Investigation of Supported Pd-Based Electrocatalysts for the Oxygen Reduction Reaction: Performance, Durability and Methanol Tolerance
Abstract
:1. Introduction
2. Results and Discussion
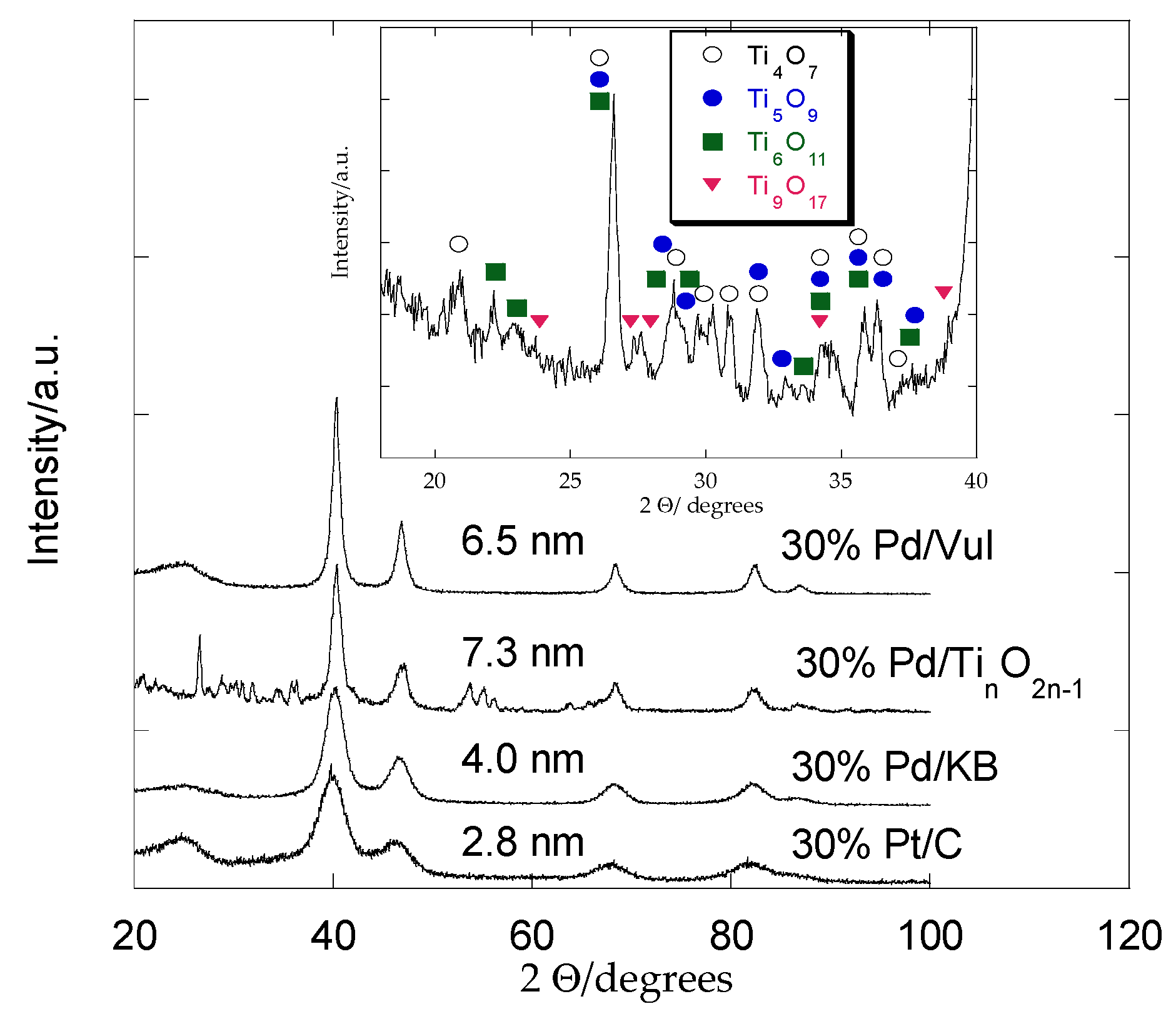
2.1. XRD Analysis
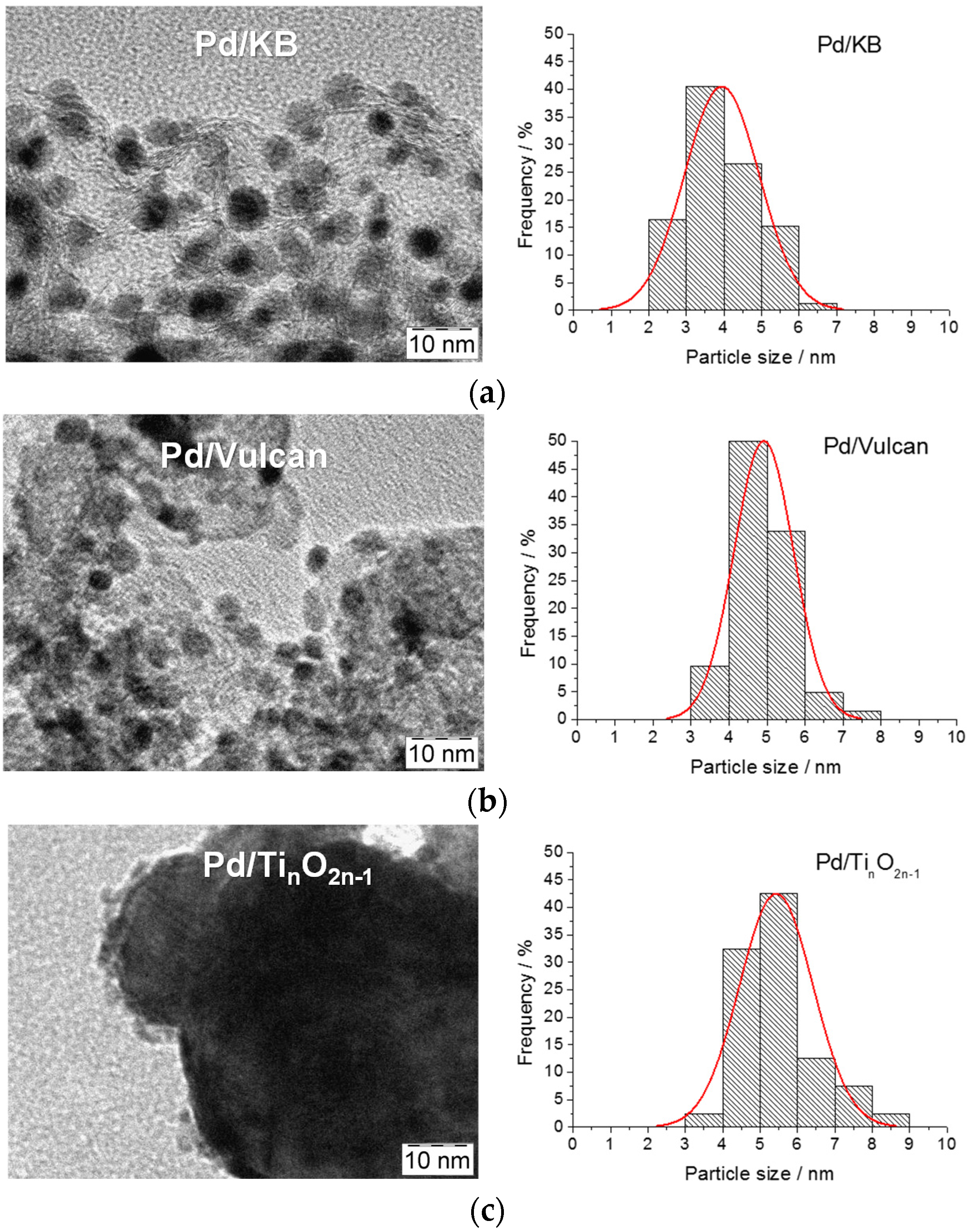
2.2. TEM Analysis
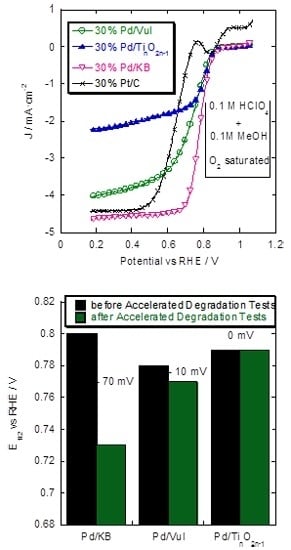
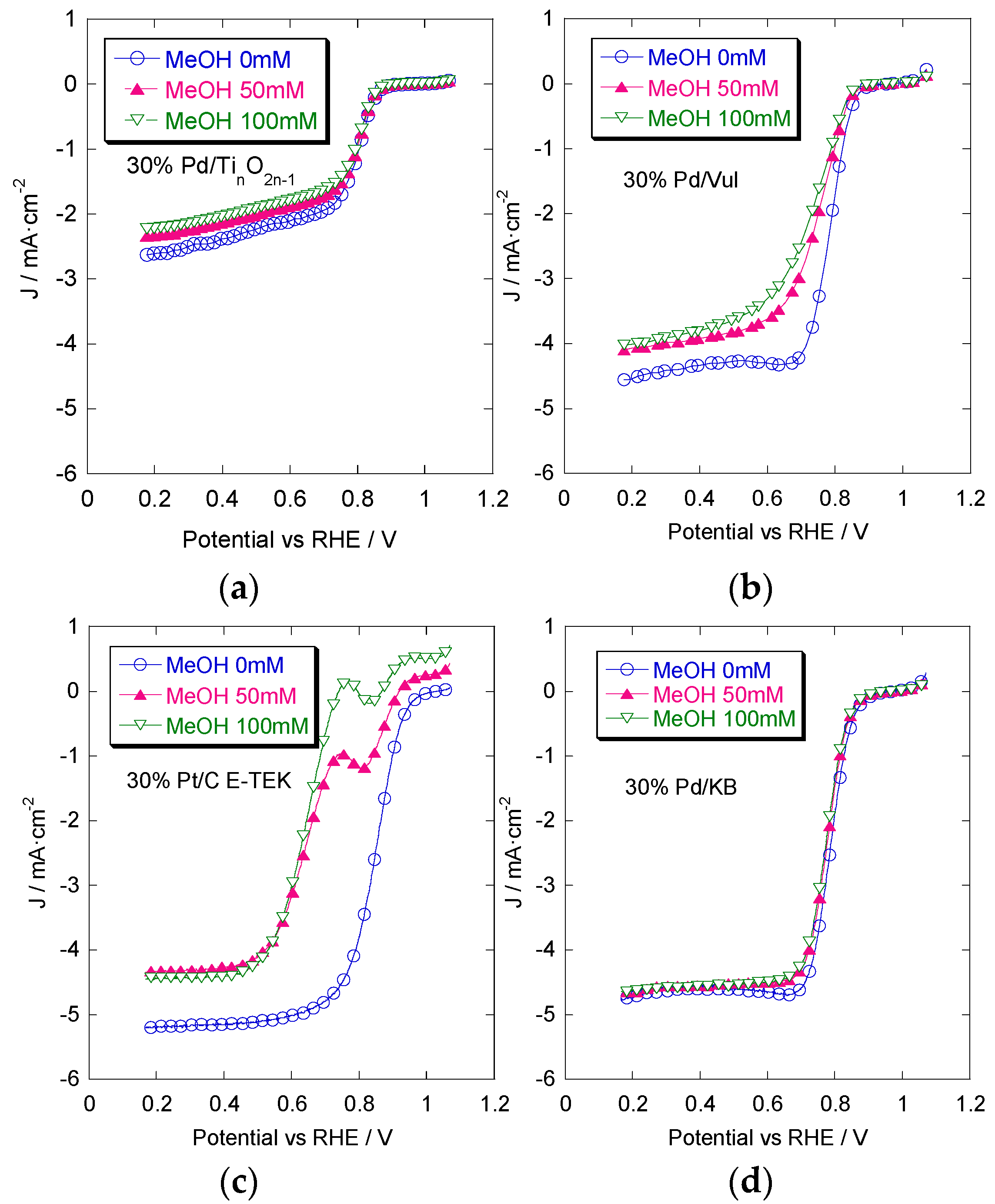
2.3. Rotating Disk Electrode (RDE) Measurements
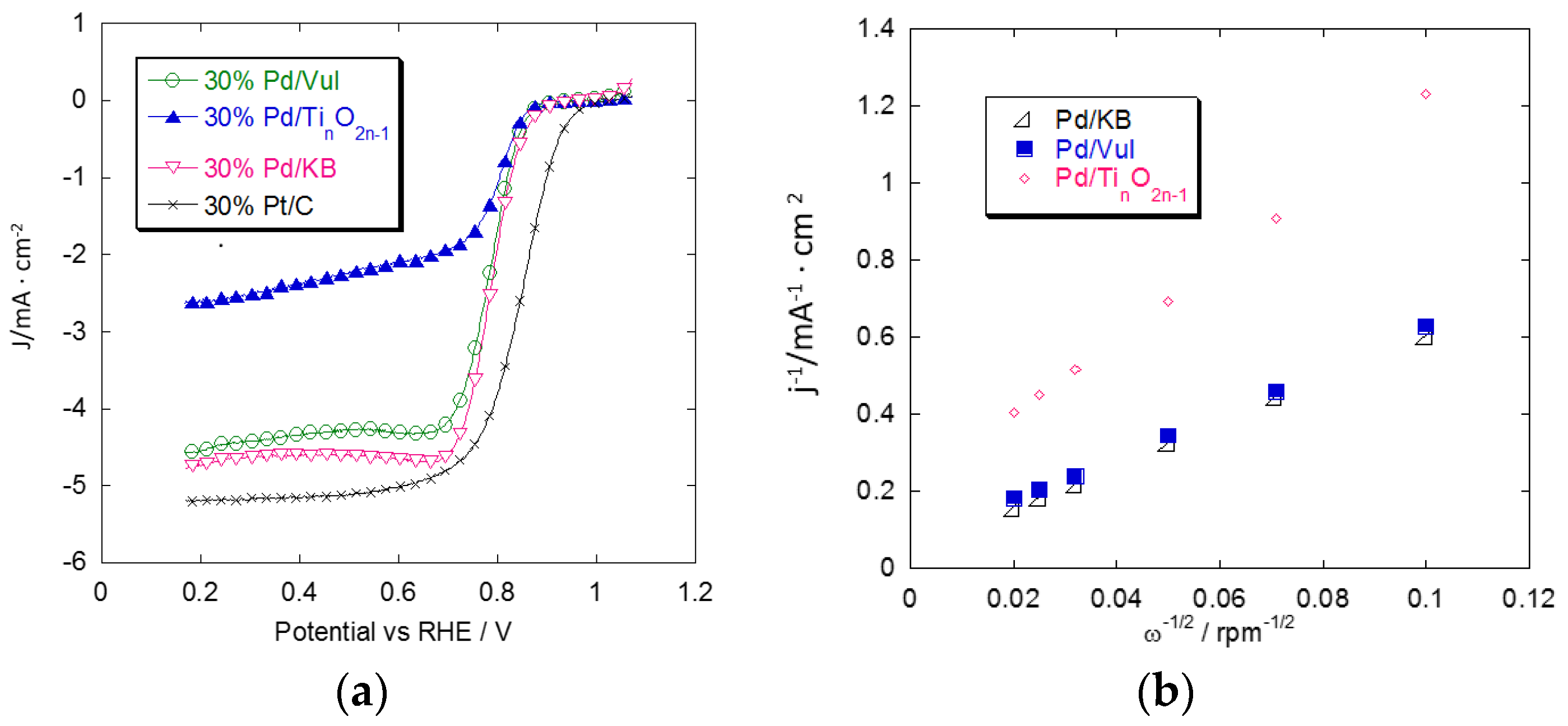
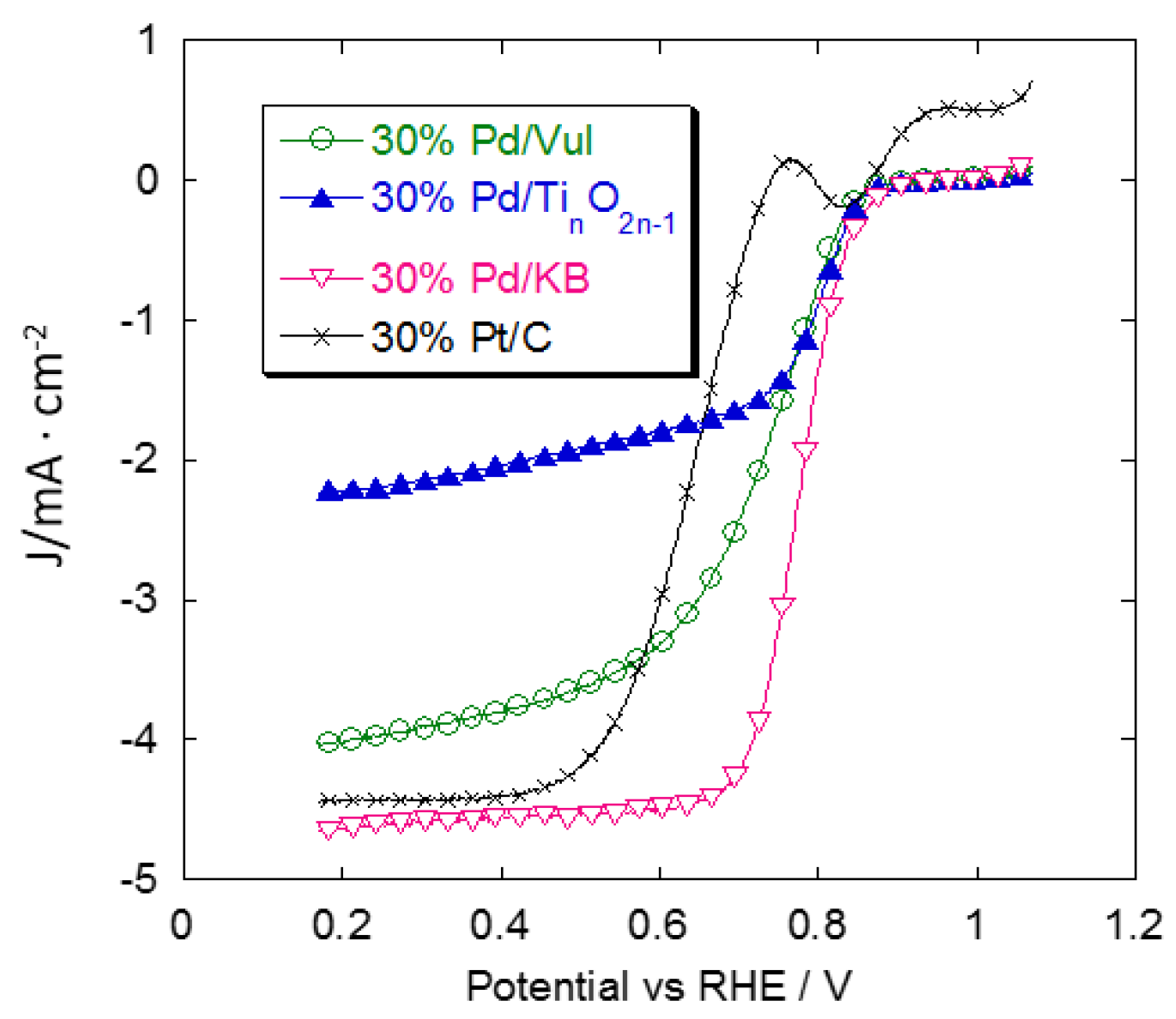
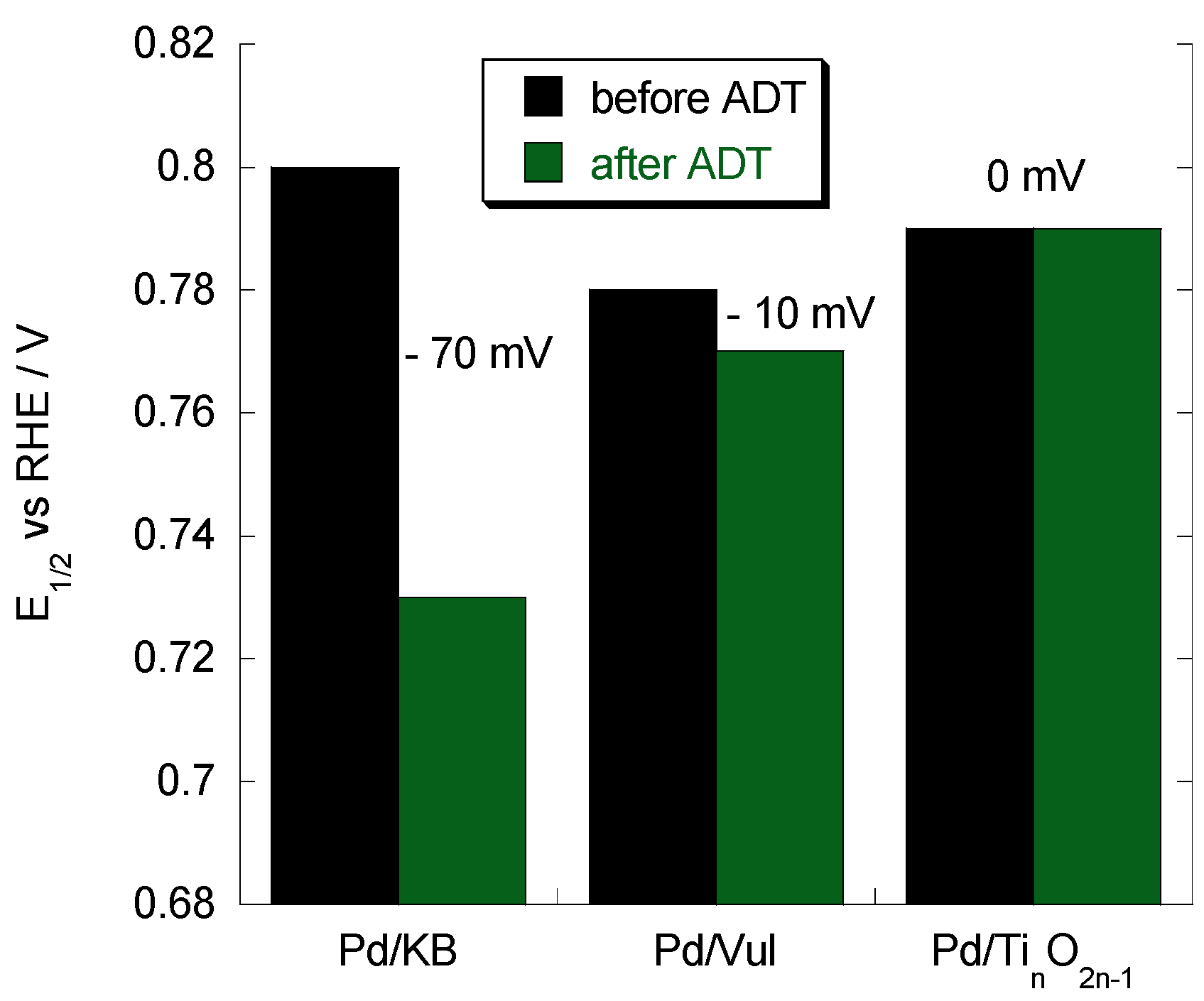
2.4. Accelerated Degradation Tests (ADTs)
| Catalyst | ECSA/m2·g−1 | Tafel Slope I Region/mV·dec−1 | Tafel slope II Region/mV·dec−1 | E1/2/V | SA at 0.85 V vs. RHE/mA·cm−2 |
|---|---|---|---|---|---|
| Before ADT | |||||
| Pd/Vul | 37.8 | 54.8 | 126.7 | 0.78 | 0.022 |
| Pd/KB | 39.0 | 63.5 | 143.0 | 0.80 | 0.033 |
| Pd/TinO2n–1 | 9.10 | 48.8 | 126.9 | 0.79 | 0.056 |
| After ADT | |||||
| Pd/Vul | 15.2 | 48.6 | 133.5 | 0.77 | 0.054 |
| Pd/KB | 11.2 | 72.4 | 127.1 | 0.73 | 0.039 |
| Pd/TinO2n–1 | 7.30 | 48.6 | 124.6 | 0.79 | 0.043 |
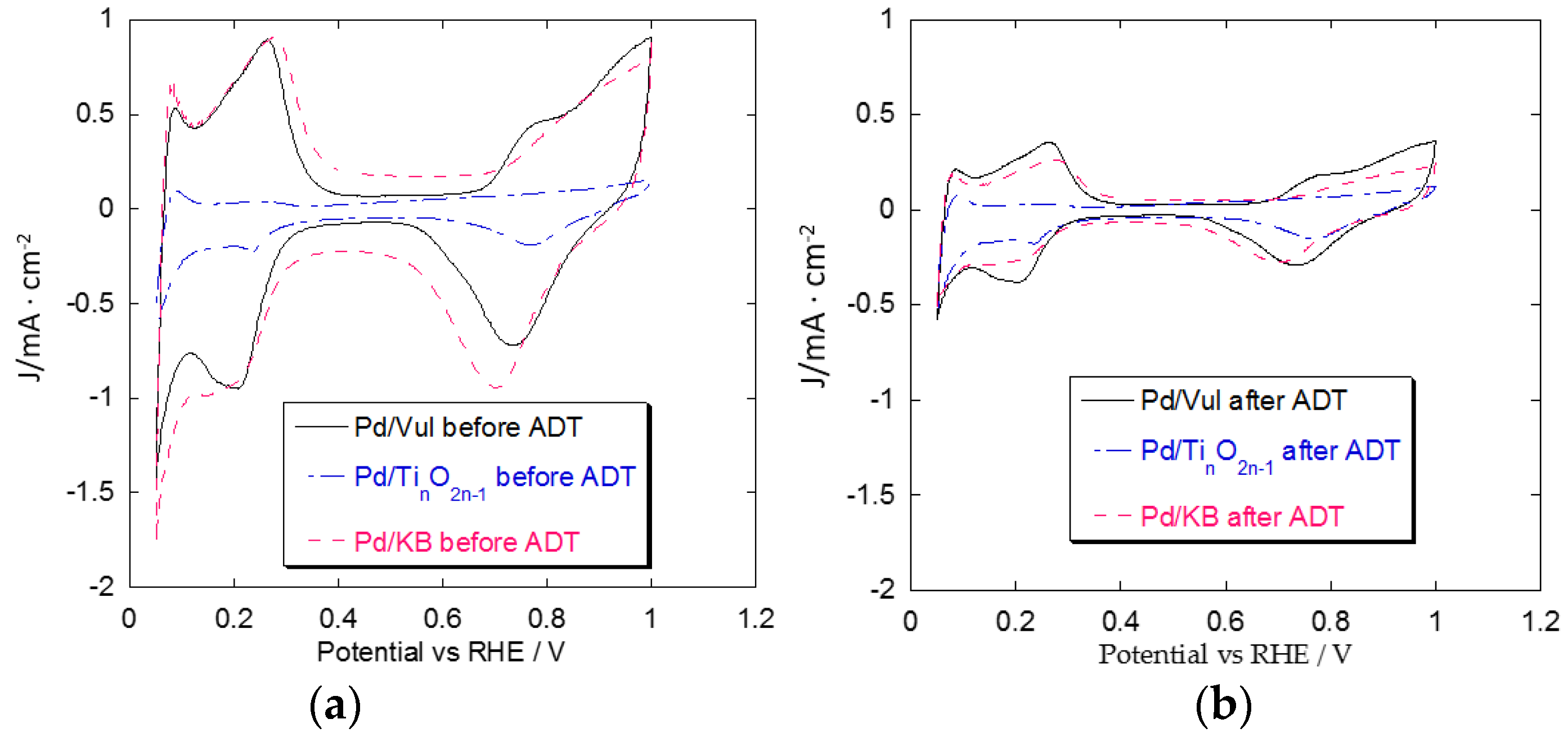
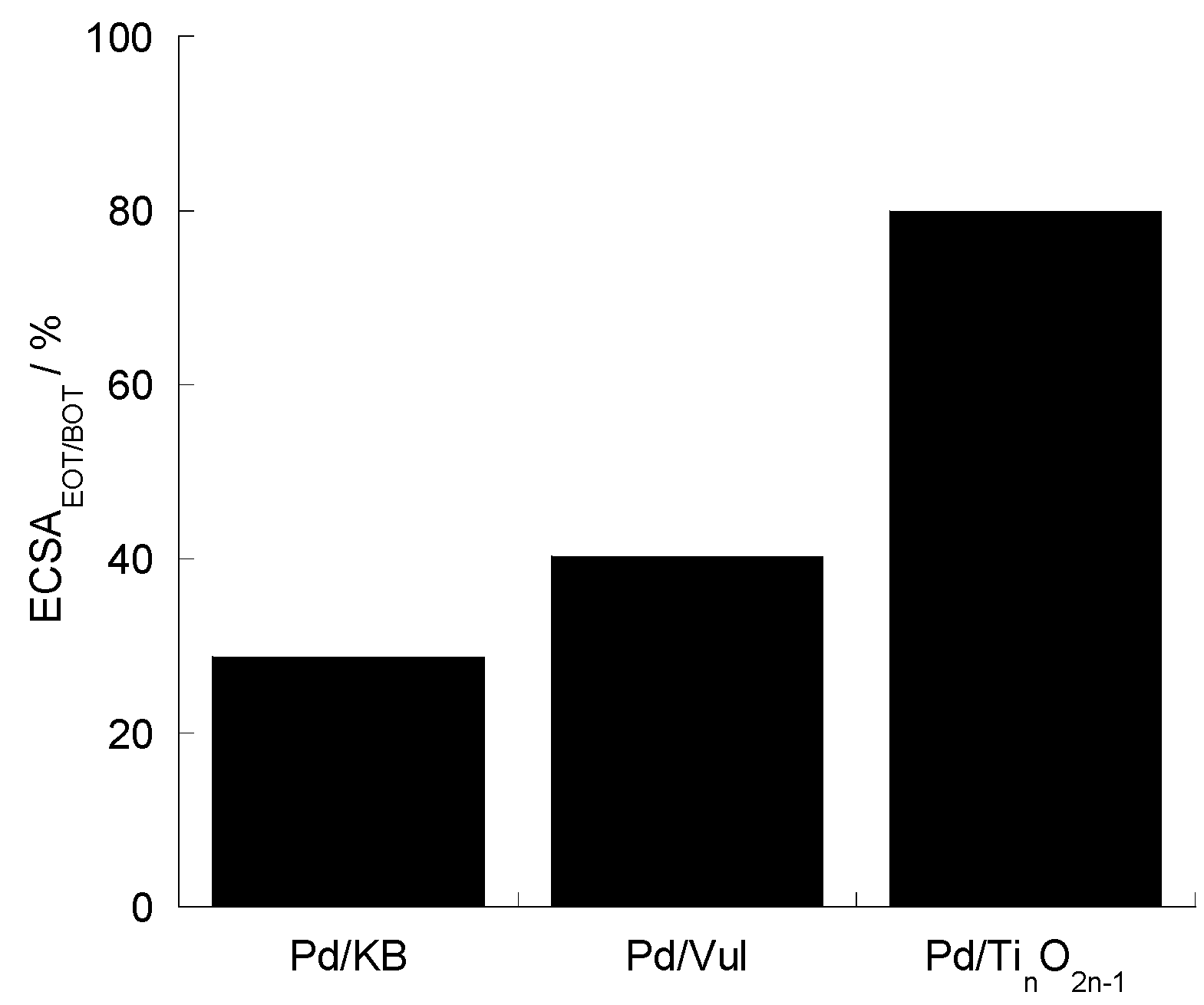

3. Materials and Methods
3.1. Pd-Based Electrocatalyst Preparation
3.2. Physico-Chemical Characterization
3.3. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel cells: Principles, types, fuels, and applications. Chem. Phys. Chem. 2000, 1, 163–193. [Google Scholar]
- Boudghene Stambouli, A.; Traversa, E. Fuel cells, an alternative to standard sources of energy. Renew. Sustain. Energy Rev. 2002, 6, 297–306. [Google Scholar] [CrossRef]
- Wee, J.H. A feasibility study of direct methanol fuel cells for laptop computers based on a cost comparison with lithium ion batteries. J. Power Sources 2007, 173, 424–436. [Google Scholar] [CrossRef]
- Zhao, T.S.; Chen, R.; Yang, W.W.; Xu, C. Small direct methanol fuel cells with passive supply of reactants. J. Power Sources 2009, 191, 185–202. [Google Scholar] [CrossRef]
- Castro Luna, A.M.; Bonesi, A.; Triaca, W.E.; Baglio, V.; Antonucci, V.; Aricò, A.S. Pt-Fe cathode catalysts to improve the oxygen reduction reaction and methanol tolerance in direct methanol fuel cells. J. Solid State Electrochem. 2008, 12, 643–649. [Google Scholar] [CrossRef]
- Park, A.R.; Lee, Y.W.; Kwak, D.H.; Roh, B.; Hwang, I.; Park, K.W. Enhanced electrocatalytic activity and stability of PdCo@Pt core-shell nanoparticles for oxygen reduction reaction. J. Appl. Electrochem. 2014, 44, 1219–1223. [Google Scholar] [CrossRef]
- Kolary-Zurowska, A.; Zieleniak, A.; Miecznikowski, K.; Baranowska, B.; Lewera, A.; Fiechter, S.; Bogdanoff, P.; Dorbandt, I.; Marassi, R.; Kulesza, P.J. Activation of methanol-tolerant carbon-supported RuSex electrocatalytic nanoparticles towards more efficient oxygen reduction. J. Solid State Electrochem. 2007, 11, 915–921. [Google Scholar] [CrossRef]
- Carrera-Cerritos, R.; Baglio, V.; Aricò, A.S.; Ledesma-Garcia, J.; Sgroi, M.F.; Pullini, D.; Pruna, A.J.; Mataix, D.B.; Fuentes-Ramirez, R.; Arriaga, L.G. Improved Pd electrocatalysis for oxygen reduction reaction in direct methanol fuel cell by reduced graphene oxide. Appl. Catal. B Environ. 2014, 144, 544–560. [Google Scholar] [CrossRef]
- Shao, M. Palladium based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Golikand, A.N.; Lohrasbi, E.; Maragheh, M.G.; Asgari, M. Carbon nano-tube supported Pt-Pd as methanol-resistant oxygen reduction electrocatalyts for enhancing catalytic activity in DMFCs. J. Appl. Electrochem. 2009, 39, 2421–2431. [Google Scholar] [CrossRef]
- Limpattayanate, S.; Hunsom, M. Effect of supports on activity and stability of Pt-Pd catalysts for oxygen reduction reaction in proton exchange membrane fuel cells. J. Solid State Electrochem. 2013, 17, 1221–1231. [Google Scholar] [CrossRef]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Alegre, C.; Gálvez, M.E.; Moliner, R.; Baglio, V.; Aricò, A.S.; Lázaro, M.J. Towards an optimal synthesis route for the preparation of highly mesoporous carbon xerogel-supported Pt catalysts for the oxygen reduction reaction. Appl. Catal. B Environ. 2014, 147, 947–957. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemistry of graphene, graphene oxide and other graphenoids: Review. Electrochem. Commun. 2013, 36, 14–18. [Google Scholar] [CrossRef]
- Sebastián, D.; Alegre, C.; Gálvez, M.E.; Moliner, R.; Lázaro, M.J.; Aricò, A.S.; Baglio, V. Towards new generation fuel cell electrocatalysts based on xerogel-nanofiber carbon composites. J. Mater. Chem. A 2014, 2, 13713–13722. [Google Scholar] [CrossRef]
- Zeng, J.; Francia, C.; Dumitrescu, M.A.; Monteverde Videla, A.H.A.; Ijeri, V.S.; Specchia, S.; Spinelli, P. Electrochemical performance of Pt-based catalysts supported on different ordered mesoporous carbons (Pt/OMCs) for oxygen reduction reaction. Ind. Eng. Chem. Res. 2012, 51, 7500–7509. [Google Scholar] [CrossRef]
- Sebastián, D.; Garcìa Ruiz, A.; Suelves, I.; Moliner, R.; Lázaro, M.J.; Baglio, V.; Stassi, A.; Aricò, A.S. Enhanced oxygen reduction reaction activity and durability of Pt catalysts supported on carbon nanofibers. Appl. Catal. B Environ. 2012, 115–116, 269–275. [Google Scholar] [CrossRef]
- Ferreira, P.J.; Shao-Horn, Y.; Morgan, D.; Makharia, S.; Kocha, S.; Gasteiger, H.A. Instability of Pt/C electrocatalysts in proton exchange membrane fuel cells: A mechanistic investigation. J. Electrochem. Soc. 2005, 152, A2256–A2271. [Google Scholar] [CrossRef]
- Stassi, A.; Gatto, I.; Baglio, V.; Passalacqua, E.; Aricò, A.S. Oxide-supported PtCo alloy catalyst for intermediate temperature polymer electrolyte fuel cells. Appl. Catal. B Environ. 2013, 142–143, 15–24. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wills, R.G.A. The continuing development of Magnéli phase titanium sub-oxides and Ebonex® electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; D’Urso, C.; Antonucci, V.; Aricò, A.S. Preparation and characterization of titanium suboxides as conductive supports of IrO2 electrocatalysts for application in SPE electrolyzers. Electrochim. Acta 2009, 54, 6292–6299. [Google Scholar] [CrossRef]
- Denaro, T.; Baglio, V.; Girolamo, M.; Antonucci, V.; Aricò, A.S.; Matteucci, F.; Ornelas, R. Investigation of low cost carbonaceous materials for application as counter electrode in dye sensitized solar cells. J. Appl. Electrochem. 2009, 39, 2173–2179. [Google Scholar] [CrossRef]
- Brocato, S.; Serov, A.; Atanassov, P. pH dependence of catalytic activity for ORR of the non-PGM catalyst derived from heat-treated Fe-phenantroline. Eletrochim. Acta. 2013, 87, 361–365. [Google Scholar] [CrossRef]
- Pattabiraman, R. Electrochemical investigations on carbon supported palladium catalysts. Appl. Catal. A Gen. 1997, 153, 9–20. [Google Scholar] [CrossRef]
- Siracusano, S.; Stassi, A.; Modica, E.; Baglio, V.; Aricò, A.S. Preparation and characterisation of Ti oxide based catalyst supports for low temperature fuel cells. Int. J. Hydrogen. Energy 2013, 38, 11600–11608. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Schalkwijk, W.V. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K. Particle size effects for oxygen reduction on highly dispersed platinum in acid electrolytes. J. Electrochem. Soc. 1990, 137, 845–848. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Sarapuu, A.; Tammeveski, K.; Vidal-Iglesias, F.J.; Solla-Gullòn, J.; Feliu, J.M. Electroreduction of oxygen on Vulcan carbon supported Pd nanoparticles and Pd-M nanoalloys in acid and alkaline solutions. Electrochim. Acta 2011, 56, 6702–6708. [Google Scholar] [CrossRef]
- Pech-Pech, I.E.; Gervasio Dominic, F.; Godìnez-Garcia, A.; Solorza-Feria, O. Nanoparticles of Ag with a Pt and Pd rich surface supported on carbon as new catalyst for the oxygen electroreduction reaction (ORR) in acid electrolytes: Part 1. J. Power Sources 2015, 276, 365–373. [Google Scholar] [CrossRef]
- Aricò, A.S.; Stassi, A.; Modica, E.; Ornelas, R.; Gatto, I.; Passalacqua, E.; Antonucci, V. Performance and degradation of high temperature polymer electrolyte fuel cell catalysts. J. Power Sources 2008, 178, 525–536. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, J.; Wang, Y.; Lin, Y. Novel catalyst support materials for PEM fuel: Current status and future prospects. J. Mater. Chem. 2009, 19, 46–59. [Google Scholar] [CrossRef]
- Aricò, A.S.; Stassi, A.; D’Urso, C.; Sebastiàn, D.; Baglio, V. Synthesis of Pd3Co1@Pt/C core-shell catalysts for methanol-tolerant cathodes of direct methanol fuel cells. Chem. Eur. J. 2014, 20, 10679–10684. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Vecchio, C.; Alegre, C.; Sebastián, D.; Stassi, A.; Aricò, A.S.; Baglio, V. Investigation of Supported Pd-Based Electrocatalysts for the Oxygen Reduction Reaction: Performance, Durability and Methanol Tolerance. Materials 2015, 8, 7997-8008. https://doi.org/10.3390/ma8125438
Lo Vecchio C, Alegre C, Sebastián D, Stassi A, Aricò AS, Baglio V. Investigation of Supported Pd-Based Electrocatalysts for the Oxygen Reduction Reaction: Performance, Durability and Methanol Tolerance. Materials. 2015; 8(12):7997-8008. https://doi.org/10.3390/ma8125438
Chicago/Turabian StyleLo Vecchio, Carmelo, Cinthia Alegre, David Sebastián, Alessandro Stassi, Antonino S. Aricò, and Vincenzo Baglio. 2015. "Investigation of Supported Pd-Based Electrocatalysts for the Oxygen Reduction Reaction: Performance, Durability and Methanol Tolerance" Materials 8, no. 12: 7997-8008. https://doi.org/10.3390/ma8125438
APA StyleLo Vecchio, C., Alegre, C., Sebastián, D., Stassi, A., Aricò, A. S., & Baglio, V. (2015). Investigation of Supported Pd-Based Electrocatalysts for the Oxygen Reduction Reaction: Performance, Durability and Methanol Tolerance. Materials, 8(12), 7997-8008. https://doi.org/10.3390/ma8125438