Influence of Aggregate Coated with Modified Sulfur on the Properties of Cement Concrete
Abstract
:1. Introduction
2. Test Results and Discussion
2.1. Properties of Hardened Concrete
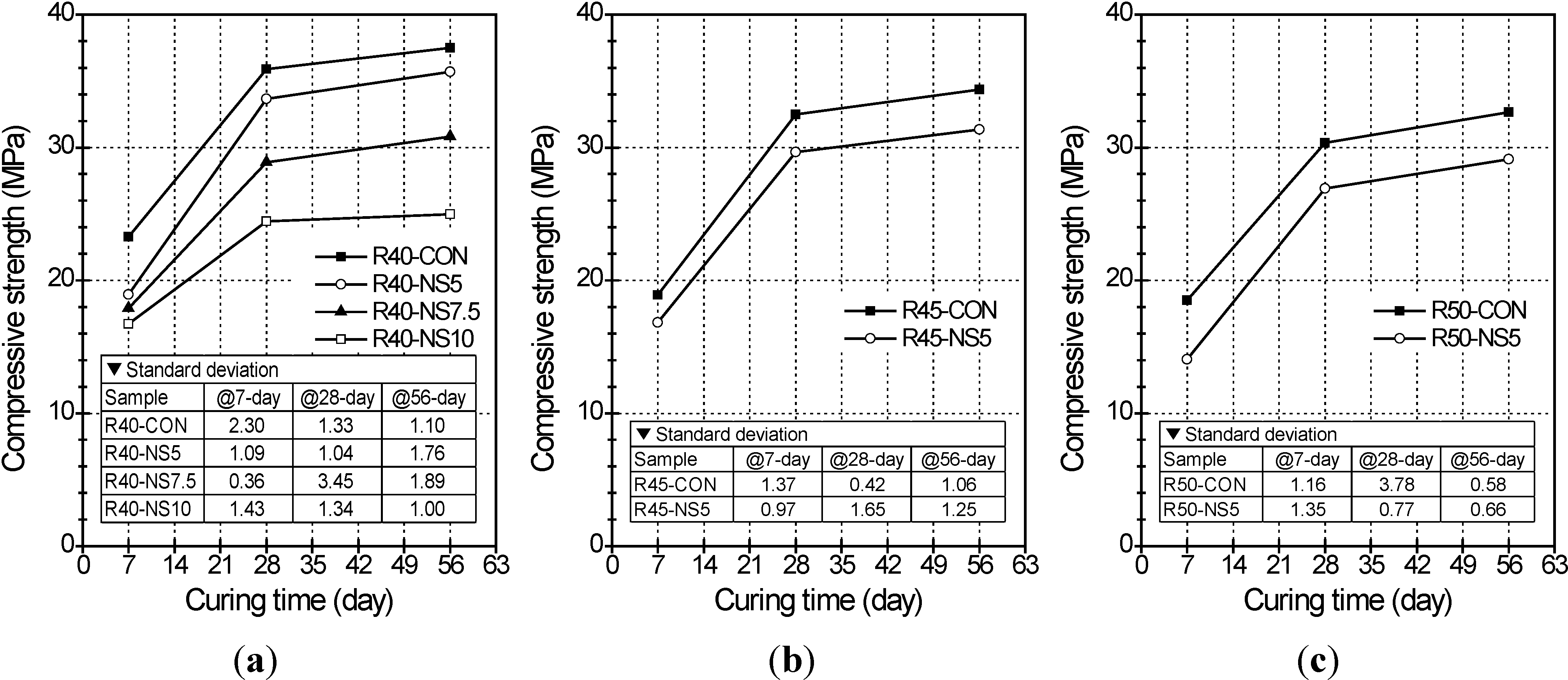
2.2. Compressive Strength
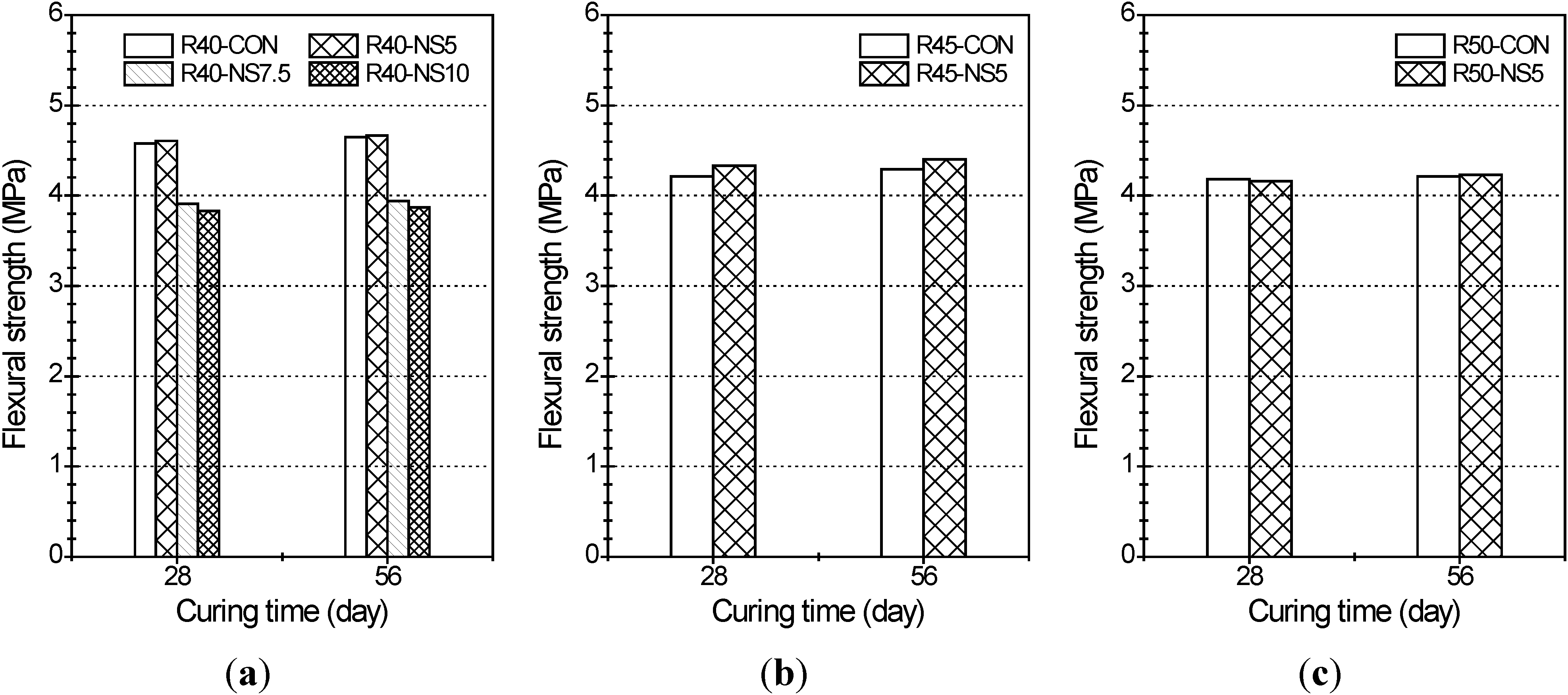
2.3. Flexural Strength
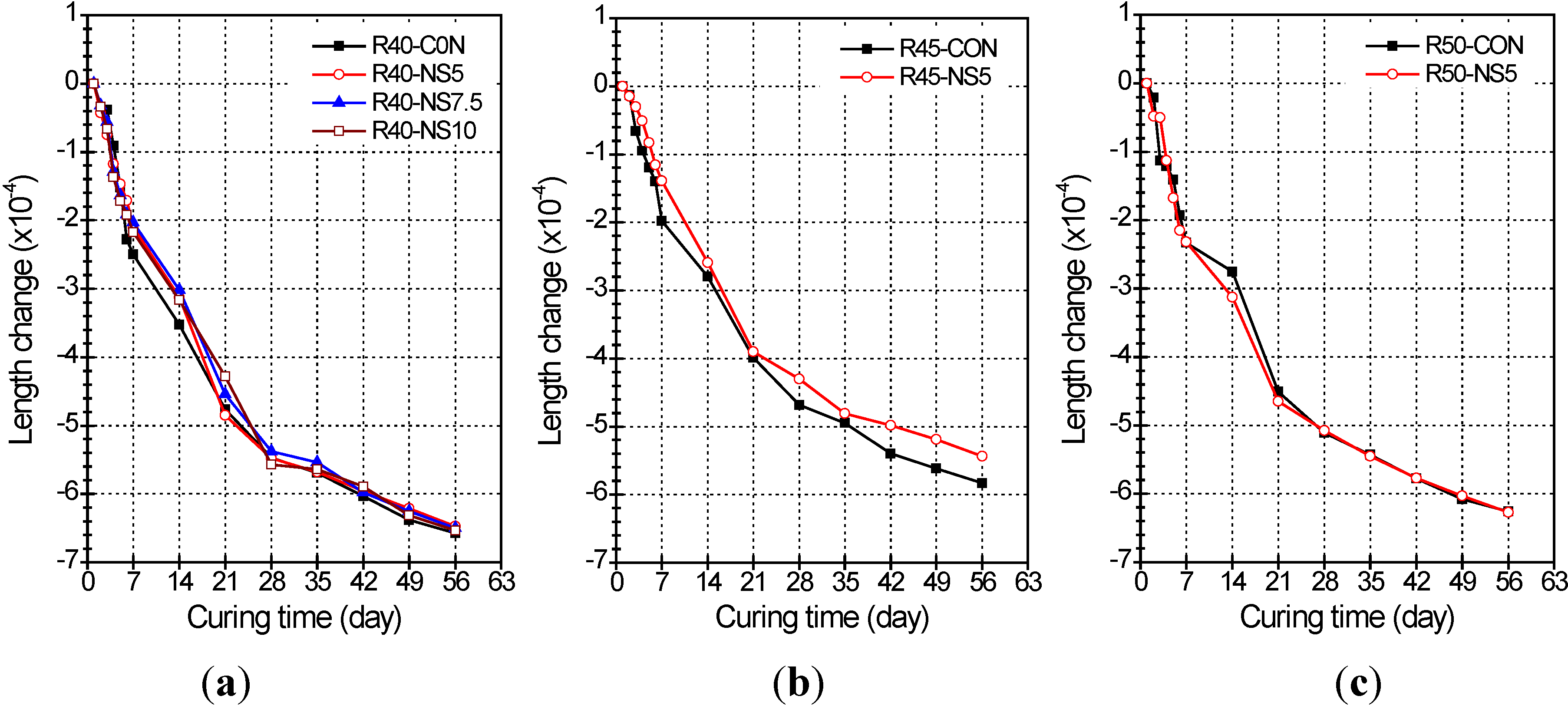
2.4. Length Change
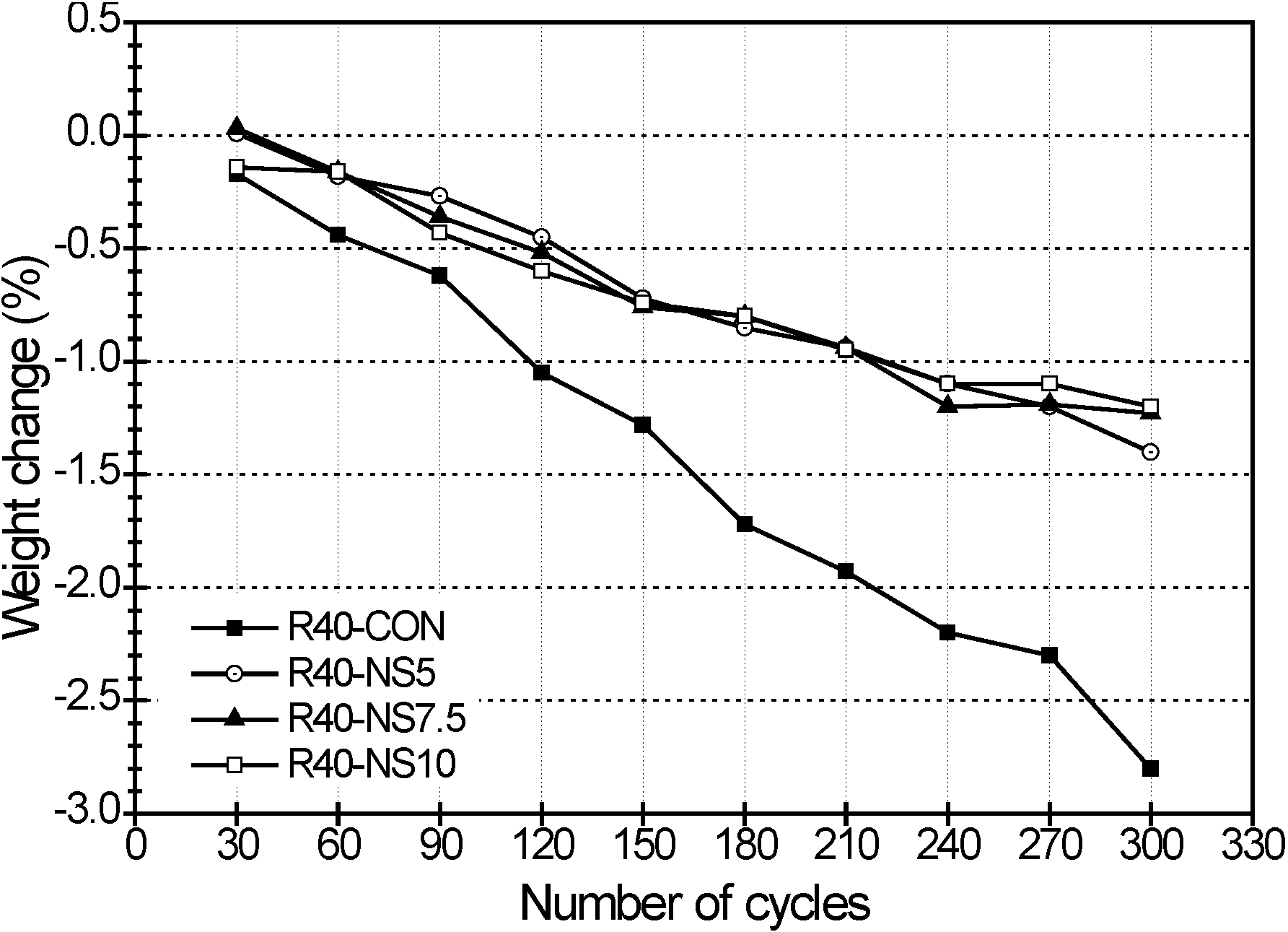
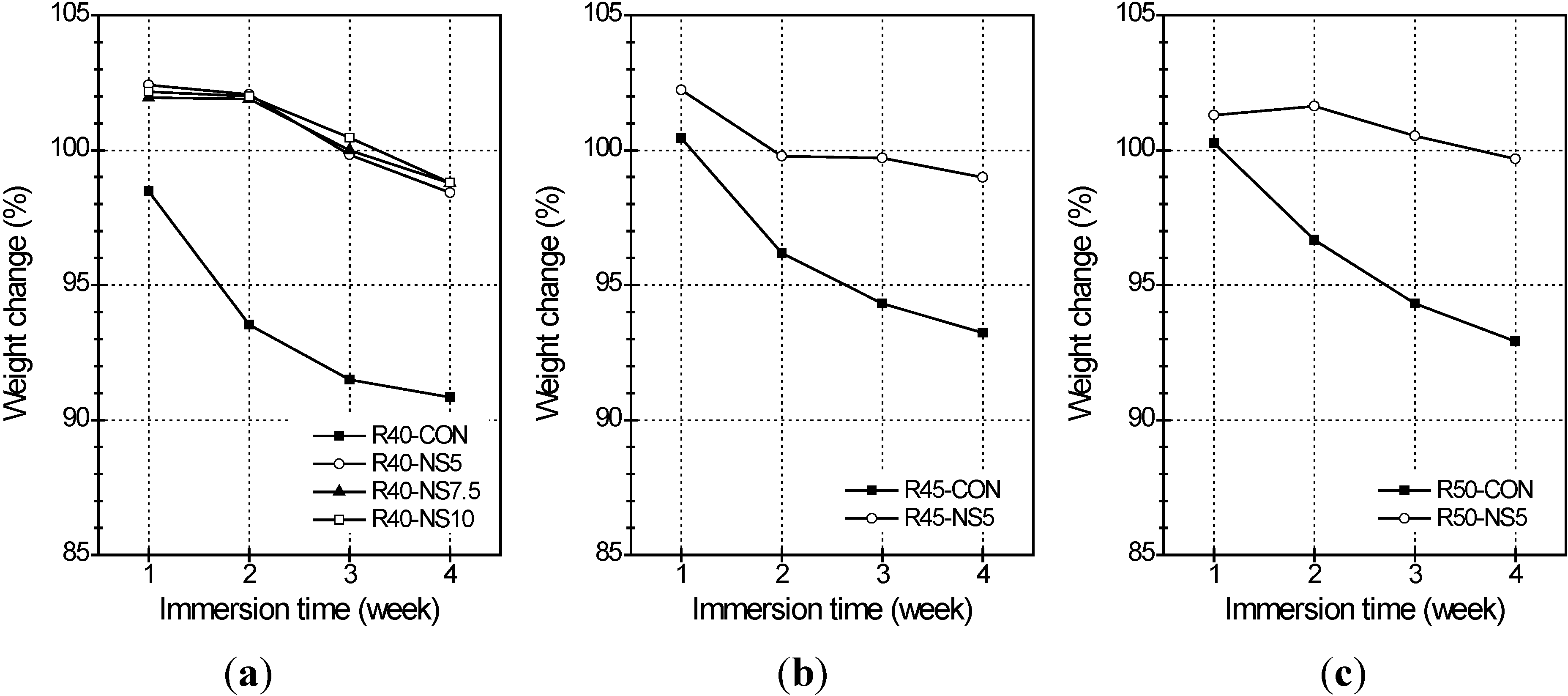
2.5. Freezing and Thawing

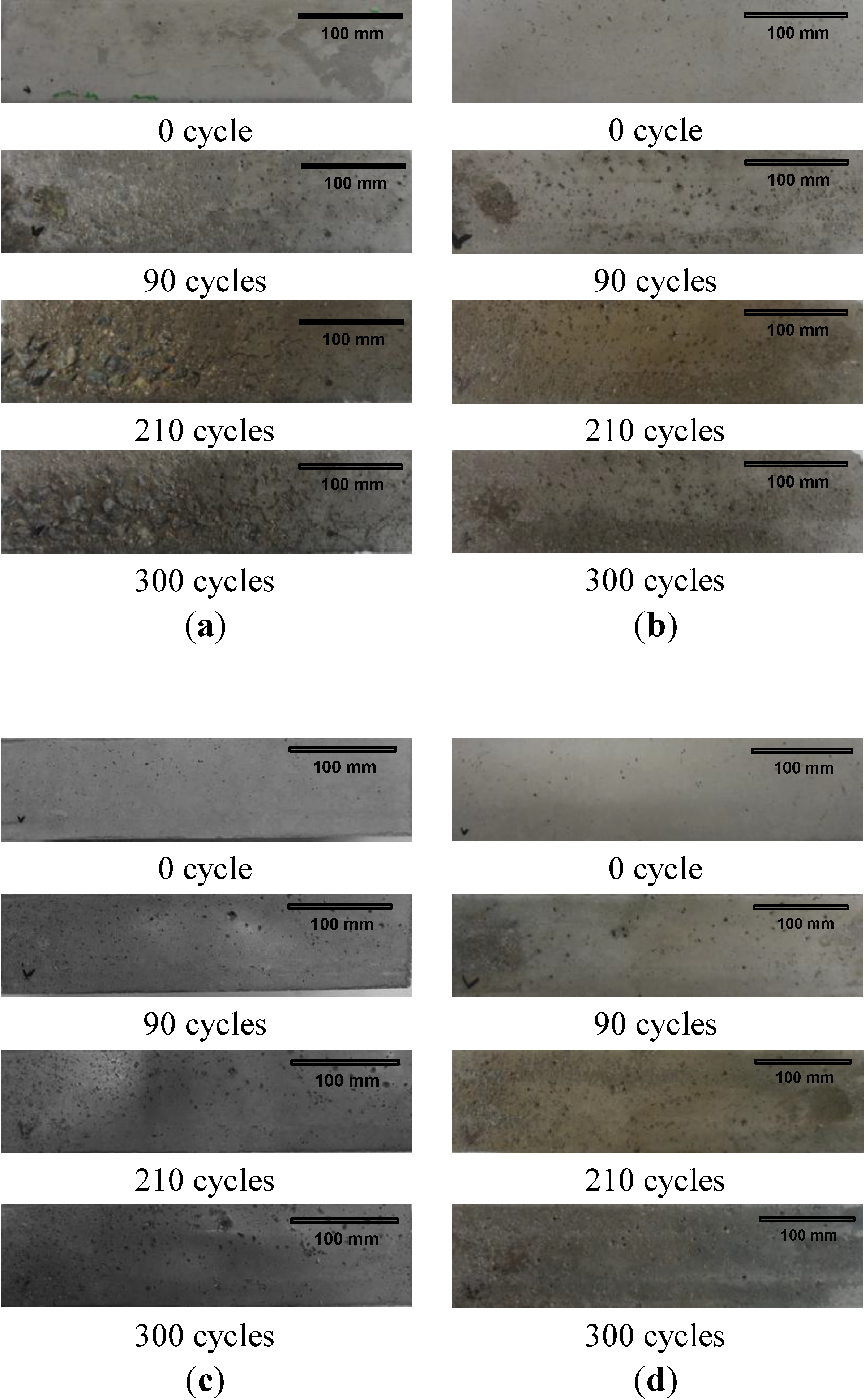
2.6. Sulfate Resistance

3. Experimental Program
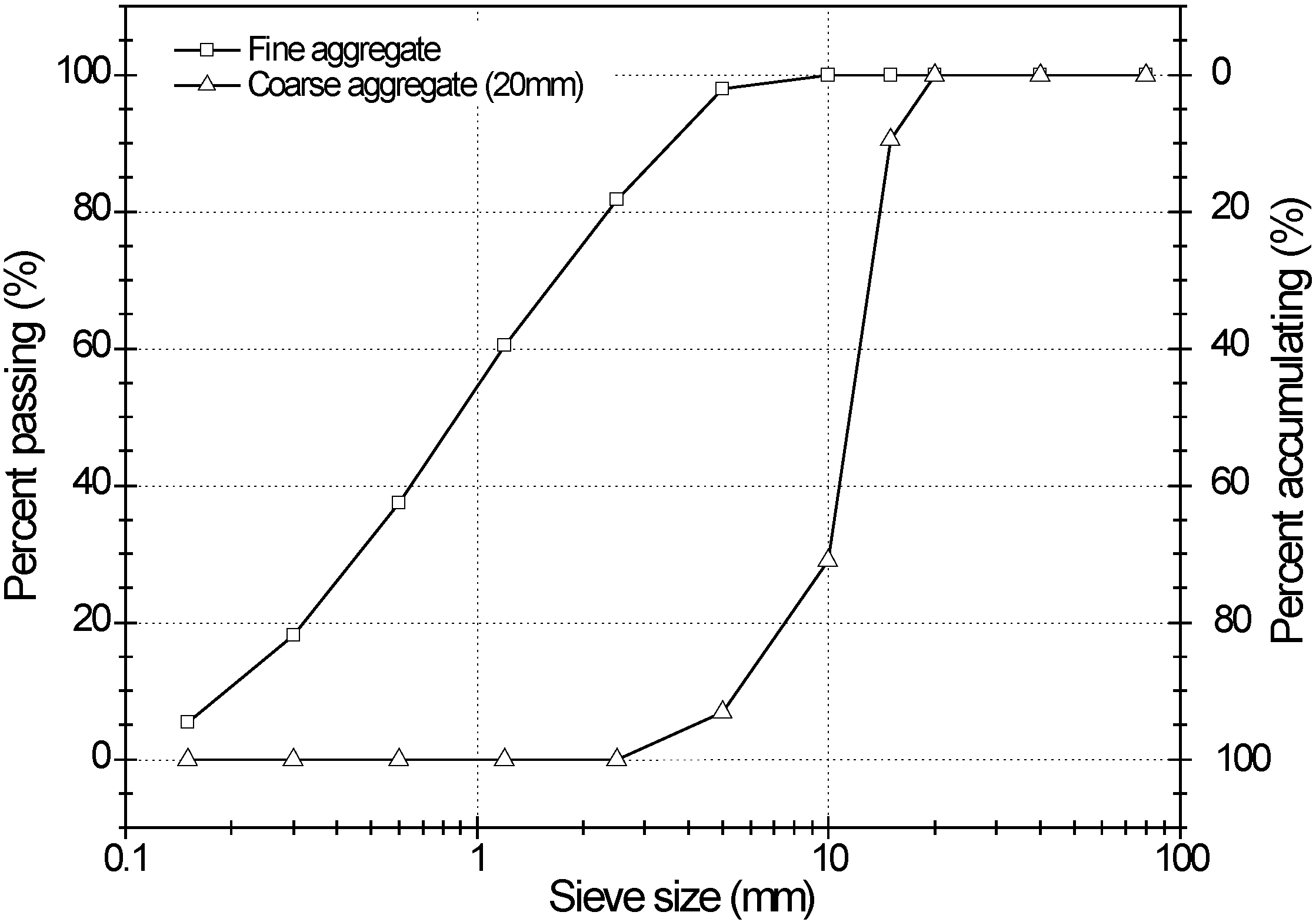
3.1. Materials
| Chemical composition (%) | Loss Ignition | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | SO3 | K2O | Na2O | |
| 21.74 | 62.79 | 5 | 3.17 | 2.97 | 1.67 | 1.36 | 0.11 | 1.19 |
| Setting time (min) | Compressive strength (MPa) | Blaine specificsurface area (cm2/g) | Specific gravity | |||
|---|---|---|---|---|---|---|
| Initial Setting | Final Setting | @ 3-day | @ 7-day | @ 28-day | ||
| 221 | 303 | 30.32 | 41.50 | 50.20 | 3340 | 3.15 |
| Samples | Specific gravity | Viscosity (mPa·s) | Polymerization degree (g/mol) | Melting point (°C) | * Ratio of modified sulfur (%) |
|---|---|---|---|---|---|
| NS | 1.75 | 0.045 at 85 °C | 300–1200 | 80 | 99.3 |
| Type | Maximum size (mm) | Specific gravity | Fineness modulus | Water absorption (%) |
|---|---|---|---|---|
| Fine aggregate | – | 2.6 | 2.99 | 2.9 |
| Coarse aggregate | 20 | 2.63 | 7.03 | 1.01 |
| Specific gravity (g/mL) | pH value | Temperature stability (°C) | Usage (%) |
|---|---|---|---|
| 120 ± 0.2 | 7 ± 2 | 5–50 | *C × (0.1–3.0) |
3.2. Mixture Proportion
| Mixture | W/C (%) | S/a (%) | Air (%) | Unit weight (kg/m3) | Sulfur (C%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| W | C | S | G | AD | |||||
| R40-CON | 40 | 45 | 5 | 185 | 462 | 737 | 917 | 2.5 | 0.0 |
| R40-NS5 | 4.3 | 5.0 | |||||||
| R40-NS7.5 | 4.6 | 7.5 | |||||||
| R40-NS10 | 4.9 | 10.0 | |||||||
| R45-CON | 45 | 45 | 5 | 185 | 411 | 756 | 942 | 2.2 | 0.0 |
| R45-NS5 | 3.7 | 5.0 | |||||||
| R50-CON | 50 | 45 | 5 | 185 | 370 | 771 | 961 | 1.0 | 0.0 |
| R50-NS5 | 3.1 | 5.0 | |||||||
3.3. Specimen Preparation
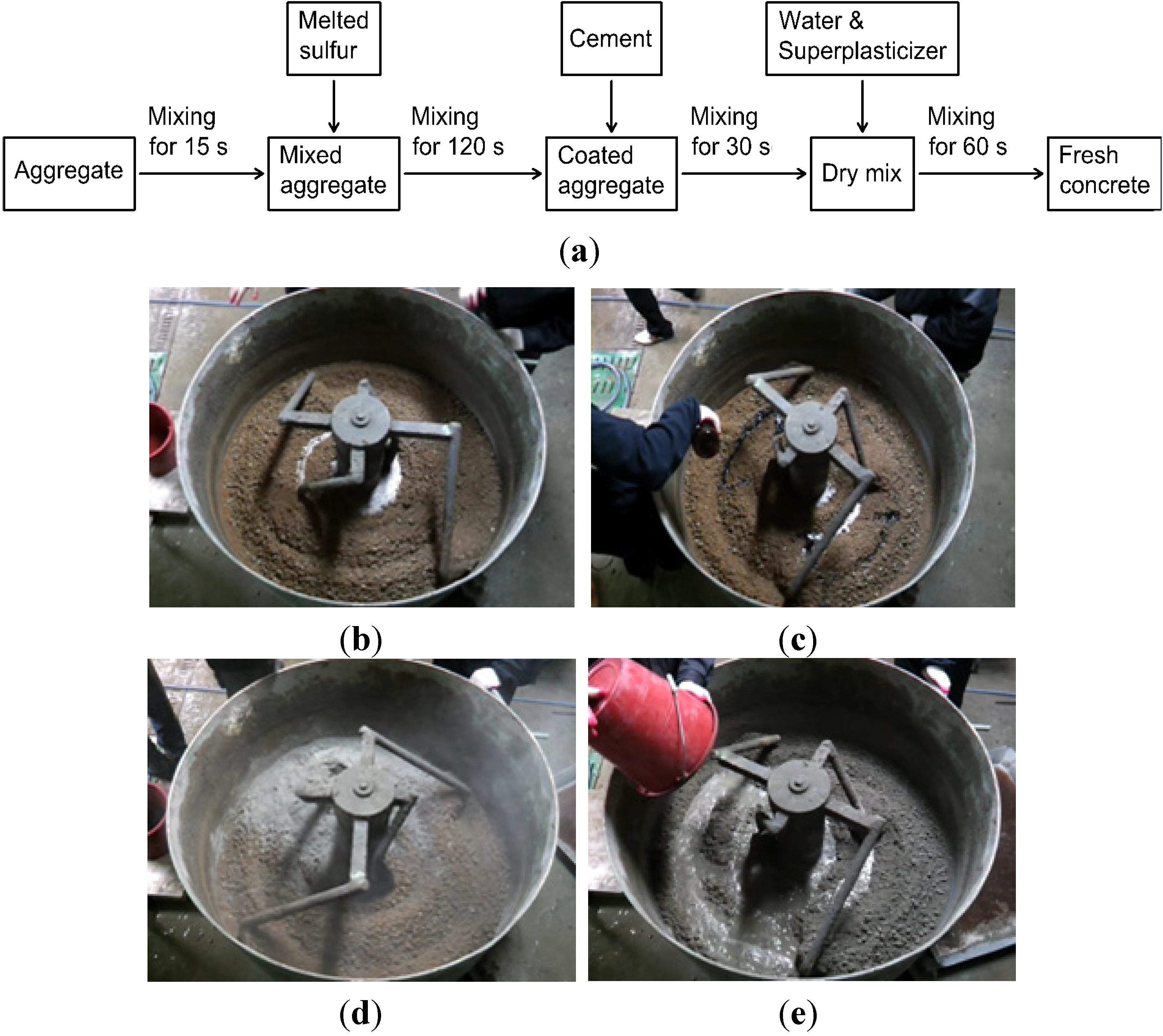
3.4. Tests
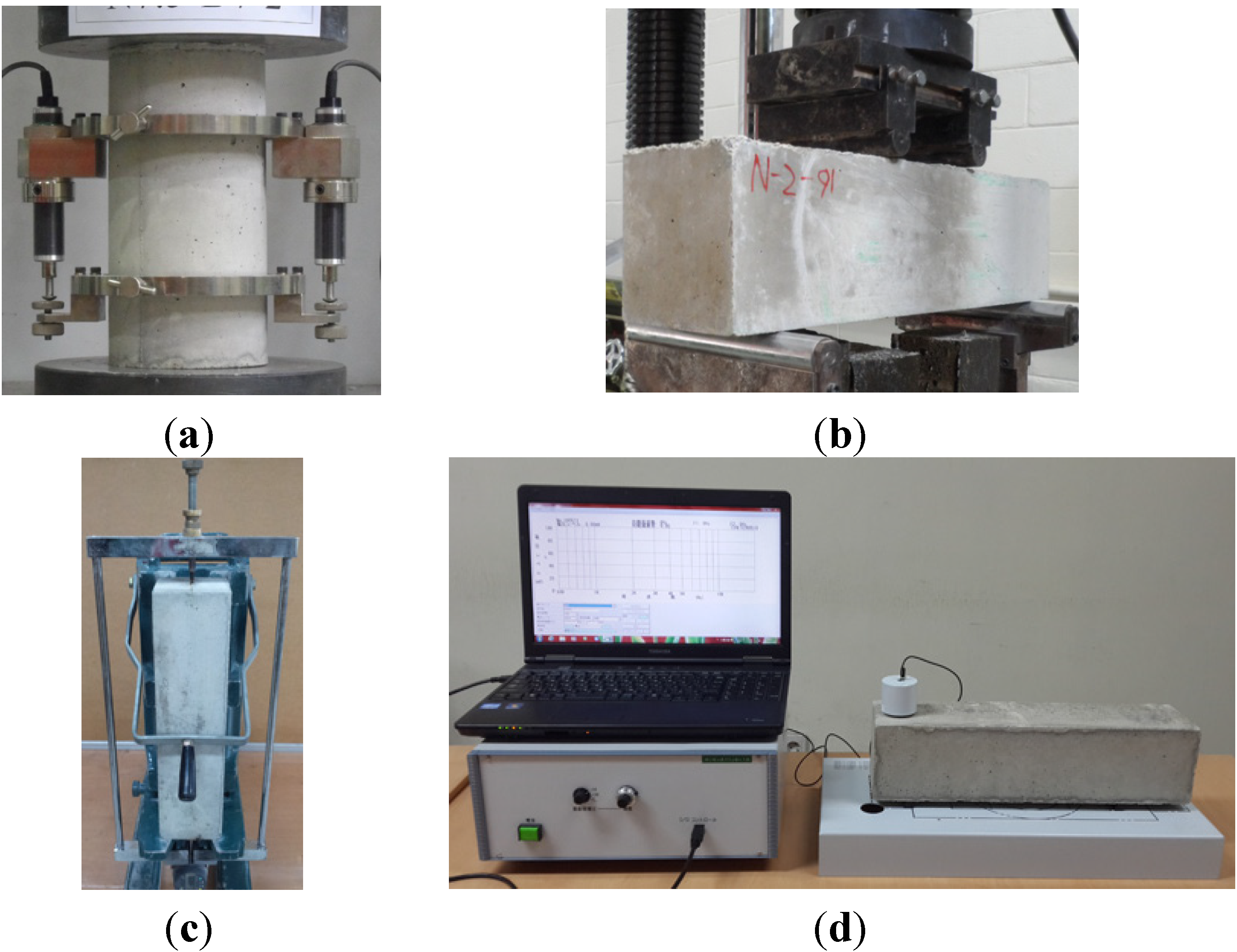
4. Conclusions
- The greater the amount of modified sulfur used to coat the aggregate, the lower the compressive strength of the concrete was. This is because the modified sulfur remaining after coating can act as impurities or light-weight aggregate in the concrete. Five percent modified sulfur of MSCA barely affected the flexural strength of the concrete regardless of the water-cement ratio, while addition of more than 7.5% of modified sulfur led to quickly decreased strength.
- Use of MSCA in concrete slightly reduced the length change of concrete as compared with concrete not having MSCA, but the change rates did not significantly differ with variation of the melted amount of sulfur in the MSCA.
- Also, the MSCA considerably improved the resistance to freezing-thawing and sulfuric acid because its use resulted in high porosity and prevented a chemical reaction between acid and basic oxides. The dosage of sulfur, however, did not greatly affect the resistance.
- Amounts of modified sulfur exceeding 5% had bad effects on mechanical strength through a compressive and flexural test. And the modified sulfur enhanced the durability in sulfuric acid and freezing-thawing, whereas the improvement did not strikingly vary according to the amount of modified sulfur. Synthetically, in terms of improvement of durability, using 5% modified sulfur was suggested restrictively in the MSCA concrete.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neville, A.M. Properties of Concrete, 4th ed.; Pearson Education Limited: England, UK, 2008. [Google Scholar]
- Wang, X.; Nguyen, M.; Stewart, M.G.; Syme, M.; Leitch, A. Analysis of Climate Change Impacts on the Deterioration of Concrete Infrastructure—Part 1: Mechanisms, Practices, Modelling and Simulations—A Review; CSIRO: Canberra, Australia, 2010. [Google Scholar]
- Wang, X.; Nguyen, M.; Stewart, M.G.; Syme, M.; Leitch, A. Analysis of Climate Change Impacts on the Deterioration of Concrete Infrastructure—Part 2: Modelling and Simulation of Deterioration and Adaptation Options; CSIRO: Canberra, Australia, 2010. [Google Scholar]
- Wang, X.; Nguyen, M.; Stewart, M.G.; Syme, M.; Leitch, A. Analysis of Climate Change Impacts on the Deterioration of Concrete Infrastructure—Part 3: Case Studies of Concrete Deterioration and Adaptation; CSIRO: Canberra, Australia, 2010. [Google Scholar]
- Wang, X.; Nguyen, M.; Stewart, M.G.; Syme, M.; Leitch, A. Analysis of Climate Change Impacts on the Deterioration of Concrete Infrastructure—Synthesis Report; CSIRO: Canberra, Australia, 2010. [Google Scholar]
- Ajdukiewicz, A.; Kliszczewicz, A. Influence of recycled aggregates on mechanical properties of HS/HPC. Cem. Concr. Compos. 2002, 24, 269–279. [Google Scholar] [CrossRef]
- Mohamed, A.M.; El Gamal, M.M. Solidification of cement kiln dust using sulfur binder. J. Hazard. Mater. 2011, 192, 576–584. [Google Scholar] [CrossRef]
- Sagoe-Crentsil, K.K.; Brown, T.; Taylor, A.H. Performance of concrete made with commercially produced coarse recycled concrete aggregate. Cem. Concr. Res. 2011, 31, 707–712. [Google Scholar] [CrossRef]
- Sandrolini, F.; Saccani, A. The effect of polymer addition on the electrical behavior of ordinary and pulverized fly ash modified cement mortars. Mater. Struct. 1997, 30, 412–417. [Google Scholar] [CrossRef]
- Bacon, R.F.; Davis, H.S. Recent advances in the American sulfur industry. Chem. Metall. Eng. 1921, 24, 65–72. [Google Scholar]
- Beaudoin, J.J.; Feldmant, R.F. Durability of porous systems impregnated with dicyclopentadiene-modified sulfur. Int. J. Cem. Compos. Lightweight Concr. 1984, 6, 13–17. [Google Scholar] [CrossRef]
- Bordoloi, B.K.; Pierce, E.M. Plastic sulfur stabilization by copolymerization of sulfur with dicyclopentadiene. Adv. Chem. Ser. 1978, 165, 31–53. [Google Scholar] [CrossRef]
- Lin, S.-L.; Lai, J.S.; Chian, E.S.K. Modification of sulfur polymer cement (SPC) stabilization and solidification (S/S) process. Waste Mang. 1995, 15, 441–447. [Google Scholar] [CrossRef]
- American Concrete Institute (ACI) Committee. Guide for Mixing and Placing Sulfur Concrete in Construction (Report 548.2R-93); American Concrete Institute (ACI) Committee: Farmington Hills, MI, USA, 1993. [Google Scholar]
- Grugel, R.N.; Toutanji, H. Sulfur concrete for lunar applications-sublimation concerns. Adv. Space Res. 2008, 41, 103–112. [Google Scholar] [CrossRef]
- Mohamed, A.-M.O.; El Gamal, M. Hydro-mechanical behavior of a newly developed sulfur polymer concrete. Cem. Concr. Compos. 2009, 31, 186–194. [Google Scholar]
- Thomas, C.; Cimentada, A.; Polanco, J.A.; Setién, J.; Méndez, D.; Rico, J. Influence of recycled aggregates containing sulphur on properties of recycled aggregate mortar and concrete. Compos. B Eng. 2013, 45, 474–485. [Google Scholar] [CrossRef]
- Vlahovic, M.M.; Martinovic, S.P.; Boljanac, T.D.; Jovanic, P.B.; Volkov-Husovic, T.D. Durability of sulfur concrete in various aggressive environments. Constr. Build. Mater. 2011, 25, 3926–3934. [Google Scholar] [CrossRef]
- Li, J.; Xiao, H.; Zhou, Y. Influence of coating recycled aggregate surface with pozzolanic powder on properties of recycled aggregate concrete. Constr. Build. Mater. 2009, 23, 1287–1291. [Google Scholar] [CrossRef]
- Kong, D.; Lei, T.; Zheng, J.; Ma, C.; Jiang, J.; Jiang, J. Effect and mechanism of surface-coating pozzalanics materials around aggregate on properties and ITZ microstructure of recycled aggregate concrete. Constr. Build. Mater. 2010, 24, 701–708. [Google Scholar]
- Morin, V.; Moevus, M.; Dubois-Brugger, I.; Gartner, E. Effect of polymer modification of the paste–aggregate interface on the mechanical properties of concretes. Cem. Concr. Res. 2011, 41, 459–466. [Google Scholar]
- Zhihui, Z.; Shoude, W; Lingchao, L.; Chenchen, G. Evaluation of pre-coated recycled aggregate for concrete and mortar. Constr. Build. Mater. 2013, 43, 191–196. [Google Scholar] [CrossRef]
- Standard Test Method for Density, Relative Density (Specific Gravity), and Absorption of Fine Aggregate (ASTM C128); American Society for Testing and Material (ASTM) International: West Conshohocken, PA, USA, 2012.
- Standard Test Method for Density, Absorption, and Voids in Hardened Concrete (ASTM C642); American Society for Testing and Material (ASTM) International: West Conshohocken, PA, USA, 2013.
- Standard Test Method for Compressive Strength of Cylinder Concrete Specimens (ASTM C39/C39M); American Society for Testing and Material (ASTM) International: West Conshohocken, PA, USA, 2012.
- Standard Test Method for Flexural Strength of Concrete (Using Simple Beam with Third-Point Loading) (ASTM C78/C78M); American Society for Testing and Material (ASTM) International: West Conshohocken, PA, USA, 2010.
- Standard Test Method for Length Change of Hardened Hydraulic-Cement Mortar and Concrete (ASTM C157/C157M); American Society for Testing and Material (ASTM) International: West Conshohocken, PA, USA, 2008.
- Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing (ASTM C666/C666M); American Society for Testing and Material (ASTM) International: West Conshohocken, PA, USA, 2008.
- Japan Society for Testing Materials (JSTM). Chemical Resistance Test Method of Concrete by Solution Immersion (JSTM C7401); Japan Testing Center for Construction Materials: Tokyo, Japan, 1999. (In Japanese) [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, S.-H.; Hong, K.-N.; Park, J.-K.; Ko, J. Influence of Aggregate Coated with Modified Sulfur on the Properties of Cement Concrete. Materials 2014, 7, 4739-4754. https://doi.org/10.3390/ma7064739
Lee S-H, Hong K-N, Park J-K, Ko J. Influence of Aggregate Coated with Modified Sulfur on the Properties of Cement Concrete. Materials. 2014; 7(6):4739-4754. https://doi.org/10.3390/ma7064739
Chicago/Turabian StyleLee, Swoo-Heon, Ki-Nam Hong, Jae-Kyu Park, and Jung Ko. 2014. "Influence of Aggregate Coated with Modified Sulfur on the Properties of Cement Concrete" Materials 7, no. 6: 4739-4754. https://doi.org/10.3390/ma7064739
APA StyleLee, S.-H., Hong, K.-N., Park, J.-K., & Ko, J. (2014). Influence of Aggregate Coated with Modified Sulfur on the Properties of Cement Concrete. Materials, 7(6), 4739-4754. https://doi.org/10.3390/ma7064739




