Hydrogen Production by Steam Reforming of Ethanol over Nickel Catalysts Supported on Sol Gel Made Alumina: Influence of Calcination Temperature on Supports
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Catalyst Characterization
- = Metal dispersion
- = Chemisorption volume (mol g−1)
- = Stoichiometry factor = 1
- = Supported metal atomic weight
- = Supported metal weight, wt %
- = Metal surface area (per g catalyst)
- = Supported metal cross section area = 0.0649 nm atom−1
- = Mean particle diameter (nm)
- = Supported metal density
2.3. Catalyst Performance Test
3. Results and Discussion
3.1. Support Physical Properties
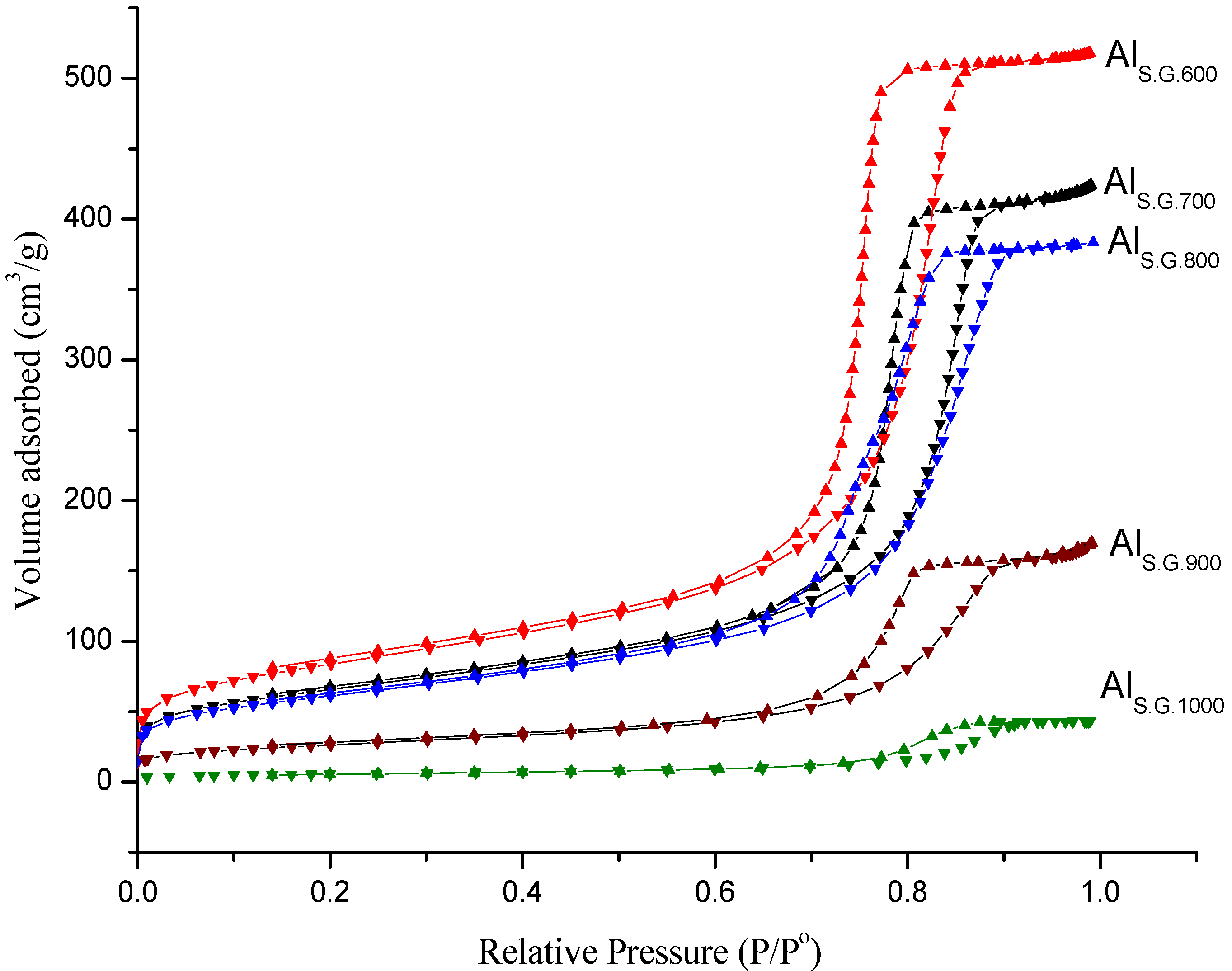
| Catalyst name | BET (m2/g) a | Pore volume(cm3/g) b | Pore diameter(nm) c |
|---|---|---|---|
| AlS.G.600 | 292 | 0.81 | 7.91 |
| AlS.G.700 | 230 | 0.67 | 8.46 |
| AlS.G.800 | 214 | 0.60 | 9.06 |
| AlS.G.900 | 92 | 0.27 | 9.10 |
| AlS.G.1000 | 19 | 0.07 | 10.18 |
3.2. Effect of Calcination Temperature on the Structure of the Supports and Supported Ni Catalysts
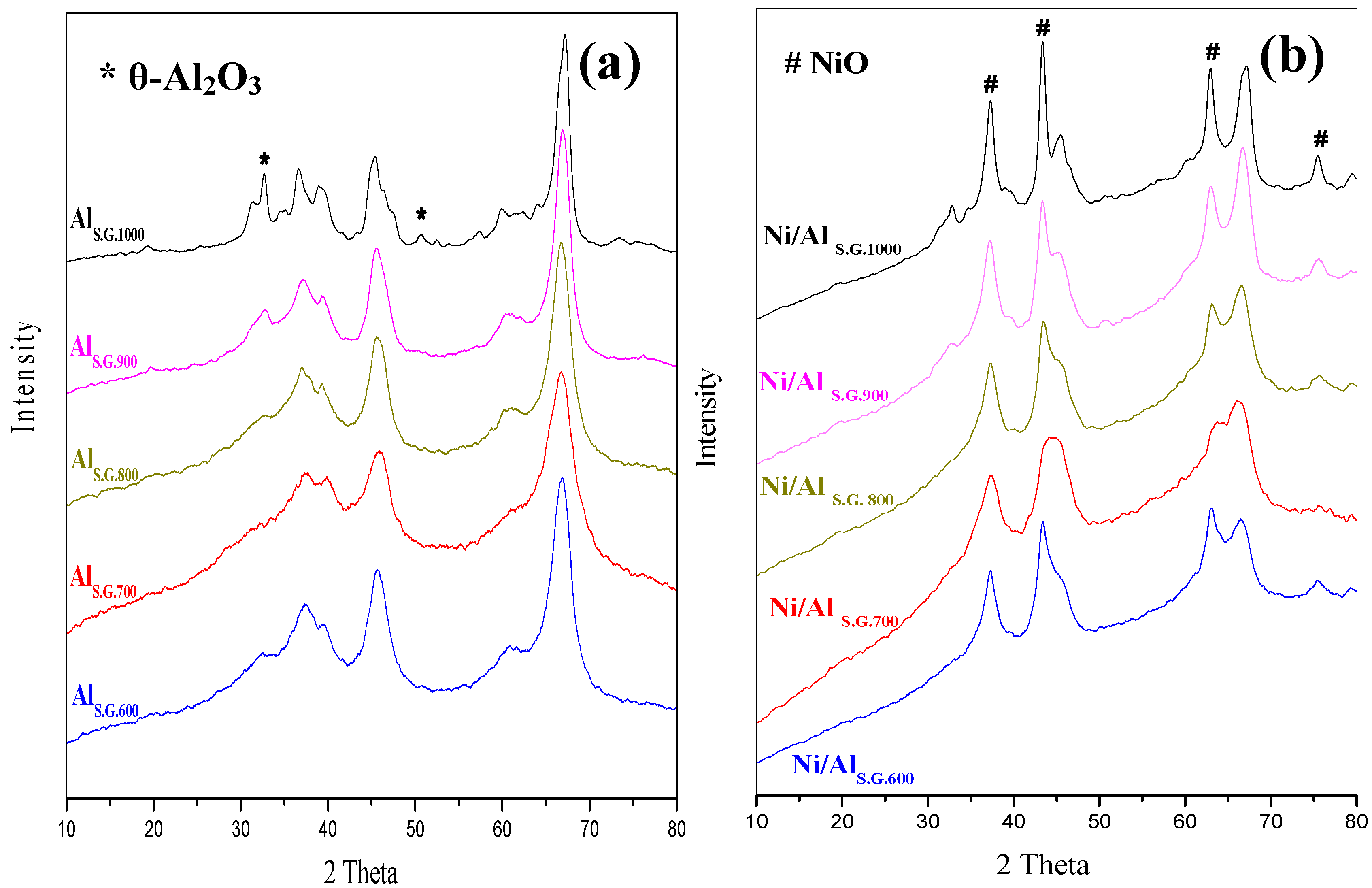
3.3. Metal–Support Interaction
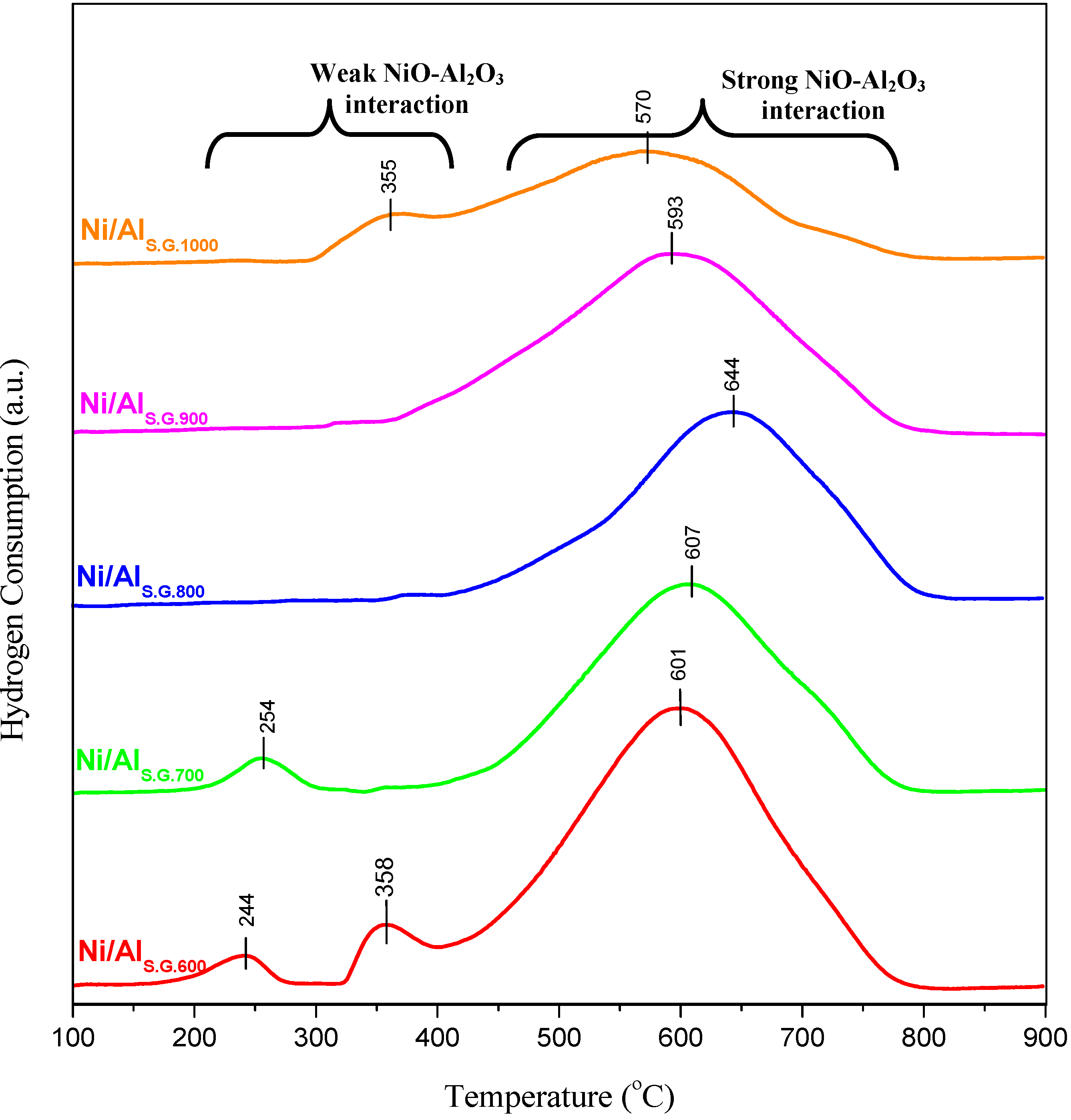
3.4. Hydrogen Chemisorption Measurements
| Catalyst name | Amount of H2 uptake (μmol g−1) | Nickel dispersion (%) * | Nickel surface area (m2 g−1) * | Mean particle diameter (nm) |
|---|---|---|---|---|
| Ni/AlS.G.600 | 45 | 4.40 | 1.75 | 22.9 |
| Ni/AlS.G.700 | 254 | 24.8 | 9.90 | 4.1 |
| Ni/AlS.G.800 | 566 | 55.4 | 22.1 | 1.8 |
| Ni/AlS.G.900 | 489 | 47.8 | 19.1 | 2.1 |
| Ni/AlS.G.1000 | 310 | 30.3 | 12.1 | 3.3 |
3.5. Steam Reforming of Ethanol over the Supported Ni Catalysts
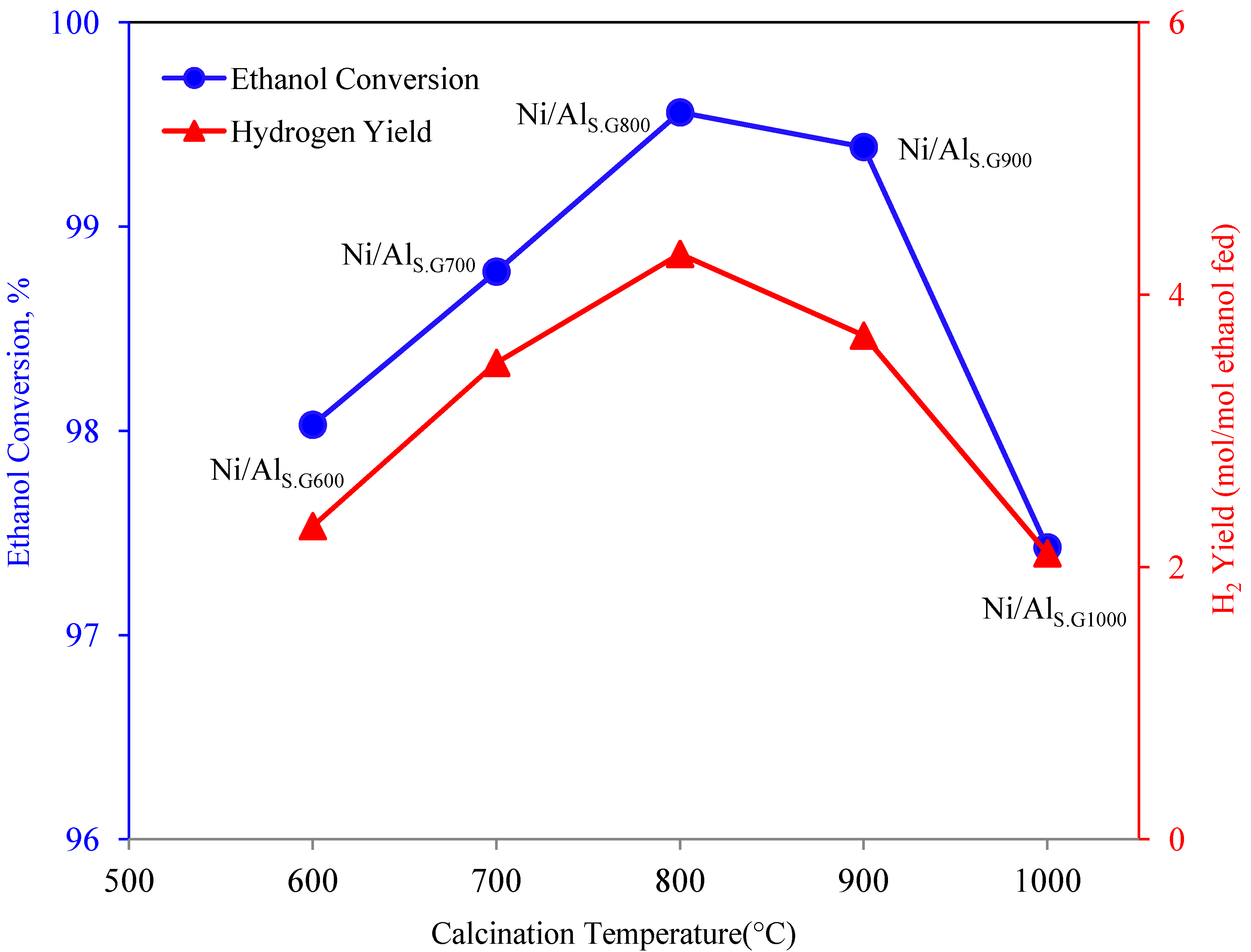
| Catalyst name | Amount of carbon deposition (wt %) |
|---|---|
| Ni/AlS.G.600 | 0.26 |
| Ni/AlS.G.700 | 0.20 |
| Ni/AlS.G.800 | 0.14 |
| Ni/AlS.G.900 | 0.11 |
| Ni/AlS.G.1000 | 0.68 |
4. Conclusions
Acknowledgments
References
- Mariño, F.; Boveri, M.; Baronetti, G.; Laborde, M. Hydrogen production from steam reforming of bioethanol using Cu/Ni/K/γ-Al2O3 catalysts. Effect of Ni. Int. J. Hydrog. Energy 2001, 26, 665–668. [Google Scholar] [CrossRef]
- Llorca, J.; de la Piscina, P.R.; Dalmon, J.-A.; Sales, J.; Homs, N. CO-free hydrogen from steam-reforming of bioethanol over ZnO-supported cobalt catalysts: Effect of the metallic precursor. Appl. Catal. B Environ. 2003, 43, 355–369. [Google Scholar] [CrossRef]
- Bshish, A.; Yaakob, Z.; Narayanan, B.; Ramakrishnan, R.; Ebshish, A. Steam-reforming of ethanol for hydrogen production. Chem. Pap. 2011, 65, 251–266. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhou, X.; Li, C.; Ding, J. Hydrogen production by steam reforming of ethanol over copper doped Ni/CeO2 catalysts. J. Rare Earths 2011, 29, 872–877. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Gutiérrez-Martínez, A.; Palacios, J.; Vega-Hernández, M.; Rodríguez-Lugo, V. Hydrogen production by oxidative steam reforming of methanol over Ni/CeO2–ZrO2 catalysts. Int. J. Hydrog. Energy 2011, 36, 6601–6608. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Zhang, C.; Wang, S.; Ma, X.; Gong, J. Steam reforming of ethanol over Ni/ZrO2 catalysts: Effect of support on product distribution. Int. J. Hydrog. Energy 2012, 37, 2940–2949. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Zhang, L.; Liu, S.; Lu, G. Steam reforming of acetic acid over Ni/ZrO2 catalysts: Effects of nickel loading and particle size on product distribution and coke formation. Appl. Catal. A Gen. 2012, 417–418, 281–289. [Google Scholar] [CrossRef]
- Rossetti, I.; Biffi, C.; Bianchi, C.L.; Nichele, V.; Signoretto, M.; Menegazzo, F.; Finocchio, E.; Ramis, G.; Di Michele, A. Ni/SiO2 and Ni/ZrO2 catalysts for the steam reforming of ethanol. Appl. Catal. B Environ. 2012, 117–118, 384–396. [Google Scholar] [CrossRef]
- Garbarino, G.; Lagazzo, A.; Riani, P.; Busca, G. Steam reforming of ethanol–phenol mixture on Ni/Al2O3: Effect of Ni loading and sulphur deactivation. Appl. Catal. B Environ. 2013, 129, 460–472. [Google Scholar] [CrossRef]
- Freni, S.; Cavallaro, S.; Mondello, N.; Spadaro, L.; Frusteri, F. Production of hydrogen for MC fuel cell by steam reforming of ethanol over MgO supported Ni and Co catalysts. Catal. Commun. 2003, 4, 259–268. [Google Scholar] [CrossRef]
- Fatsikostas, A.N.; Verykios, X.E. Reaction network of steam reforming of ethanol over Ni-based catalysts. J. Catal. 2004, 225, 439–452. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Spadaro, L.; Chiodo, V.; Bonura, G.; Donato, S.; Cavallaro, S. H2 production for MC fuel cell by steam reforming of ethanol over MgO supported Pd, Rh, Ni and Co catalysts. Catal. Commun. 2004, 5, 611–615. [Google Scholar] [CrossRef]
- Mariño, F.; Boveri, M.; Baronetti, G.; Laborde, M. Hydrogen production via catalytic gasification of ethanol. A mechanism proposal over copper–nickel catalysts. Int. J. Hydrog. Energy 2004, 29, 67–71. [Google Scholar] [CrossRef]
- Homs, N.; Llorca, J.; de la Piscina, P.R. Low-temperature steam-reforming of ethanol over ZnO-supported Ni and Cu catalysts: The effect of nickel and copper addition to ZnO-supported cobalt-based catalysts. Catal. Today 2006, 116, 361–366. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Ethanol steam reforming over Ni/MxOy–Al2O3 (M = Ce, La, Zr and Mg) catalysts: Influence of support on the hydrogen production. Int. J. Hydrog. Energy 2007, 32, 1462–1471. [Google Scholar] [CrossRef]
- Denis, A.; Grzegorczyk, W.; Gac, W.; Machocki, A. Steam reforming of ethanol over Ni/support catalysts for generation of hydrogen for fuel cell applications. Catal. Today 2008, 137, 453–459. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Cho, K.M.; Park, S.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas over a nickel catalyst supported on mesoporous alumina xerogel. J. Power Sources 2007, 173, 943–949. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Park, S.; Chung, J.S.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over Ni/Al2O3–ZrO2 xerogel catalysts: Effect of calcination temperature of Al2O3–ZrO2 xerogel supports. Int. J. Hydrog. Energy 2009, 34, 3755–3763. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Park, S.; Song, I.K. Effect of calcination temperature of mesoporous alumina xerogel (AX) supports on hydrogen production by steam reforming of liquefied natural gas (LNG) over Ni/AX catalysts. Int. J. Hydrog. Energy 2008, 33, 7427–7434. [Google Scholar] [CrossRef]
- Burtin, P.; Brunelle, J.P.; Pijolat, M.; Soustelle, M. Influence of surface area and additives on the thermal stability of transition alumina catalyst supports. I: Kinetic data. Appl. Catal. 1987, 34, 225–238. [Google Scholar] [CrossRef]
- Burtin, P.; Brunelle, J.P.; Pijolat, M.; Soustelle, M. Influence of surface area and additives on the thermal stability of transition alumina catalyst supports. II: Kinetic model and interpretation. Appl. Catal. 1987, 34, 239–254. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Ramana Rao, P.V.; Venkat Rao, V. Catalytic functionalities of nickel supported on different polymorphs of alumina. Catal. Commun. 2008, 9, 886–893. [Google Scholar] [CrossRef]
- Rynkowski, J.M.; Paryjczak, T.; Lenik, M. On the nature of oxidic nickel phases in NiO/γ-Al2O3 catalysts. Appl. Catal. A Gen. 1993, 106, 73–82. [Google Scholar] [CrossRef]
- Zieliński, J. Morphology of nickel/alumina catalysts. J. Catal. 1982, 76, 157–163. [Google Scholar] [CrossRef]
- Negrier, F.; Marceau, É.; Che, M.; de Caro, D. Role of ethylenediamine in the preparation of alumina-supported Ni catalysts from [Ni(en)2(H2O)2](NO3)2: From solution properties to nickel particles. Comptes Rendus Chim. 2003, 6, 231–240. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Jing, Y.; Xie, Y. Support effects on the catalytic behavior of NiO/Al2O3 for oxidative dehydrogenation of ethane to ethylene. Appl. Catal. A Gen. 2003, 240, 143–150. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yaakob, Z.; Bshish, A.; Ebshish, A.; Tasirin, S.M.; Alhasan, F.H. Hydrogen Production by Steam Reforming of Ethanol over Nickel Catalysts Supported on Sol Gel Made Alumina: Influence of Calcination Temperature on Supports. Materials 2013, 6, 2229-2239. https://doi.org/10.3390/ma6062229
Yaakob Z, Bshish A, Ebshish A, Tasirin SM, Alhasan FH. Hydrogen Production by Steam Reforming of Ethanol over Nickel Catalysts Supported on Sol Gel Made Alumina: Influence of Calcination Temperature on Supports. Materials. 2013; 6(6):2229-2239. https://doi.org/10.3390/ma6062229
Chicago/Turabian StyleYaakob, Zahira, Ahmed Bshish, Ali Ebshish, Siti Masrinda Tasirin, and Fatah H. Alhasan. 2013. "Hydrogen Production by Steam Reforming of Ethanol over Nickel Catalysts Supported on Sol Gel Made Alumina: Influence of Calcination Temperature on Supports" Materials 6, no. 6: 2229-2239. https://doi.org/10.3390/ma6062229
APA StyleYaakob, Z., Bshish, A., Ebshish, A., Tasirin, S. M., & Alhasan, F. H. (2013). Hydrogen Production by Steam Reforming of Ethanol over Nickel Catalysts Supported on Sol Gel Made Alumina: Influence of Calcination Temperature on Supports. Materials, 6(6), 2229-2239. https://doi.org/10.3390/ma6062229




