3.1. Characterization
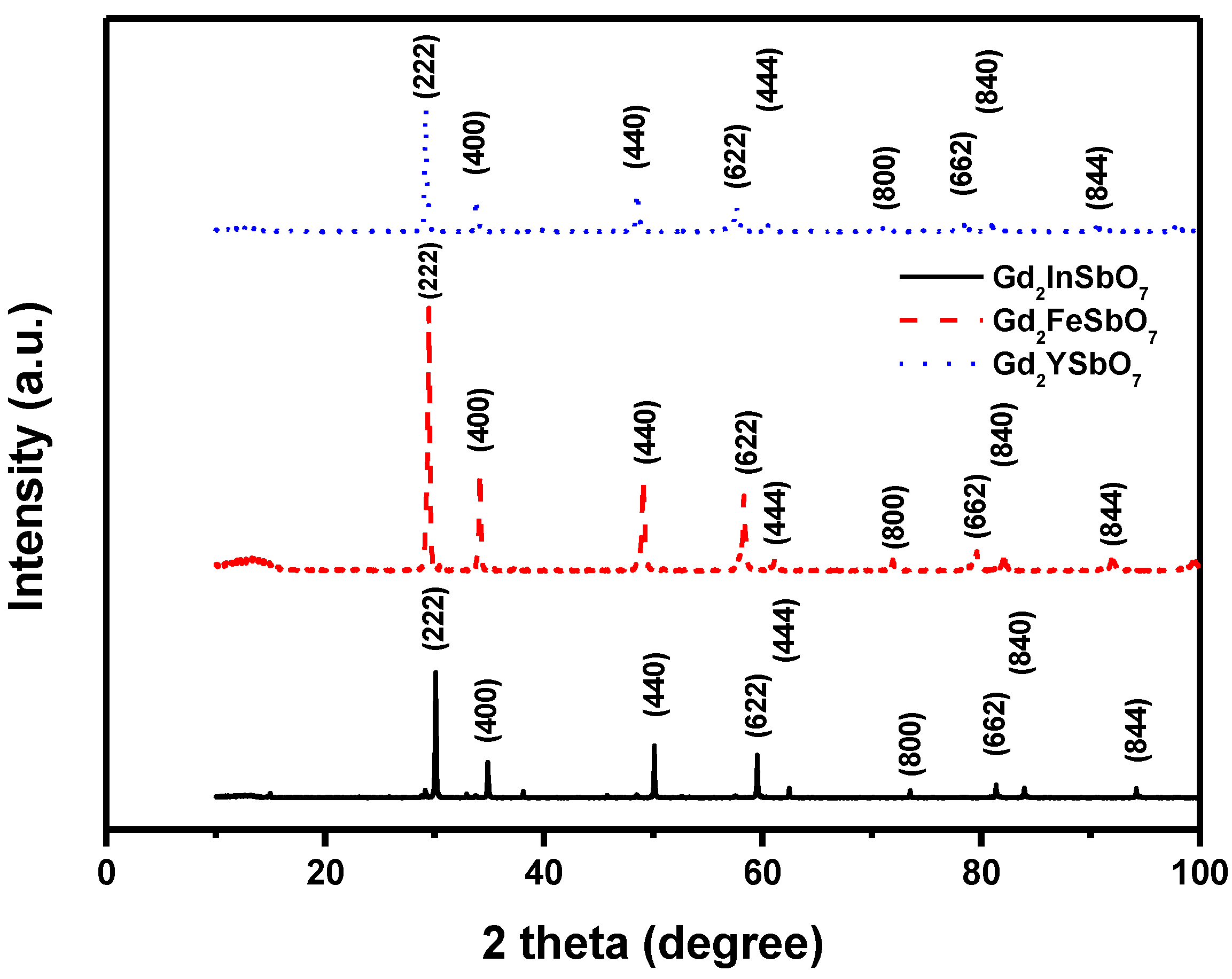
Figure 1 shows the X-ray powder diffraction patterns of Gd
2FeSbO
7, Gd
2InSbO
7 and Gd
2YSbO
7. It could be seen from
Figure 1 that Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was a single phase. The calculations of lattice parameters were performed with the program of Cambridge serial total energy package (CASTEP) and first-principles simulation. The CASTEP package was provided by Materials Studio software and the CASTEP calculation was composed of the plane-wave pseudopotential total energy method according to the density functional theory. Thus, our calculations were based on the plane-wave-based density functional theory (DFT) in generalized gradient approximations (GGA) with Perdew–Burke–Ernzerh of (PBE) exchange-correlation potential.
In order to obtain the crystal lattice parameters, Rietveld refinement from X-Ray Diffraction (XRD) data was performed with DBWS software, experimental XRD data and simulation XRD data. The uncertainty of the refined lattice parameters lay in the estimated standard deviation (e.s.d.), calculated by the full pattern fitting program. However, e.s.d. was a measure of precision rather than of accuracy, and these two terms must not be confused. For a sound estimation of the measurement uncertainty of lattice parameters that were refined from XRD data, more information was needed than just the e.s.d. that was provided by the Rietveld refinement of the diffraction pattern of the sample. The outcome of refinements for Gd
2InSbO
7 generated the unweighted
R factors,
Rp = 12.13% with space group Fd3m. As for Gd
2YSbO
7,
Rp was 12.16% with space group Fd3m. As for Gd
2FeSbO
7,
Rp was 16.20% with space group Fd3m. According to the Rietveld analysis, Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 had the pyrochlore-type structure and a cubic crystal system which owned a space group Fd3m. The atomic coordinates and structural parameters of Gd
2FeSbO
7, Gd
2InSbO
7 and Gd
2YSbO
7 are listed in
Table 1,
Table 2 and
Table 3, respectively. The lattice parameter
a for Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was 10.276026 Å, 10.449546 Å or 10.653651 Å. Moreover, the XRD results showed that two theta angles of each reflection of Gd
2FeSbO
7 changed with Fe
3+ being substituted by In
3+ or Y
3+. The lattice parameter α increased from α = 10.276026 Å for Gd
2FeSbO
7 to α = 10.449546 Å for Gd
2InSbO
7, which indicated a decrease in the lattice parameter of the photocatalyst with a decrease of the M ionic radii, Fe
3+ (0.78 Å) < In
3+ (0.92 Å). The lattice parameter α also increased from α = 10.276026 Å for Gd
2FeSbO
7 to α = 10.653651 Å for Gd
2YSbO
7, which indicated a decrease in lattice parameter of the photocatalyst with decrease of the M ionic radii, Fe
3+ (0.78 Å) < Y
3+ (1.019 Å). Meanwhile, The lattice parameter α also increased from α = 10.449546 Å for Gd
2InSbO
7 to α = 10.653651 Å for Gd
2YSbO
7, which indicated a decrease in the lattice parameter of the photocatalyst with a decrease of the M ionic radii, In
3+ (0.92 Å) < Y
3+ (1.019 Å).
Figure 1.
X-ray powder diffraction pattern of Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 prepared by a solid-state reaction method at 1250 °C, 1320 °C or 1320 °C
Figure 1.
X-ray powder diffraction pattern of Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 prepared by a solid-state reaction method at 1250 °C, 1320 °C or 1320 °C
Table 1.
Structural parameters of Gd2YSbO7 prepared by solid-state reaction method. x, y and z refer to the position coordinates of different atoms in one unit cell, respectively.
Table 1.
Structural parameters of Gd2YSbO7 prepared by solid-state reaction method. x, y and z refer to the position coordinates of different atoms in one unit cell, respectively.
| Atom | x | y | z | Occupation Factor |
|---|
| Gd | 0.00000 | 0.00000 | 0.00000 | 1.0 |
| Y | 0.50000 | 0.50000 | 0.50000 | 0.5 |
| Sb | 0.50000 | 0.50000 | 0.50000 | 0.5 |
| O(1) | −0.16519 | 0.12500 | 0.12500 | 1.0 |
| O(2) | 0.12500 | 0.12500 | 0.12500 | 1.0 |
Table 2.
Structural parameters of Gd2BiSbO7 prepared by solid-state reaction method. x, y and z refer to the position coordinates of different atoms in one unit cell, respectively.
Table 2.
Structural parameters of Gd2BiSbO7 prepared by solid-state reaction method. x, y and z refer to the position coordinates of different atoms in one unit cell, respectively.
| Atom | x | y | z | Occupation Factor |
|---|
| Gd | 0.00000 | 0.00000 | 0.00000 | 1.0 |
| Bi | 0.50000 | 0.50000 | 0.50000 | 0.5 |
| Sb | 0.50000 | 0.50000 | 0.50000 | 0.5 |
| O(1) | −0.14538 | 0.12500 | 0.12500 | 1.0 |
| O(2) | 0.12500 | 0.12500 | 0.12500 | 1.0 |
Table 3.
Structural parameters of Gd2FeSbO7 prepared by solid-state reaction method. x, y and z refer to the position coordinates of different atoms in one unit cell, respectively.
Table 3.
Structural parameters of Gd2FeSbO7 prepared by solid-state reaction method. x, y and z refer to the position coordinates of different atoms in one unit cell, respectively.
| Atom | x | y | z | Occupation Factor |
|---|
| Gd | 0.00000 | 0.00000 | 0.00000 | 1.0 |
| Fe | 0.50000 | 0.50000 | 0.50000 | 0.5 |
| Sb | 0.50000 | 0.50000 | 0.50000 | 0.5 |
| O(1) | −0.20249 | 0.12500 | 0.12500 | 1.0 |
| O(2) | 0.12500 | 0.12500 | 0.12500 | 1.0 |
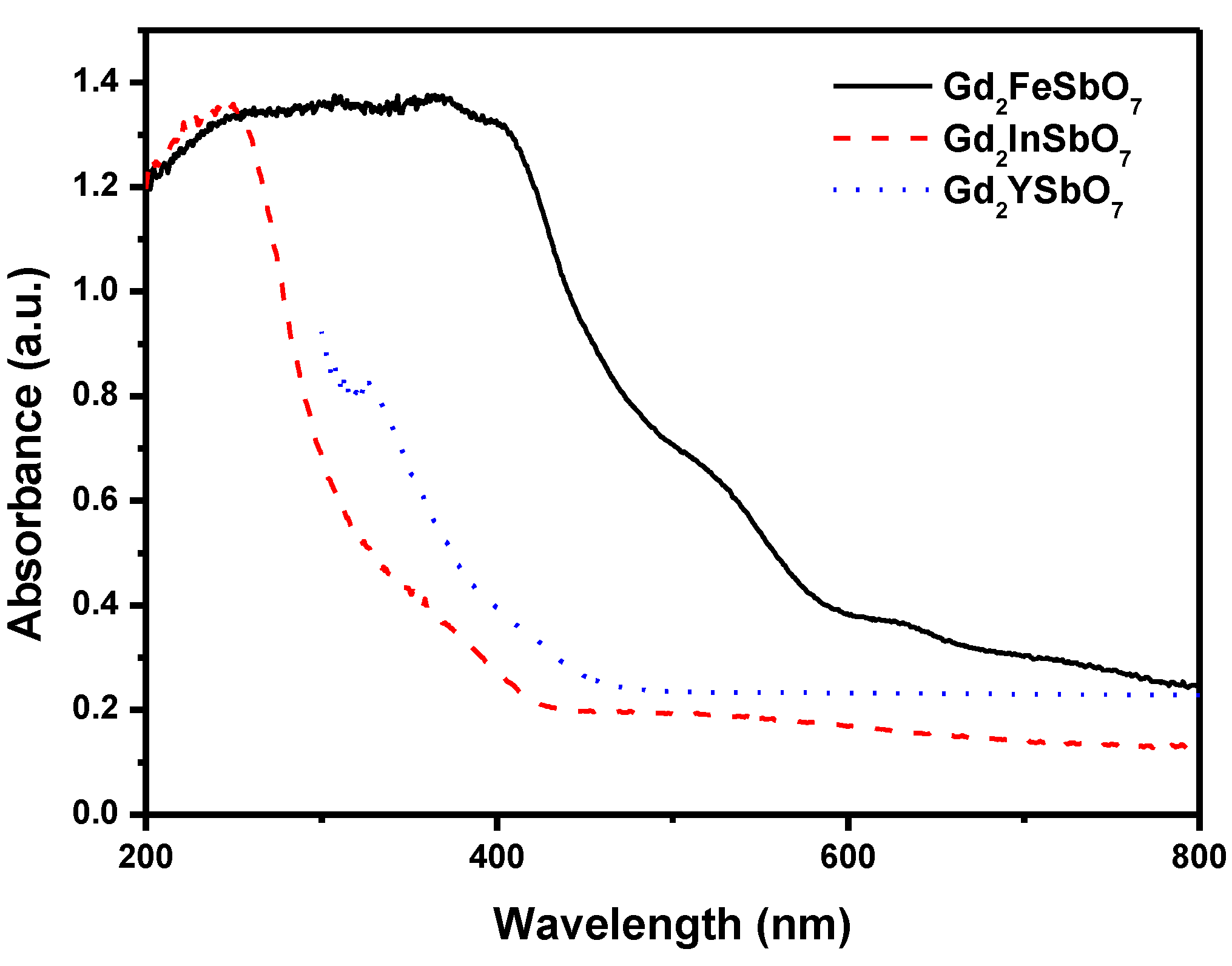
Figure 2 represents the diffuse reflection spectra of Gd
2FeSbO
7, Gd
2InSbO
7 and Gd
2YSbO
7. Compared with well-known photocatalyst TiO
2 whose absorption edge was only 380 nm, the absorption band edge of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was found to be 586 nm, 428 nm or 479 nm. Clearly, the obvious absorption did not result from reflection and scattering. Consequently, the apparent absorbance at sub-band gap wavelengths (600–800 nm for Gd
2FeSbO
7, and 425–800 nm for Gd
2InSbO
7, and 490–700 nm for Gd
2YSbO
7) was higher than zero.
Figure 2.
The diffuse reflection spectrum of Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7.
Figure 2.
The diffuse reflection spectrum of Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7.
For a crystalline semiconductor, the optical absorption near the band edge followed the equation:
αhν =
A (
hν −
Eg)
n [
22,
23]. Here,
A, α,
Eg and ν were proportional constant, absorption coefficient, band gap and light frequency, respectively.
Eg and
n could be calculated by the following steps: (i) plotting ln(α
hν)
vs. ln(
hν −
Eg) by assuming an approximate value of
Eg; (ii) deducing the value of
n according to the slope in this graph; (iii) refining the value of
Eg by plotting (α
hν)
1/n vs. hν and extrapolating the plot to (α
hν)
1/n = 0. According to the above method, the band gap of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was estimated to be 2.151 eV, 2.897 eV or 2.396 eV.
3.2. Photocatalytic Activity of Gd2FeSbO7, Gd2InSbO7 and Gd2YSbO7
Generally speaking, the semiconductor photocatalysis started from the direct absorption of supra-band gap photons and the generation of electron–hole pairs in the semiconductor particles. Subsequently, the diffusion of the charge carriers to the surface of the semiconductor particle was followed. Under visible light irradiation, we measured H2 or O2 evolution rate by using Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as photocatalyst from CH3OH/H2O or AgNO3/H2O solution, respectively. The wavelength (λ) dependence on the photocatalytic activity under light irradiation from full arc up to λ = 420 nm was measured by using different cut-off filters.
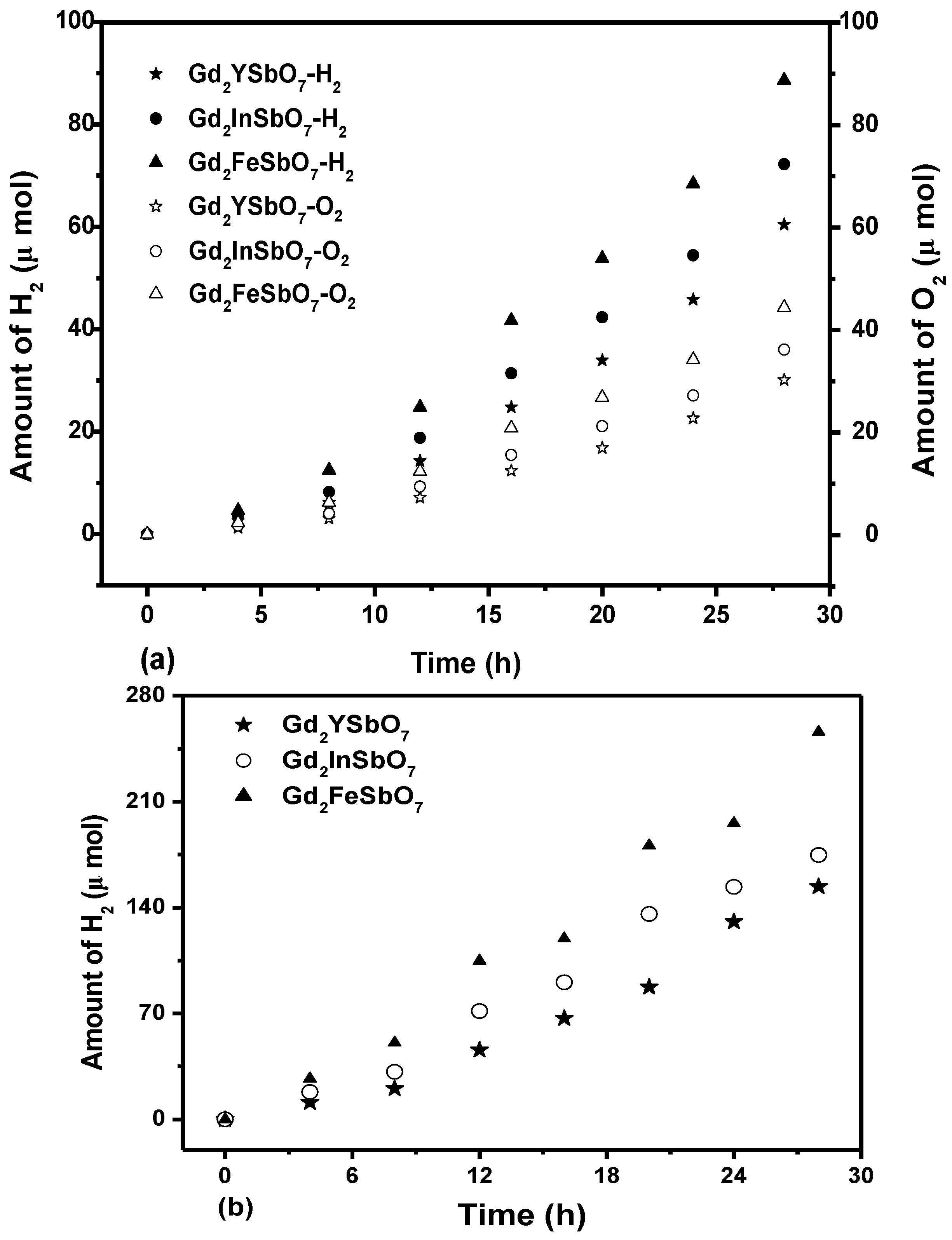
Figure 3a shows the photocatalytic H
2 evolution from pure water with Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g powder sample, 250 mL pure water). It could be found from
Figure 3a that under visible light irradiation, the rate of H
2 evolution in the first 28 h with Gd
2FeSbO
7 as catalyst was 6.329 μmol h
−1 g
−1, and that with Gd
2InSbO
7 as catalyst was 5.157 μmol h
−1 g
−1, and that with Gd
2YSbO
7 as catalyst was 4.314 μmol h
−1 g
−1. Besides, under dark condition, no H
2 evolution was detected from pure water with above three catalysts, which reflected the phocatalytic H
2 evolution activities from pure water of three synthesized catalysts. The reason that water could be split for H
2 evolution from pure water with Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 as catalyst under visible light irradiation (λ > 420 nm) was as following: Water could be split at a wavelength higher than 420 nm. However, the wavelength was not cut in exactly at 420 nm, in fact, the wavelength was cut by +50 or −50 nm, which meant that the wavelength up to 370 nm was probably absorbed by Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7, which could split water to provide tiny amounts of hydrogen generation in our experiment. The recycling experiments were performed three times with the same experimental conditions of
Figure 3a, and the results were almost the same as the above results in
Figure 3a. It could be seen that the photocatalysts we had produced had good stability and were thus desirably recyclable.
Figure 3b shows the photocatalytic O
2 evolution from pure water with Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g powder sample, 250 mL pure water). It could be found from
Figure 3b that under visible light irradiation, the rate of O
2 evolution in the first 28 h with Gd
2FeSbO
7 as catalyst was 3.158 μmol h
−1 g
−1, and that with Gd
2InSbO
7 as catalyst was 2.574 μmol h
−1 g
−1, and that with Gd
2YSbO
7 as catalyst was 2.149 μmol h
−1 g
−1.
Figure 3c shows the photocatalytic H
2 evolution from aqueous methanol solution with Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL methanol solution, 200 mL pure water). It could be found from
Figure 3c that under visible light irradiation, the rate of H
2 evolution in the first 28 h with Gd
2FeSbO
7 as catalyst was 18.271 μmol h
−1 g
−1, and that with Gd
2InSbO
7 as catalyst was 12.479 μmol h
−1 g
−1, and that with Gd
2YSbO
7 as catalyst was 10.986 μmol h
−1 g
−1, indicating that the photocatalytic activity of Gd
2FeSbO
7 was much higher than that of Gd
2InSbO
7 or Gd
2YSbO
7.
Figure 3.
(a) Photocatalytic H2 evolution and photocatalytic O2 evolution from pure water with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g powder sample, 250 mL pure water). Light source: 300 W Xe lamp; (b) Photocatalytic H2 evolution from aqueous methanol solution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL methanol solution, 200 mL pure water). Light source: 300 W Xe lamp.
Figure 3.
(a) Photocatalytic H2 evolution and photocatalytic O2 evolution from pure water with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g powder sample, 250 mL pure water). Light source: 300 W Xe lamp; (b) Photocatalytic H2 evolution from aqueous methanol solution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL methanol solution, 200 mL pure water). Light source: 300 W Xe lamp.
We would estimate apparent quantum yield in this paper because scattering effects were assumed to be the same for all the photocatalysts and our system was a suspension rather than a homogeneous solution. The apparent quantum yield for hydrogen evolution at 420 nm with Gd
2FeSbO
7 as catalyst was 0.446%, and that with Gd
2InSbO
7 as catalyst was 0.305% and that with Gd
2YSbO
7 as catalyst was 0.268% under visible light irradiation. Moreover, Gd
2InSbO
7 showed higher photocatalytic activity than Gd
2YSbO
7. This also proved that the conduction band level of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was more negative than the reduction potential of H
2O for forming H
2. The formation rate of H
2 increased with decreasing the M ionic radii within Gd
2MSbO
7 (M = Fe, In, Y), Fe
3+ (0.78 Å) < In
3+ (0.92 Å) < Y
3+ (1.019 Å). The reason was that the surface area of the photocatalyst increased with decreasing the M ionic radii, and the creation of more active sites was realized. As a result, the hydrogen generation rate increased. Moreover, the decrease of the M ionic radii would result in a decrease for the migration distance of photogenerated electrons and holes to reach the reaction site on the photocatalyst surface. Thus, the photogenerated electrons and holes could get to the photocatalyst surface more quickly. Above factors would suppress the electron–hole recombination, therefore, the photocatalytic activity would be enhanced. Such results were in good agreement with the optical absorption property of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 (see
Figure 2). The rate of H
2 evolution also increased with increasing illumination time. The photocatalytic activity of Gd
2FeSbO
7 increased by about 166% than that of Gd
2YSbO
7.
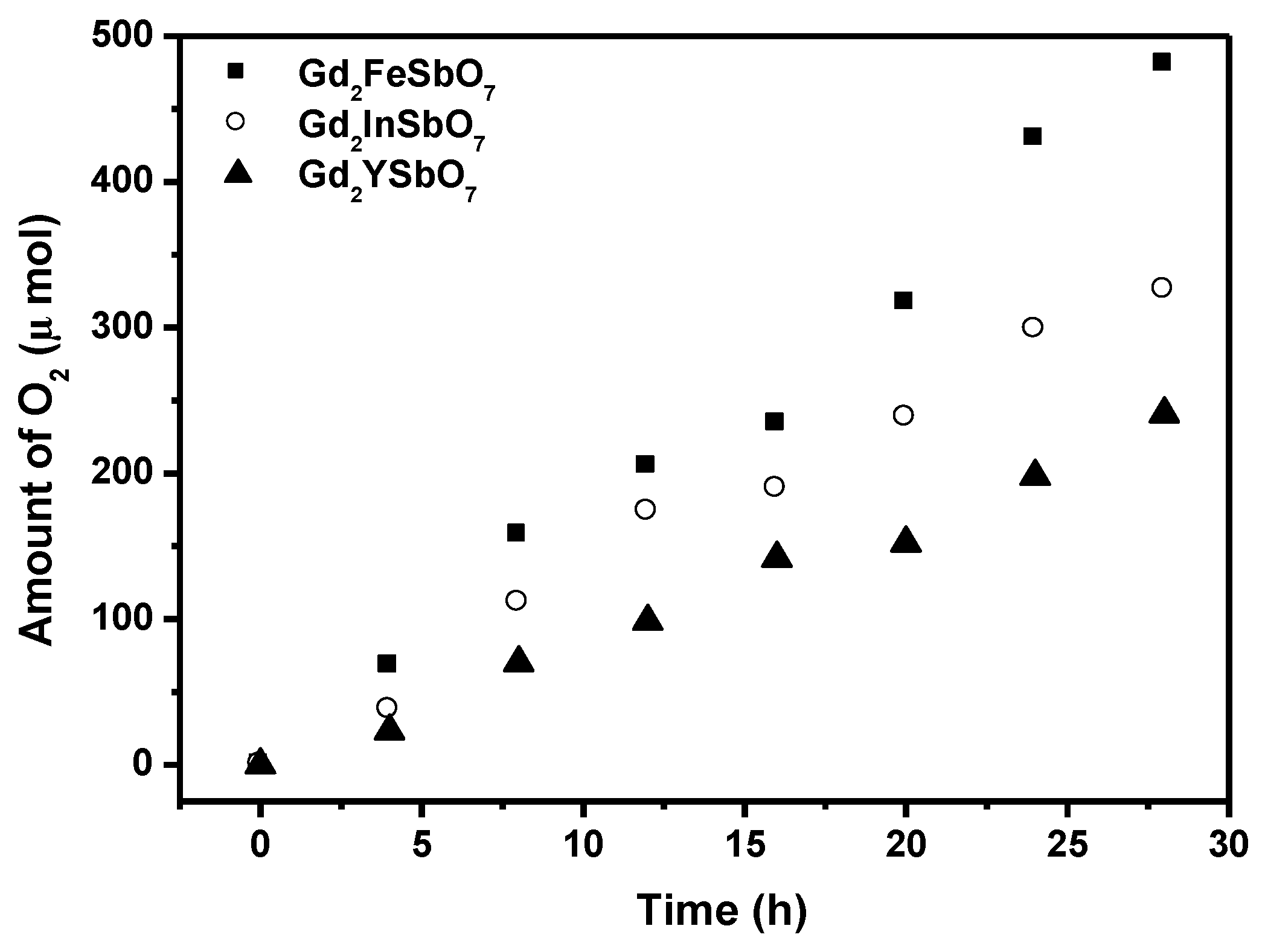
Figure 4 shows the photocatalytic O
2 evolution from AgNO
3 solution with Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g photocatalyst, 1 mmol AgNO
3, 270 mL pure water). It could be seen from
Figure 4 that under visible light irradiation, the rate of O
2 evolution in the first 28 h with Gd
2FeSbO
7 as catalyst was 34.329 μmol h
−1 g
−1, and that with Gd
2InSbO
7 as catalyst was 23.264 μmol h
−1 g
−1, and that with Gd
2YSbO
7 as catalyst was 17.200 μmol h
−1g
−1, indicating that the valence band level of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was more positive than the oxidation potential of H
2O for forming O
2. The formation rate of O
2 increased with decreasing the M ionic radii within Gd
2MSbO
7 (M = Fe, In, Y), Fe
3+ (0.78 Å) < In
3+ (0.92 Å) < Y
3+ (1.019 Å). The apparent quantum yield for the oxygen evolution at 420 nm with Gd
2FeSbO
7 as catalyst was 1.677%, and that with Gd
2InSbO
7 as catalyst was 1.136%, and that with Gd
2YSbO
7 as catalyst is 0.840% under visible light irradiation.
Figure 4.
Photocatalytic O2 evolution from AgNO3 solution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g photocatalyst, 1 mmol AgNO3, 270 mL pure water). Light source: 300 W Xe lamp.
Figure 4.
Photocatalytic O2 evolution from AgNO3 solution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under visible light irradiation (λ > 420 nm, 0.5 g photocatalyst, 1 mmol AgNO3, 270 mL pure water). Light source: 300 W Xe lamp.
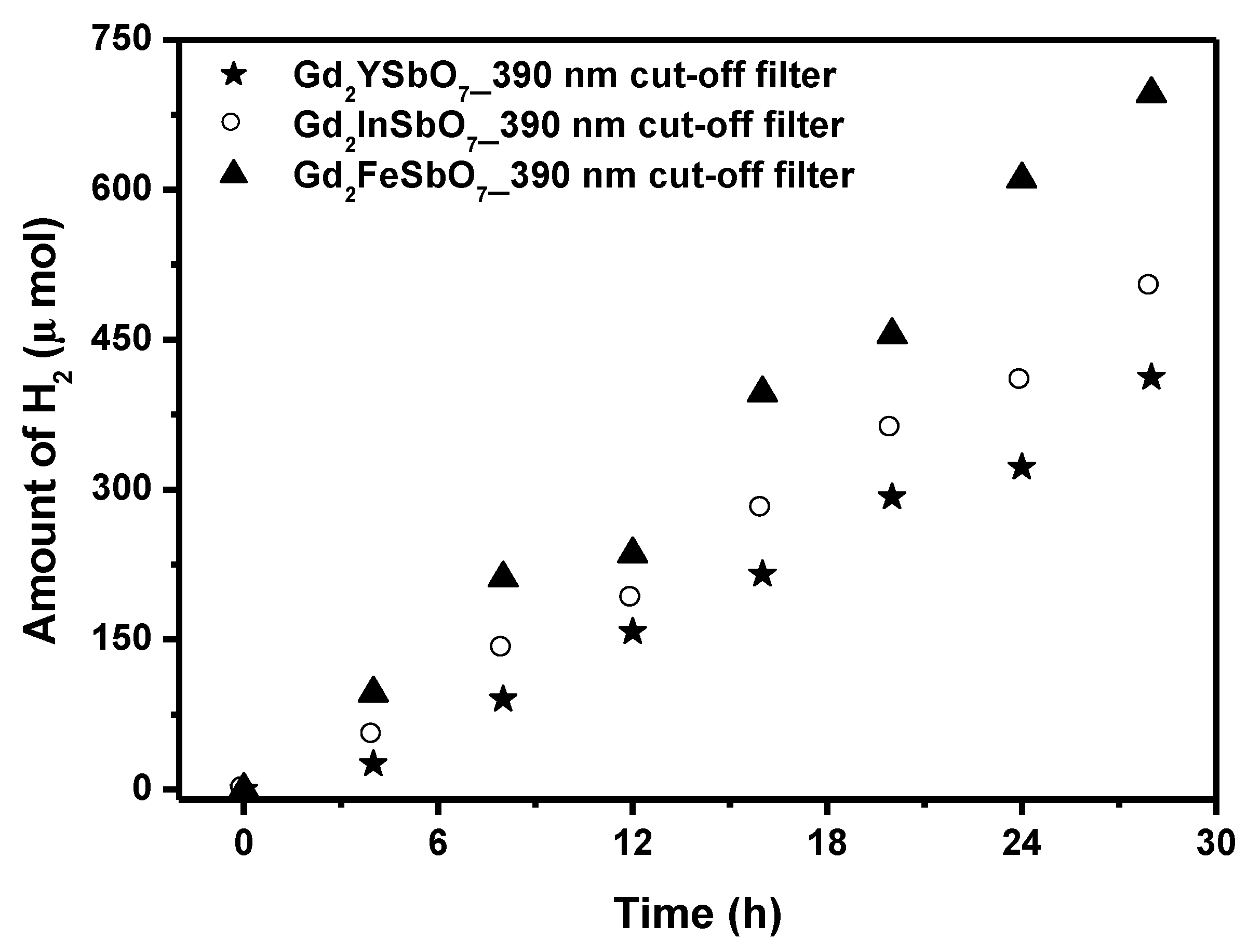
Figure 5 shows the photocatalytic H
2 evolution from aqueous methanol solution with Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 as catalyst under light irradiation (390 nm cut-off filter, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL CH
3OH, 200 mL pure water). It was depicted in
Figure 5 that under light irradiation (390 nm cut-off filter), the rate of H
2 evolution in the first 28 h with Gd
2FeSbO
7 as catalyst was 49.707 μmol h
−1 g
−1, and that with Gd
2InSbO
7 as catalyst was 35.900 μmol h
−1 g
−1, and that with Gd
2YSbO
7 as catalyst was 29.457 μmol h
−1 g
−1, indicating that the effect of wavelength (λ) dependence on the photocatalytic activity was very important. The apparent quantum yield for hydrogen evolution at 390 nm with Gd
2FeSbO
7 as catalyst was 0.871%, and that with Gd
2InSbO
7 as catalyst was 0.629% and that with Gd
2YSbO
7 as catalyst was 0.516% under light irradiation (390 nm cut-off filter).
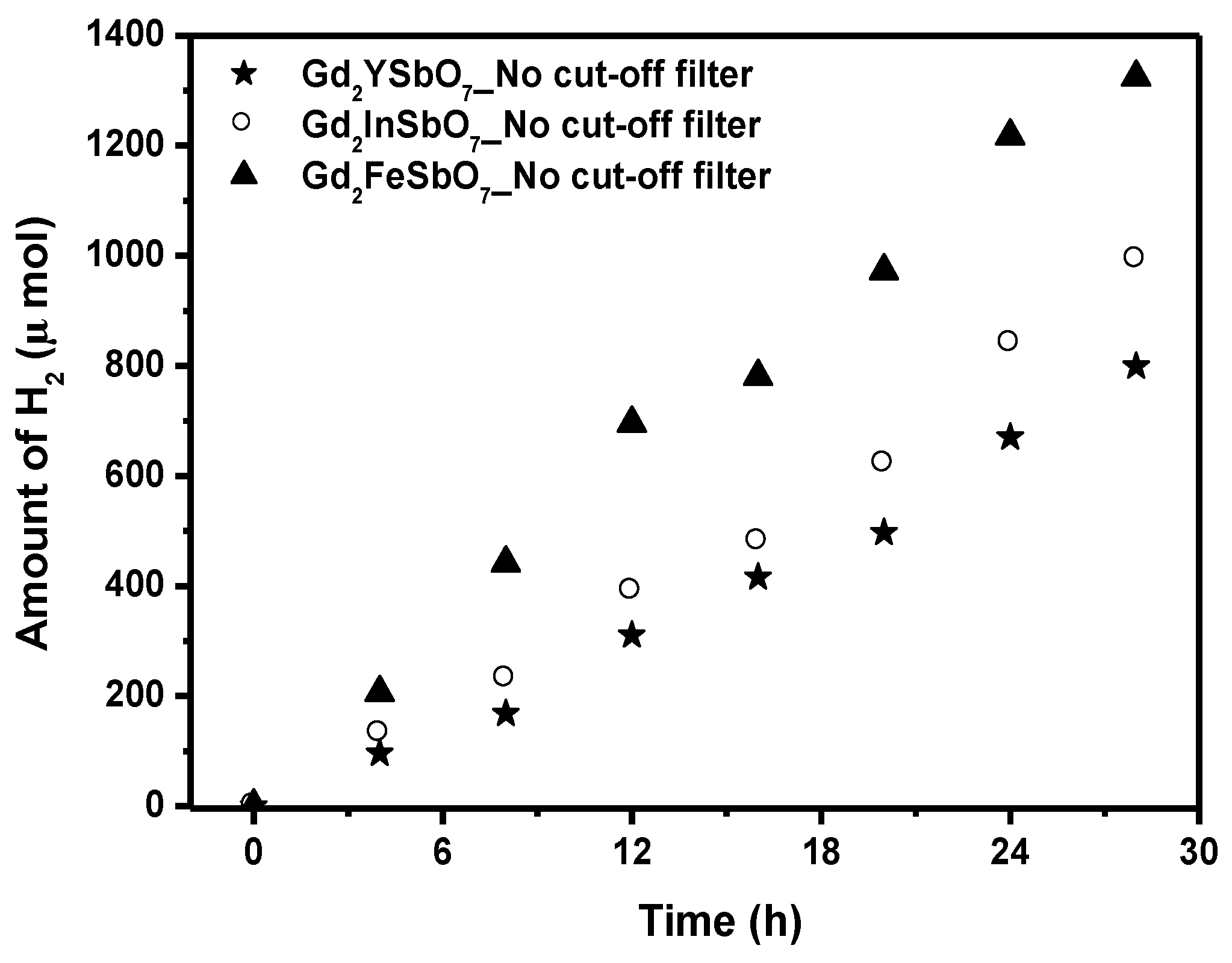
The photocatalytic H
2 evolution from aqueous methanol solution with Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 as catalyst under light irradiation (No cut-off filter, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL CH
3OH, 200 mL pure water) are shown in
Figure 6. It could be found from
Figure 6 that under light irradiation without using any filters, the rate of H
2 evolution in the first 28 h with Gd
2FeSbO
7 as catalyst was 94.614 μmol h
−1 g
−1, and that with Gd
2InSbO
7 as catalyst was 70.893 μmol h
−1 g
−1, and that with Gd
2YSbO
7 as catalyst was 57.100 μmol h
−1 g
−1, indicating that Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 shows high photocatalytic activity under full arc irradiation. The apparent quantum yield for hydrogen evolution at 420 nm with Gd
2FeSbO
7 as catalyst was 2.311%, and that with Gd
2InSbO
7 as catalyst was 1.731%, and that with Gd
2YSbO
7 as catalyst was 1.394% under light irradiation without using any filters. The photocatalytic activity decreased with increasing incident wavelength λ. As to Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7, the turnover number—the ratio of total amount of gas evolves to catalyst—exceeded 1 for Gd
2FeSbO
7 after 46 h reaction time, exceeded 1 for Gd
2InSbO
7 after 57 h reaction time, and exceeded 1 for Gd
2YSbO
7 after 66 h reaction time under visible light irradiation (λ > 420 nm). Under the condition of full arc irradiation, after 28 h of reaction time, the turnover number exceeded 1.60 for Gd
2FeSbO
7, and the turnover number exceeded 1.32 for Gd
2InSbO
7, and the turnover number exceeded 1.02 as to Gd
2YSbO
7. Above results were enough to prove that the reaction occurred catalytically. The reaction stopped when the light was turned off in this experiment, showing the obvious light response.
Figure 5.
Photocatalytic H2 evolution from aqueous methanol solution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under light irradiation (390 nm cut-off filter, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL CH3OH, 200 mL pure water). Light source: 300 W Xe lamp.
Figure 5.
Photocatalytic H2 evolution from aqueous methanol solution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under light irradiation (390 nm cut-off filter, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL CH3OH, 200 mL pure water). Light source: 300 W Xe lamp.
Figure 6.
Photocatalytic H2 evolution from aqueous methanol solution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under light irradiation (No cut-off filter, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL CH3OH, 200 mL pure water). Light source: 300 W Xe lamp.
Figure 6.
Photocatalytic H2 evolution from aqueous methanol solution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst under light irradiation (No cut-off filter, 0.5 g 0.1 wt% Pt-loaded powder sample, 50 mL CH3OH, 200 mL pure water). Light source: 300 W Xe lamp.
It was known that TiO2 has very high photocatalytic activity under ultraviolet light irradiation. By contrast, the photocatalytic activity was not obtained with Pt/TiO2 as catalyst under visible light irradiation (λ > 420 nm), while an obvious photocatalytic activity was observed with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst, showing that Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 could respond to visible light irradiation. The formation rate of H2 evolution with Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 as catalyst was much larger than that with TiO2 as catalyst under visible light irradiation. This indicated that the photocatalytic activity of Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 for decomposing CH3OH/H2O solution was higher than that of TiO2. The structure of Gd2FeSbO7, Gd2InSbO7 or Gd2YSbO7 after photocatalytic reaction was also checked by using X-ray diffraction method, and no change in their structures was observed during this reaction, which indicated that the H2 evolution was induced from the photocatalytic reaction of H2O.
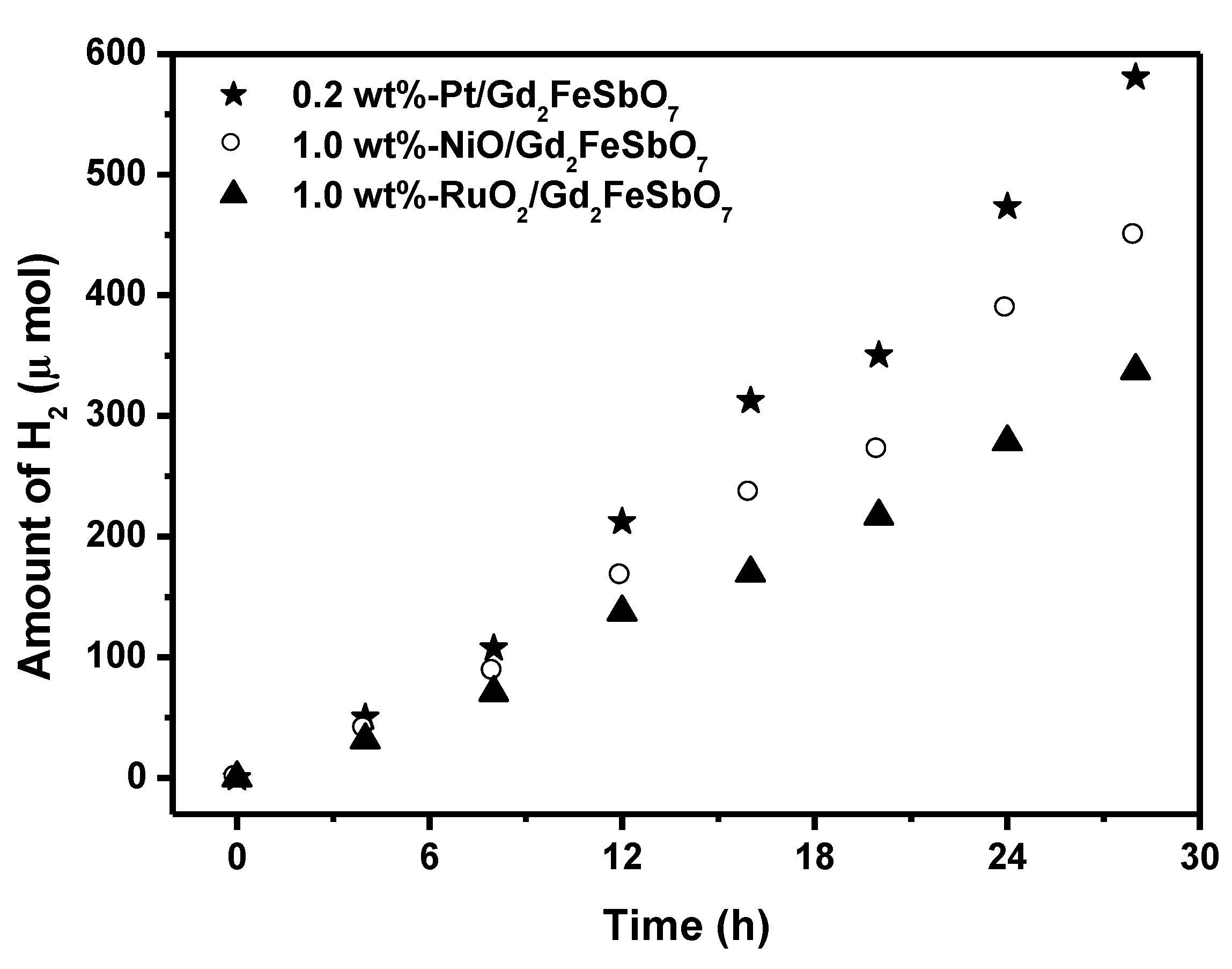
Figure 7 shows the effect of Pt, NiO and RuO
2 co-catalysts on the photoactivity of Gd
2FeSbO
7 under visible light irradiation (λ > 420 nm, 0.5 g powder sample, 50 mL methanol solution, 200 mL pure water). In principle, the photoinduced electrons preferentially enriched on the surface of co-catalyst particles and the recombination of the photoinduced electrons with the photoinduced holes was therefore markedly suppressed. It could be found from
Figure 7 that in the first 28 h under visible light irradiation, the rate of H
2 evolution was estimated to be 41.471 μmol h
−1 g
−1 with 0.2 wt%-Pt/Gd
2FeSbO
7 as catalyst, and that was estimated to be 32.064 μmol h
−1 g
−1 with 1.0 wt%-NiO/Gd
2FeSbO
7 as catalyst, and that was estimated to be 24.114 μmol h
−1 g
−1 with 1.0 wt%-RuO
2/Gd
2FeSbO
7 as catalyst, indicating that the photocatalytic activities could be further improved under visible light irradiation with Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 being loaded by Pt, NiO or RuO
2. The apparent quantum yield for hydrogen evolution at 420 nm with 0.2 wt%-Pt/Gd
2FeSbO
7 as catalyst was 1.013%, and that with 1.0 wt%-NiO/Gd
2FeSbO
7 as catalyst was 0.783%, and that with 1.0 wt%-RuO
2/Gd
2FeSbO
7 as catalyst was 0.589% under visible light irradiation (λ > 420 nm). The effect of Pt was better than that of NiO or RuO
2 for improving the photocatalytic activity of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7.
Figure 7.
Effect of Pt, NiO and RuO2 co-catalysts on the photoactivity of Gd2FeSbO7 under visible light irradiation (λ > 420 nm, 0.5 g powder sample, 50 mL methanol solution, 200 mL pure water). Light source: 300 W Xe lamp.
Figure 7.
Effect of Pt, NiO and RuO2 co-catalysts on the photoactivity of Gd2FeSbO7 under visible light irradiation (λ > 420 nm, 0.5 g powder sample, 50 mL methanol solution, 200 mL pure water). Light source: 300 W Xe lamp.
It was known that the process for photocatalysis of semiconductors was the direct absorption of photon by band gap of the materials and generated electron–hole pairs in the semiconductor particles, and the excitation of an electron from the valence band to the conduction band was initiated by light absorption with energy equal to or greater than the band gap of the semiconductor. Upon excitation of photon, the separated electron and hole could follow the solid surface. This suggested that the narrow band gap could more easily excite an electron from the valence band to the conduction band. If the conduction band potential level of the semiconductor was more negative than that of H
2 evolution, and the valence band potential level was more positive than that of O
2 evolution, decomposition of water could occur even without applying electric power [
1]. According to the above analysis, the photon absorption of Gd
2FeSbO
7 was much easier than that of the Gd
2InSbO
7 or Gd
2YSbO
7, which resulted in higher photocatalytic activity of Gd
2FeSbO
7.
The information about the specific surface area, pore volume, average pore size of Gd
2FeSbO
7, Gd
2InSbO
7 and Gd
2YSbO
7 are presented in
Table 4, from which we could see that the specific surface area of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was measured to be 4.12 m
2 g
−1, 3.26 m
2 g
−1 or 1.28 m
2 g
−1, which was significantly smaller than that of TiO
2 photocatalyst (53.8 m
2 g
−1). Above results indicated much higher potential efficiency of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7. Although the surface area of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was smaller than that of TiO
2, but Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 showed higher photocatalytic activity for H
2 evolution under visible light irradiation, which indicated that the high photocatalytic activity of the Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7 was not owing to a big surface area, but rather due to the narrow band gap. It was obvious that further increase in photocatalytic activity might be prospected from increasing the surface area of Gd
2FeSbO
7, Gd
2InSbO
7 or Gd
2YSbO
7. Since an efficient photocatalytic reaction process occurred on the photocatalyst surface, the increase of the surface area for the photocatalysts might lead to the increase of their photocatalytic activity.
Table 4.
The detailed information about the specific surface area, pore volume, average pore size of Gd2FeSbO7, Gd2InSbO7 and Gd2YSbO7 catalysts.
Table 4.
The detailed information about the specific surface area, pore volume, average pore size of Gd2FeSbO7, Gd2InSbO7 and Gd2YSbO7 catalysts.
| Catalyst | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) |
|---|
| Gd2FeSbO7 | 4.12 | 0.034 | 33 |
| Gd2InSbO7 | 3.26 | 0.033 | 41 |
| Gd2YSbO7 | 1.28 | 0.019 | 59 |