Surface Characteristics and Catalytic Activity of Copper Deposited Porous Silicon Powder
Abstract
:1. Introduction
2. Results and Discussion
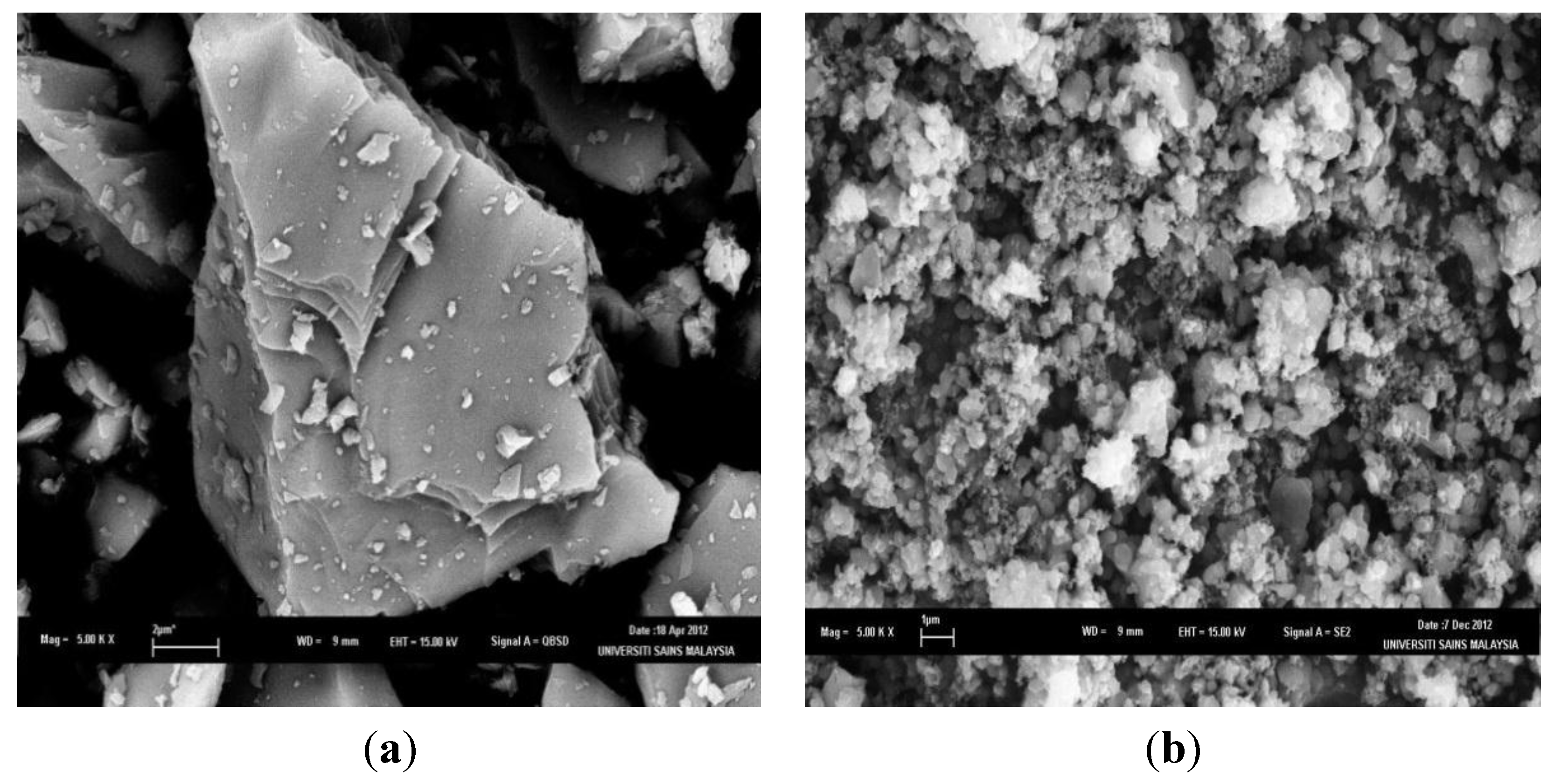
2.1. Synthesis of PS
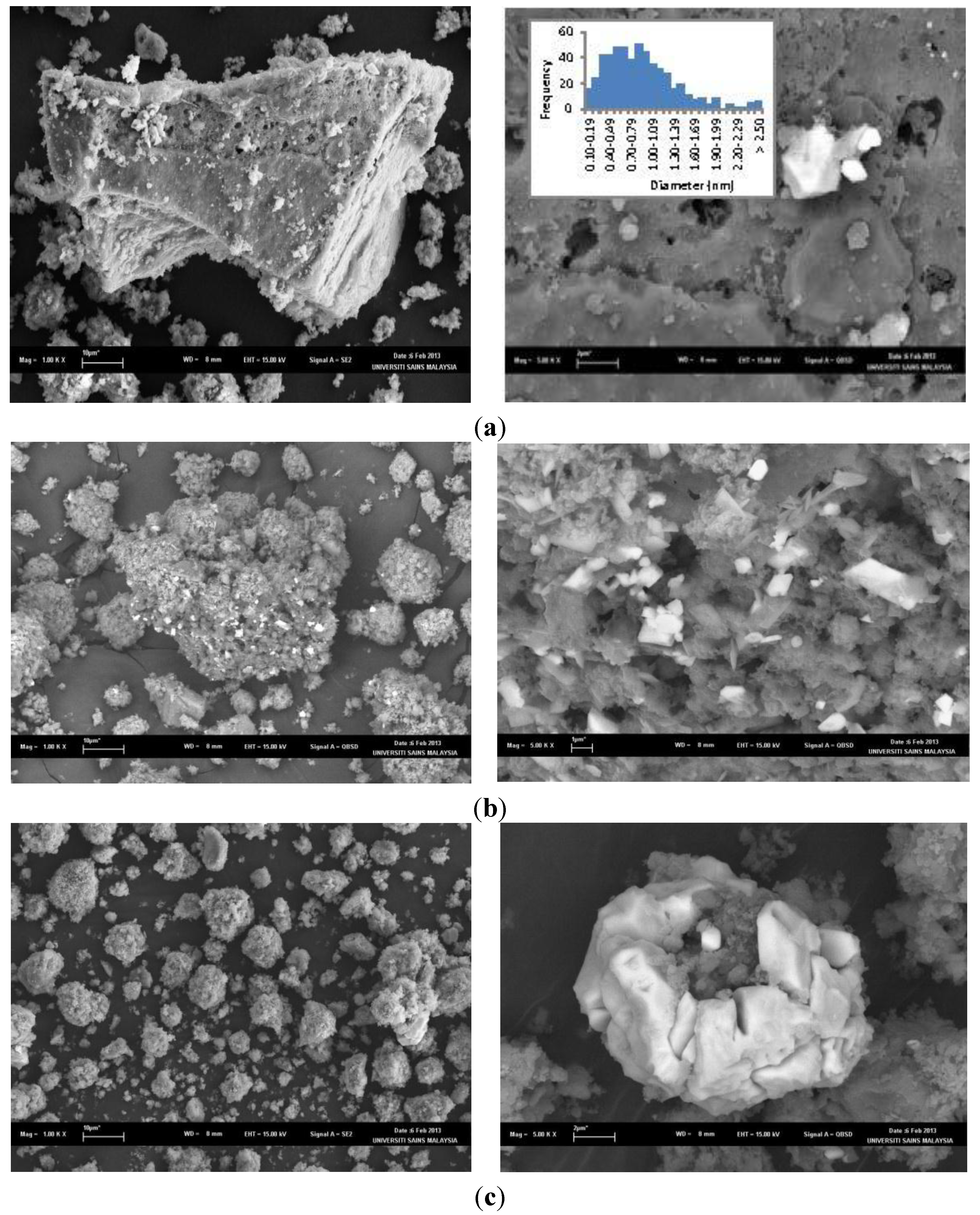
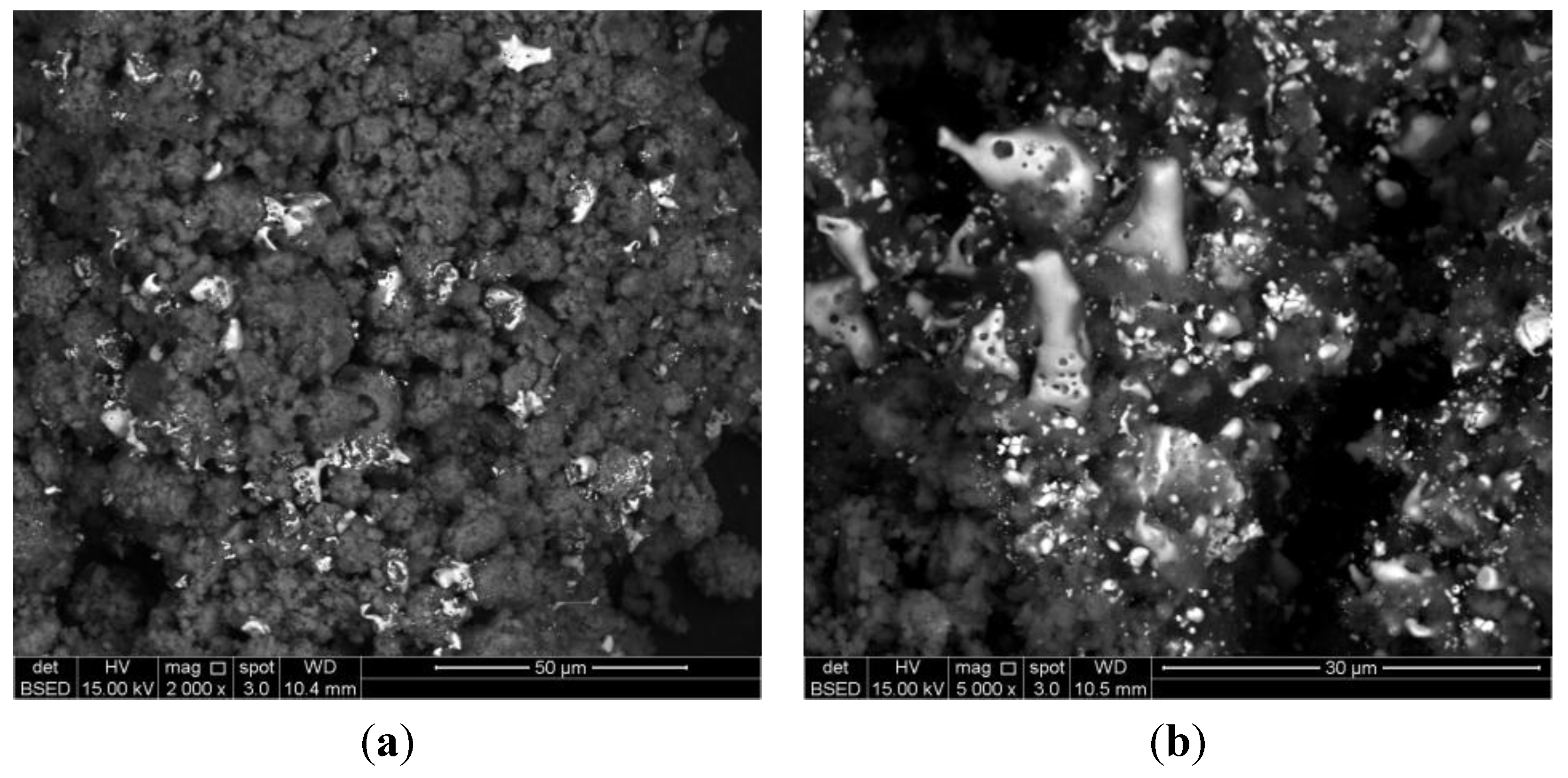
2.2. Synthesis of Cu-PS
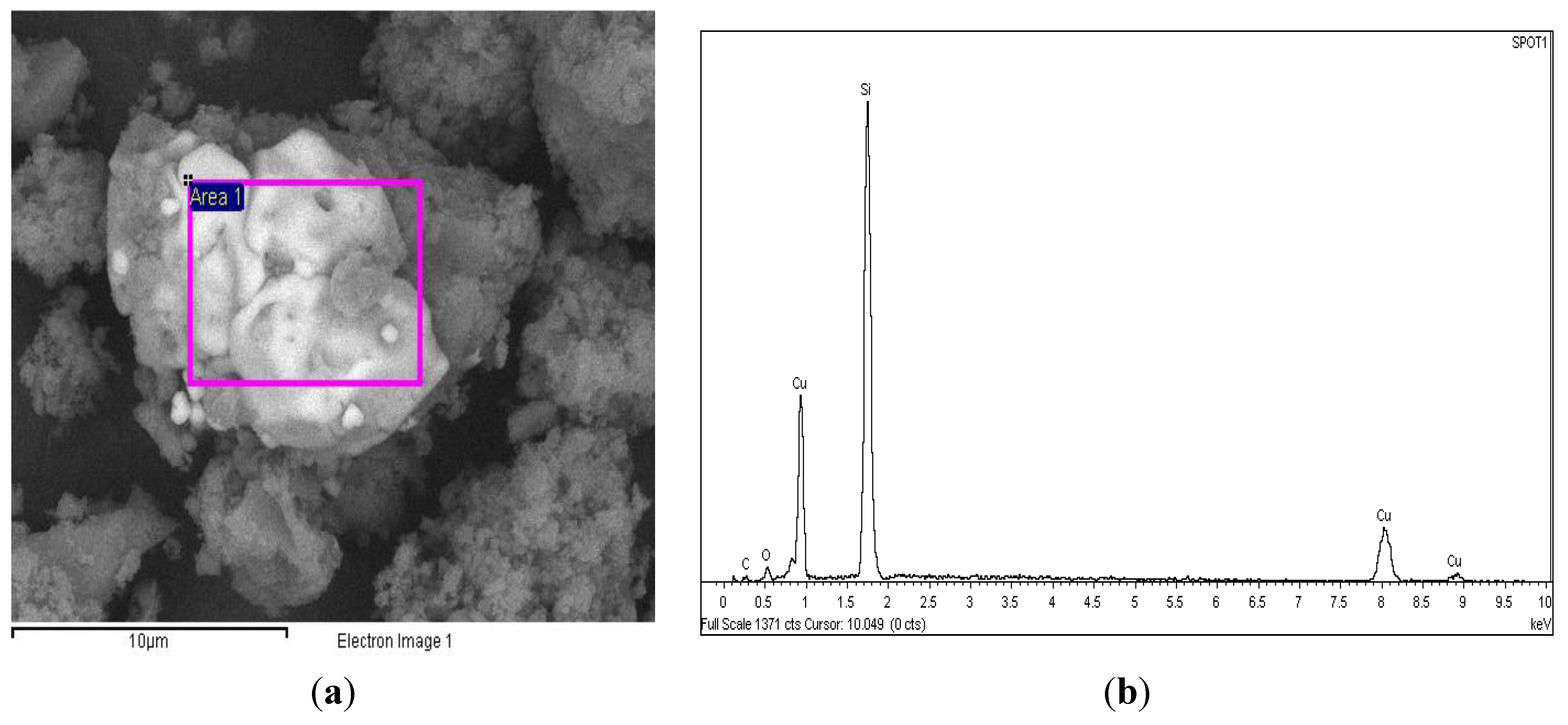
| Notation | Cu° Content * (wt%) | BET Surface Area (m2·g−1) | Cu° Particle Size | H2 Consumed (mol·gmetal−1) | H2 Produced (mol·gmetal−1) |
|---|---|---|---|---|---|
| PS | 0 | 3.10 | N/A | Nil | Nil |
| 3.3 wt% Cu-PS | 3.3 | 5.42 | 0.9 ± 0.5 μm | 5.70 × 10−3 | 3.27 × 10−3 |
| 7.8 wt% Cu-PS | 7.8 | 20.52 | 4.1 ± 1.0 μm | 4.41 × 10−3 | 3.33 × 10−4 |
| 9.8 wt% Cu-PS | 9.8 | 3.51 | Highly agglomerated | 2.88 × 10−3 | 9.59 × 10−4 |
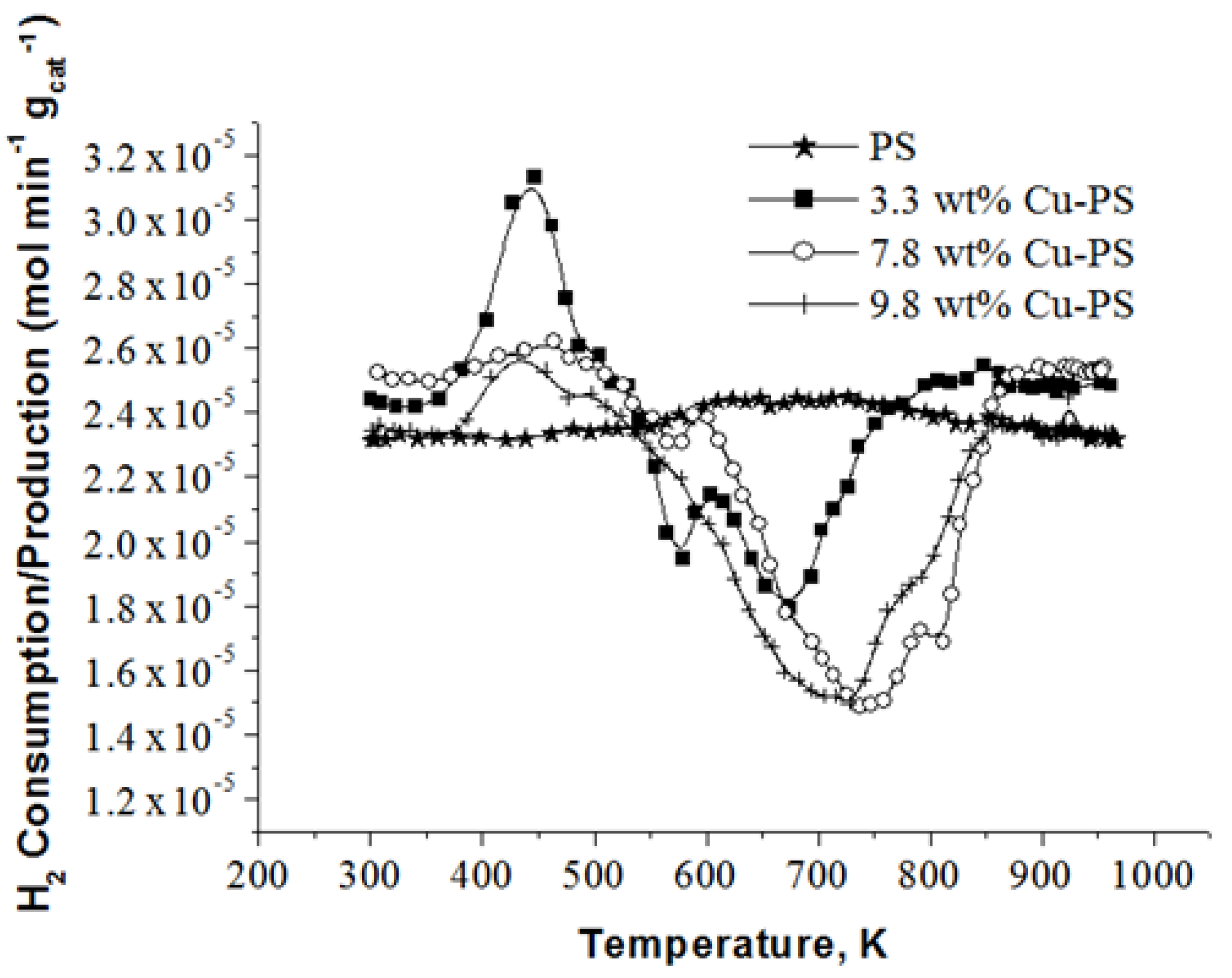
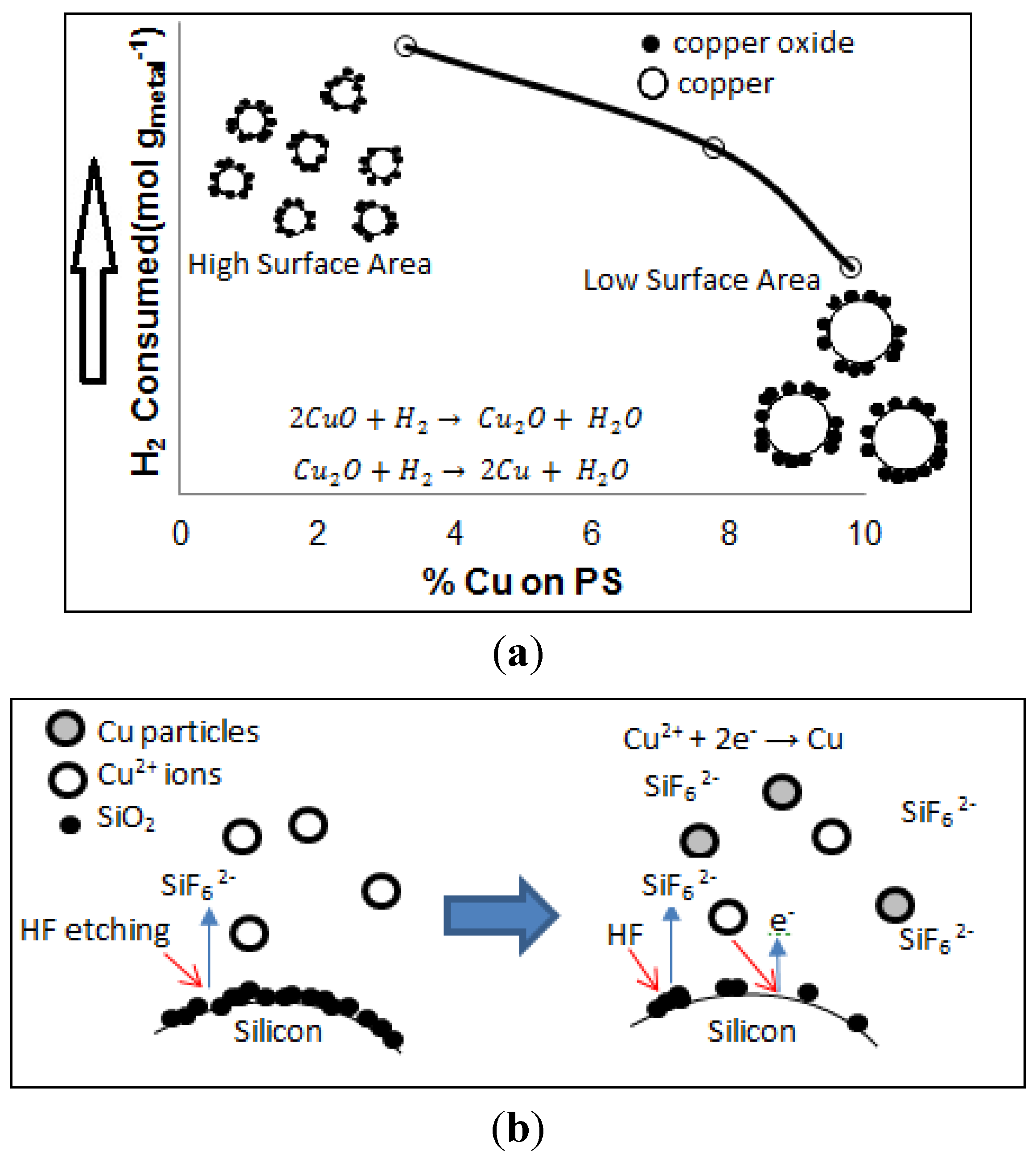
2.3. H2-TPR Analysis of Cu-PS
2.4. Catalytic Test
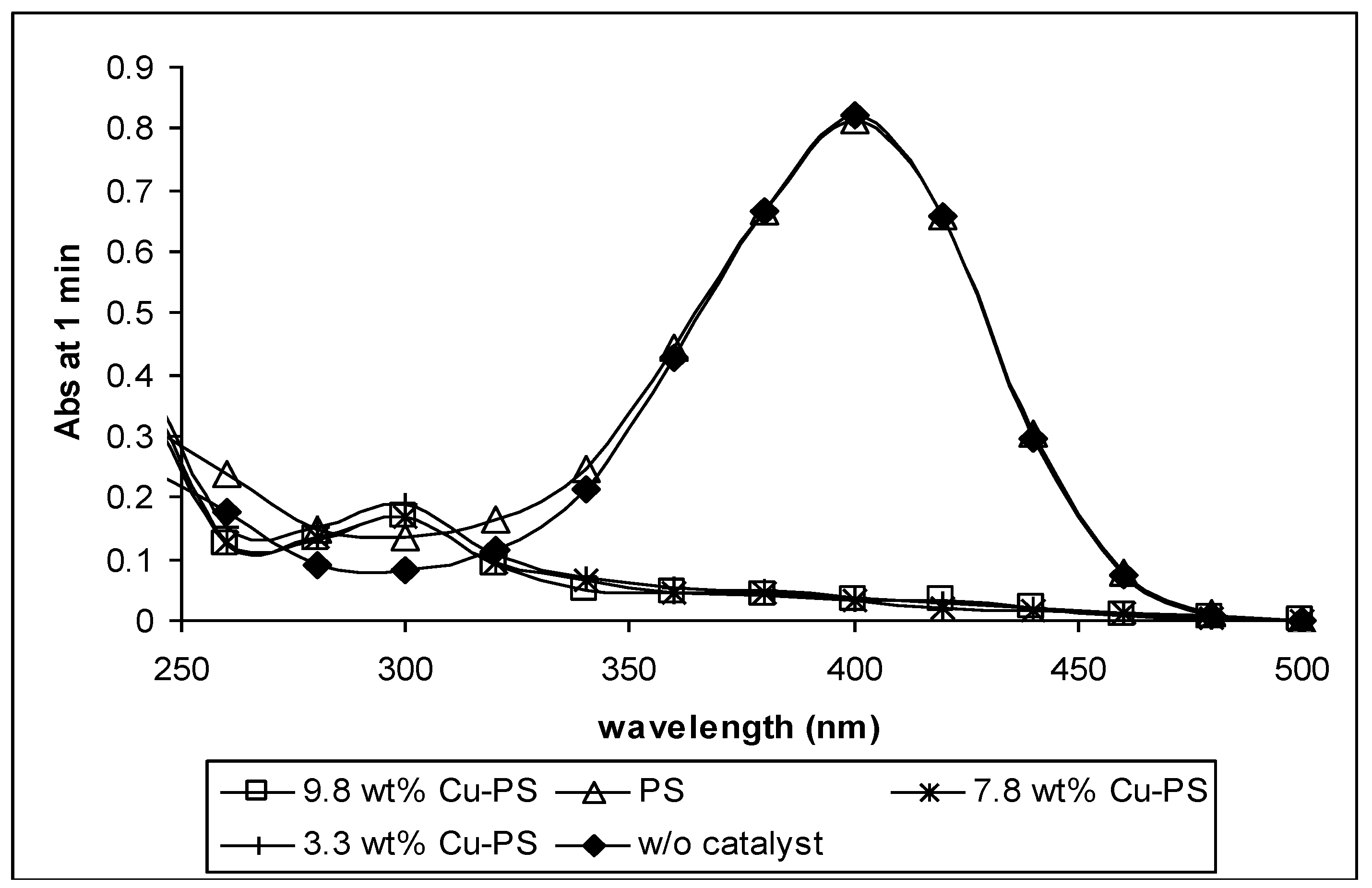
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Porous Silicon (PS) Powder
3.2.2. Synthesis of Copper Deposited PS (Cu-PS) Powder
3.2.3. Catalytic Reduction of P-Nitrophenol
3.3. Characterizations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henstock, J.R.; Ruktanonchai, U.R.; Canham, L.T.; Anderson, S.I. Porous silicon confers bioactivity to polycaprolactone composites in vitro. J. Mater. Sci. Mater. Med. 2014, 25, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- De la Mora, M.B.; Jaramillo, O.A.; Nava, R.; Taguena-Martınez, J.; del Rıo, J.A. Viability study of porous silicon photonic mirrors as secondary reflectors for solar concentration systems. Sol. Energy Mater. Sol. Cells 2009, 93, 1218–1224. [Google Scholar]
- Polisski, S.; Goller, B.; Wilson, K.; Kovalev, D.; Zaikowskii, V.; Lapkin, A. In situ synthesis and catalytic activity in CO oxidation of metal nanoparticles supported on porous nanocrystalline silicon. J. Catal. 2010, 271, 59–66. [Google Scholar] [CrossRef]
- Yaacob, S.; Abu Bakar, M.; Abu Bakar, N.H.H.; Mahmud, M.; Ismail, J.; Ibrahim, K. Immersion plating of Cu onto N-type porous silicon: From sparsely distributed cubes to closed packed spheres. Arch. Des. Sci. 2013, 66, 544–555. [Google Scholar]
- Llorca, J.; Casanovas, A.; Trifonav, T.; Rodriguez, A.; Alcubilla, R. First use of macroporous silicon loaded with catalyst film for a chemical reaction: A microreformer for producing hydrogen from ethanol steam reforming. J. Catal. 2008, 255, 228–233. [Google Scholar] [CrossRef]
- Polisski, S. Porous Silicon/Metal Nanocomposites for Catalytic Applications. Ph.D. Thesis, University of Bath, Bath, UK, 2010. [Google Scholar]
- Liu, X.; Cheng, H.; Ciu, P. Catalysis by silver nanoparticles/porous silicon for the reduction of nitroaromatics in the presence of sodium borohydride. Appl. Surf. Sci. 2014, 292, 695–701. [Google Scholar] [CrossRef]
- Yashtulov, N.A.; Gavrin, S.S.; Bondarenko, V.P.; Kholostov, K.I.; Revina, A.A.; Flid, V.R. Formation of nanocomposite platinum catalysts on porous silicon. Russ. Chem. Bull. Int. Ed. 2011, 60, 434–439. [Google Scholar] [CrossRef]
- Feng, J.; Sua, L.; Maa, Y.; Ren, C.; Guo, Q.; Chen, X. CuFe2O4 magnetic nanoparticles: A simple and efficient catalyst for the reduction of nitrophenol. Chem. Eng. J. 2013, 221, 16–24. [Google Scholar] [CrossRef]
- Xu, R.; Bi, H.; He, G.; Zhu, J.; Chen, H. Synthesis of Cu-Fe3O4@Graphene composite: A magnetically separable and efficient catalyst for the reduction of 4-nitrophenol. Mater. Res. Bull. 2014, 57, 190–196. [Google Scholar] [CrossRef]
- Nemanashi, M.; Meijboom, R. Synthesis and characterization of Cu, Ag and Au dendrimer-encapsulated nanoparticles and their application in the reduction of 4-nitrophenol to 4-aminophenol. J. Colloid Inter. Sci. 2013, 389, 260–267. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; González, V.R. Silver nanoparticles loaded on Cu-doped TiO2 for the effective reduction of nitro-aromatic contaminants. Chem. Eng. J. 2014. [Google Scholar] [CrossRef]
- Patra, A.K.; Dutta, A.; Bhaumik, A. Cu nanorods and nanospheres and their excellent catalytic activity in chemoselective reduction of nitrobenzenes. Catal. Commun. 2010, 11, 651–655. [Google Scholar] [CrossRef]
- Chartier, C.; Bastide, S.; Levy-Clement, C. Metal-assisted chemical etching of silicon in HF–H2O2. Electrochim. Acta 2008, 53, 5509–5516. [Google Scholar] [CrossRef]
- Lee, J.I.; Park, S. High-performance porous silicon monoxide anodes synthesized via metal-assisted chemical etching. Nano Energy 2013, 2, 146–152. [Google Scholar] [CrossRef]
- Fellahi, O.; Hadjersi, T.; Ouadah, Y. Elaboration of one-dimensional photonic structure on silicon by electrochemical etching. Phys. Procedia 2009, 2, 759–764. [Google Scholar] [CrossRef]
- Kolasinski, K.W. Silicon nanostructures from electroless electrochemical etching. Curr. Opin. Solid State Mater. Sci. 2005, 9, 73–83. [Google Scholar] [CrossRef]
- Schwartz, B.; Robbins, H. Chemical etching of silicon: III. A temperature study in the acid system. J. Electrochem. Soc. 1961, 108, 365–372. [Google Scholar] [CrossRef]
- Kolasinski, K.W. Charge transfer and nanostructure formation during electroless etching of silicon. J. Phys. Chem. C 2010, 114, 22098–22105. [Google Scholar] [CrossRef]
- Kanugo, J.; Saha, H.; Basu, S. Effect of porosity on the performance of surface modified porous silicon hydrogen sensors. Sens. Actuators B Chem. 2010, 147, 145–151. [Google Scholar] [CrossRef]
- Coulthard, I.; Sham, T.K. Novel preparation of noble metal nanostructures utilizing porous silicon. Solid State Commun. 1998, 105, 751–754. [Google Scholar] [CrossRef]
- Sham, T.K.; Coulthard, I.; Lorimer, J.W.; Hiraya, A.; Watanabe, M. Reductive deposition of Cu on porous silicon from aqueous solutions: An x-ray absorption study at the Cu L3,2 edge. Chem. Mater. 1994, 6, 2085–2091. [Google Scholar] [CrossRef]
- Tsuboi, T.; Sakka, T.; Ogata, Y.H. Metal deposition into a porous silicon layer by immersion plating: Influence of halogen ions. J. Appl. Phys. 1998, 83, 4501–4506. [Google Scholar] [CrossRef]
- Dudley, M.E.; Kolasinski, K.W. Structure and photoluminescence studies of porous silicon formed in ferric ion containing stain etchants. Phys. Status Solidi A 2009, 206, 1240–1244. [Google Scholar] [CrossRef]
- Chang, F.W.; Kuo, W.Y.; Lee, K.C. Dehydrogenation of ethanol over copper catalysts on rice husk ash prepared by incipient wetness impregnation. Appl. Catal. 2003, 246, 253–264. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.; Xu, J.; Shim, L.; Li, J.; Zhang, Y. Inspired superhydrophobic surfaces by a double-metal-assisted chemical etching route. Mater. Res. Bull. 2012, 47, 1687–1692. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Cunningham, J. Selective catalytic reduction of NO with C2H4 over Cu/ZSM-5: Influences of oxygen partial pressure and incorporated rhodia. Appl. Catal. B Environ. 1998, 15, 275–289. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Kim, J.Y.; Hanson, J.C.; Parez, M.; Frenkel, I. Reduction of CuO in H2: In situ Time-resolved XRD Studies. Catal. Lett. 2003, 85, 247–254. [Google Scholar] [CrossRef]
- Aguila, G.; Gracia, F.; Cortes, J.; Araya, P. Effect of copper species and the presence of reaction products on the activity of methane oxidation on supported CuO catalysts. Appl. Catal. B Environ. 2008, 77, 325–338. [Google Scholar] [CrossRef]
- Hoang, D.L.; Dang, T.T.H.; Engeldinger, J.; Schneider, M.; Radnik, J.; Richter, M.; Martin, A. TPR investigations on the reducibility of Cu supported on Al2O3, Zeolite Y and SAPO-5. J. Solid State Chem. 2011, 184, 1915–1923. [Google Scholar] [CrossRef]
- Du, X.; He, J.; Zhu, J.; Sun, L.; An, S. Ag-deposited silica-coated Fe3O4 magnetic nanoparticles catalyzed reduction of p-nitrophenol. Appl. Surf. Sci. 2012, 258, 2717–2723. [Google Scholar] [CrossRef]
- Lin, C.; Tao, K.; Hua, D.; Ma, Z.; Zhou, S. Size effect of gold nanoparticles in catalytic reduction of p-nitrophenol with NaBH4. Molecules 2013, 18, 12609–12620. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, W.; Tan, F.; Gu, J.; Qiao, X.; Chen, J. Facile route to hierarchical silver microstructures with high catalytic activity for the reduction of p-nitrophenol. Mater. Res. Bull. 2014, 49, 138–143. [Google Scholar] [CrossRef]
- Hwang, C.G.; Sang-Ho, K.; Hoon, O.J.; Kim, M.R.; Choi, S.H. Reduction of aromatic nitro compounds on Pd colloids prepared by γ-irradiation. J. Ind. Eng. Chem. 2008, 14, 864–868. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Chakraborty, M. Synthesis of colloidal CuO/γ-Al2O3 by microemulsion and its catalytic reduction of aromatic nitro compounds. Chin. J. Catal. 2012, 33, 1532–1541. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halim, M.Y.A.; Tan, W.L.; Bakar, N.H.H.A.; Bakar, M.A. Surface Characteristics and Catalytic Activity of Copper Deposited Porous Silicon Powder. Materials 2014, 7, 7737-7751. https://doi.org/10.3390/ma7127737
Halim MYA, Tan WL, Bakar NHHA, Bakar MA. Surface Characteristics and Catalytic Activity of Copper Deposited Porous Silicon Powder. Materials. 2014; 7(12):7737-7751. https://doi.org/10.3390/ma7127737
Chicago/Turabian StyleHalim, Muhammad Yusri Abdul, Wei Leng Tan, Noor Hana Hanif Abu Bakar, and Mohamad Abu Bakar. 2014. "Surface Characteristics and Catalytic Activity of Copper Deposited Porous Silicon Powder" Materials 7, no. 12: 7737-7751. https://doi.org/10.3390/ma7127737
APA StyleHalim, M. Y. A., Tan, W. L., Bakar, N. H. H. A., & Bakar, M. A. (2014). Surface Characteristics and Catalytic Activity of Copper Deposited Porous Silicon Powder. Materials, 7(12), 7737-7751. https://doi.org/10.3390/ma7127737




