Nanoporous Anodic Alumina: A Versatile Platform for Optical Biosensors
Abstract
:1. Introduction
2. Fabrication and Structural Engineering of NAA
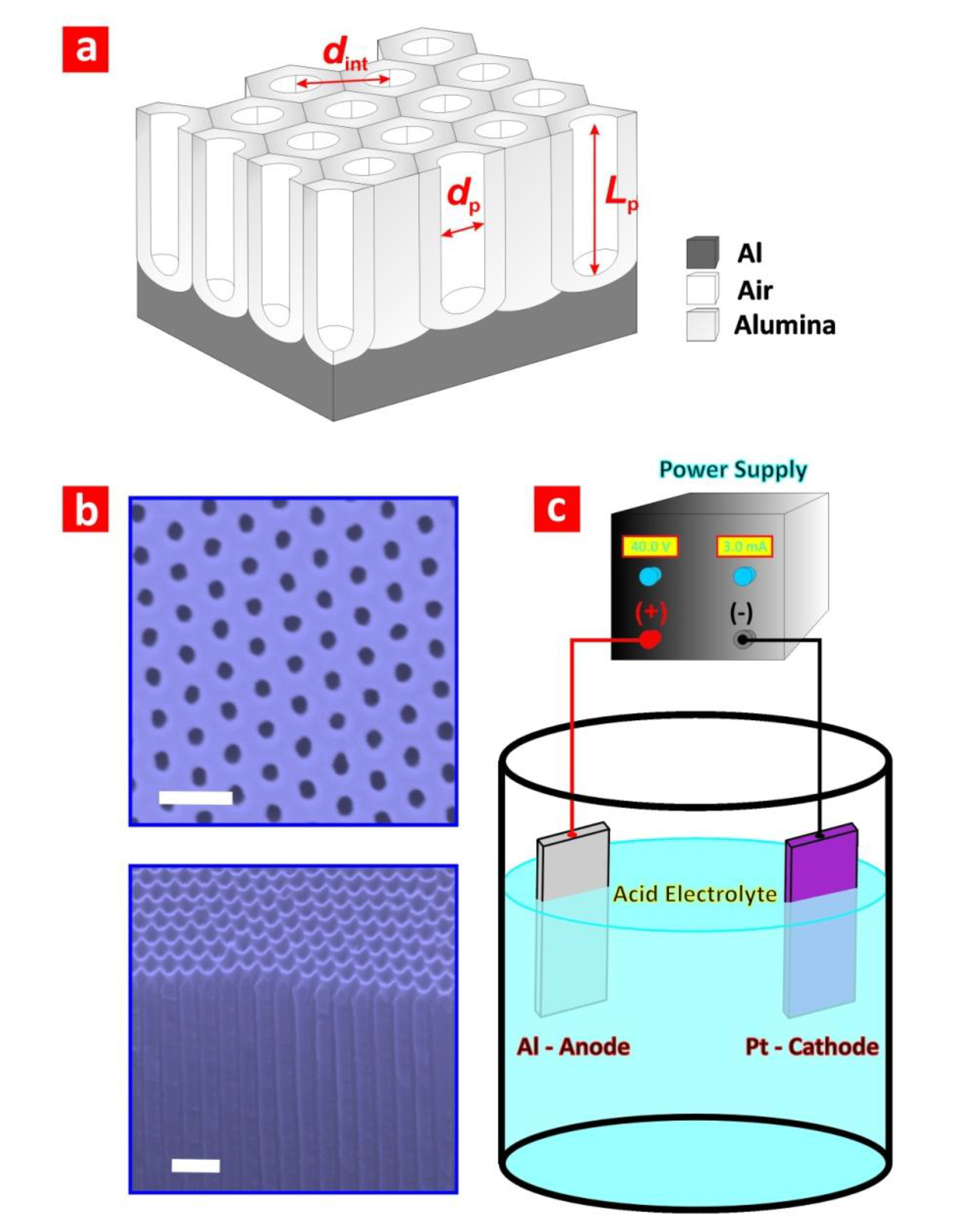
2.1. Fabrication of NAA
- (i)
- Formation of alumina (aluminium-alumina interface (anode))2Al(s) + 3H2O(1) ↔ Al2O3(s) + 6H+(aq) + 6e−
- (ii)
- Dissolution of alumina (alumina-electrolyte interface (anode))Al2O3(s) + 6H+(aq) → 2Al3+(aq) + 3H2O(1)
- (iii)
- Diffusion of aluminium cations (within oxide barrier layer (anode))2Al(s) → 2Al3+(aq) + 6e−
- (iv)
- Hydrogen evolution (electrolyte-cathode interface (cathode))6H+(aq) + 6e− → 3H2(g)
| Acid electrolyte | Anodisation regime | V (V) | T (°C) | dp (nm) | dint (nm) | Growth rate (µm·h−1) | References |
|---|---|---|---|---|---|---|---|
| H2SO4 0.3 M | MA | 25 | 5–8 | 25 | 63 | 7.5 | [12] |
| H2SO4 0.3 M | HA | 40 | 0–1 | 30 | 78 | 85 | [18] |
| H2C2O4 0.3 M | MA | 40 | 5–8 | 30 | 100 | 3.5 | [11] |
| H2C2O4 0.3 M | HA | 140 | 0–1 | 50 | 280 | 50 | [4] |
| H3PO4 0.1 M | MA | 195 | 0–1 | 160 | 500 | 2 | [13] |
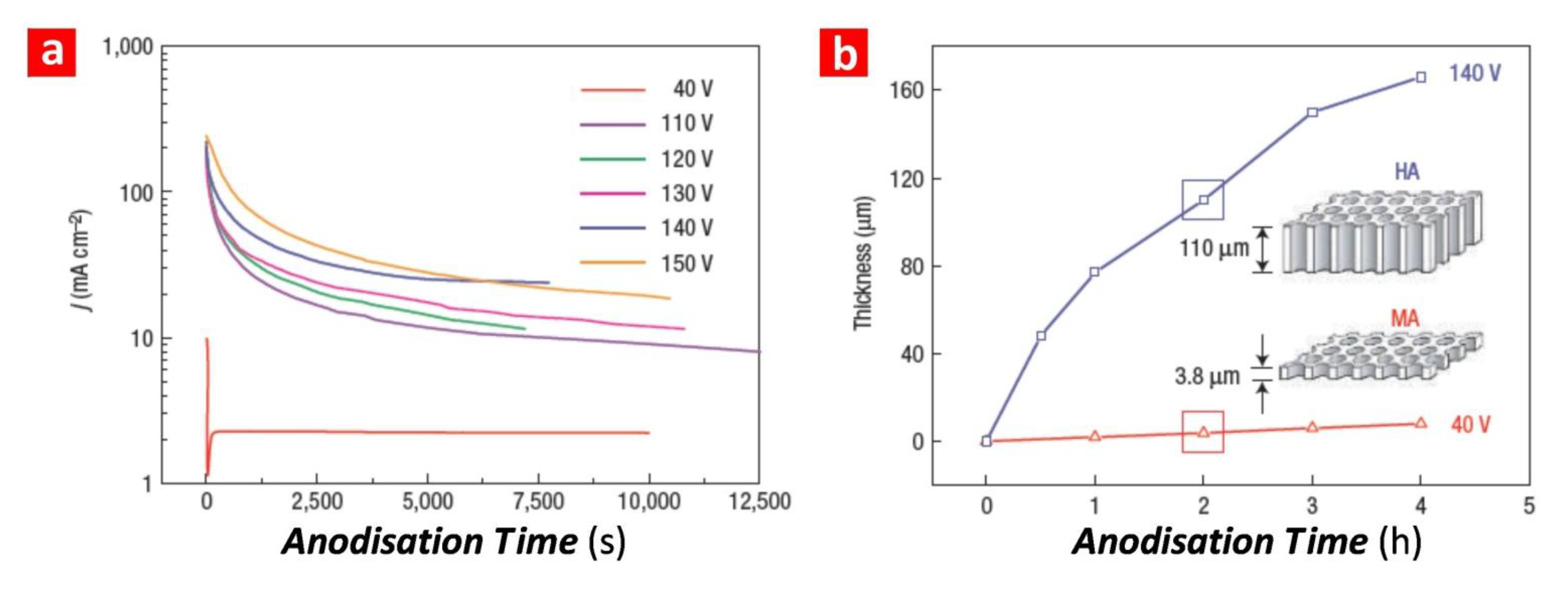
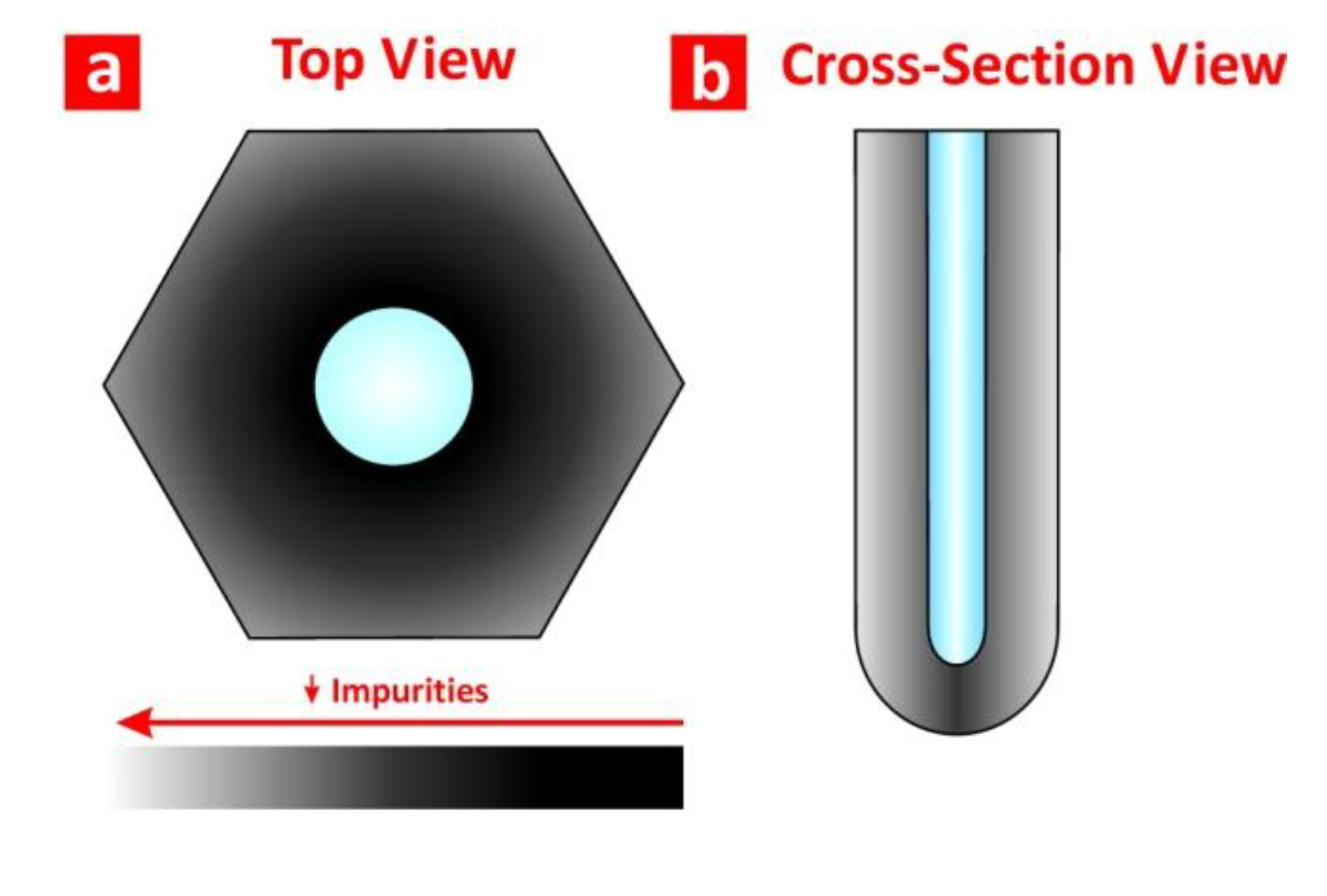
2.2. Structural and Optical Engineering of NAA
| Type of NAA | Electrochemical approach | References |
|---|---|---|
| Modulated | modulated anodisation profile | [18,21,22] |
| Hierarchical | asymmetric two-step anodisation | [23,24] |
| Serrated | anodisation at low voltage and high temperature | [25,26] |
| Three-dimensional | modulated anodisation profile and final wet chemical etching | [27,28] |
| Multilayered | stepwise or pulse anodisation | [29,30,31,32,33] |
| Funnel-like | combination of multiple anodisation and pore widening steps | [34,35,36,37,38,39] |
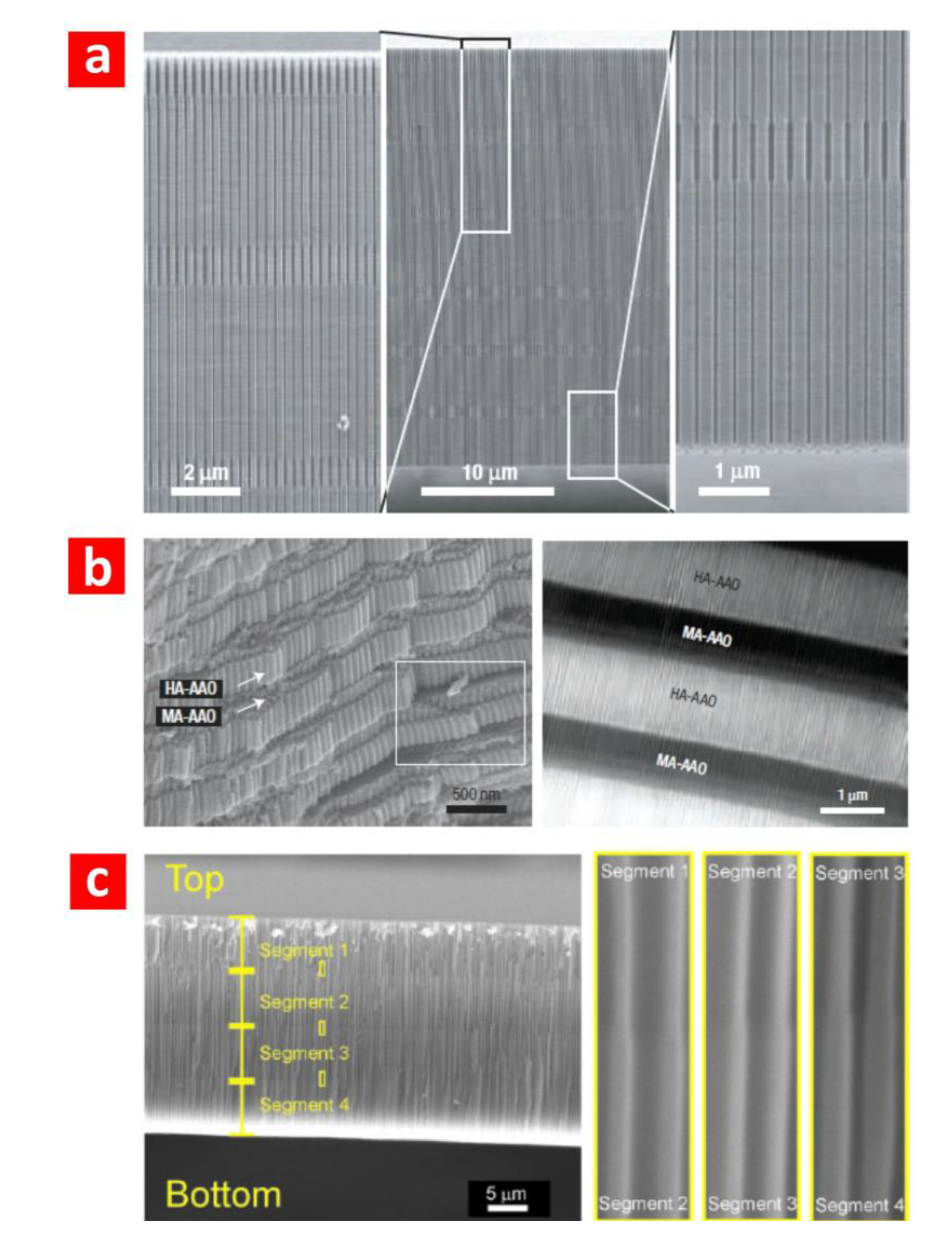
3. Optical Biosensors Based on NAA
| Sensing Platform | Analyte | Detection Limit | References |
|---|---|---|---|
| SPR-NAA | avidin | 10 µg·mL−1 | [44] |
| anti-5-fluorouracil | 100 mg·mL−1 | [44] | |
| melittin | 100 ng·mL−1 | [44] | |
| Ru[BPhen3]2+ | 2 µM | [45] | |
| Fe[Phen3]2+ | 1 µM | [45] | |
| BSA | 60 nM | [46] | |
| invertase | 10 nM | [36] | |
| SERS-NAA | p-aminothiophenol | 0.5 M | [47] |
| 4-mercaptopyridine | 1 × 10−6 M | [48] | |
| 3-mercaptobenzoic | 3 mM | [49] | |
| benzenethiol | 500 ppb | [50] | |
| N-methyl-4-nitroaniline | 3 ppb | [50] | |
| PL-NAA | morin | 5 × 10−6 M | [51] |
| trypsin | 40 µg·mL−1/0.1 mg·mL−1 | [51,52] | |
| DNA | 100 mM | [53] | |
| oxazine 170 | 6.5 × 10−3 M | [54] | |
| glucose | 0.1 M | [54] | |
| glucose | 10 mM | [55] | |
| cysteine | 5 mM | [55] | |
| RIfS-NAA | H2S | 0.5 v% | [56] |
| DNA | 2 nmol·cm−2 | [57] | |
| circulating tumour cells | 1000 cells·mL−1 | [58] | |
| immunoglobulin | 0.1 mg·mL−1 | [59] | |
| glucose | 100 mM | [55] | |
| cysteine | 5 mM | [55] | |
| gold(III) | 0.1 µM | [60] | |
| BSA | 1 mg·mL−1 | [61] | |
| glucose | 0.01 M | [2] | |
| BSA | 15 µM | [58] | |
| human IgG | 600 nM | [62] |
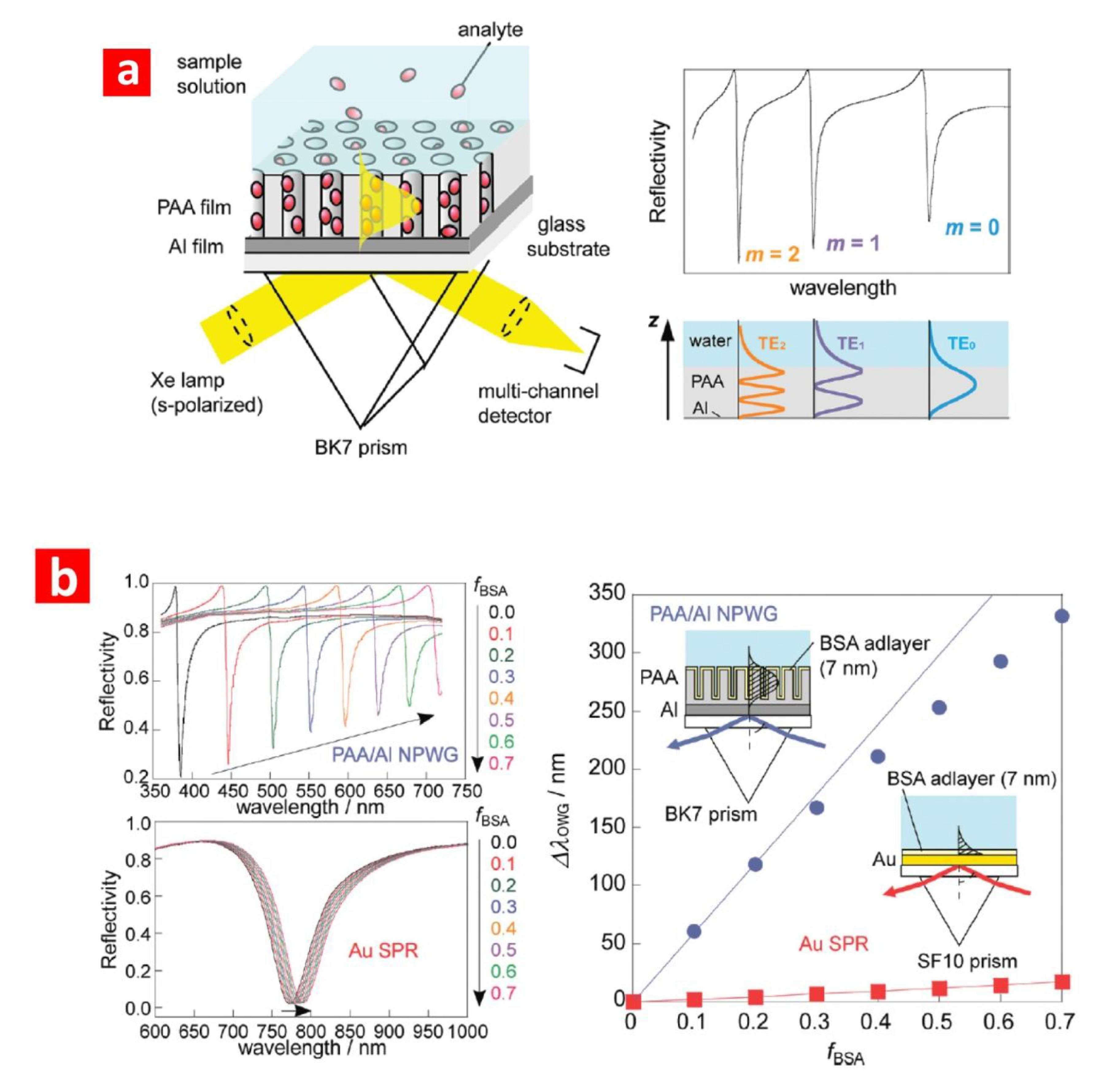
3.1. Surface Plasmon Resonance (SPR)
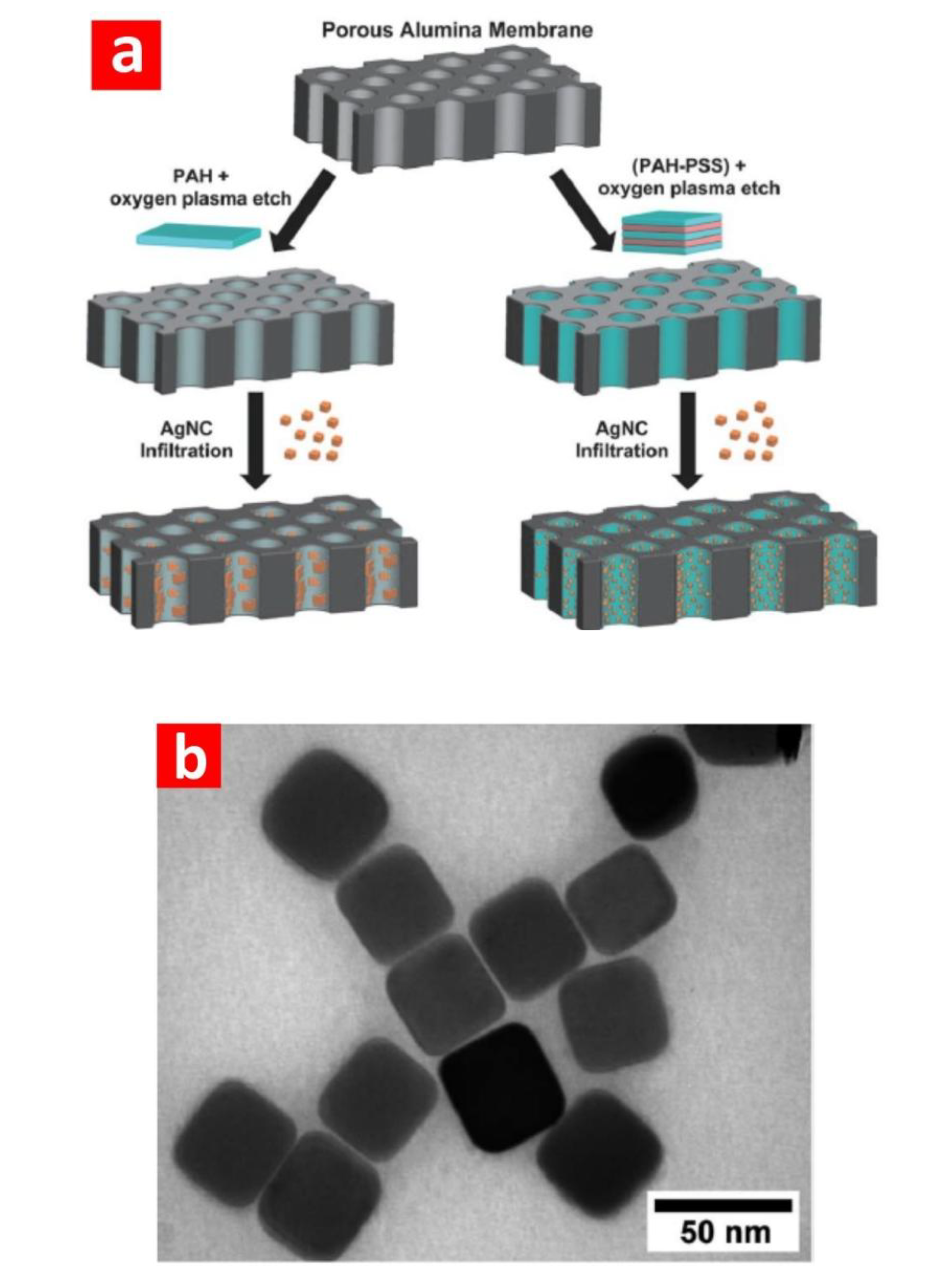
3.2. Surface-Enhanced Raman Scattering (SERS)
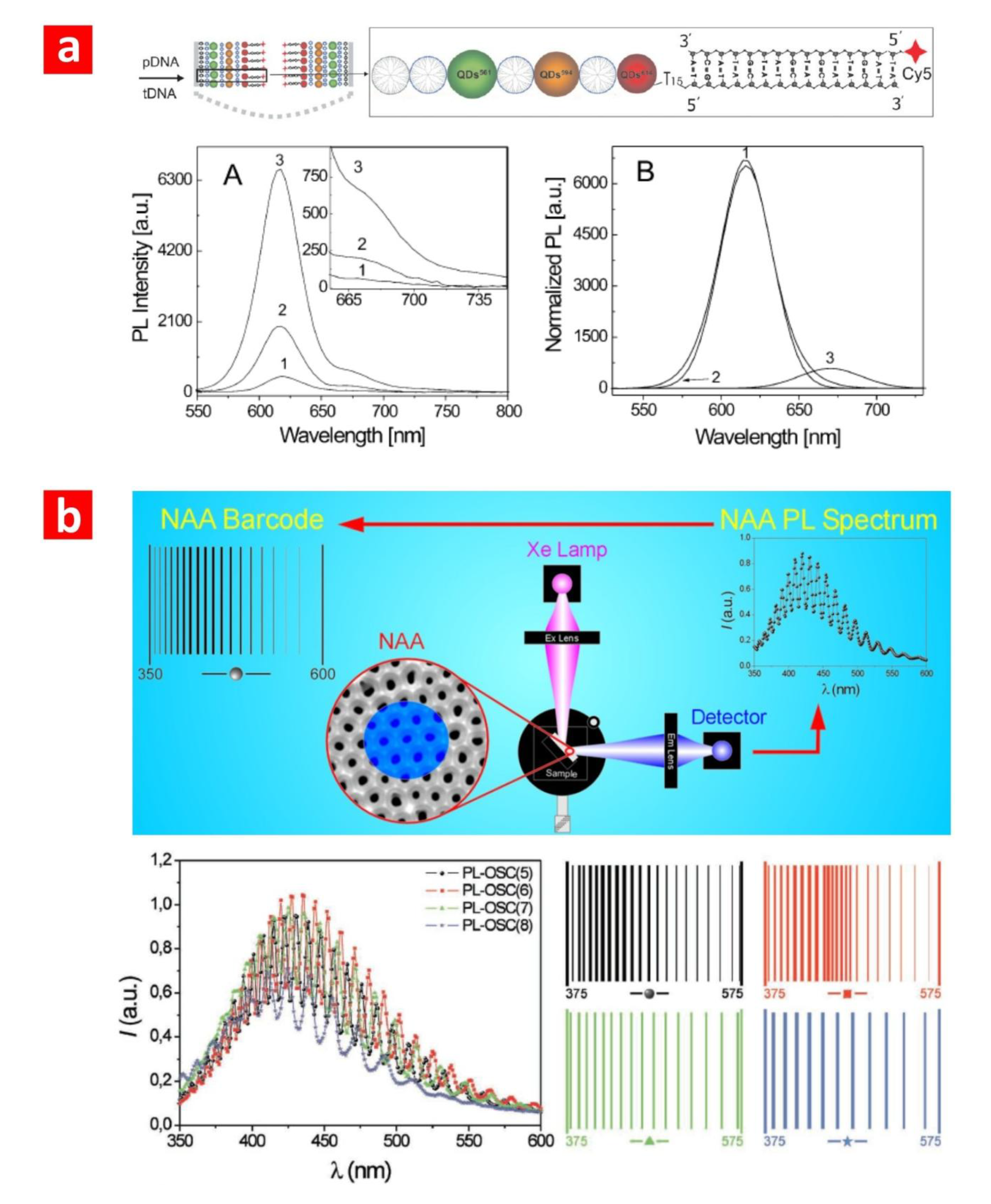
3.3. Photoluminescence (PL)
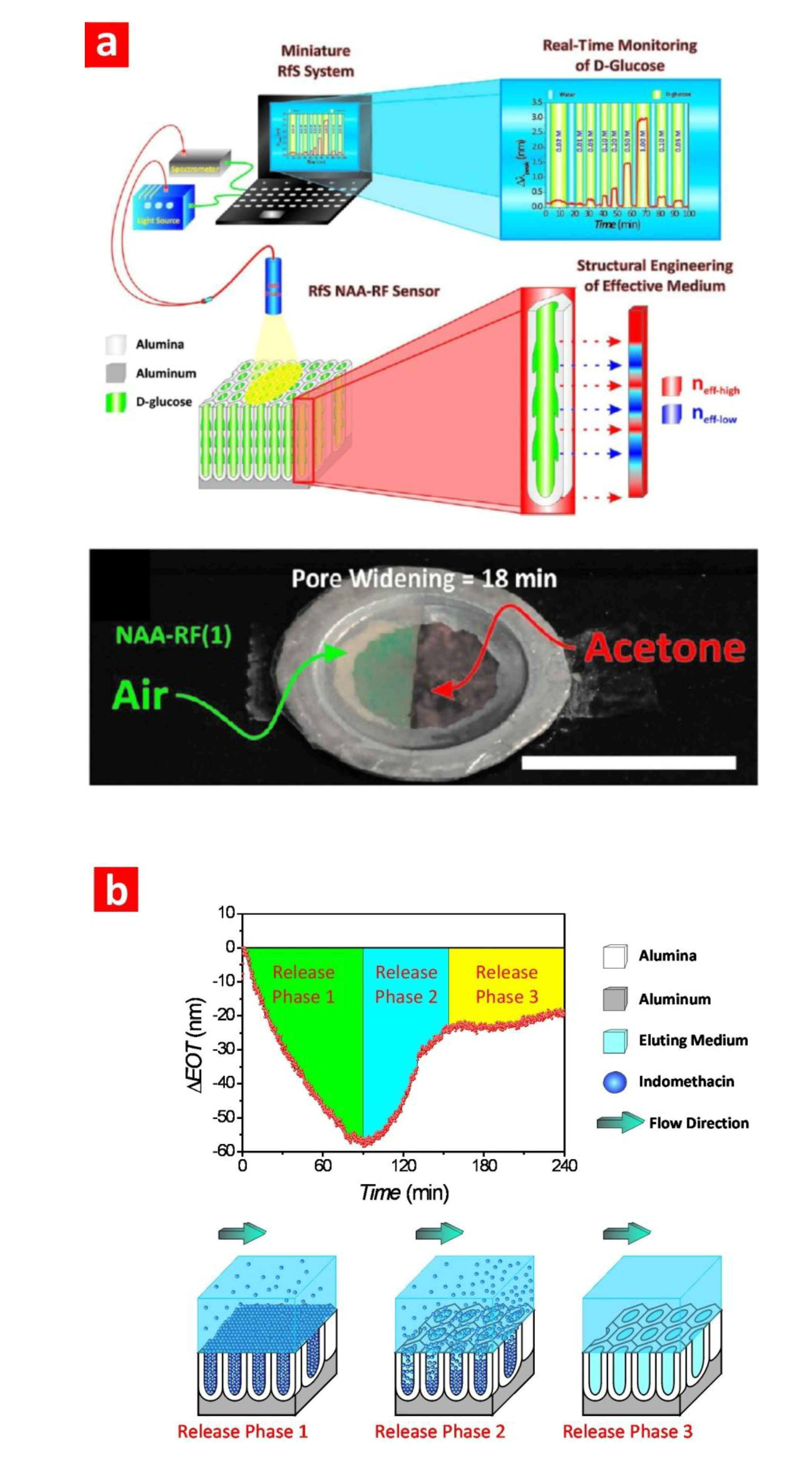
3.4. Reflectometric Interference Spectroscopy (RIfS)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F.; Losic, D. Structural and optical nanoengineering of nanoporous anodic alumina rugate filters for real-time and label-free biosensing applications. Anal. Chem. 2014, 86, 1837–1844. [Google Scholar] [CrossRef]
- Md Jani, A.M.; Kempson, I.M.; Losic, D.; Voelcker, N.H. Dressing in layers: Layering surface functionalities in nanoporous aluminum oxide membranes. Angew. Chem. Int. Ed. 2010, 49, 7933–7937. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- O’Sullivan, J.P.; Wood, G.C. The morphology and mechanism of formation of porous anodic films on aluminium. Proc. R. Soc. Lond. Ser. A Math. Phys. 1970, 317, 511–543. [Google Scholar] [CrossRef]
- Field, D.J. Aluminium alloys—Contemporary research and applications. In Treatise on Materials Science and Technology; Vasudevan, A.K., Doherty, R.D., Eds.; Elsevier: Amsterdam, the Netherland, 1989; Volume 31, pp. 523–537. [Google Scholar]
- Thompson, G.E.; Xu, Y.; Skeldon, P.; Shimizu, K.; Han, S.H.; Wood, G.C. Anodic oxidation of aluminium. Philos. Mag. Part B 1987, 55, 651–667. [Google Scholar] [CrossRef]
- Shimizu, K.; Kobayashi, K.; Thompson, G.E.; Wood, G.C. A novel marker for the determination of transport during anodic barrier oxide growth on aluminium. Philos. Mag. Part B 1991, 64, 345–353. [Google Scholar] [CrossRef]
- Wood, G.C.; Skeldon, P.; Thompson, G.E.; Shimizu, K. A model for the incorporation of electrolyte species into anodic alumina. J. Electrochem. Soc. 1996, 143, 74–83. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar]
- Masuda, H.; Hasegwa, F. Self-ordering of cell arrangement of anodic porous alumina formed in sulfuric acid solution. J. Electrochem. Soc. 1997, 144, L127–L130. [Google Scholar] [CrossRef]
- Masuda, H.; Yada, K.; Osaka, A. Self-ordering of cell configuration of anodic porous alumina with large-size pores in phosphoric acid solution. Jpn. J. Appl. Phys. 1998, 37, L1340–L1342. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B.; Gösele, U. Self-ordering regimes of porous alumina: The 10% porosity rule. Nano Lett. 2002, 2, 677–680. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly ordered anodic porous alumina formation by self-organized anodizing. In Nanostructured Materials in Electrochemistry; Eftekahari, A., Ed.; Wiley-VCH Verlag GmbH and Co. KGaA: Weinhem, Germany, 2008; Volume 1, pp. 1–116. [Google Scholar]
- Li, A.P.; Müller, F.; Birner, A.; Nielsch, K.; Gösele, U. Hexagonal pore arrays with a 50–420 nm interpore distance formed by self-organization in anodic alumina. J. Appl. Phys. 1998, 84, 6023–6026. [Google Scholar] [CrossRef]
- Jessensky, O.; Müller, F.; Gösele, U. Self-organized formation of hexagonal pore arrays in anodic alumina. Appl. Phys. Lett. 1998, 72, 1173–1175. [Google Scholar] [CrossRef]
- Schwirn, K.; Lee, W.; Hillebrand, R.; Steinhart, M.; Nielsch, K.; Gösele, U. Self-ordered anodic aluminum oxide formed by H2SO4 hard anodization. ACS Nano 2008, 2, 302–310. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Baba, N.; Tajima, S. Coloured materials and photoluminescence centres in anodic film on aluminium. Nature 1981, 289, 572–574. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Wang, Y.; Losic, D. In situ monitored engineering of inverted nanoporous anodic alumina funnels: On the precise generation of 3D optical nanostructures. Nanoscale 2014. [Google Scholar] [CrossRef]
- Pitzschel, K.; Montero-Moreno, J.M.; Escrig, J.; Albrecht, O.; Nielsch, K.; Bachmann, J. Controlled introduction of diameter modulations in arrayed magnetic iron oxide nanotubes. ACS Nano 2009, 3, 3463–3468. [Google Scholar]
- Losic, D.; Lillo, M.; Losic, D., Jr. Porous alumina with shaped pore geometries and complex pore architectures fabricated by cyclic anodization. Small 2009, 5, 1392–1397. [Google Scholar] [CrossRef]
- Montero-Moreno, J.M.; Sarret, M.; Müller, C. Some considerations on the influence of voltage in potentiostatic two-step anodizing of AA1050. J. Electrochem. Soc. 2007, 154, C169–C174. [Google Scholar] [CrossRef]
- Santos, A.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Hierarchical nanoporous anodic alumina templates by asymmetric two-step anodization. Phys. Status Solidi A 2011, 208, 668–674. [Google Scholar]
- Li, D.; Zhao, L.; Jiang, C.; Lu, J.G. Formation of anodic aluminum oxide with serrated nanochannels. Nano Lett. 2010, 10, 2766–2771. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, L.; Song, Y.; Jia, H.; Yu, H.; Xiao, X.; Yang, X. Oxygen bubble mould effect: Serrated nanopore formation and porous alumina growth. Monatshefte Chem. 2008, 139, 999–1003. [Google Scholar]
- Losic, D.; Losic, D., Jr. Preparation of porous anodic alumina with periodically perforated pores. Langmuir 2009, 25, 5426–5431. [Google Scholar] [CrossRef]
- Santos, A.; Vojkuvka, L.; Alba, M.; Balderrama, V.S.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Understanding and morphology control of pore modulations in nanoporous anodic alumina by discontinuous anodization. Phys. Status Solidi A 2012, 209, 2045–2048. [Google Scholar] [CrossRef]
- Lee, W.; Schwirn, K.; Steinhart, M.; Pippel, E.; Scholz, R.; Gösele, U. Structural engineering of nanoporous anodic aluminium oxide by pulse anodization of aluminium. Nat. Nanotechnol. 2008, 3, 234–239. [Google Scholar] [CrossRef]
- Sulka, G.D.; Hnida, K. Distributed Bragg reflector base on porous anodic alumina fabricated by pulse anodization. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Zheng, W.J.; Fei, G.T.; Wang, B.; Zhang, L.D. Modulation of transmission spectra of anodized alumina membrane distributed Bragg reflector by controlling anodization temperature. Nanoscale Res. Lett. 2009, 4, 665–667. [Google Scholar] [CrossRef]
- Santos, A.; Montero-Moreno, J.M.; Bachmann, J.; Nielsch, K.; Formentín, P.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Understanding pore rearrangement during mild to hard transition in bilayered porous anodic alumina membranes. ACS Appl. Mater. Interfaces 2011, 3, 1925–1932. [Google Scholar] [CrossRef]
- Rahman, M.M.; Marsal, L.F.; Pallarès, J.; Ferré-Borrull, J. Tuning the photonic stop bands of nanoporous anodic alumina-based distributed Bragg reflectors by pore widening. ACS Appl. Mater. Interfaces 2013, 5, 13375–13381. [Google Scholar] [CrossRef]
- He, B.; Son, S.J.; Lee, S.B. Suspension array with shape-coded silica nanotubes for multiplexed immunoassays. Anal. Chem. 2007, 79, 5257–5263. [Google Scholar] [CrossRef]
- Nagaura, T.; Takeuchi, F.; Inoue, S. Fabrication and structural control of anodic alumina films with inverted cone porous structure using multi-step anodizing. Electrochim. Acta 2008, 53, 2109–2114. [Google Scholar]
- Nagaura, T.; Takeuchi, F.; Yamauchi, Y.; Wada, K.; Inoue, S. Fabrication of ordered Ni nanocones using porous anodic alumina template. Electrochem. Commun. 2008, 10, 681–685. [Google Scholar] [CrossRef]
- Yanagishita, T.; Kondo, T.; Nishio, K.; Masuda, H. Optimization of antireflection structures of polymer based on nanoimprinting using anodic porous alumina. J. Vac. Sci. Technol. B 2008, 26, 1856–1859. [Google Scholar] [CrossRef]
- Santos, A.; Formentín, P.; Pallarès, J.; Ferré-Borrull, J.; Marsal, L.F. Structural engineering of nanoporous anodic alumina funnels with high aspect ratio. J. Electroanal. Chem. 2011, 655, 73–78. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Chen, C.; Hao, Q.; Wang, Z.; Zhu, J.; Gao, X. Facile method for modulating the profiles and periods of self-ordered three-dimensional alumina taper-nanopores. ACS Appl. Mater. Interfaces 2012, 4, 5678–5683. [Google Scholar] [CrossRef]
- Janshoff, A.; Dancil, K.P.S.; Steinem, C.; Greiner, D.P.; Lin, V.S.Y.; Gurtner, C.; Motesharei, K.; Sailor, M.J.; Ghadiri, M.R. Macroporous p-type silicon Fabry–Perot layers. Fabrication, characterization, and applications in biosensing. J. Am. Chem. Soc. 1998, 120, 12108–12116. [Google Scholar] [CrossRef]
- Lin, V.S.Y.; Motesharei, K.; Dancil, K.P.S.; Sailor, M.J.; Ghadiri, M.R. A porous silicon-based optical interferometric biosensor. Science 1997, 278, 840–843. [Google Scholar] [CrossRef]
- Stephan, M.; Mey, I.; Steinem, C.; Janshoff, A. Combining reflectometry and fluorescence microscopy: An assay for the investigation of leakage processes across lipid membranes. Anal. Chem. 2014, 86, 1366–1371. [Google Scholar] [CrossRef]
- Neubacher, H.; Mey, I.; Carnarius, C.; Lazzara, T.D.; Steinem, C. Permeabilization assay for antimicrobial peptides based on pore-spanning lipid membranes on nanoporous alumina. Langmuir 2014, 30, 4767–4774. [Google Scholar] [CrossRef]
- Hiep, H.M.; Yoshikawa, H.; Tamiya, E. Interference localized surface plasmon resonance nanosensor tailored for the detection of specific biomolecular interactions. Anal. Chem. 2010, 82, 1221–1227. [Google Scholar] [CrossRef]
- Hotta, K.; Yamaguchi, A.; Teramae, N. Nanoporous waveguide sensor with optimized nanoarchitectures for highly sensitive label-free biosensing. ACS Nano 2012, 6, 1541–1547. [Google Scholar] [CrossRef]
- Lau, K.H.A.; Tan, L.S.; Tamada, K.; Sander, M.S.; Knoll, W. Highly sensitive detection of processes occurring inside nanoporous anodic alumina templates: A waveguide optical study. J. Phys. Chem. B 2004, 108, 10812–10818. [Google Scholar] [CrossRef]
- Lu, Z.; Ruan, W.; Yang, J.; Xu, W.; Zhao, C.; Zhao, B. Deposition of Ag nanoparticle on porous anodic alumina for surface enhanced Raman scattering substrate. J. Raman Spectrosc. 2009, 40, 112–116. [Google Scholar] [CrossRef]
- Ji, N.; Ruan, W.; Wang, C.; Lu, Z.; Zhao, B. Fabrication of silver decorated anodic aluminum oxide substrate and its optical properties on surface-enhanced Raman scattering and thin film interference. Langmuir 2009, 25, 11869–11873. [Google Scholar] [CrossRef]
- Velleman, L.; Bruneel, J.L.; Guillaume, F.; Losic, D.; Shapter, J.G. Raman spectroscopy probing of self-assembled monolayers inside the pores of gold nanotube membranes. PhysChemChemPhys 2011, 13, 19587–19593. [Google Scholar]
- Kodiyath, R.; Malak, S.T.; Combs, Z.A.; Koenig, T.; Mahmoud, M.A.; El-Sayed, M.A.; Tsukruk, V.V. Assemblies of silver nanocubes for highly sensitive SERS chemical vapor detection. J. Mater. Chem. A 2013, 1, 2777–2788. [Google Scholar]
- Jia, R.P.; Shen, Y.; Luo, H.Q.; Chen, X.G.; Hu, Z.D.; Xue, D.S. Enhanced photoluminescence properties of morin and trypsin absorbed on porous alumina films with ordered pore array. Solid State Commun. 2004, 130, 367–372. [Google Scholar] [CrossRef]
- Santos, A.; Macias, G.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Photoluminescent enzymatic sensor based on nanoporous anodic alumina. ACS Appl. Mater. Interfaces 2012, 4, 3584–3588. [Google Scholar]
- Feng, C.L.; Zhong, X.; Steinhart, M.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Graded-bandgap quantum-dot-modified nanotubes: A sensitive biosensor for enhanced detection of DNA hybridization. Adv. Mater. 2007, 19, 1933–1936. [Google Scholar] [CrossRef]
- Santos, A.; Balderrama, V.S.; Alba, M.; Formentín, P.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Nanoporous anodic alumina barcodes: Toward smart optical biosensors. Adv. Mater. 2012, 24, 1050–1054. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Optically optimized photoluminescent and interferometric biosensors base on nanoporous anodic alumina: A comparison. Anal. Chem. 2013, 85, 7904–7911. [Google Scholar] [CrossRef]
- Kumeria, T.; Losic, D. Reflective interferometric gas sensing using nanoporous anodic aluminium oxide (AAO). Phys. Status Solidi RRL 2011, 5, 10–11. [Google Scholar] [CrossRef]
- Pan, S.; Rothberg, L.J. Interferometric sensing of biomolecular binding using nanoporous aluminum oxide templates. Nano Lett. 2003, 3, 811–814. [Google Scholar] [CrossRef]
- Kumeria, T.; Kurkuri, M.D.; Diener, K.R.; Parkinson, L.; Losic, D. Label-free reflectometric interference microchip biosensor based on nanoporous alumina for detection of circulating tumour cells. Biosens. Bioelectron. 2012, 35, 167–173. [Google Scholar] [CrossRef]
- Alvarez, S.D.; Li, C.P.; Chiang, C.E.; Schuller, I.K.; Sailor, M.J. A label-free porous alumina interferometric immunosensor. ACS Nano 2009, 3, 3301–3307. [Google Scholar] [CrossRef]
- Kumeria, T.; Santos, A.; Losic, D. Ultrasensitive nanoporous interferometric sensor for label-free detection of gold(III) ions. ACS Appl. Mater. Interfaces 2013, 5, 11783–11790. [Google Scholar] [CrossRef]
- Macias, G.; Hernández-Eguía, L.P.; Ferré-Borrull, J.; Pallares, J.; Marsal, L.F. Gold-coated ordered nanoporous anodic alumina bilayers for future label-free interferometric biosensors. ACS Appl. Mater. Interfaces 2013, 5, 8093–8098. [Google Scholar] [CrossRef]
- Dronov, R.; Jane, A.; Shapter, J.G.; Hodges, A.; Voelcker, N.H. Nanoporous alumina-based interferometric transducers ennobled. Nanoscale 2011, 3, 3109–3114. [Google Scholar] [CrossRef]
- Green, R.J.; Frazier, R.A.; Shakesheff, K.M.; Davies, M.C.; Roberts, C.J.; Tendler, S.J.B. Surface plasmon resonance analysis of dynamic biological interactions with biomaterials. Biomaterials 2000, 21, 1823–1835. [Google Scholar] [CrossRef]
- Koutsioubas, A.G.; Spiliopoulos, N.; Anastassopoulos, D.; Vradis, A.A.; Priftis, G.D. Nanoporous alumina enhanced surface plasmon resonance sensors. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Dhathathreyan, A. Real-time monitoring of invertase activity immobilized in nanoporous aluminium oxide. J. Phys. Chem. B 2011, 115, 6678–6682. [Google Scholar] [CrossRef]
- Lau, K.H.A.; Duran, H.; Knoll, W. In situ characterization of N-carboxy anhydride polymerization in nanoporous anodic alumina. J. Phys. Chem. B 2009, 113, 3179–3189. [Google Scholar] [CrossRef]
- Ko, H.; Tsukruk, V.V. Nanoparticle-decorated nanocanals for surface-enhanced Raman scattering. Small 2008, 4, 1980–1984. [Google Scholar]
- Lee, S.J.; Guan, Z.; Xu, H.; Moskovits, M. Surface-enhanced Raman spectroscopy and nanogeometry: The plasmonic origin of SERS. J. Phys. Chem. C 2007, 111, 17985–17988. [Google Scholar] [CrossRef]
- Santos, A.; Alba, M.; Rahman, M.M.; Formentín, P.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Structural tuning of photoluminescence in nanoporous anodic alumina by hard anodization in oxalic and malonic acids. Nanoscale Res. Lett. 2012, 7, 228. [Google Scholar] [CrossRef]
- Li, Y.B.; Zheng, M.J.; Ma, L. High-speed growth and photoluminescence of porous anodic alumina with controllable interpore distances over a large range. Appl. Phys. Lett. 2007, 91, 073109:1–073109:3. [Google Scholar]
- Du, Y.; Cai, W.L.; Mo, C.M.; Chen, J.; Zhang, L.D.; Zhu, X.G. Preparation and photoluminescence of alumina membranes with ordered pore arrays. Appl. Phys. Lett. 1999, 74, 2951–2953. [Google Scholar] [CrossRef]
- Huang, G.S.; Wu, X.L.; Mei, Y.F.; Shao, X.F.; Siu, G.C. Strong blue emission from anodic alumina membranes with ordered nanopore array. J. Appl. Phys. 2003, 93, 582–585. [Google Scholar] [CrossRef]
- Mukhurov, N.I.; Zhvavyi, S.P.; Terekhov, S.N.; Panarin, A.Y.; Kotova, I.F.; Pershukevich, P.P.; Khodasevich, I.A.; Gasenkova, I.V.; Orlovich, V.A. Influence of electrolyte composition on photoluminescent properties of anodic aluminum oxide. J. Appl. Spectrosc. 2008, 75, 214–218. [Google Scholar] [CrossRef]
- Li, Z.; Huang, K. Optical properties of alumina membranes prepared by anodic oxidation process. J. Lumin. 2007, 127, 435–440. [Google Scholar]
- Gauglitz, G.; Ingenhoff, J. Design of new integrated optical substrates for immuno-analytical applications. Fresenius J. Anal. Chem. 1994, 349, 355–359. [Google Scholar] [CrossRef]
- Pacholski, C.; Sartor, M.; Sailor, M.J.; Cunin, F.; Miskelly, G.M. Biosensing using porous silicon double-layer interferometers: Reflective interferometric Fourier transform spectroscopy. J. Am. Chem. Soc. 2005, 127, 11636–11645. [Google Scholar]
- Pacholski, C.; Yu, C.; Miskelly, G.M.; Godin, D.; Sailor, M.J. Reflective interferometric Fourier transform spectroscopy: A self-compensating label-free immunosensor using double-layers of porous SiO2. J. Am. Chem. Soc. 2006, 128, 4250–4252. [Google Scholar]
- Orosco, M.M.; Pacholski, C.; Sailor, M.J. Real-time monitoring of enzyme activity in a mesoporous silicon double layer. Nat. Nanotechnol. 2009, 4, 255–258. [Google Scholar]
- Cunin, F.; Schmedake, T.A.; Link, J.R.; Li, Y.Y.; Koh, J.; Bhatia, S.N.; Sailor, M.J. Biomolecular screening with encoded porous silicon photonic crystals. Nat. Mater. 2002, 1, 39–41. [Google Scholar] [CrossRef]
- Jane, A.; Dronov, R.; Hodges, A.; Voelcker, N.H. Porous silicon biosensors on the advance. Trends Biotechnol. 2009, 27, 230–239. [Google Scholar] [CrossRef]
- Kumeria, T.; Losic, D. Controlling interferometric properties of nanoporous anodic aluminium oxide. Nanoscale Res. Lett. 2012, 7, 88:1–88:10. [Google Scholar]
- Casanova, F.; Chiang, C.E.; Li, C.P.; Roshchin, I.V.; Ruminski, A.M.; Sailor, M.J. Gas adsorption and capillary condensation in nanoporous alumina films. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Kumeria, T.; Gulati, K.; Santos, A.; Losic, D. Real-time and in situ drug release monitoring from nanoporous implants under dynamic flow conditions by reflectometric interference spectroscopy. ACS Appl. Mater. Interfaces 2013, 5, 5436–5442. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Santos, A.; Kumeria, T.; Losic, D. Nanoporous Anodic Alumina: A Versatile Platform for Optical Biosensors. Materials 2014, 7, 4297-4320. https://doi.org/10.3390/ma7064297
Santos A, Kumeria T, Losic D. Nanoporous Anodic Alumina: A Versatile Platform for Optical Biosensors. Materials. 2014; 7(6):4297-4320. https://doi.org/10.3390/ma7064297
Chicago/Turabian StyleSantos, Abel, Tushar Kumeria, and Dusan Losic. 2014. "Nanoporous Anodic Alumina: A Versatile Platform for Optical Biosensors" Materials 7, no. 6: 4297-4320. https://doi.org/10.3390/ma7064297
APA StyleSantos, A., Kumeria, T., & Losic, D. (2014). Nanoporous Anodic Alumina: A Versatile Platform for Optical Biosensors. Materials, 7(6), 4297-4320. https://doi.org/10.3390/ma7064297







