The Inhibition of Aluminum Corrosion in Sulfuric Acid by Poly(1-vinyl-3-alkyl-imidazolium Hexafluorophosphate)
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Characterization of PILs
| Abbreviation | Name | Structure | MW (g/mol) | DP | IR, cm−1 |
|---|---|---|---|---|---|
| PImC12 | Poly(1-vinyl-3-dodecylimidazolium hexafluorophosphate) | 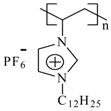 | 60,500 | 148 | 3,168, 2,934, 2,879, 1,553, 1,475, 837, 738, 555 |
| PImC8 | Poly(1-vinyl-3-octylimidazolium hexafluorophosphate) |  | 51,400 | 145 | 3,168, 2,931, 2,859, 1,554, 1,469, 1,164, 835, 736, 557 |
| PImC4 | Poly(1-vinyl-3-butylimidazolium hexafluorophosphate) | 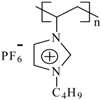 | 32,300 | 108 | 3,166, 2,969, 2,881, 1,552, 1,471, 1,162, 836, 738, 557 |
| IL | 1H NMR, ppm | 13C NMR, ppm | |||||
|---|---|---|---|---|---|---|---|
| Alkylic chain | Imidazolium ring | Polymeric chain | N-CH2 | Alkylic chain | Imidazolium ring | N-CH2 | |
| PImC12 | 0.89 (b, 3H) 1.26 (b, 18H) 1.72 (b, 2H) | 7.32 (b, 2H) 8.35 (b, 1H) | 1.86 (b, 2H) 2.95 (b, 1H) | 4.09 b, 2H | 14.30, 22.91, 26.51, 29.17 (2C), 29.60 (2C), 29.90 (3C), 32.16 | 123.83, 129.42, 136.12 | 51.60 |
| PImC8 | 0.89 (b, 3H) 1.29 (b, 10H) 1.69 (b, 2H) | 7.89 (b, 2H) 8.62 (b, 1H) | 2.28 (b, 2H) 3.24 (t, 1H) | 3.94 b, 2H | 13.57, 21.78, 25.70, 28.15, 28.41, 28.89, 30.95 | 123.83, 128.87, 136.12 | 49.52 |
| PImC4 | 0.96 (b, 3H) 1.35 (b, 2H) 1.70 (b, 2H) | 7.68 (b, 1H) 7.89 (b, 1H) 8.66 (b, 1H) | 2.27 (b, 2H) 3.24 (b, 1H) | 3.97 b, 2H | 12.88, 18.78, 30.63 | 123.44, 129.12, 134.49 | 49.08 |
2.2. Materials Preparation
2.3. Test Solution
2.4. Weight Loss Measurements
2.5. Electrochemical Test
2.6. Surface Analysis
3. Results and Discussion
3.1. Weight Loss Tests
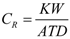
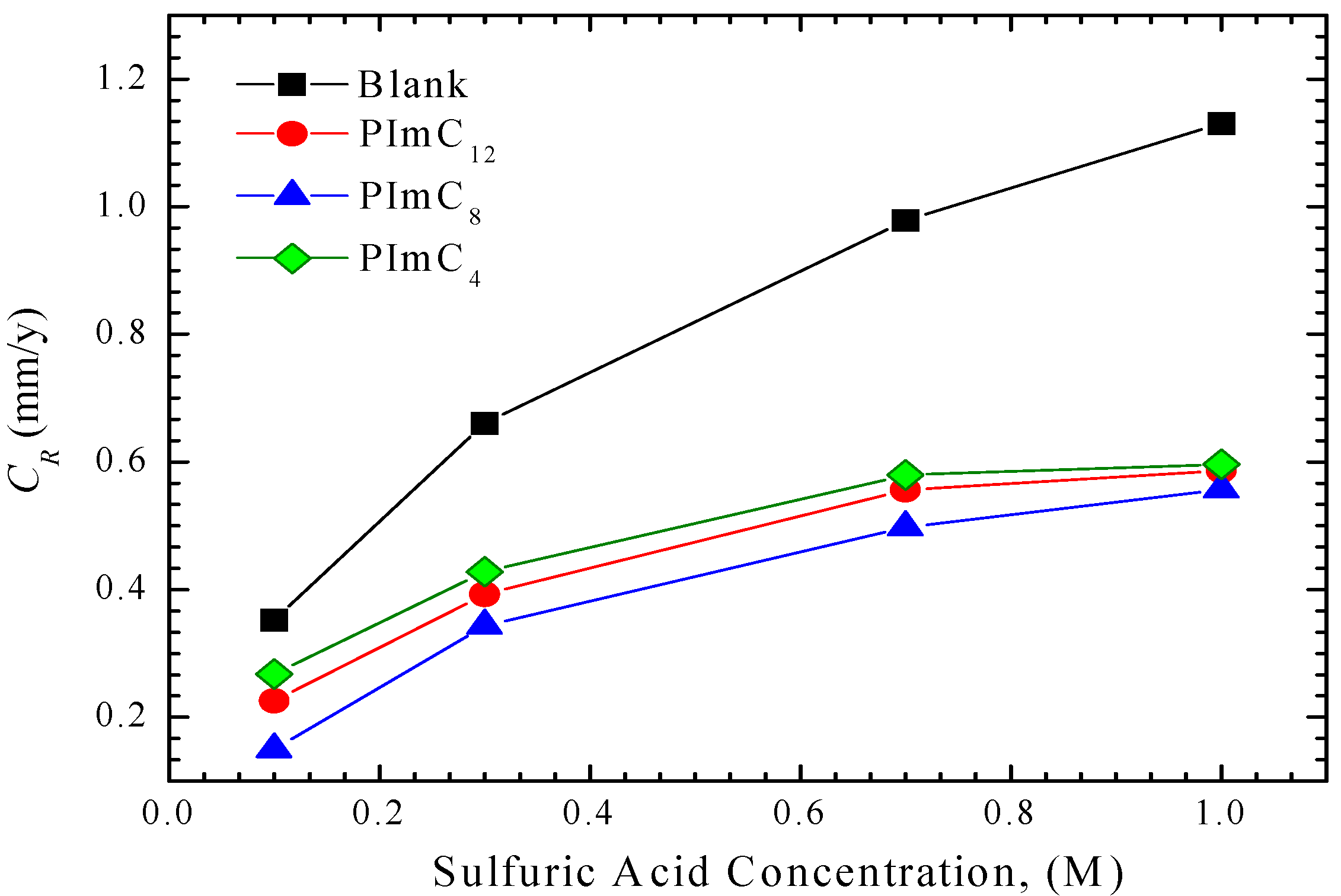
3.2. Electrochemical Test
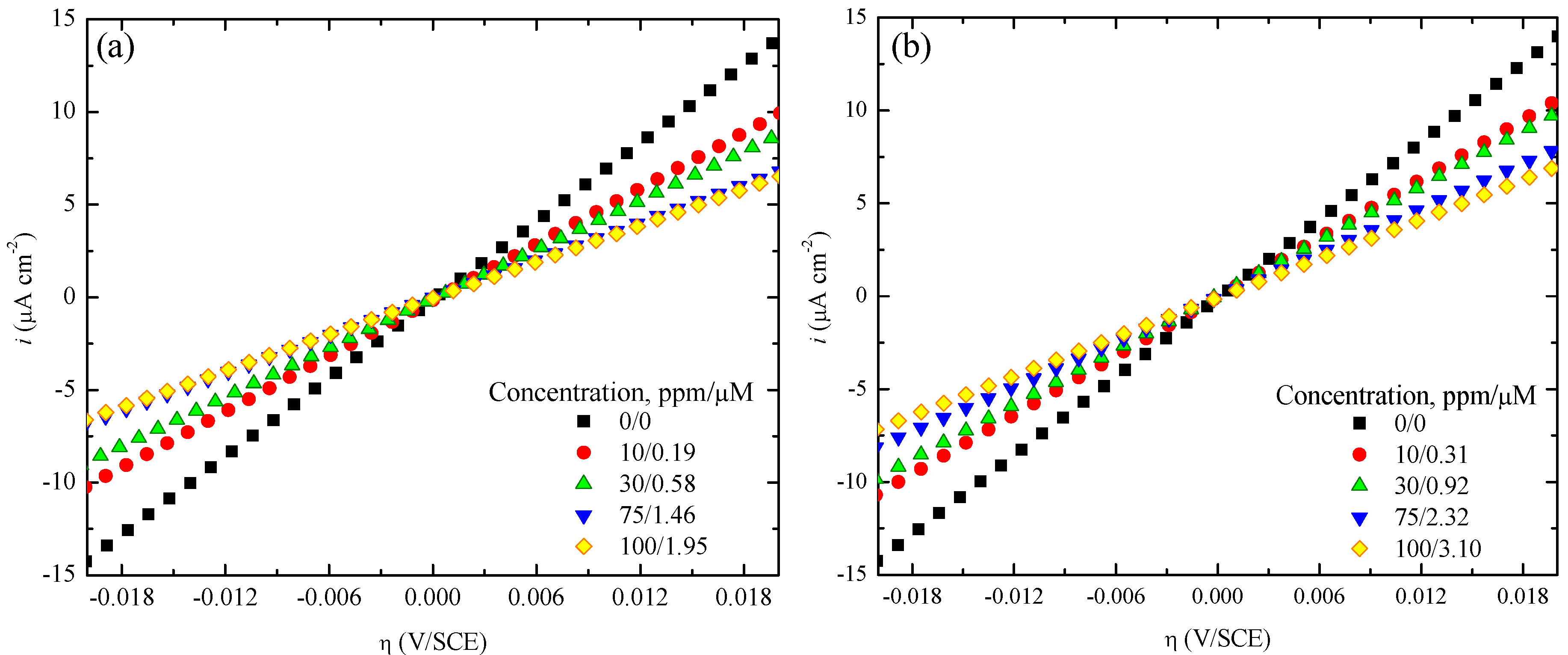
| CI | Concentration (ppm/µM) | 0.1 M H2SO4 | 0.3 M H2SO4 | 0.7 M H2SO4 | 1.0 M H2SO4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Rp (Ohm·cm2) | −Ecorr (mV) | Rp (Ω·cm2) | −Ecorr (mV) | Rp (Ω·cm2) | −Ecorr (mV) | Rp (Ω·cm2) | −Ecorr (mV) | ||
| PImC12 | Blank materialAA6061 | 1,423 | 657 | 969 | 651 | 940 | 621 | 924 | 653 |
| 10/0.16 | 2,270 | 658 | 1,340 | 647 | 1,288 | 639 | 970 | 636 | |
| 30/0.49 | 2,523 | 653 | 1,491 | 676 | 1,373 | 620 | 1,072 | 642 | |
| 75/1.24 | 3,334 | 650 | 1,952 | 660 | 1,543 | 644 | 1,203 | 659 | |
| 100/1.65 | 3,663 | 641 | 2,070 | 658 | 1,667 | 639 | 1,242 | 654 | |
| PImC8 | 10/0.19 | 1,982 | 670 | 1,313 | 646 | 1,245 | 643 | 930 | 652 |
| 30/0.58 | 2,260 | 647 | 1,405 | 655 | 1,345 | 646 | 1,013 | 650 | |
| 75/1.46 | 2,942 | 646 | 1,843 | 642 | 1,469 | 641 | 1,131 | 655 | |
| 100/1.95 | 3,052 | 642 | 2,003 | 639 | 1,543 | 644 | 1,176 | 659 | |
| PImC4 | 10/0.31 | 1,889 | 657 | 1,283 | 669 | 1,240 | 666 | 929 | 618 |
| 30/0.92 | 2,034 | 663 | 1,369 | 665 | 1,308 | 632 | 1,001 | 612 | |
| 75/2.32 | 2,495 | 646 | 1,679 | 665 | 1,434 | 643 | 1,074 | 623 | |
| 100/3.10 | 2,832 | 647 | 1,727 | 655 | 1,517 | 655 | 1,136 | 623 | |

| CI | Concentration (ppm/µM) | IE (%) | |||
|---|---|---|---|---|---|
| 0.1 M H2SO4 | 0.3 M H2SO4 | 0.7 M H2SO4 | 1.0 M H2SO4 | ||
| PImC12 | 10/0.16 | 37 | 28 | 27 | 5 |
| 30/0.49 | 44 | 35 | 31 | 14 | |
| 75/1.24 | 57 | 50 | 39 | 23 | |
| 100/1.65 | 61 | 53 | 44 | 25 | |
| PImC8 | 10/0.19 | 28 | 26 | 24 | 1 |
| 30/0.58 | 37 | 31 | 30 | 9 | |
| 75/1.46 | 52 | 47 | 36 | 18 | |
| 100/1.95 | 53 | 52 | 39 | 21 | |
| PImC4 | 10/0.31 | 25 | 24 | 24 | 1 |
| 30/0.92 | 30 | 29 | 28 | 8 | |
| 75/2.32 | 43 | 42 | 34 | 14 | |
| 100/3.10 | 50 | 44 | 38 | 19 | |
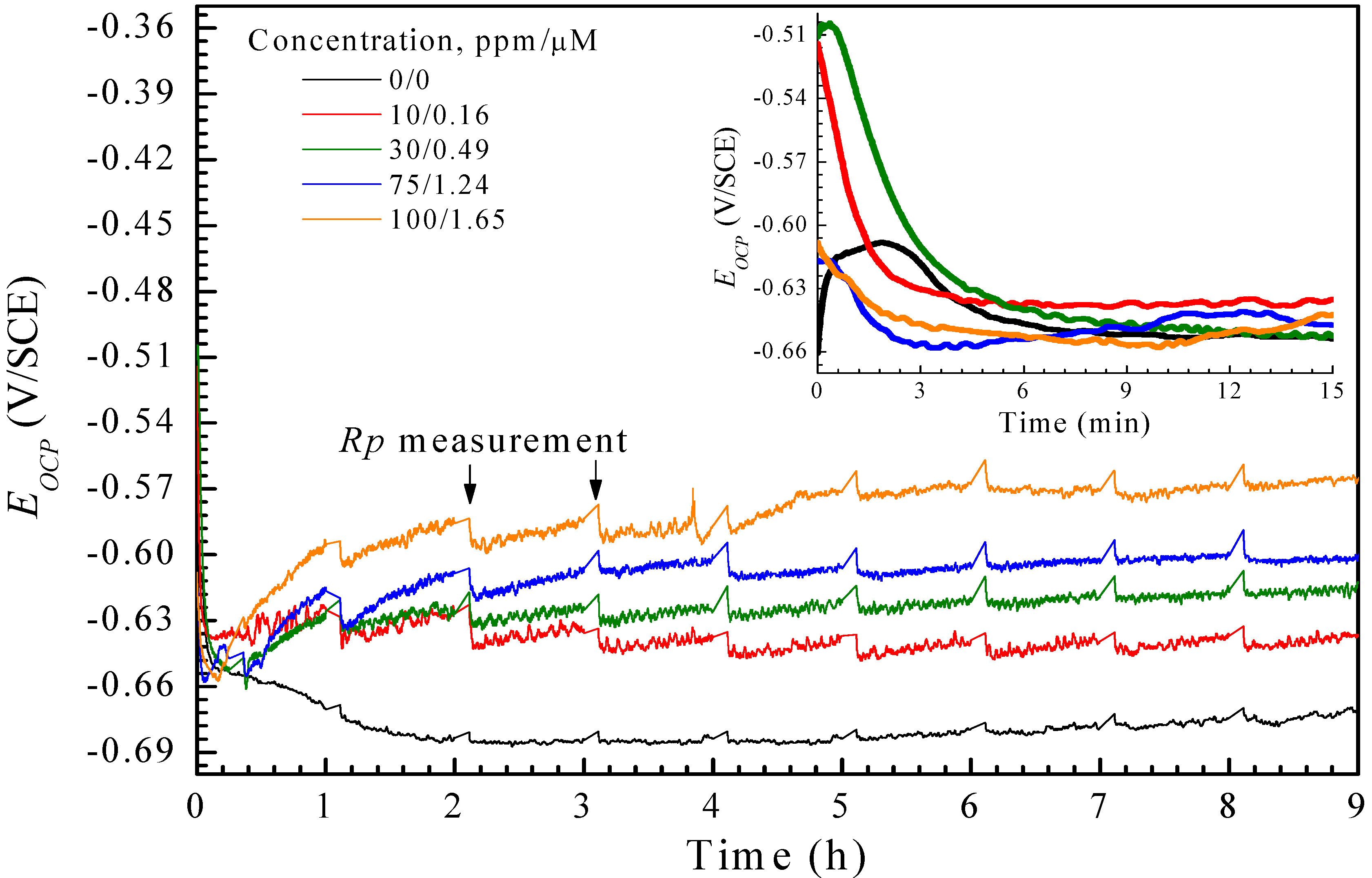
| CI | Time (h) | IE (%) | |||
|---|---|---|---|---|---|
| 10 ppm | 30 ppm | 75 ppm | 100 ppm | ||
| PImC12 | 1 | 18 | 23 | 40 | 63 |
| 3 | 24 | 30 | 37 | 66 | |
| 6 | 23 | 30 | 36 | 55 | |
| 9 | 21 | 29 | 38 | 53 | |
| PImC8 | 1 | 15 | 20 | 38 | 59 |
| 3 | 18 | 21 | 30 | 61 | |
| 6 | 20 | 26 | 29 | 47 | |
| 9 | 18 | 21 | 30 | 47 | |
| PImC4 | 1 | 14 | 16 | 26 | 44 |
| 3 | 12 | 19 | 27 | 39 | |
| 6 | 15 | 20 | 25 | 41 | |
| 9 | 10 | 15 | 30 | 41 | |
3.3. Adsorption Isotherms
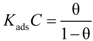
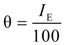
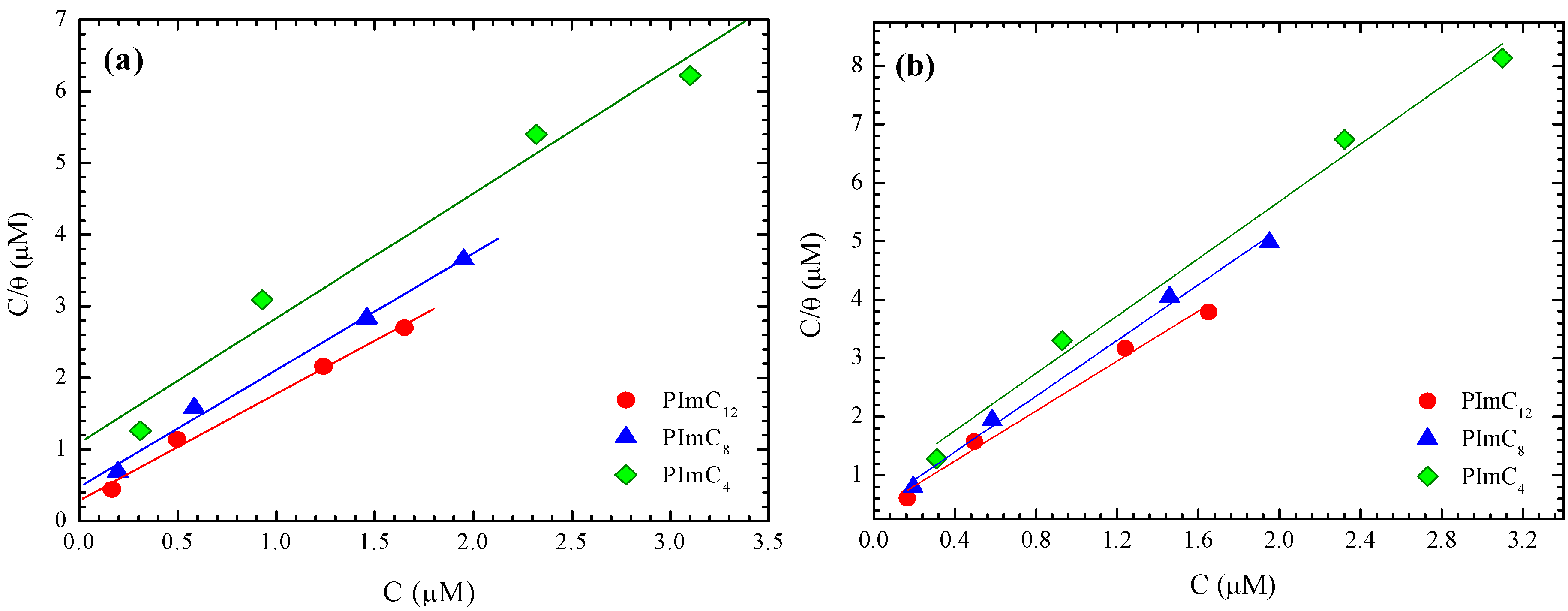
| H2SO4 Solution (M) | CI | R2 | Slope | Kads (mmol−1) | −ΔG0ads (kJ·mol−1) |
|---|---|---|---|---|---|
| 0.1 | PImC12 | 0.98 | 1.49 | 3,431 | 30.1 |
| PImC8 | 0.98 | 1.63 | 2,089 | 28.9 | |
| PImC4 | 0.95 | 1.74 | 917 | 26.9 | |
| 0.3 | PImC12 | 0.98 | 1.63 | 2,219 | 29.1 |
| PImC8 | 0.96 | 1.65 | 1,550 | 28.2 | |
| PImC4 | 0.98 | 1.99 | 1,062 | 27.2 | |
| 0.7 | PImC12 | 0.98 | 2.14 | 2,567 | 29.4 |
| PImC8 | 0.99 | 2.38 | 2,253 | 29.1 | |
| PImC4 | 0.98 | 2.45 | 1,276 | 27.7 | |
| 1.0 | PImC12 | 0.99 | 2.09 | 350 | 25.0 |
| PImC8 | 0.97 | 1.73 | 179 | 22.8 | |
| PImC4 | 0.75 | 2.21 | 96 | 21.3 |

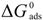 ) as indicated in the following Equation:
) as indicated in the following Equation:

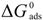 are displayed in Table 6, which showed only negative ones, suggesting that the Cis’ adsorption process occurred, that is to say, PILs formed an adsorbed film on the aluminum alloy surface. PILs in the aqueous media produced a negative change in the adsorption free energy as a result of water and ion displacement from the metallic surface. Negative values persisted even at 0.1 M H2SO4 as an indication that PILs adsorption is favored even when the number of sulfate aggressive ions and protons in solution tended to be lower.
are displayed in Table 6, which showed only negative ones, suggesting that the Cis’ adsorption process occurred, that is to say, PILs formed an adsorbed film on the aluminum alloy surface. PILs in the aqueous media produced a negative change in the adsorption free energy as a result of water and ion displacement from the metallic surface. Negative values persisted even at 0.1 M H2SO4 as an indication that PILs adsorption is favored even when the number of sulfate aggressive ions and protons in solution tended to be lower.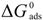 are equal to −20 kJ·mol−1 or less negative, this thermodynamic parameter suggests an adsorption process determined by the electrostatic attraction forces between the ionic charges and the dipoles of the adsorbed chemical species and the electric charge of metal in the metal-solution interface, which indicates that a physisorption process has occurred [47]. On the other hand, the
are equal to −20 kJ·mol−1 or less negative, this thermodynamic parameter suggests an adsorption process determined by the electrostatic attraction forces between the ionic charges and the dipoles of the adsorbed chemical species and the electric charge of metal in the metal-solution interface, which indicates that a physisorption process has occurred [47]. On the other hand, the 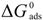 derived in values of −40 kJ·mol−1 or more negative ones suggested that a chemisorption process is under control, in which the CIs are capable of forming chemical bonds with the surface as electrons move into the metallic surface to form a coordinated type of bond with the metallic surface [48]. The values of
derived in values of −40 kJ·mol−1 or more negative ones suggested that a chemisorption process is under control, in which the CIs are capable of forming chemical bonds with the surface as electrons move into the metallic surface to form a coordinated type of bond with the metallic surface [48]. The values of 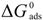 for these molecules were determined in the range of −30 to −21 kJ·mol−1, which means that compounds were adsorbed on the metallic surface by a process of physical adsorption.
for these molecules were determined in the range of −30 to −21 kJ·mol−1, which means that compounds were adsorbed on the metallic surface by a process of physical adsorption.3.4. Surface Analysis
3.4.1. Sample Surface after 3 h of Immersion
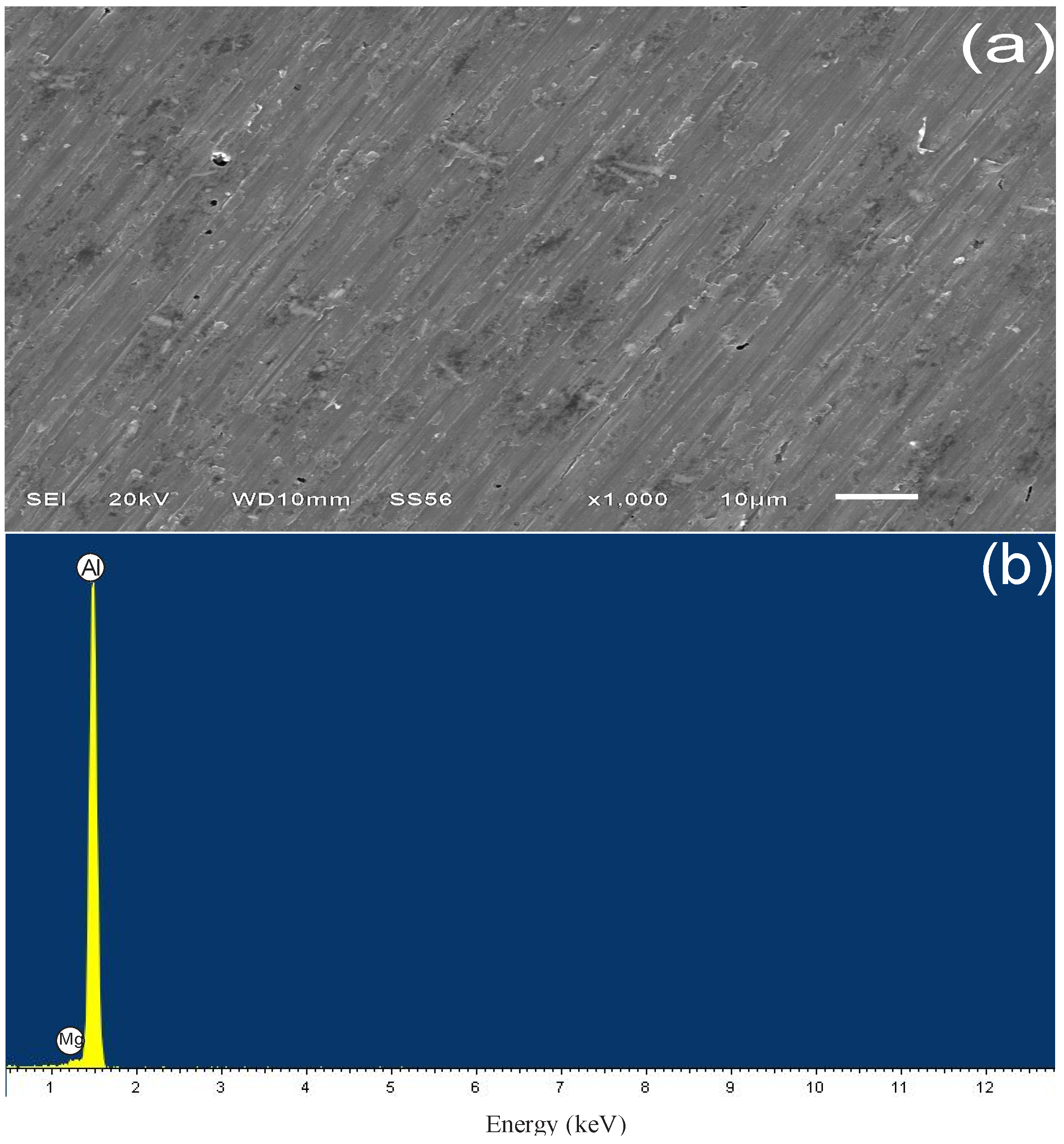
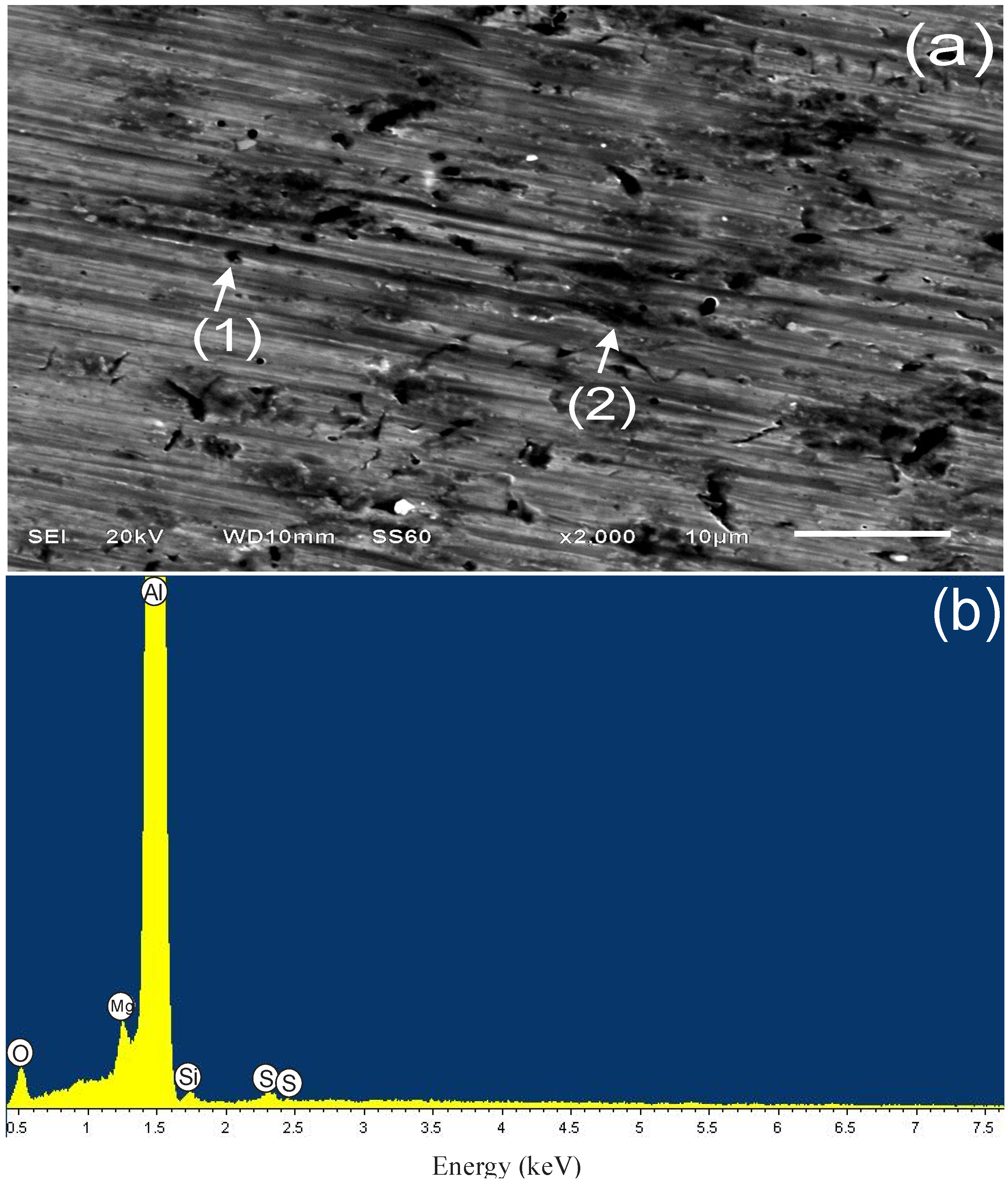
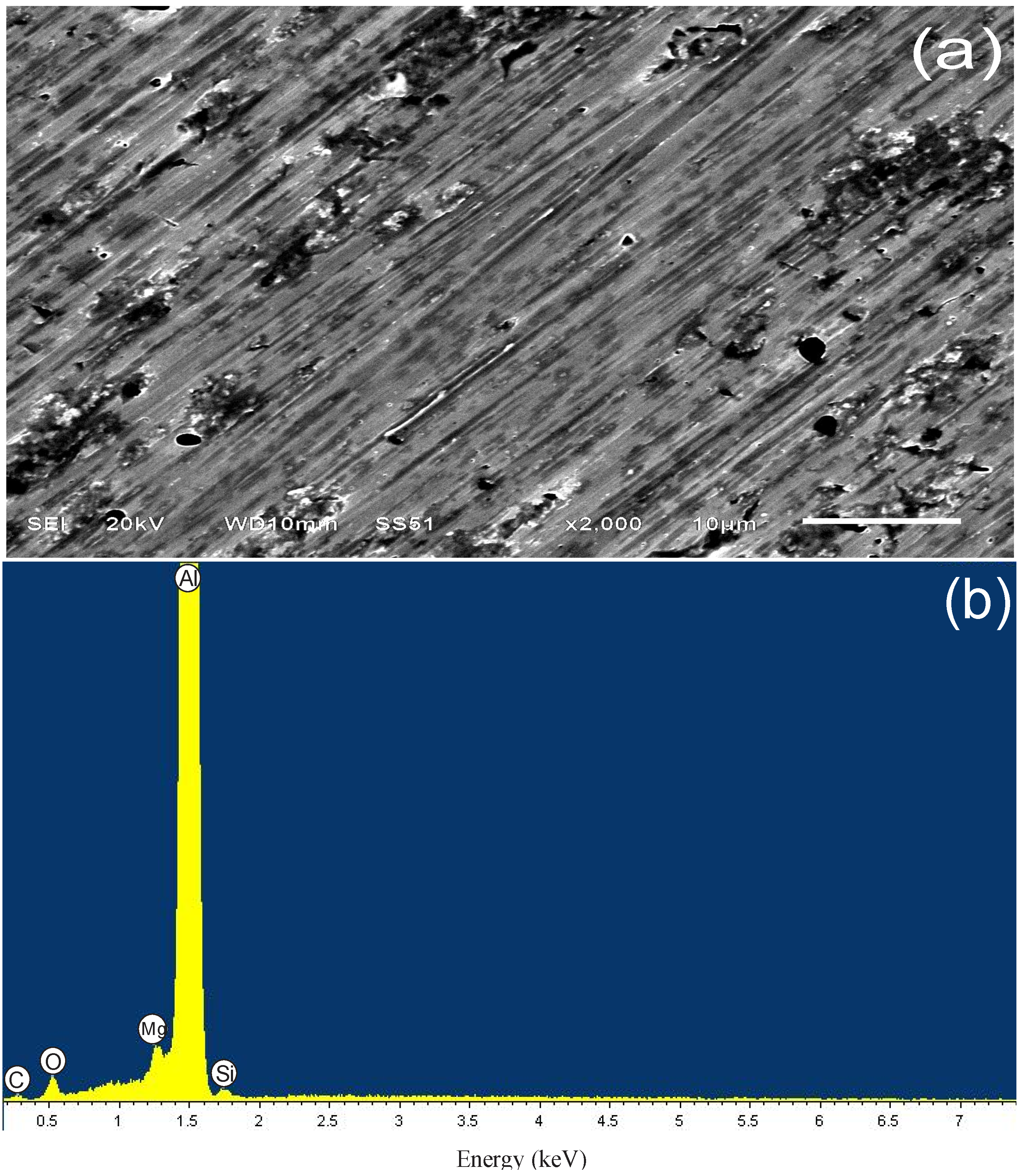
| Spectrum | Weight % | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | S | Total | |
| Without attack | – | – | 1.02 | 98.98 | – | – | 100 |
| Blank | – | 6.17 | 0.9 | 91.83 | 0.64 | 0.46 | 100 |
| PImC12 | 8.28 | 5.01 | 0.76 | 85.3 | 0.66 | – | 100 |
| PImC8 | 4.83 | 2.77 | 0.78 | 91.12 | 0.51 | – | 100 |
| PImC4 | 9.42 | 3.52 | 0.74 | 85.67 | 0.65 | – | 100 |
3.4.2. Sample Surface after 30 Days of Immersion
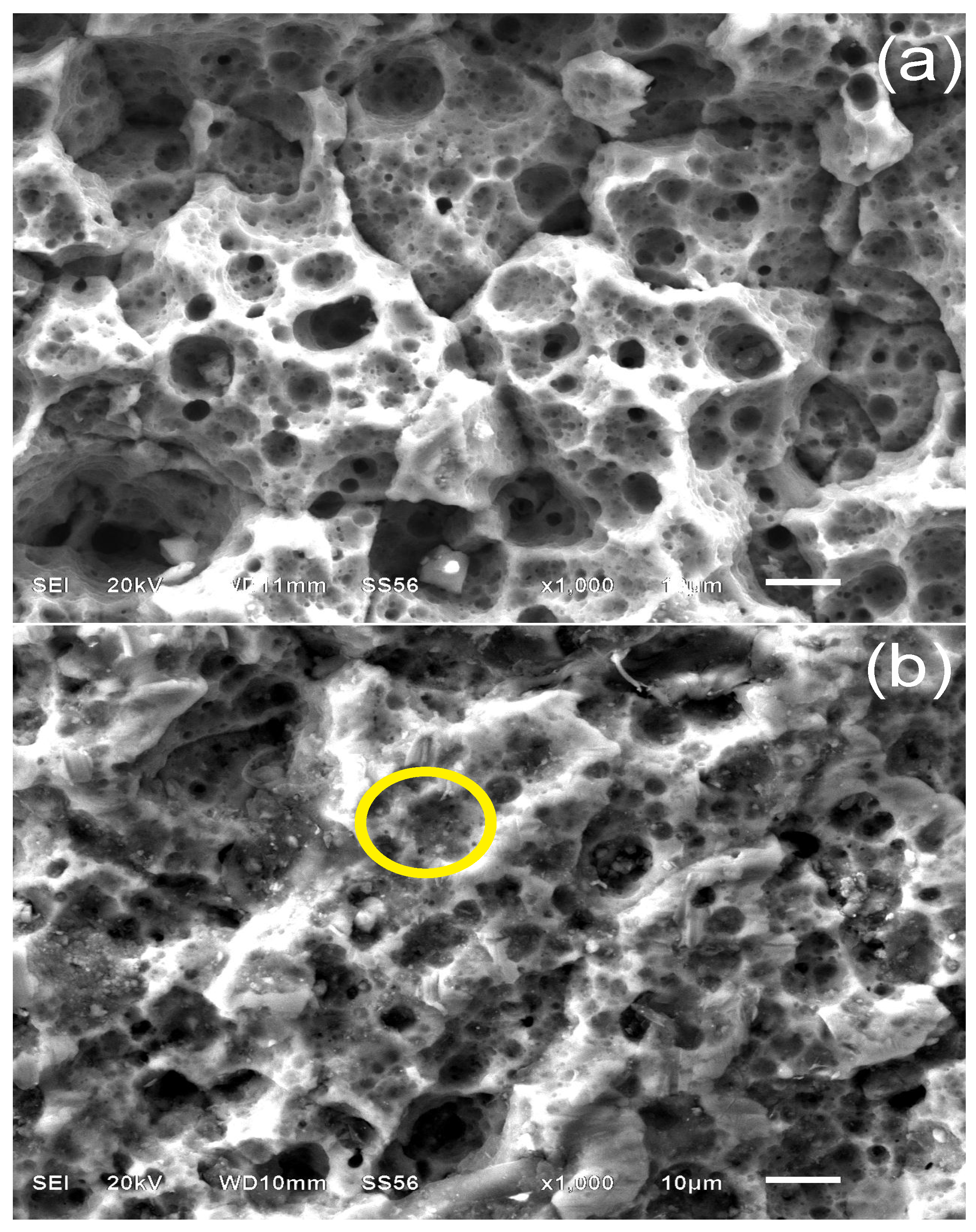
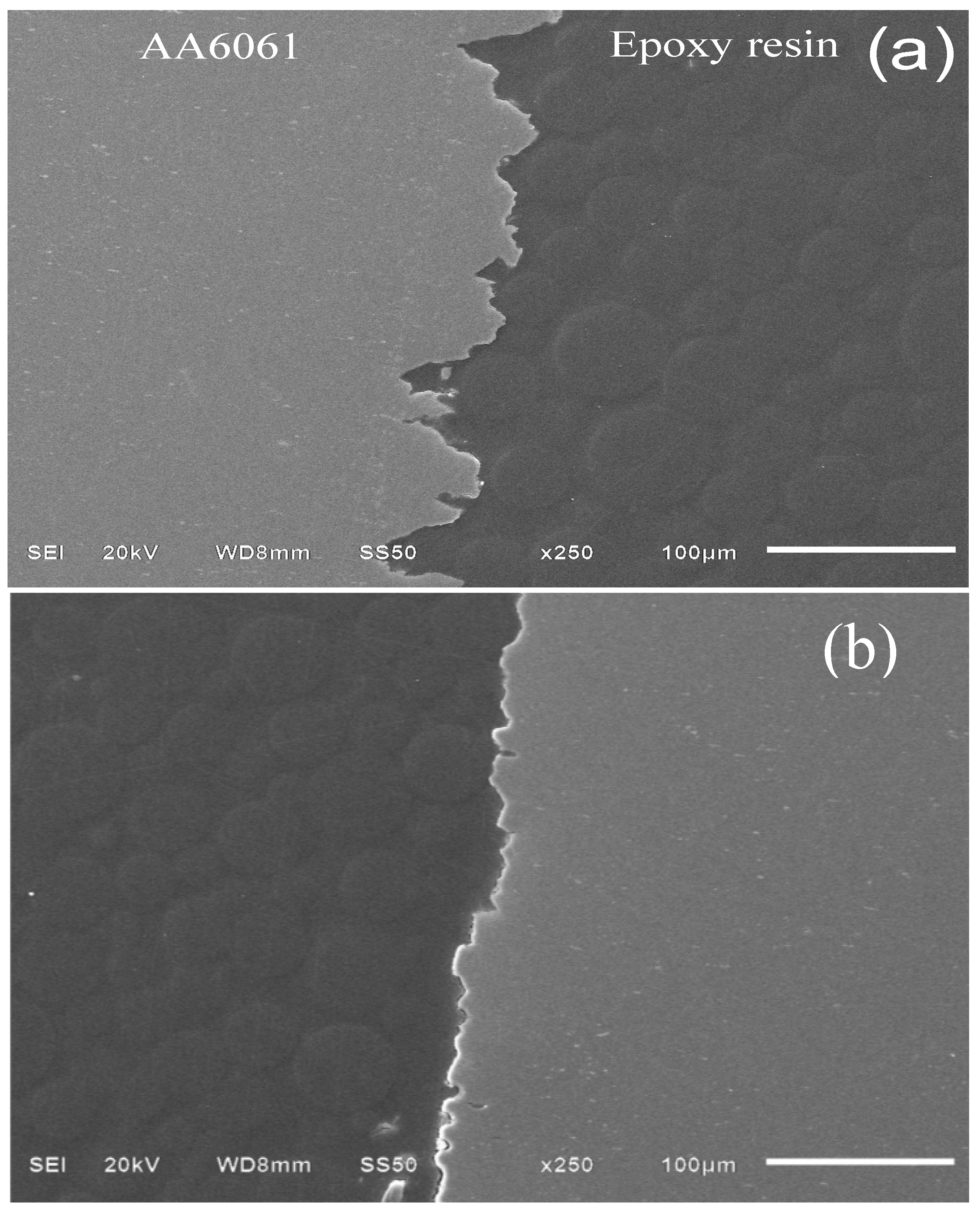
3.5. Adsorption Mechanism
3.5.1. Anodic Reactions
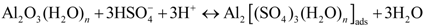
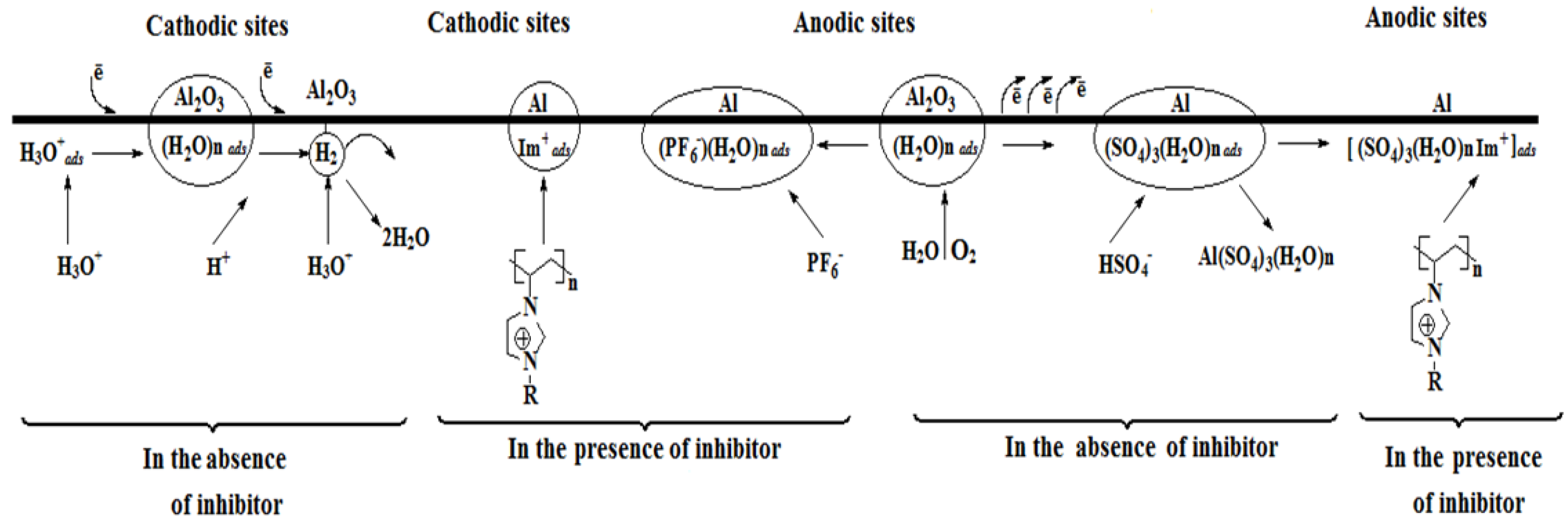
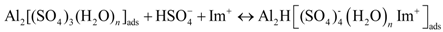

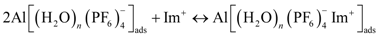
3.5.2. Cathodic Reactions
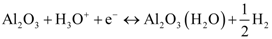
4. Conclusions
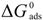 values suggested that physisorption is the process in control for inhibition.
values suggested that physisorption is the process in control for inhibition.Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, C.C. Electrodeposition of aluminum in molten AlCl3-n-butylpyridinium chloride electrolyte. Mat. Chem. Phys. 1994, 37, 355–361. [Google Scholar]
- Fellner, P.; Chrenková-Paučírová, M.; Matiašovský, K. Electrolytic aluminium plating in molten salt mixtures based on AlCl3 I: Influence of the addition of tetramethylammonium chloride. Surf. Technol. 1981, 14, 101–108. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium, 1st ed.; Elsevier: San Diego, CA, USA, 2004. [Google Scholar]
- Davis, J.R. Corrosion of Aluminium and Aluminium Alloys, 1st ed.; ASM International: Materials Park, OH, USA, 1999. [Google Scholar]
- Umoren, S.A.; Ogbobe, O.; Igwe, I.O.; Ebenso, E.E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros. Sci. 2008, 50, 1998–2006. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.H.; Mohamad, A.B.; Takrif, M.S.; Chee, E.P. Inhibition of aluminum corrosion by phthalazinone and synergistic effect of halide ion in 1.0 M HCl. Curr. Appl. Phys. 2012, 12, 325–330. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; NACE: Houston, TX, USA, 1974. [Google Scholar]
- Muniandy, M.T.; Rahim, A.A.; Osman, H.; Shah, A.M.; Yahya, S.; Raja, P.B. Investigation of some schiff bases as corrosion inhibitors for aluminium alloy in 0.5 M hydrochloric acid solutions. Surf. Rev. Lett. 2011, 18, 127–133. [Google Scholar] [CrossRef]
- Nisancioglu, K. Corrosion of aluminium alloys. In Proceedings of the ICCA3, Trondheim, Norway, 22–26 June 1992.
- Bregman, J.I. Corrosion Inhibitors; Collier-MacMillan Co.: London, UK, 1963. [Google Scholar]
- Sastri, V.S. Corrosion Inhibitors: Principles and Application; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Mourya, P.; Banerjee, S.; Singh, M.M. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014, 85, 352–363. [Google Scholar]
- Flores, E.A.; Olivares, O.; Likhanova, N.V.; Dominguez-Aguilar, M.A.; Nava, N.; Guzmán-Lucero, D.; Corrales, M. Sodium phthalamates as corrosion inhibitors for carbon steel in aqueous hydrochloric acid solution. Corros. Sci. 2012, 53, 3899–3913. [Google Scholar]
- Muralidharan, S.M.; Iyer, S.V. Influence of N-heterocyclics on corrosion inhibition and hydrogen permeation through mild steel in acidic solutions. Anti Corros. Meth. Mater. 1997, 44, 100–109. [Google Scholar]
- Khaled, K.F.; Al-Qahtani, M.M.J. The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: Chemical, electrochemical and theoretical studies. Mater. Chem. Phys. 2009, 113, 150–158. [Google Scholar]
- Lagrenée, M.; Mernari, B.; Bouanis, M.; Traisnel, M.; Bentiss, F. Study of the mechanism and inhibiting efficiency of 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole on mild steel corrosion in acidic media. Corros. Sci. 2002, 44, 573–588. [Google Scholar] [CrossRef]
- Fouda, A.S.; El-Sherbiny, M.F.; Motawea, M.M. Corrosion inhibition of aluminum alloy in H3PO4 solution using para-thiazolidinone derivatives. Desalination 2011, 30, 207–216. [Google Scholar]
- Guzman-Lucero, D.; Olivares-Xometl, O.; Martinez-Palou, R.; Likhanova, N.V.; Dominguez-Aguilar, M.A.; Garibay-Febles, V. Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind. Eng. Chem. Res. 2011, 50, 7129–7140. [Google Scholar] [CrossRef]
- Likhanova, N.V.; Olivares-Xometl, O.; Guzmán-Lucero, D.; Dominguez-Agilar, M.A.; Nava, N.; Corrales-Luna, M.; Mendoza, M.C. Corrosion inhibition of carbon steel in acidic environment by imidazolium ionic liquids containing vinyl-hexafluorophosphate as anion. Int. J. Electrochem. Sci. 2011, 6, 4514–4536. [Google Scholar]
- McCafferty, E.; Pravdic, V.; Zettlemoyer, A.C. Dielectric behaviour of adsorbed water films on the α-Fe2O3 surface. Trans. Faraday Soc. 1970, 66, 1720–1731. [Google Scholar] [CrossRef]
- El-Taib, H.F.; Haruyama, S. Impedance studies of the inhibitive effect of benzotriazole on the corrosion of copper in sodium chloride medium. Corros. Sci. 1980, 20, 887–898. [Google Scholar] [CrossRef]
- Metikos-Hukovic, M.; Grubac, Z.; Stupnisek-Lisac, E. Organic corrosion inhibitors for aluminum in perchloric acid. Corrosion 1994, 50, 146–151. [Google Scholar]
- Sezer, E.; Ustamehmetoglu, B.; Bayir, A.Z.; Coban, K.; Kalkan, A. Corrosion inhibition effect of 4-(2-Diethylamino-Ethylsulfonyl)-Phthalonitrile and 4,5-Bis(Hexylsulfonyl)-Phthalonitrile. Int. J. Electrochem. 2010, 2011. [Google Scholar] [CrossRef]
- Ren, Y.; Luo, Y.; Zhang, K.; Zhu, G.; Tan, X. Lignin terpolymer for corrosion inhibition of mild steel in 10% hydrochloric acid medium. Corros. Sci. 2008, 50, 3147–3153. [Google Scholar] [CrossRef]
- Abed, Y.; Arrar, Z.; Aounit, A.; Hammaouti, B.; Kertit, S.; Mansri, A. An electrochemical study of the action of poly(4-vinylpyridinepolyoxyethylene) as inhibitor for iron in sulfuric acid solution. J. Chem. Phys. 1999, 95, 1347–1355. [Google Scholar]
- Bereket, G.; Yurt, A.; Turk, H. Inhibition of corrosion of low carbon steel in acidic solution by selected polyelectrolytes and polymers. Anti-Corros. Methods Mater. 2003, 50, 422–535. [Google Scholar]
- Kralijie, M.; Mandie, Z.; Duie, L.J. Inhibition of steel corrosion by polyaniline coating. Corros. Sci. 2003, 45, 98–181. [Google Scholar]
- Umoren, S.A.; Ogbobe, O.; Okafor, P.C.; Ebenso, E.E. Polyethylene glycol and polyvinyl alcohol as corrosion inhibitors for aluminium in acidic medium. J. Appl. Polym. Sci. 2007, 105, 3363–3370. [Google Scholar]
- Kroschwitz, J.I.; Mark, H.F.; Bikales, N.M.; Overberger, C.G.; Menges, G. Encyclopaedia of Polymer Science and Technology, 2nd ed.; Wiley-Interscience Publication: Chichester, UK, 1964; Volume 1. [Google Scholar]
- Gao, B.; Zhang, X.; Sheng, Y. Studies on preparing and corrosion inhibition behaviour of quaternized polyethyleneimine for low carbon steel in sulfuric acid. Mater. Chem. Phys. 2008, 108, 375–381. [Google Scholar]
- Amin, M.A.; El-Rehim, S.S.A.; El-Sherbini, E.E.F.; Hazzazi, O.A.; Abbas, M.N. Polyacrylic acid as a corrosion inhibitor for aluminium in weakly alkaline solutions. Part I: Weight loss, polarization, impedance EFM and EDX studies. Corros. Sci. 2009, 51, 658–667. [Google Scholar] [CrossRef]
- Rajendran, S.; Sridevi, S.P.; Anthony, N.; Amalraj, A.J.; Sundaravadivelu, M. Corrosion behaviour of carbon steel in polyvinyl alcohol. Anti-Corros. Methods Mater. 2005, 52, 102–107. [Google Scholar]
- Olivares-Xometl, O.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Arce, E.; Dorantes, H.; Arellanes-Lozada, P. Synthesis and corrosion inhibition of α amino acid alkylamids for mild steel in acidic environment. Mat. Chem. Phys. 2008, 110, 344–351. [Google Scholar]
- Marcilla, R.; Blazquez, J.; Pomposo, J.; Mecerreyes, D. Tuning the solubility of polymerized ionic liquids by simple anion-exchange reactions. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 208–212. [Google Scholar]
- Inductively Coupled Plasma-Atomic Emission Spectrometry; EPA 6010C Method, revision 3; 2007. Available online: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/6010c.pdf (accessed on 23 July 2014).
- Baboian, R.; Dean, S.W., Jr.; Hack, H.P.; Hibner, E.L.; Scully, J.R. Corrosion Test and Standards: Application and Interpretation, 2nd ed.; ASTM International Standards Worldwide: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Durnie, W.; de Marco, R.; Jefferson, A.; Kinsella, B. Development of structure-activity relationships for oil field corrosion inhibitors. J. Electrochem. Soc. 1999, 146, 1751–1756. [Google Scholar] [CrossRef]
- Döner, A.; Kardas, G. N-Aminorhodanine as an effective corrosion inhibitor for mild steel in 0.5 M H2SO4. Corros. Sci. 2011, 53, 4223–4232. [Google Scholar] [CrossRef]
- El-Etre, A.Y. Inhibition of aluminum corrosion using Opuntia extract. Corros. Sci. 2003, 45, 2485–2495. [Google Scholar] [CrossRef]
- El-Etre, A.Y. Inhibition of acid corrosion of aluminum using vanillin. Corros. Sci. 2001, 43, 1031–1039. [Google Scholar] [CrossRef]
- Döner, A.; Solmaz, R.; Ozcan, M.; Kardas, G. Experimental and theoretical studies of Thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution. Corros. Sci. 2011, 53, 2902–2913. [Google Scholar] [CrossRef]
- Amin, M.A.; Ahmed, M.A.; Arida, H.A.; Kandemirli, F.; Saracoglu, M.; Arslan, T.; Basaran, M.A. Monitoring corrosion and corrosion control of iron in HCl by non-ionic surfactants of the TRITON-X series—Part III. Immersion time effects and theoretical studies. Corros. Sci. 2011, 53, 1895–1909. [Google Scholar] [CrossRef]
- Amin, M.A. A newly synthesized glycine derivative to control uniform and pitting corrosion processes of Al induced by SCN- anions—Chemical, electrochemical and morphological studies. Corros. Sci. 2010, 52, 3243–3257. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. Antifungal drugs as corrosion inhibitors for aluminium in 0.1 M HCl. Corros. Sci. 2009, 51, 1868–1875. [Google Scholar] [CrossRef]
- Abd El Rehim, S.S.; Amin, M.A.; Moussa, S.O.; Ellithy, A.S. The corrosion inhibition of aluminum and its copper alloys in 1.0 M H2SO4 solution using linear-sodium dodecyl benzene sulfonate as inhibitor. Mater. Chem. Phys. 2008, 112, 898–906. [Google Scholar]
- Pierre, R.R. Handbook of Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Ashassi-Sorkhabi, H.; Es’haghi, M. Corrosion inhibition of mild steel in acidic media by [BMIm]Br Ionic liquid. Mat. Chem. Phys. 2009, 114, 267–271. [Google Scholar]
- Li, X.; Deng, S.; Fu, H. Inhibition by tetradecylpyridinium bromide of the corrosion of aluminium in hydrochloric acid solution. Corros. Sci. 2011, 53, 1529–1536. [Google Scholar] [CrossRef]
- Mercier, D.; Herinx, M.; Barthés-Labrousse, M.G. Influence of 1,2-diaminoethane on the mechanism of aluminium corrosion in sulphuric acid solutions. Corros. Sci. 2010, 52, 3405–3412. [Google Scholar] [CrossRef]
- Boukerche, I.; Djerad, S.; Benmansour, L.; Tifouti, L.; Saleh, K. Degradability of aluminum in acid and alkaline solutions. Corros. Sci. 2014, 78, 343–352. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. The synergistic inhibitive effect and some quantum chemical parameters of 2,3-diaminonaphthalene and iodide ions on the hydrochloride acid corrosion of aluminum. Corros. Sci. 2009, 51, 276–282. [Google Scholar] [CrossRef]
- Amin, M.A. Understanding the inhibitory effect of sodium oleate on the corrosion of Al and Al-Cu alloys in 1.0 M H3PO4 solution-Polarization studies. J. Appl. Electrochem. 2009, 39, 689–696. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arellanes-Lozada, P.; Olivares-Xometl, O.; Guzmán-Lucero, D.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Lijanova, I.V.; Arce-Estrada, E. The Inhibition of Aluminum Corrosion in Sulfuric Acid by Poly(1-vinyl-3-alkyl-imidazolium Hexafluorophosphate). Materials 2014, 7, 5711-5734. https://doi.org/10.3390/ma7085711
Arellanes-Lozada P, Olivares-Xometl O, Guzmán-Lucero D, Likhanova NV, Domínguez-Aguilar MA, Lijanova IV, Arce-Estrada E. The Inhibition of Aluminum Corrosion in Sulfuric Acid by Poly(1-vinyl-3-alkyl-imidazolium Hexafluorophosphate). Materials. 2014; 7(8):5711-5734. https://doi.org/10.3390/ma7085711
Chicago/Turabian StyleArellanes-Lozada, Paulina, Octavio Olivares-Xometl, Diego Guzmán-Lucero, Natalya V. Likhanova, Marco A. Domínguez-Aguilar, Irina V. Lijanova, and Elsa Arce-Estrada. 2014. "The Inhibition of Aluminum Corrosion in Sulfuric Acid by Poly(1-vinyl-3-alkyl-imidazolium Hexafluorophosphate)" Materials 7, no. 8: 5711-5734. https://doi.org/10.3390/ma7085711
APA StyleArellanes-Lozada, P., Olivares-Xometl, O., Guzmán-Lucero, D., Likhanova, N. V., Domínguez-Aguilar, M. A., Lijanova, I. V., & Arce-Estrada, E. (2014). The Inhibition of Aluminum Corrosion in Sulfuric Acid by Poly(1-vinyl-3-alkyl-imidazolium Hexafluorophosphate). Materials, 7(8), 5711-5734. https://doi.org/10.3390/ma7085711





