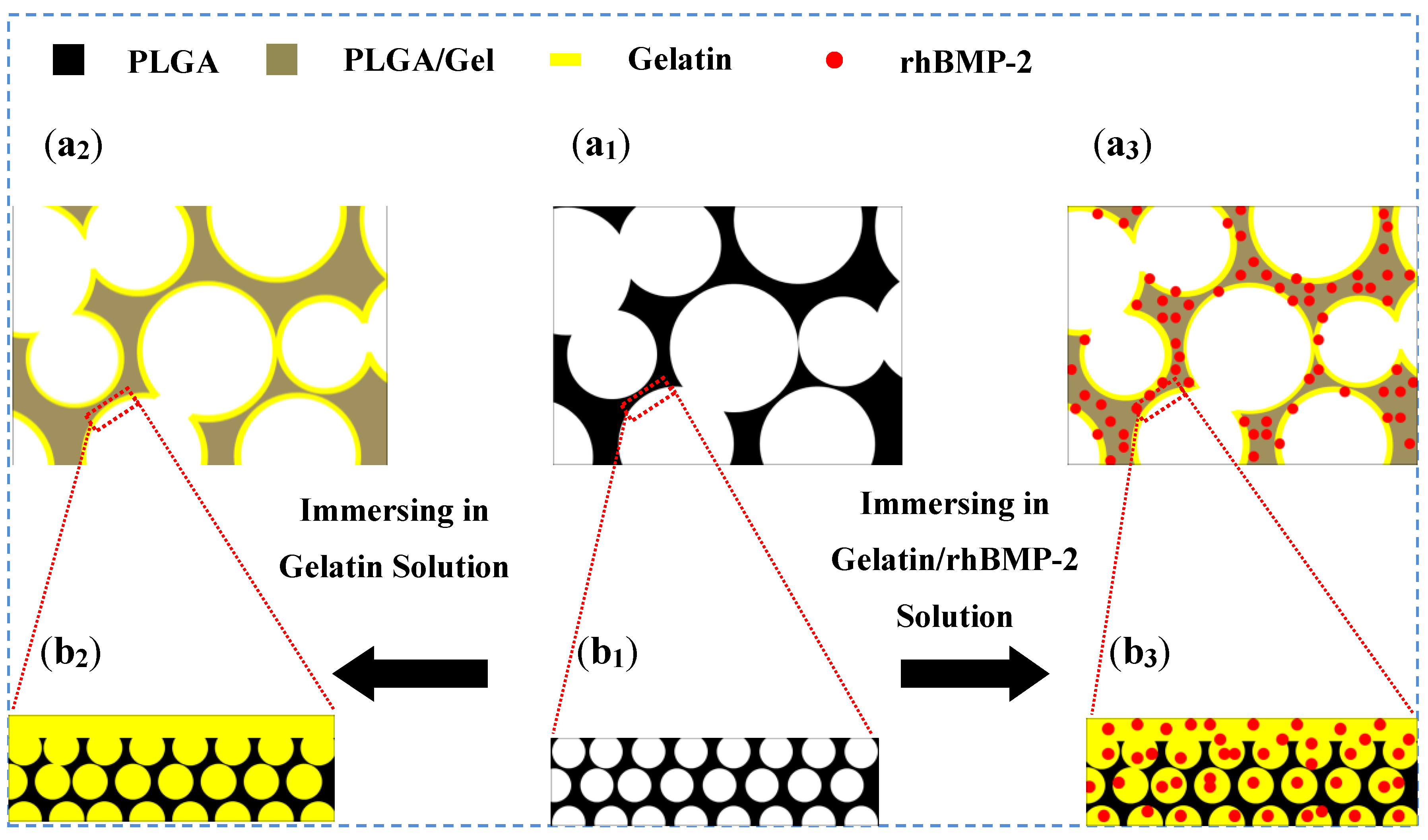
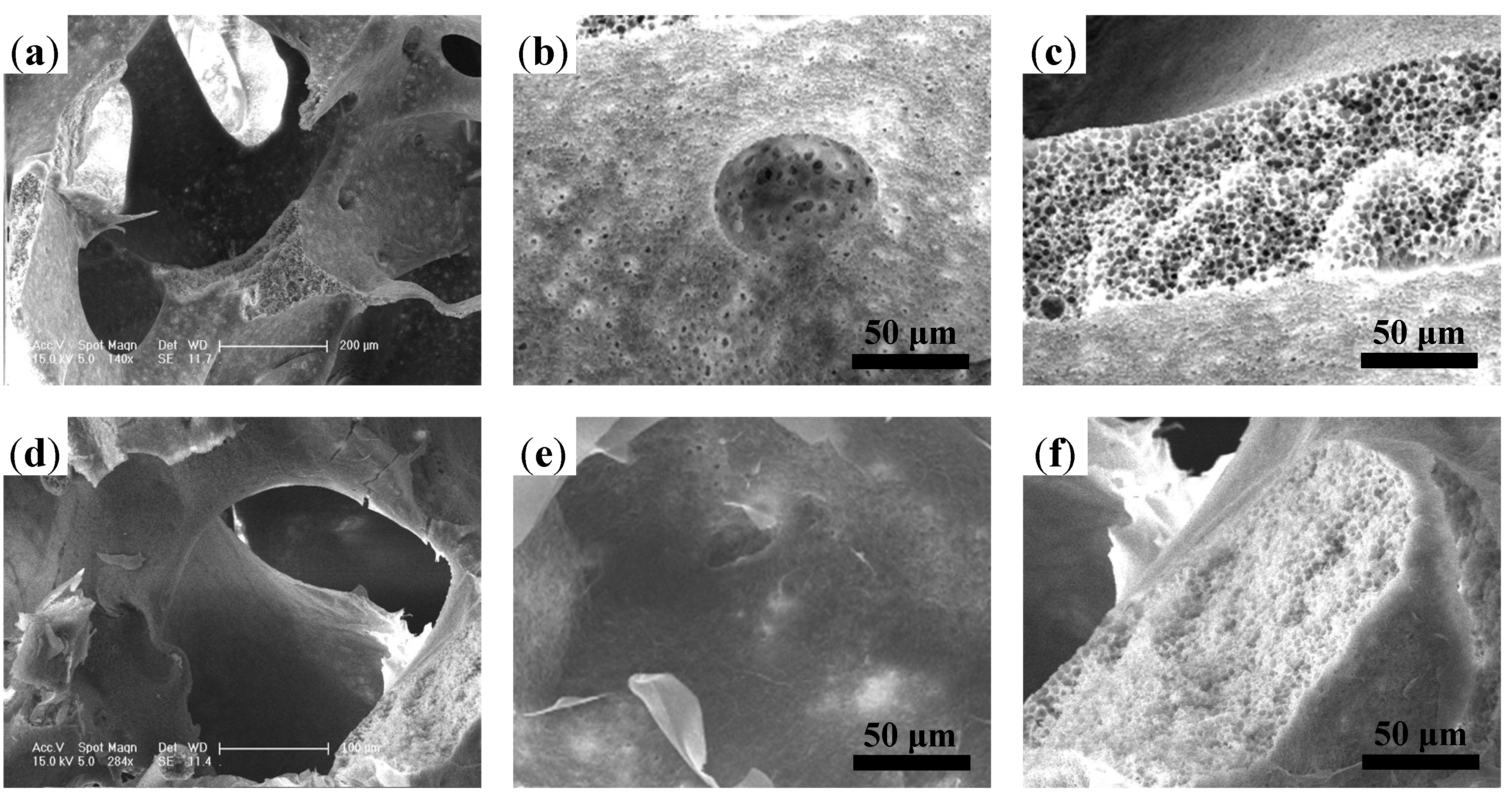
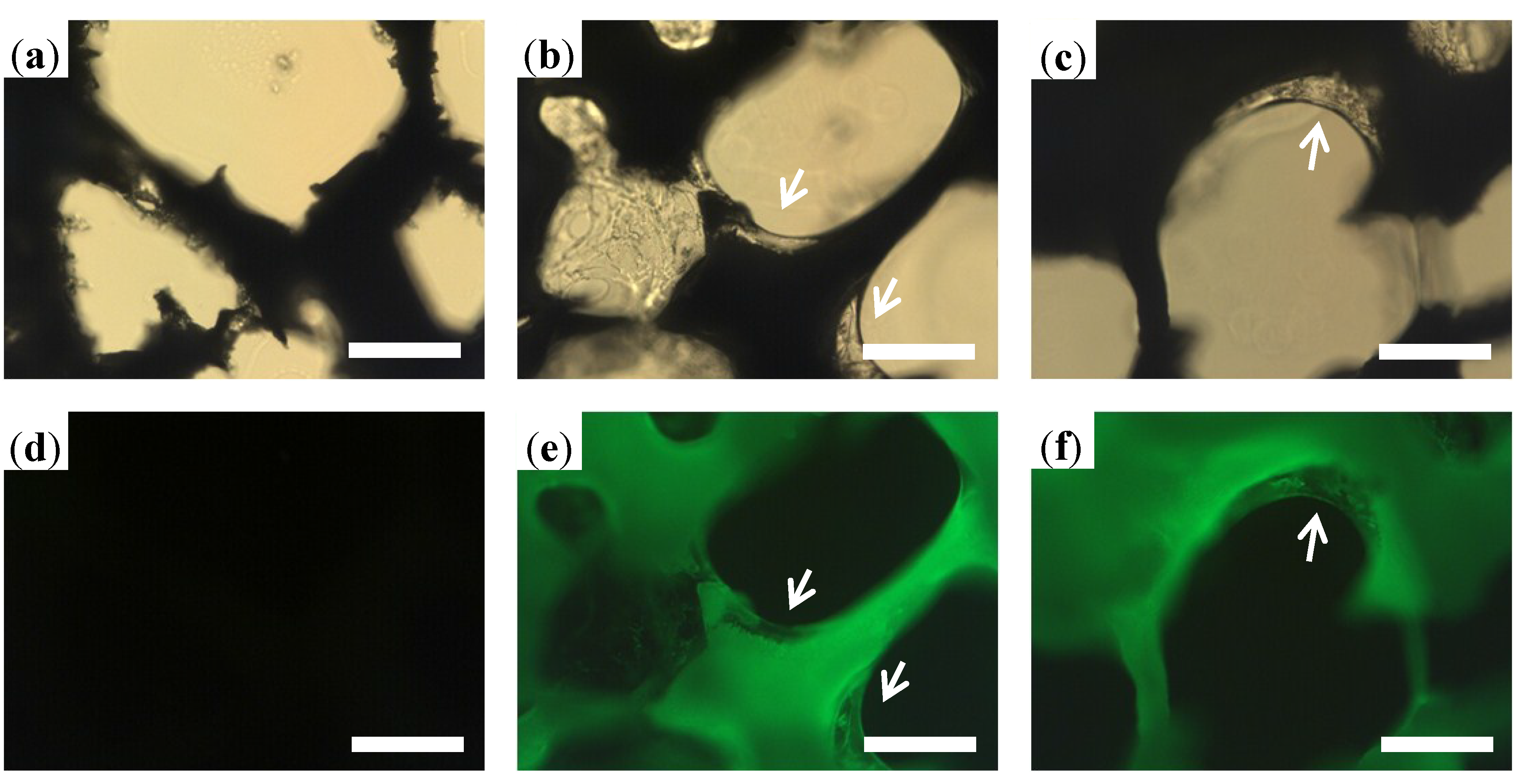
2.1.1. Microstructure Detections of 3D Porous Scaffolds
| Scaffold | Gelatin content (wt%) | Porosity (%) | Pore diameter (μm) |
|---|---|---|---|
| PLGA | 0 | 89.1 ± 8.3 | 243.6 ± 72.8 |
| PLGA/Gel | 13.8 ± 3.7 | 74.7 ± 10.1 | 219.8 ± 97.5 |
| PLGA/Gel/rhBMP-2 | 14.5 ± 4.1 | 75.5 ± 7.9 | 214.4 ± 106.3 |
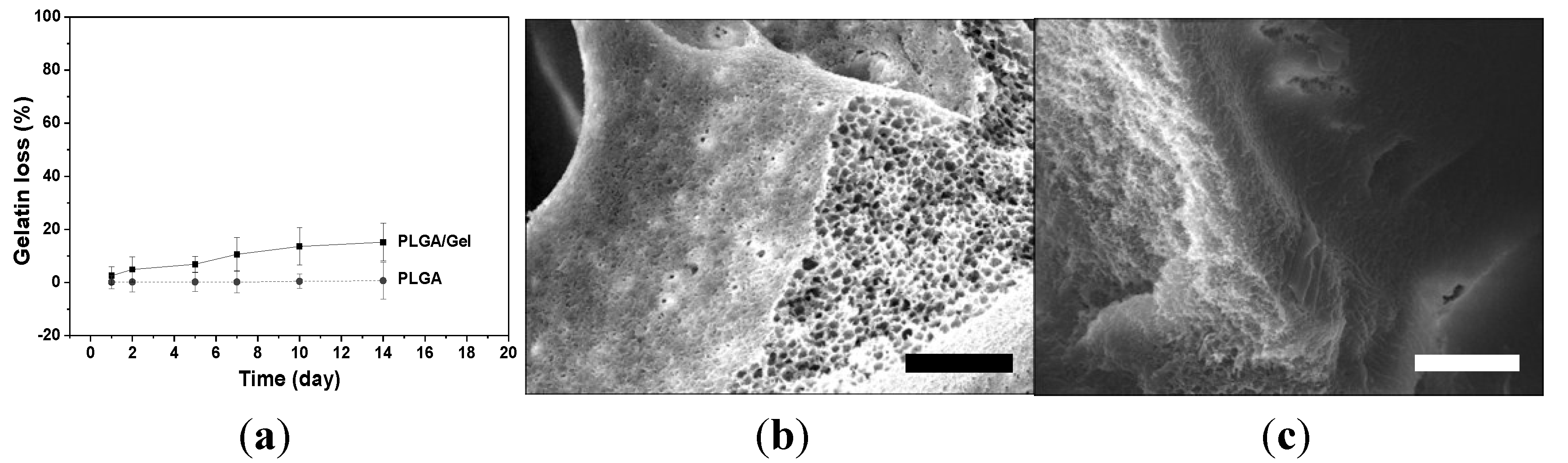
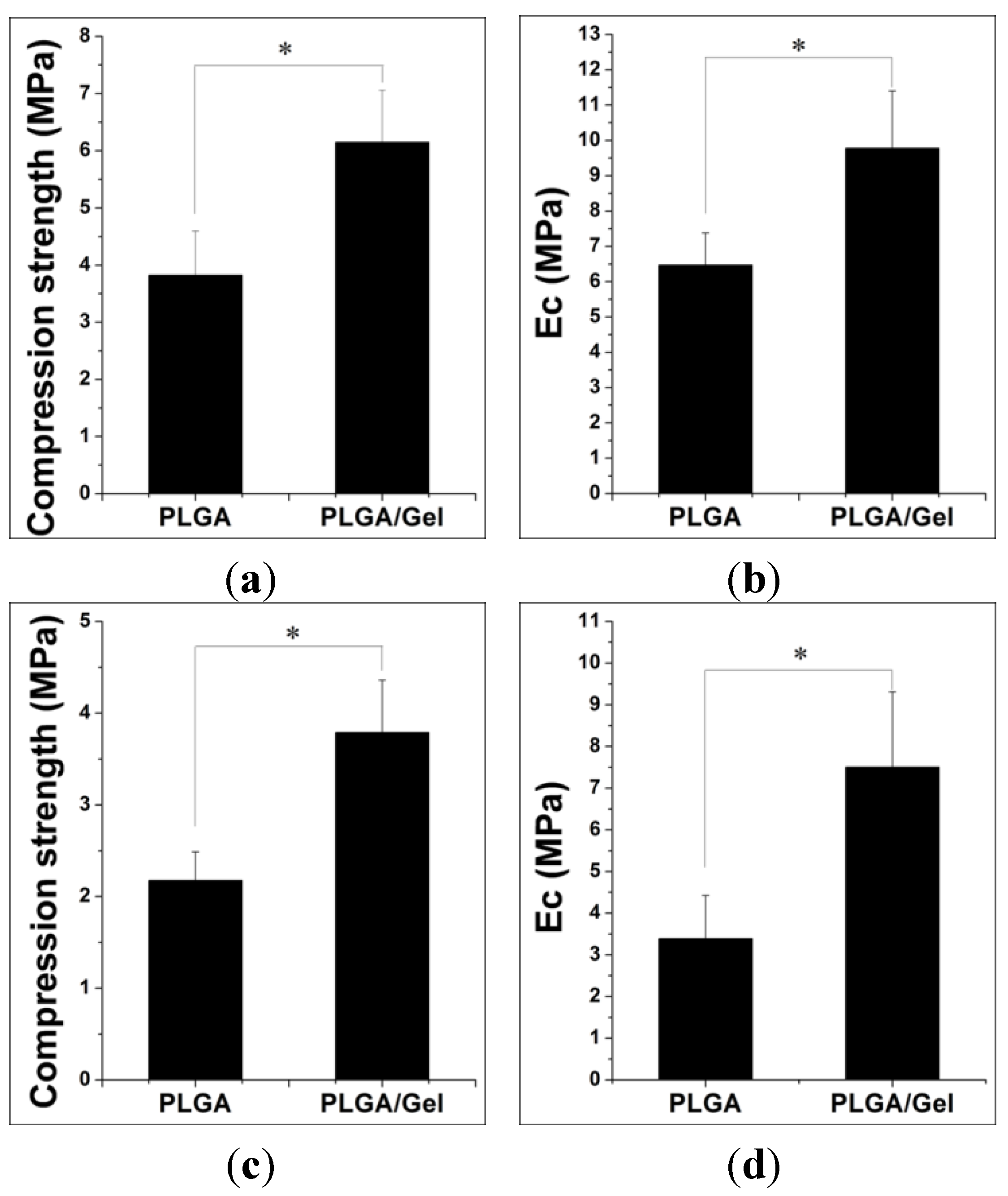
2.1.2. Mechanical Property Analyses
2.1.3. Hydrophilicity Assessments
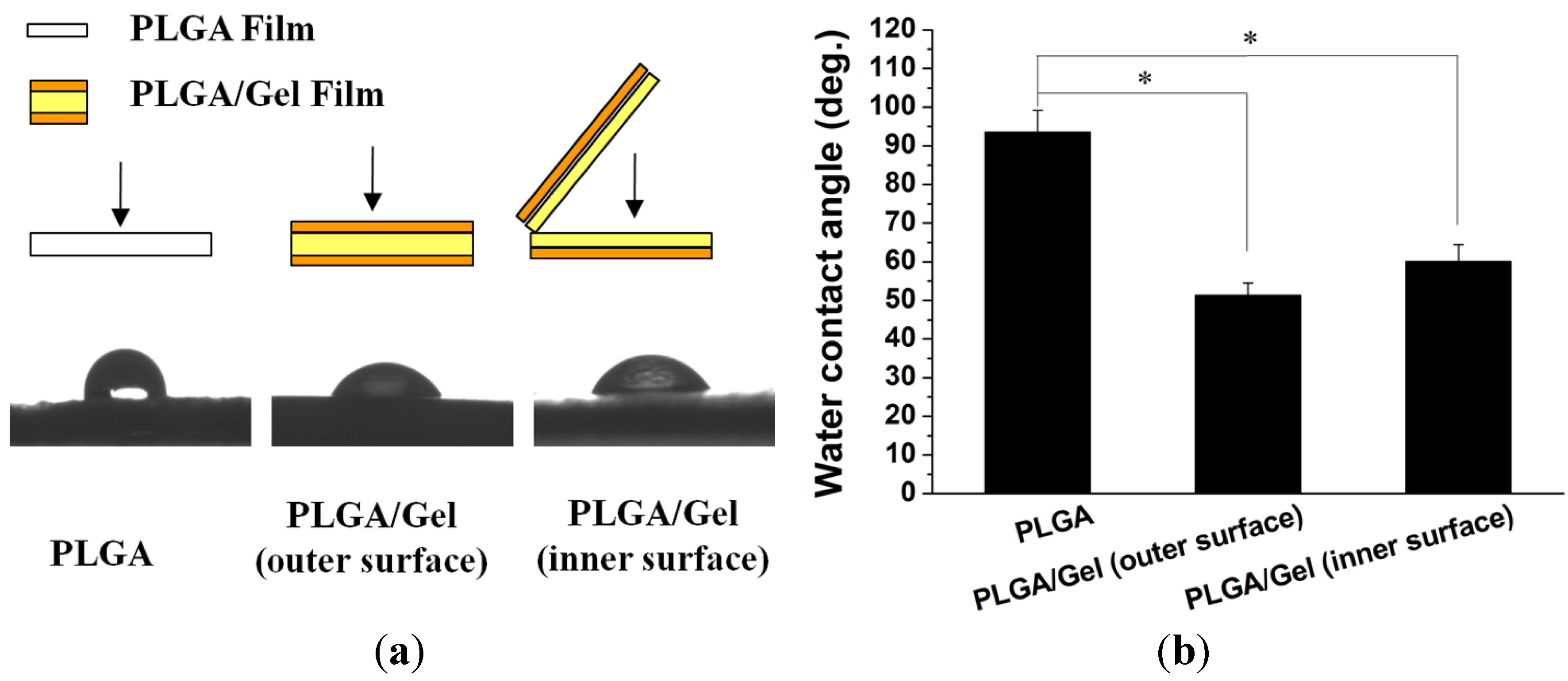
2.2. Release Kinetics of rhBMP-2

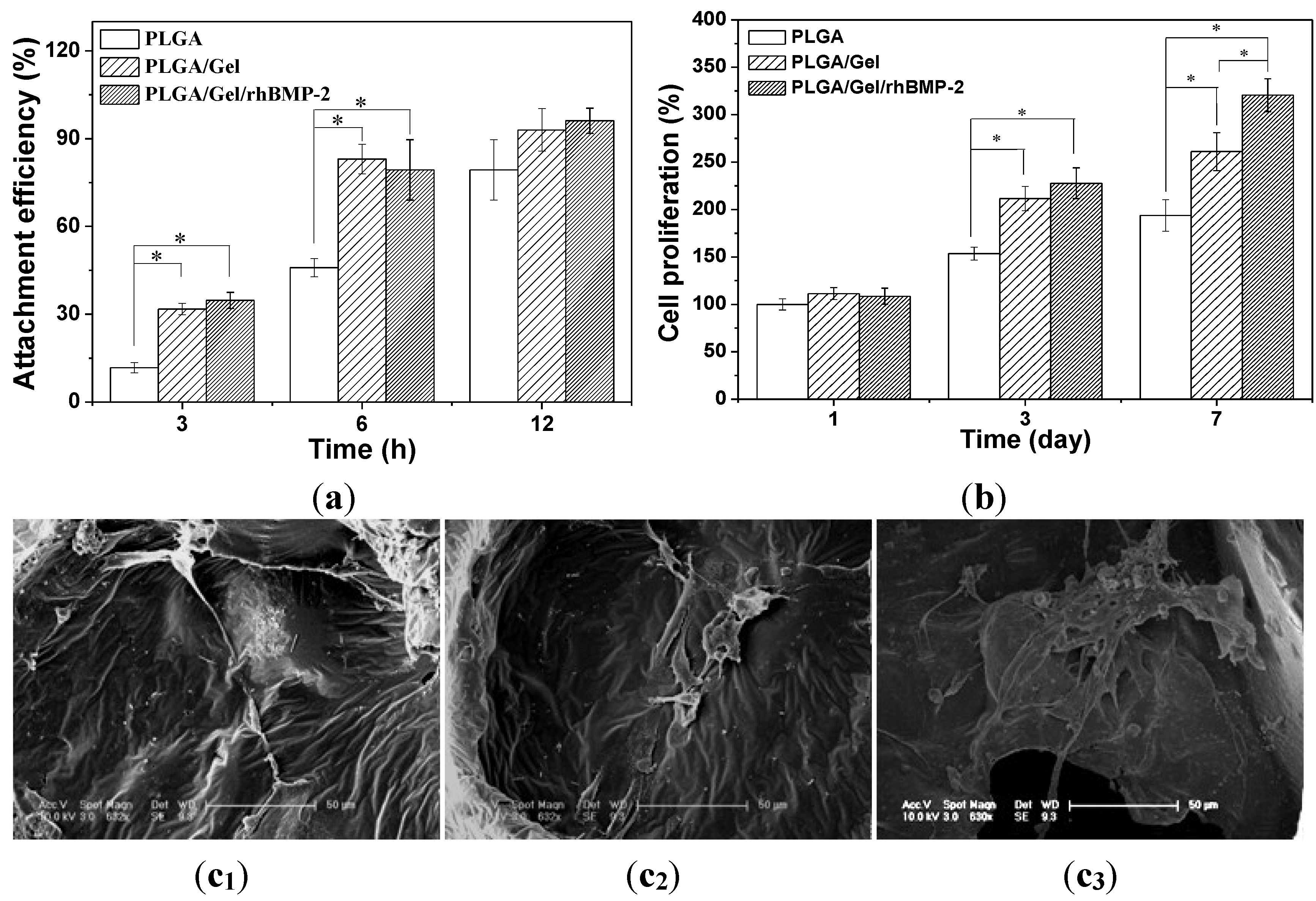
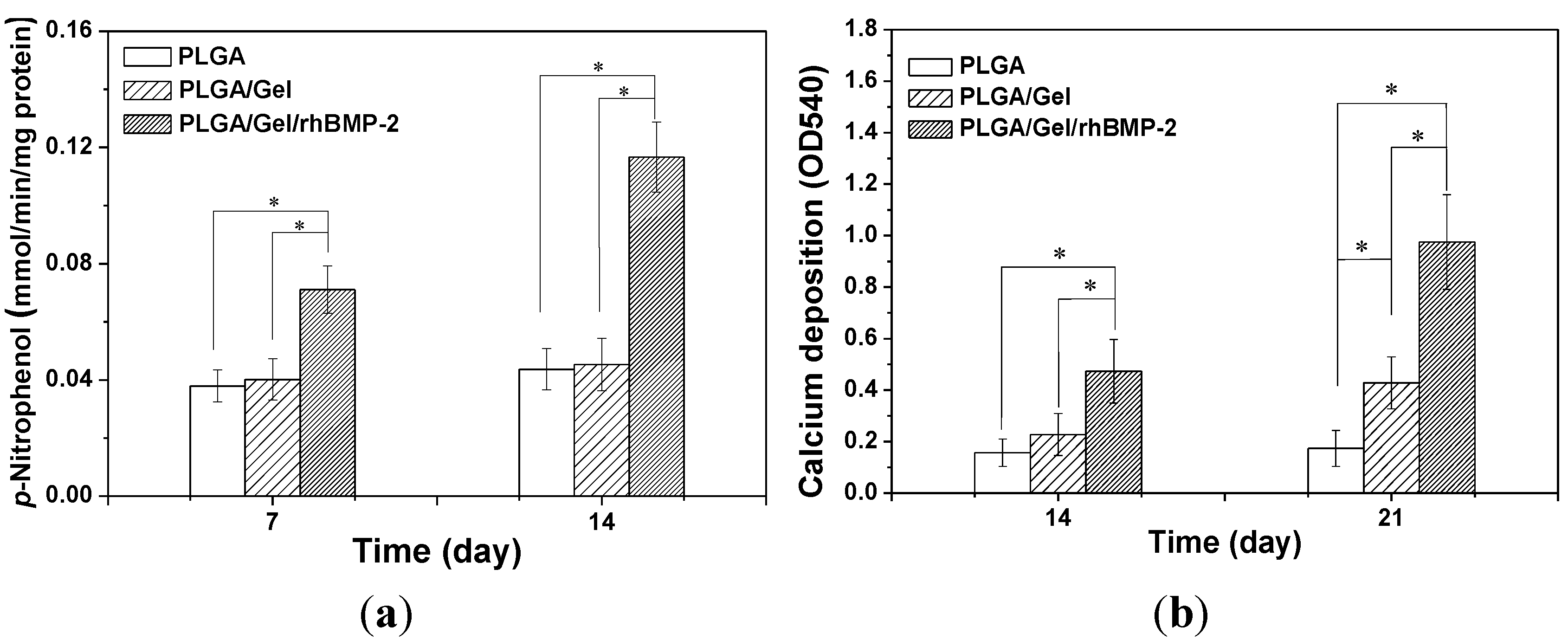
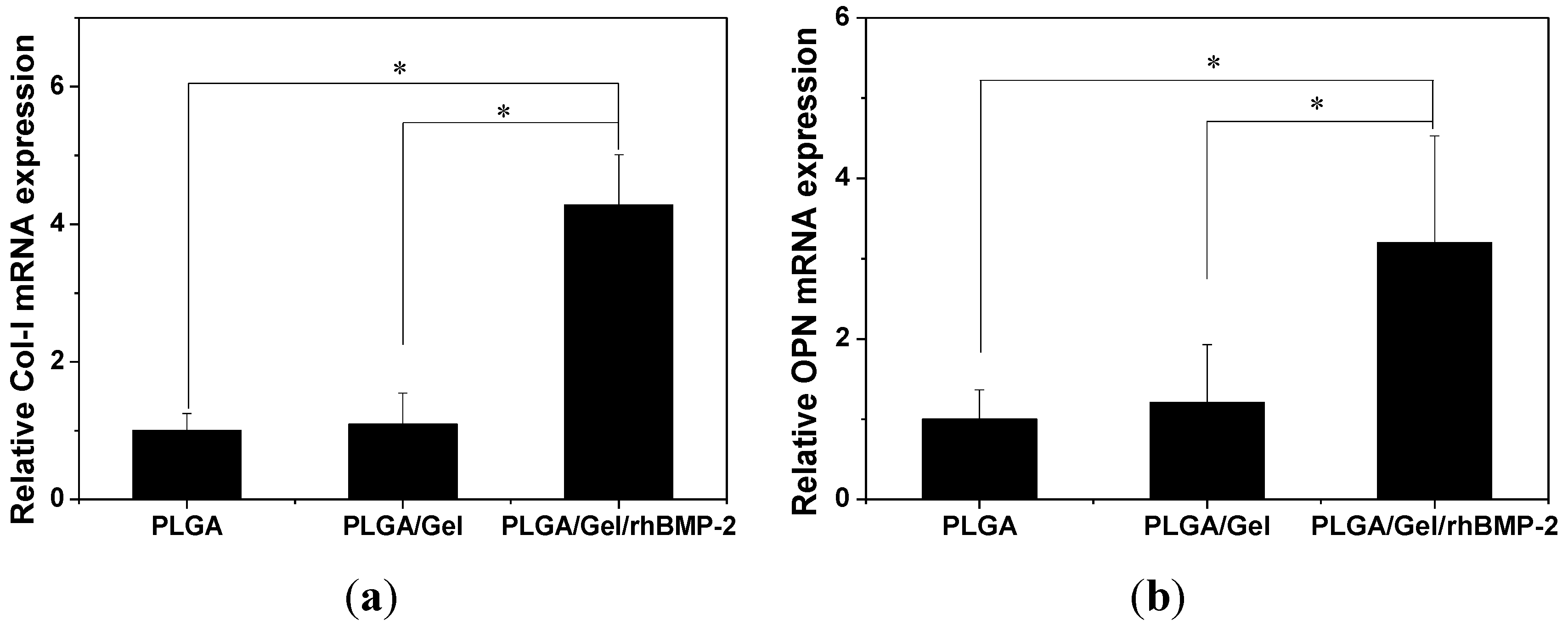
2.3. Cell Adhesion, Proliferation, and Differentiation in Scaffolds
3. Experimental Section
3.1. PLGA Scaffold Fabrication
3.2. PLGA/Gelatin (PLGA/Gel) and PLGA/Gel/rhBMP-2 Hybrid Scaffold Fabrication
3.3. Characterization of Scaffolds
3.4. In Vitro Release Study
3.5. Cell Adhesion, Proliferation, and Differentiation Assays
3.5.1. Bone Mesenchymal Stem Cell (BMSC) Isolation
3.5.2. Cell Adhesion and Proliferation Assays
3.5.3. Cell Differentiation Assays
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| COL-I | 5′-CTCGCTCACCACCTTCTC-3′ | 5′-TAACCACTGCTCCACTCTG-3′ |
| OPN | 5′-CGTGGATGATATTGATGAGGATG-3′ | 5′-TCGTCGGAGTGGTGAGAG-3′ |
| GAPDH | 5′-GATGGTGAAGGTCGGAGTG-3′ | 5′-TGTAGTGGAGGTCAATGAATGG-3′ |
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Katthagen, B.D.; Pruss, A. Bone allografting. Orthopade 2008, 37, 764–771. [Google Scholar]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Rel. Res. 1996, 329, 300–309. [Google Scholar]
- Benichou, G. Direct and indirect antigen recognition: The pathways to allograft immune rejection. Front. Biosci. 1999, 4, D476–D480. [Google Scholar]
- Shuang, F.; Hou, S.X.; Zhao, Y.T.; Zhong, H.B.; Xue, C.; Zhu, J.L.; Bu, G.Y.; Cao, Z. Characterization of an injectable chitosan-demineralized bone matrix hybrid for healing critical-size long-bone defects in a rabbit model. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 740–752. [Google Scholar]
- Zhao, X.X.; Lui, Y.S.; Toh, P.W.J.; Loo, S.C.J. Sustained release of hydrophilic L-ascorbic acid 2-phosphate magnesium from electrospun polycaprolactone scaffold-A study across blend, coaxial, and emulsion electrospinning techniques. Materials 2014, 7, 7398–7408. [Google Scholar]
- Liu, Y.; Cui, H.; Zhuang, X.; Wei, Y.; Chen, X. Electrospinning of aniline pentamer-graft-gelatin/PLLA nanofibers for bone tissue engineering. Acta Biomater. 2014, 10, 5074–5080. [Google Scholar]
- Liu, X.H.; Holzwarth, J.M.; Ma, P.X. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar]
- Asadinezhad, A.; Lehocky, M.; Saha, P.; Mozetic, M. Recent progress in surface modification of polyvinyl chloride. Materials 2012, 5, 2937–2959. [Google Scholar]
- Sengel-Turk, C.T.; Hascicek, C.; Dogan, A.L.; Esendagli, G.; Guc, D.; Gonul, N. Surface modification and evaluation of PLGA nanoparticles: The effects on cellular uptake and cell proliferation on the HT-29 cell line. J. Drug. Deliv. Sci. Tec. 2014, 24, 166–172. [Google Scholar]
- Wang, S.G.; Cui, W.J.; Bei, J.Z. Bulk and surface modifications of polylactide. Anal. Bioanal. Chem. 2005, 381, 547–556. [Google Scholar]
- Deng, C.; Tian, H.Y.; Zhang, P.B.; Sun, J.; Chen, X.S.; Jing, X.B. Synthesis and characterization of RGD peptide grafted poly(ethylene glycol)-b-poly(L-lactide)-b-poly(L-glutamic acid) triblock copolymer. Biomacromolecules 2006, 7, 590–596. [Google Scholar]
- Karde, V.; Ghoroi, C. Influence of surface modification on wettability and surface energy characteristics of pharmaceutical excipient powders. Int. J. Pharm. 2014, 475, 351–363. [Google Scholar]
- Shin, Y.M.; Jo, S.Y.; Park, J.S.; Gwon, H.J.; Jeong, S.I.; Lim, Y.M. Synergistic effect of dual-functionalized fibrous scaffold with BCP and RGD containing peptide for improved osteogenic differentiation. Macromol. Biosci. 2014, 14, 1190–1198. [Google Scholar]
- Dunn, R.L.; Cowsar, D.R.; Vanderbilt, D.P. Biodegradable in situ forming implants and methods of producing the same. U.S. Patent No. 4938763, 3 July 1990. [Google Scholar]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar]
- Ellis, M.J.; Chaudhuri, J.B. Poly(lactic-co-glycolic acid) hollow fibre membranes for use as a tissue engineering scaffold. Biotechnol. Bioeng. 2007, 96, 177–187. [Google Scholar]
- Oh, S.H.; Lee, J.H. Fabrication and characterization of hydrophilized porous PLGA nerve guide conduits by a modified immersion precipitation method. J. Biomed. Mater. Res. A 2007, 80A, 530–538. [Google Scholar]
- Amirian, J.; Linh, N.T.B.; Min, Y.K.; Lee, B.T. The effect of BMP-2 and VEGF loading of gelatin-pectin-BCP scaffolds to enhance osteoblast proliferation. J. Appl. Polym. Sci. 2015, 132, 41241:1–41241:9. [Google Scholar]
- Tan, S.; Fang, J.Y.; Yang, Z.; Nimni, M.E.; Han, B. The synergetic effect of hydrogel stiffness and growth factor on osteogenic differentiation. Biomaterials 2014, 35, 5294–5306. [Google Scholar]
- Zhang, Q.; Tan, K.; Zhang, Y.; Ye, Z.; Tan, W.S.; Lang, M. In situ controlled release of rhBMP-2 in gelatin-coated 3D porous poly(ε-caprolactone) scaffolds for homogeneous bone tissue formation. Biomacromolecules 2014, 15, 84–94. [Google Scholar]
- Fan, H.B.; Hu, Y.Y.; Zhang, C.L.; Li, X.S.; Lv, R.; Qin, L.; Zhu, R. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 2006, 27, 4573–4580. [Google Scholar]
- Shen, H.; Hu, X.X.; Yang, F.; Bel, J.Z.; Wang, S.G. Combining oxygen plasma treatment with anchorage of cationized gelatin for enhancing cell affinity of poly(lactide-co-glycolide). Biomaterials 2007, 28, 4219–4230. [Google Scholar]
- Chen, C.H.; Lee, M.Y.; Shyu, V.B.H.; Chen, Y.C.; Chen, C.T.; Chen, J.P. Surface modification of polycaprolactone scaffolds fabricated via selective laser sintering for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 389–397. [Google Scholar]
- Schloegl, W.; Marschall, V.; Witting, M.Y.; Volkmer, E.; Drosse, I.; Leicht, U.; Schieker, M.; Wiggenhorn, M.; Schaubhut, F.; Zahler, S.; et al. Porosity and mechanically optimized PLGA based in situ hardening systems. Eur. J. Pharm. Biopharm. 2012, 82, 554–562. [Google Scholar]
- Krebs, M.D.; Sutter, K.A.; Lin, A.S.P.; Guldberg, R.E.; Alsberg, E. Injectable poly(lactic-co-glycolic) acid scaffolds with in situ pore formation for tissue engineering. Acta Biomater. 2009, 5, 2847–2859. [Google Scholar]
- Hakimimehr, D.; Liu, D.M.; Troczynski, T. In-situ preparation of poly(propylene fumarate)-hydroxyapatite composite. Biomaterials 2005, 26, 7297–7303. [Google Scholar]
- Kempe, S.; Mader, K. In situ forming implants–An attractive formulation principle for parenteral depot formulations. J. Control. Release 2012, 161, 668–679. [Google Scholar]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. A 2006, 76, 264–271. [Google Scholar]
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar]
- Liebschner, M.A.K. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 2004, 25, 1697–1714. [Google Scholar]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar]
- Mckay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE (R) Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol. Lett. 2009, 31, 1825–1835. [Google Scholar]
- Yamamoto, M.; Ikada, Y.; Tabata, Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J. Biomater. Sci. Polym. Ed. 2001, 12, 77–88. [Google Scholar]
- Song, Y.; Ju, Y.; Morita, Y.; Xu, B.; Song, G. Surface functionalization of nanoporous alumina with bone morphogenetic protein 2 for inducing osteogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 120–126. [Google Scholar]
- Rowland, T.J.; Miller, L.M.; Blaschke, A.J.; Doss, E.L.; Bonham, A.J.; Hikita, S.T.; Johnson, L.V.; Clegg, D.O. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 2010, 19, 1231–1240. [Google Scholar]
- Abraham, S.; Kogata, N.; Fassler, R.; Adams, R.H. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ. Res. 2008, 102, 562–570. [Google Scholar]
- Hanson, S.; D’Souza, R.N.; Hematti, P. Biomaterial-mesenchymal stem cell constructs for immunomodulation in composite tissue engineering. Tissue Eng. Part A 2014, 20, 2162–2168. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, D.; Li, T.; Ding, J.; Liu, J.; Li, B.; Chen, X. Gelatin Tight-Coated Poly(lactide-co-glycolide) Scaffold Incorporating rhBMP-2 for Bone Tissue Engineering. Materials 2015, 8, 1009-1026. https://doi.org/10.3390/ma8031009
Wang J, Li D, Li T, Ding J, Liu J, Li B, Chen X. Gelatin Tight-Coated Poly(lactide-co-glycolide) Scaffold Incorporating rhBMP-2 for Bone Tissue Engineering. Materials. 2015; 8(3):1009-1026. https://doi.org/10.3390/ma8031009
Chicago/Turabian StyleWang, Juan, Dongsong Li, Tianyi Li, Jianxun Ding, Jianguo Liu, Baosheng Li, and Xuesi Chen. 2015. "Gelatin Tight-Coated Poly(lactide-co-glycolide) Scaffold Incorporating rhBMP-2 for Bone Tissue Engineering" Materials 8, no. 3: 1009-1026. https://doi.org/10.3390/ma8031009
APA StyleWang, J., Li, D., Li, T., Ding, J., Liu, J., Li, B., & Chen, X. (2015). Gelatin Tight-Coated Poly(lactide-co-glycolide) Scaffold Incorporating rhBMP-2 for Bone Tissue Engineering. Materials, 8(3), 1009-1026. https://doi.org/10.3390/ma8031009