Engineering Cellular Photocomposite Materials Using Convective Assembly
Abstract
:1. Introduction
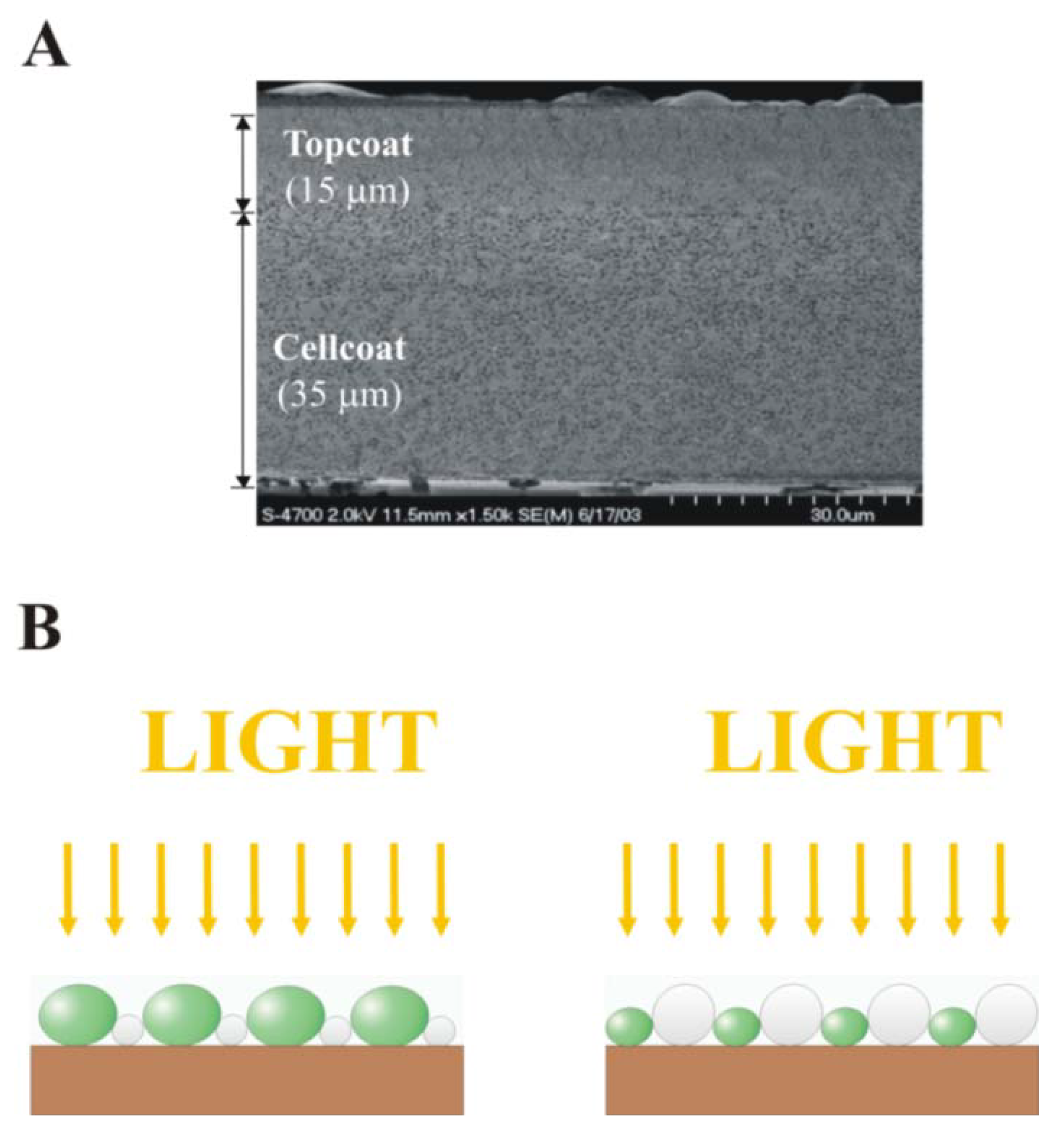
2. Composite Cellular Coating Fabrication Methods
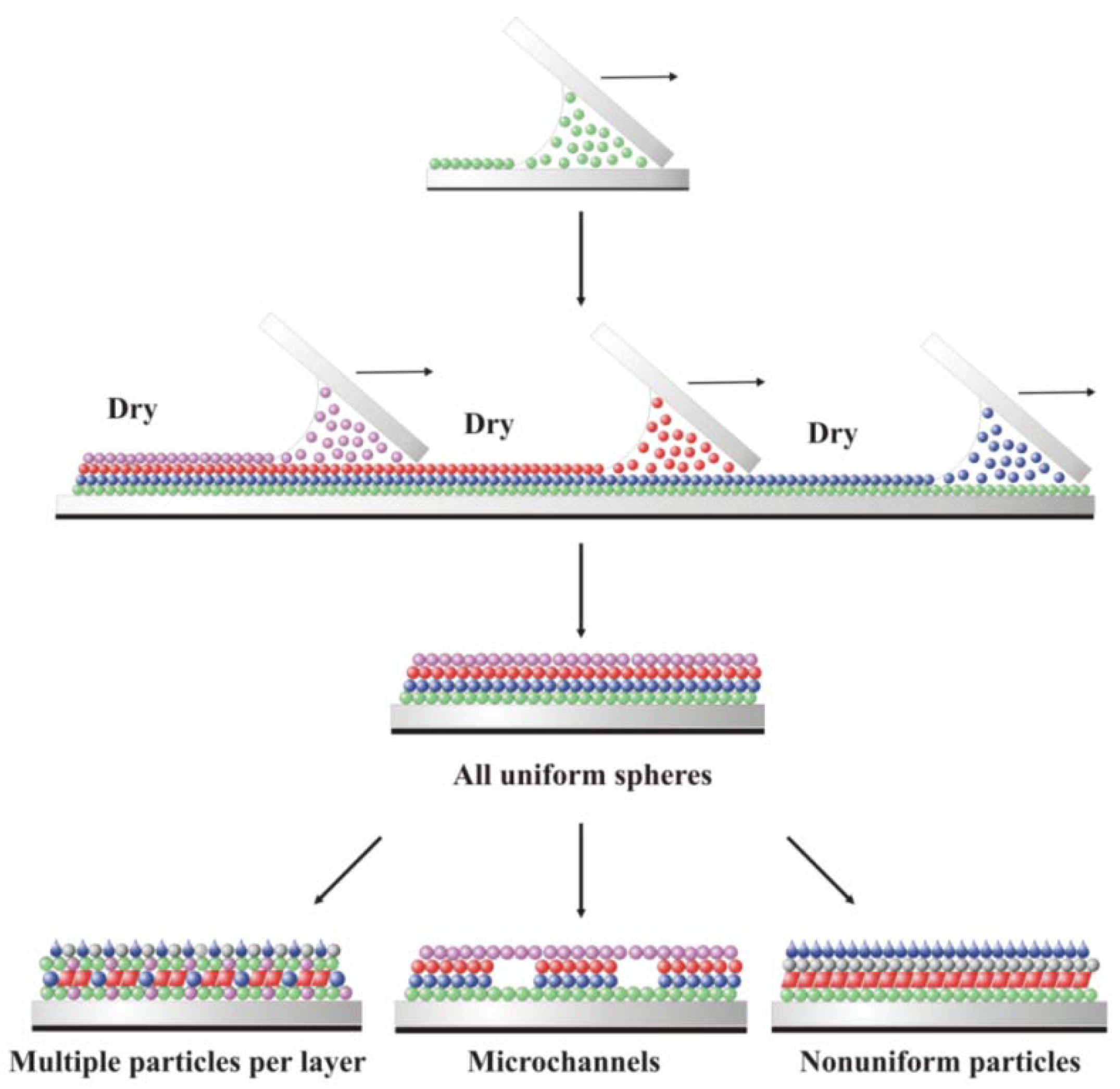
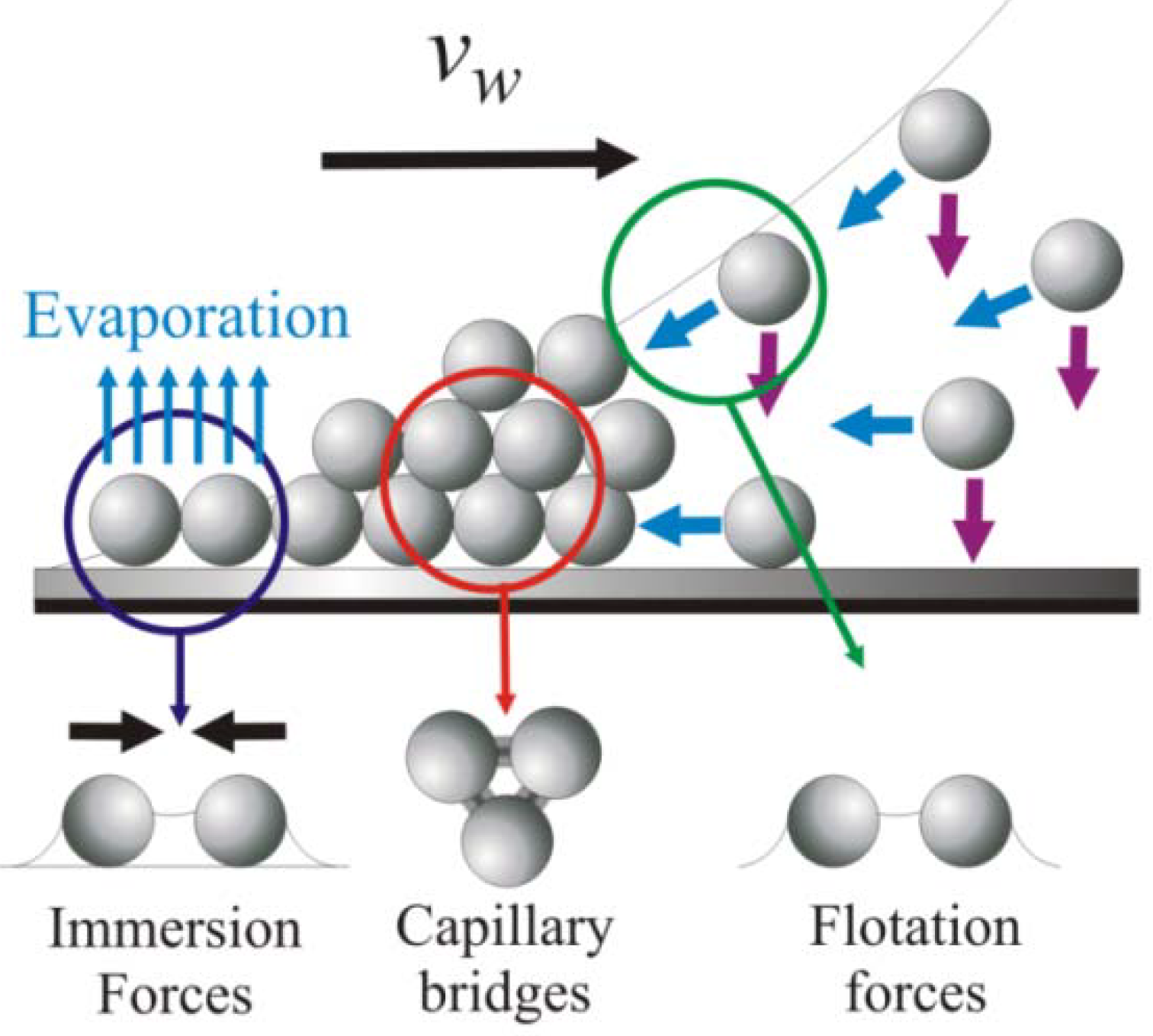
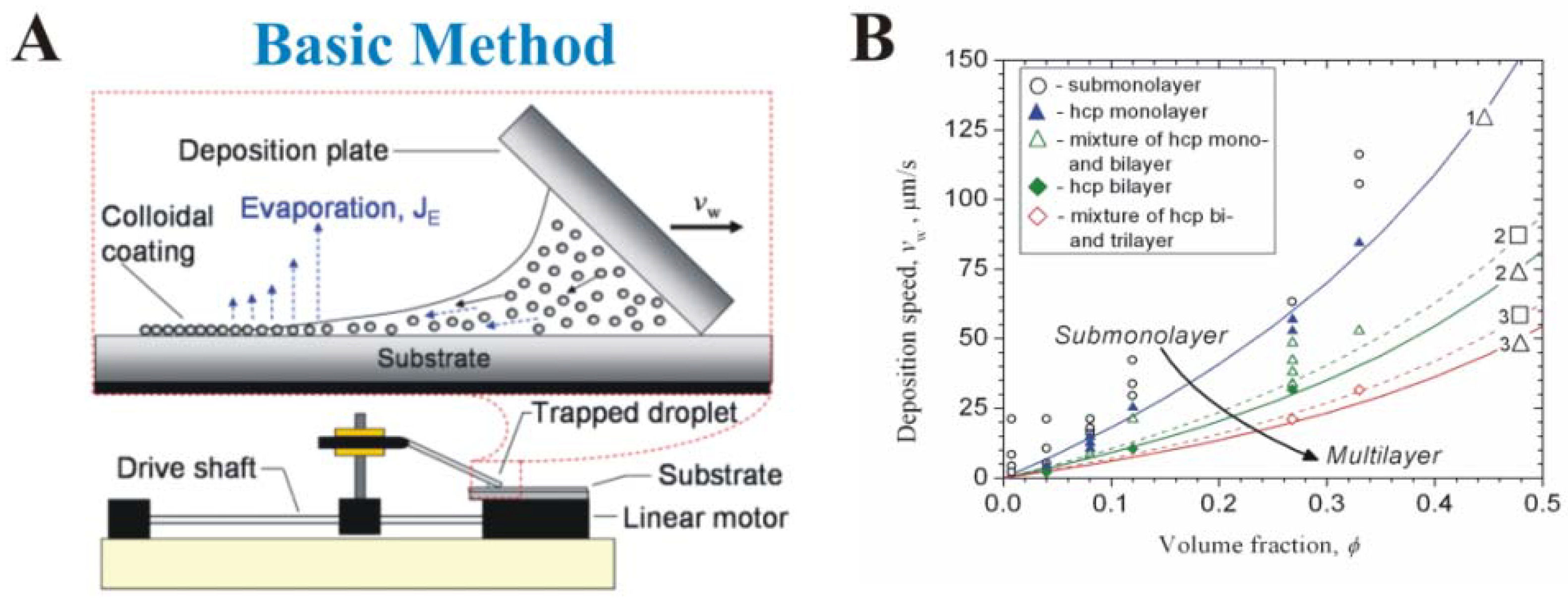
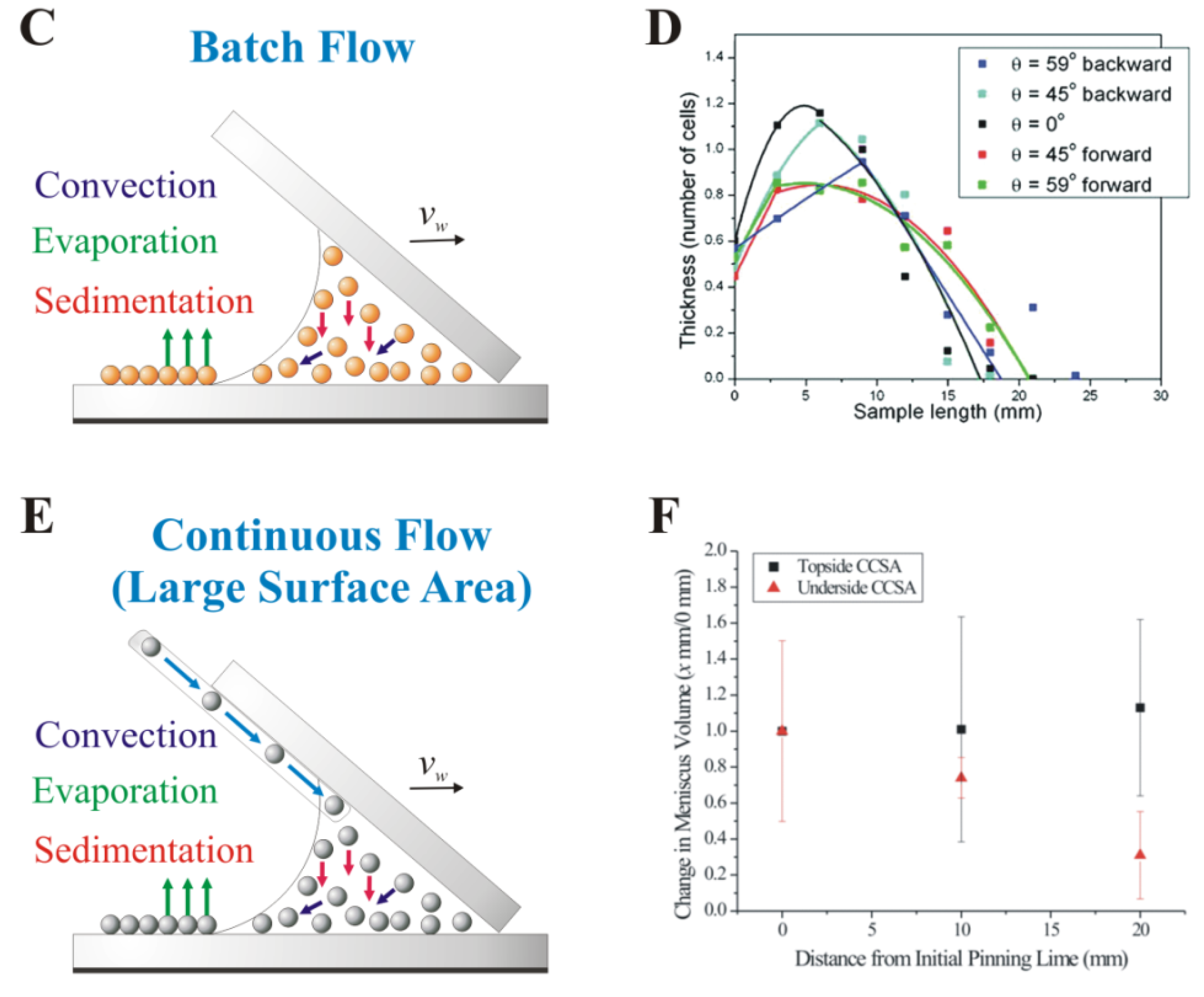
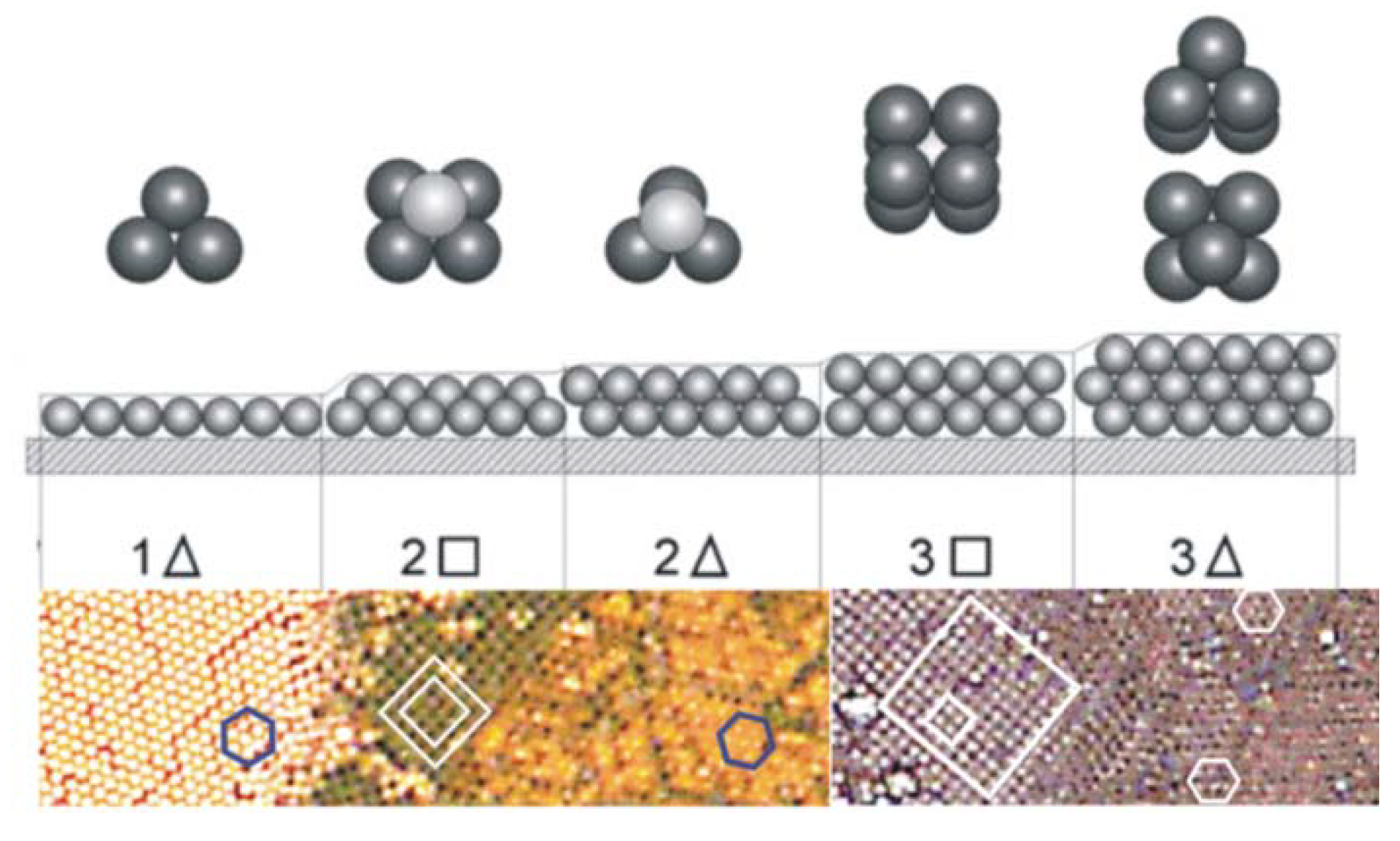
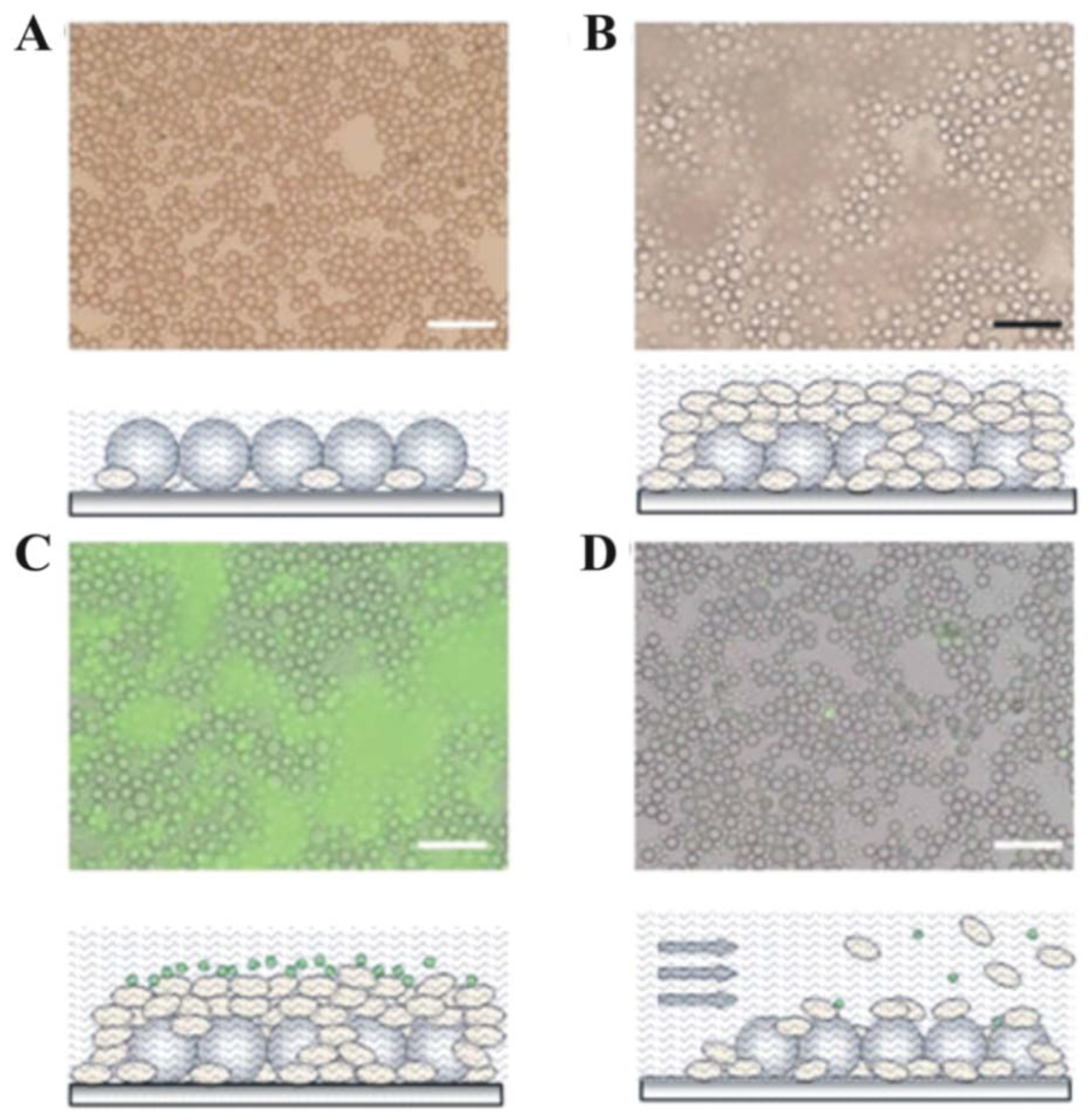
3. Early Cellular and Particle Array Composite Coating Systems
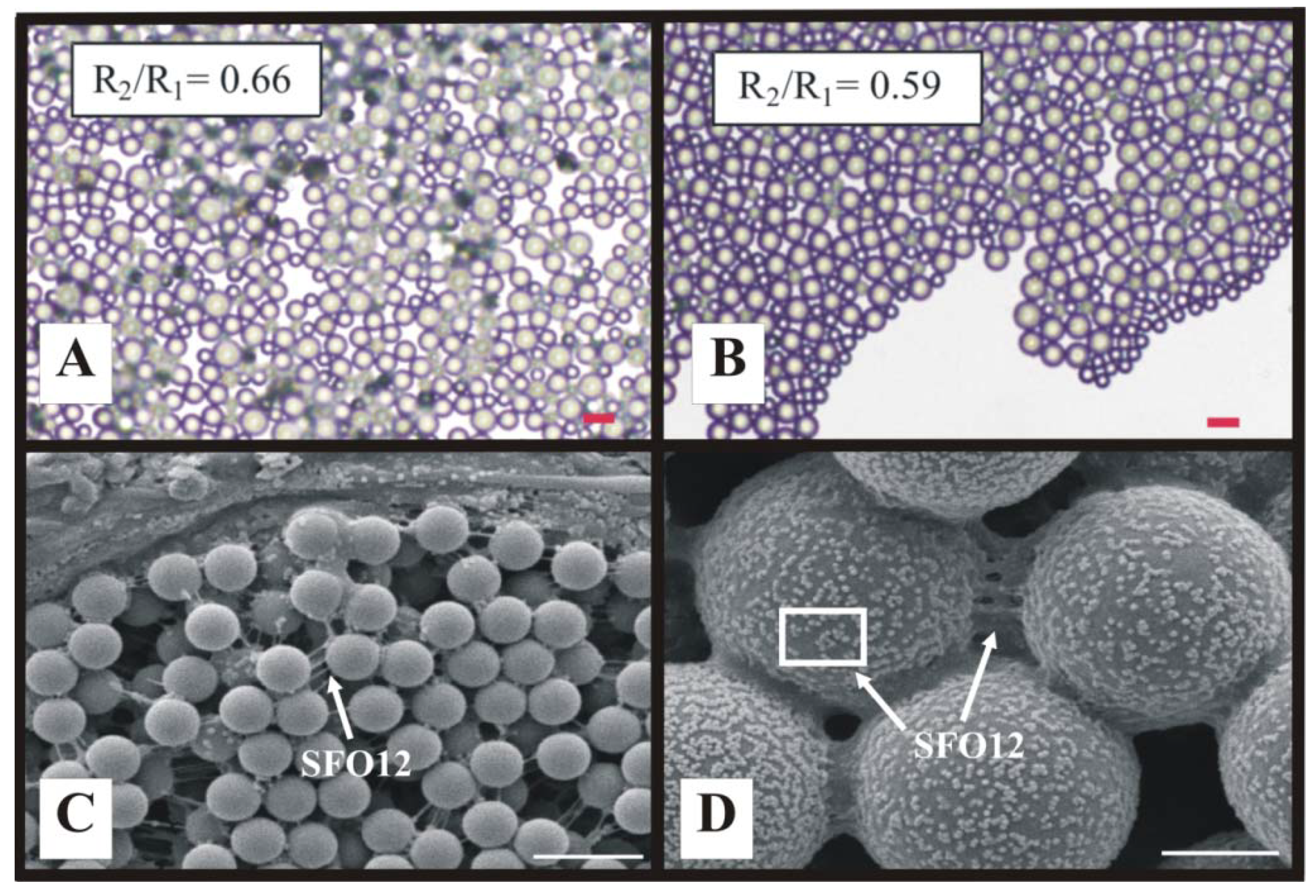
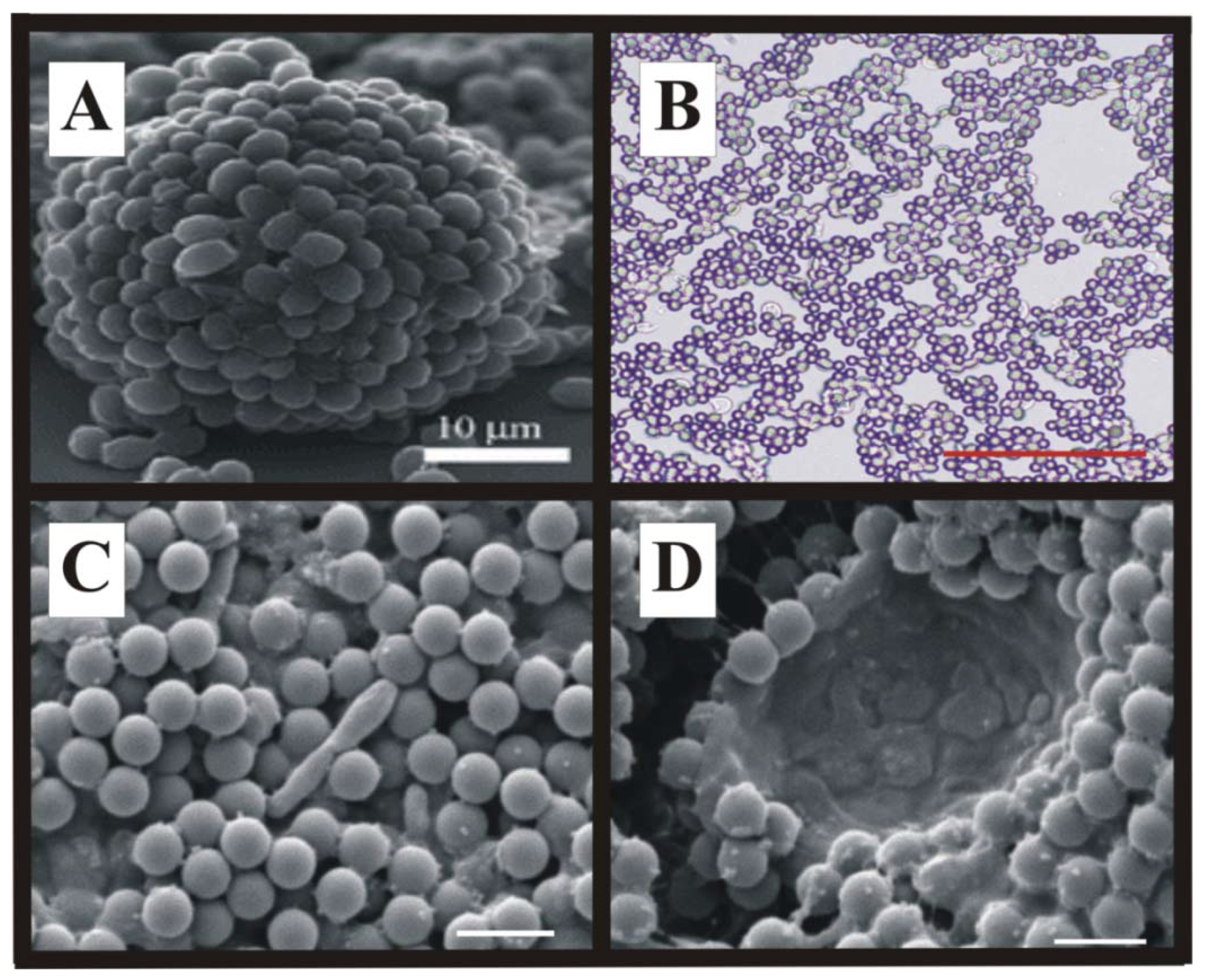
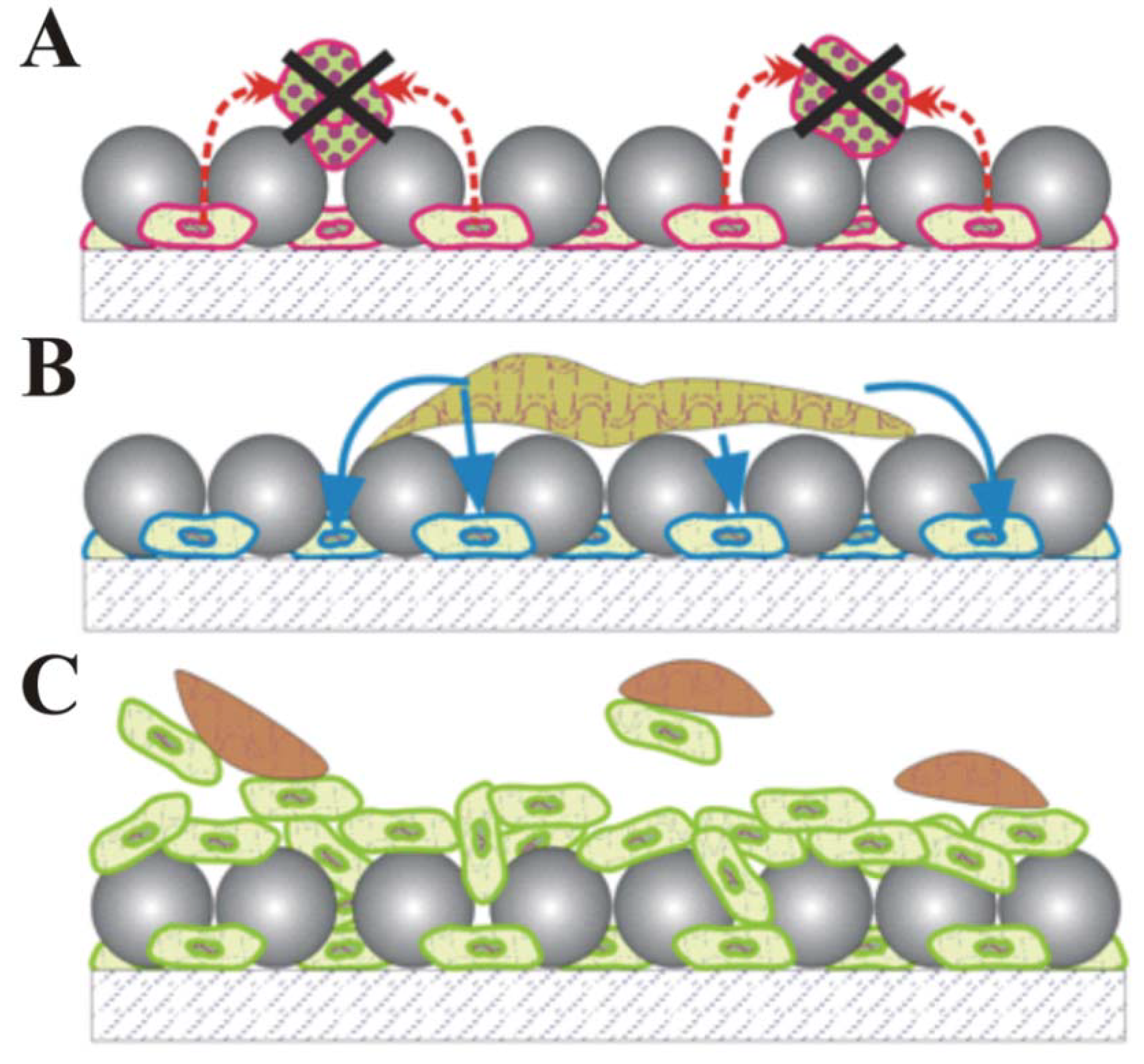
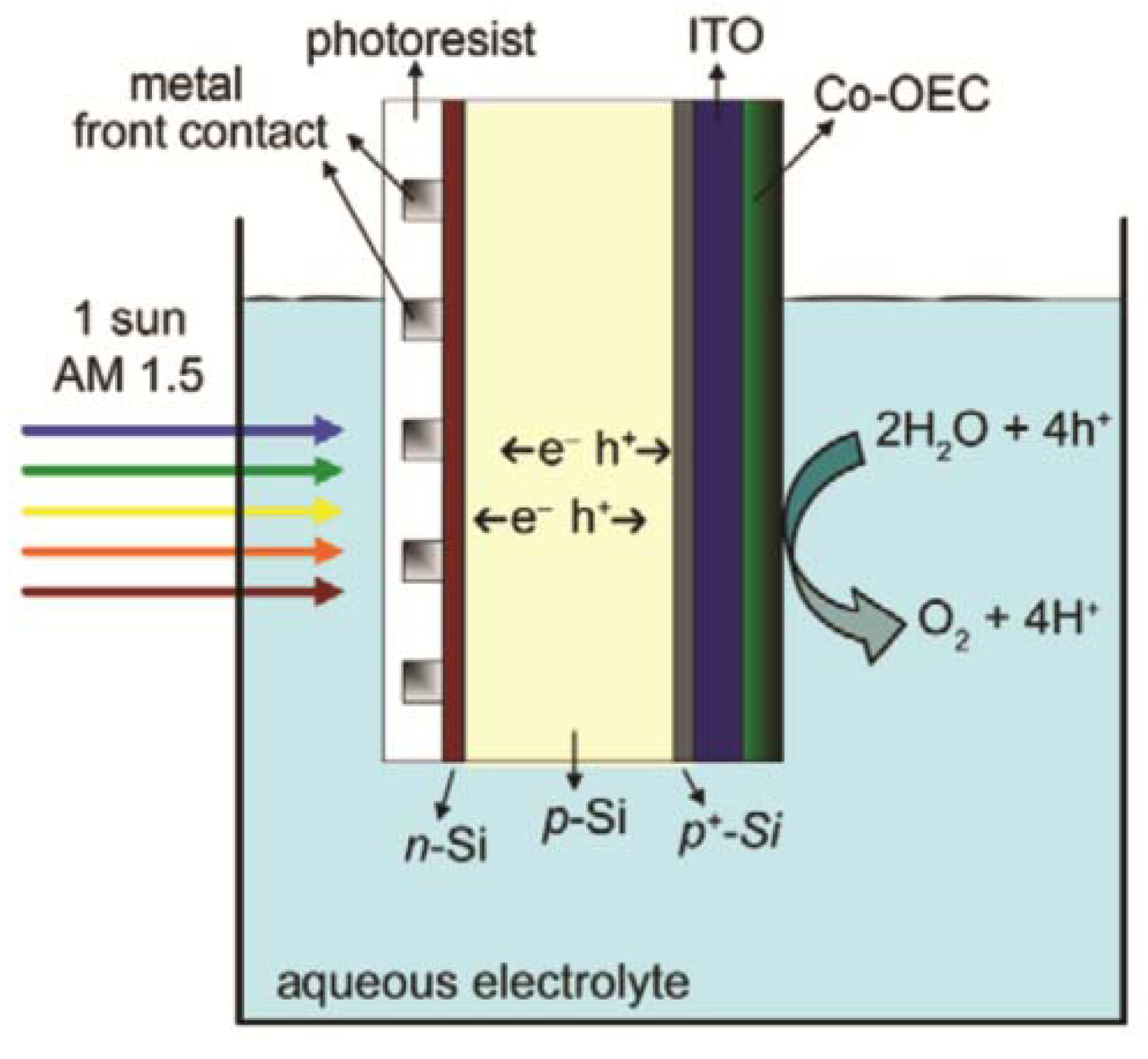
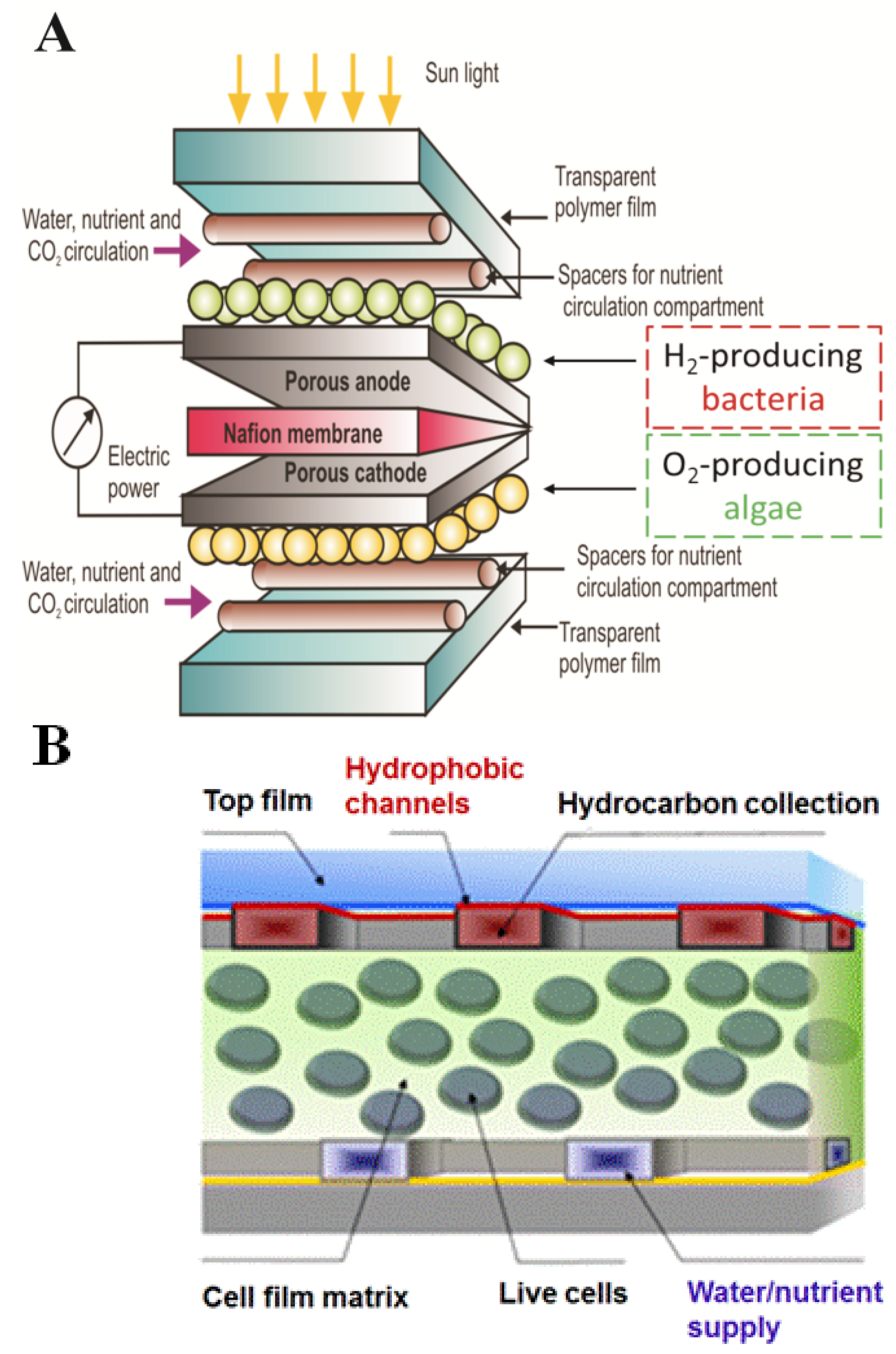
4. Future Cellular Composite Materials: Self Cleaning Composites, Artificial Leaves and Biomimetic Energetic Materials
| Matrix | Cell system | Application | Reference |
|---|---|---|---|
| Silica | Candida tropicalis Pseudomonas | Phenol and PCB degradation | [77] |
| Silica | Mixed bacteria | Sulfate reduction | [78] |
| Sol-gel | Escherichia coli | Galactose production Alkane and dicylcopropyl detection | [80,81] |
| Ceramics | Rhodococcos rhodochrous Aspergillus versicolor | Phenol and glycol degradation | [83] |
5. Conclusions
Acknowledgments
References
- Gosse, J.; Chinn, M.; Grunden, A.; Bernal, O.; Jenkins, J.; Yeager, C.; Kosourov, S.; Seibert, M.; Flickinger, M. A versatile method for preparation of hydrated microbial-latex biocatalytic coatings for gas absorption and gas evolution. J. Ind. Microbiol. Biotechnol. 2012, 39, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Gosse, J.L.; Engel, B.J.; Hui, J.C.; Harwood, C.S.; Flickinger, M.C. Progress toward a biomimetic leaf: 4000 h of hydrogen production by coating-stabilized nongrowing photosynthetic Rhodopseudomonas palustris. Biotechnol. Prog. 2010, 26, 907–918. [Google Scholar] [PubMed]
- Gosse, J.L.; Engel, B.J.; Rey, F.E.; Harwood, C.S.; Scriven, L.E.; Flickinger, M.C. Hydrogen production by photoreactive nanoporous latex coatings of nongrowing Rhodopseudomonas palustris CGA009. Biotechnol. Prog. 2007, 23, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, M.L.; Dubini, A.; Yu, J.; Maness, P. Photobiological hydrogen-producing systems. Chem. Soc. Rev. 2009, 38, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; de Groot, H.; Holzwarth, A. Harnessing Solar Energy for the Production of Clean Fuels; Leiden Institute of Chemistry: Leiden, The Netherlands, 2006. [Google Scholar]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Efimenko, K.; Finlay, J.; Callow, M.E.; Callow, J.A.; Genzer, J. Development and testing of hierarchically wrinkled coatings for marine antifouling. ACS Appl. Mater. Interfaces 2009, 1, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.F. Industrial manufacture and applications of tributyltin compounds. In Tributyltin: Case Study of an Environmental Contaminant; de Mora, S.J., Ed.; Cambridge University Press: Cambridge, UK, 1996; pp. 21–61. [Google Scholar]
- Xu, Q.; Barrios, C.; Cutright, T.; Newby, B. Assessment of antifouling effectiveness of two natural product antifoulants by attachment study with freshwater bacteria. Environ. Sci. Pollut. Res. 2005, 12, 278–285. [Google Scholar] [CrossRef]
- Weiss, K.D. Paint and coatings: A mature industry in transition. Prog. Poly. Sci. 1997, 22, 203–245. [Google Scholar] [CrossRef]
- Lyngberg, O.; Solheid, C.; Charaniya, S.; Ma, Y.; Thiagarajan, V.; Scriven, L.; Flickinger, M. Permeability and reactivity of Thermotoga maritima in latex bimodal blend coatings at 80 °C: A model high temperature biocatalytic coating. Extremophiles 2005, 9, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Swope, K.L. Manipulation of Specific Enzyme Activity in Recombinant E. coli after Immobilization in Thin Copolymer Films: A Model System for Extended Biocatalysis. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 1995. [Google Scholar]
- Swope, K.; Flickinger, M.C. Activation and regeneration of whole cell biocatalysts: Initial and periodic induction behavior in starved Escherichia coli after immobilization in thin synthetic filmss. Biotechnol. Bioeng. 1996, 51, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Piskorska, M.; Soule, T.; Gosse, J.L.; Milliken, C.; Flickinger, M.C.; Smith, G.W.; Yeager, C.M. Preservation of H2 production activity in nanoporous latex coatings of Rhodopseudomonas palustris CGA009 during dry storage at ambient temperatures. Microb. Biotechnol. 2013, in press. [Google Scholar]
- Julsing, M.K.; Kuhn, D.; Schmid, A.; Bühler, B. Resting cells of recombinant E. coli show high epoxidation yields on energy source and high sensitivity to product inhibition. Biotechnol. Bioeng. 2011, 109, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Philips, E.J.; Mitsui, A. Characterization and optimization of hydrogen production by a salt water blue-green alga oscillatoria sp. Miami BG 7. II. Use of immobilization for enhancement of hydrogen production. Int. J. Hydrog. Energy 1986, 11, 83–89. [Google Scholar] [CrossRef]
- Bagai, R.; Madamwar, D. Long-term photo-evolution of hydrogen in a packed bed reactor containing a combination of Phormidium valderianum, Halobacterium halobium, and Escherichia coli immobilized in polyvinyl alcohol. Int. J. Hydrog. Energy 1999, 24, 311–317. [Google Scholar] [CrossRef]
- Dickson, D.J.; Page, C.J.; Ely, R.L. Photobiological hydrogen production from Synechocystis sp. PCC 6803 encapsulated in silica sol-gel. Int. J. Hydrog. Energy 2009, 34, 204–215. [Google Scholar] [CrossRef]
- Leonard, A.; Dandoy, P.; Danloy, E.; Leroux, G.; Meunier, C.F.; Rooke, J.C.; Su, B.-L. Whole-cell based hybrid materials for green energy production, environmental remediation and smart cell-therapy. Chem Soc. Rev. 2011, 40, 860–885. [Google Scholar]
- Flickinger, M.C.; Schottel, J.L.; Bond, D.R.; Aksan, A.; Scriven, L.E. Painting and printing living bacteria: Engineering nanoporous biocatalytic coatings to preserve microbial viability and intensify reactivity. Biotechnol. Prog. 2007, 23, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, M.C.; Fidaleo, M.; Gosse, J.; Polzin, K.; Charaniya, S.; Solheid, C.; Lyngberg, O.K.; Laudon, M.; Ge, H.; Schottel, J.L.; et al. Engineering nanoporous bioactive smart coatings containing microorganisms: fundamentals and emerging applications. In Smart Coatings II; Provder, T., Baghdachi, J., Eds.; American Chemical Society: Washington, DC, USA, 2009; pp. 52–94. [Google Scholar]
- Jerrim, L.B.; Velev, O.D. Deposition of coatings from live yeast cells and large particles by “convective-sedimentation” assembly. Langmuir 2009, 25, 5692–5702. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.S.; Flickinger, M.C.; Velev, O.D. Continuous convective-sedimentation assembly of colloidal microsphere coatings for biotechnology applications. Coatings 2013, 3, 26–48. [Google Scholar] [CrossRef]
- Fidaleo, M.; Flickinger, M.C. Engineering and modeling of thin, adhesive, microbial biocatalytic coatings for high intensity oxidations in multi-phase microchannel bioreactors. Chem. Eng. Sci. 2011, 66, 3251–3257. [Google Scholar] [CrossRef]
- Scriven, L.E.S. Fine–structured materials by continuous coating and drying or curing of liquid precursors. In Chemical Engineering; Galan, M.A., del Valle, M., Eds.; John Wiley: Hoboken, NJ, USA, 2005; pp. 229–266. [Google Scholar]
- Lyngberg, O.; Ng, C.; Thiagarajan, V.; Scriven, L.; Flickinger, M. Engineering the microstructure and permeability of thin multilayer latex biocatalytic coatings containing E. Coli. Biotechnol. Prog. 2001, 17, 1169–1171. [Google Scholar]
- Jenkins, J.S.; Flickinger, M.C.; Velev, O.D. Deposition of composite coatings from particle-particle and particle-yeast blends by convective-sedimentation assembly. J. Colloid Interface Sci. 2012, 38, 192–200. [Google Scholar] [CrossRef]
- Kuncicky, D.M.; Naik, R.R.; Velev, O.D. Rapid deposition and long-range alignment of nanocoatings and arrays of electrically conductive wires from tobacco mosaic virus. Small 2006, 2, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Kumnorkaew, P.; Ee, Y.; Tansu, N.; Gilchrist, J.F. Investigation of the deposition of microsphere monolayers for fabrication of microlens arrays. Langmuir 2008, 24, 12150–12157. [Google Scholar] [CrossRef] [PubMed]
- Prevo, B.; Velev, O. Controlled rapid deposition of structured coatings from micro-and nanoparticle suspensions. Langmuir 2004, 16, 2099–2107. [Google Scholar] [CrossRef]
- Hosein, I.D.; Lee, S.H.; Liddell, C.M. Dimer-based three-dimensional photonic crystals. Adv. Funct. Mater. 2010, 20, 3085–3091. [Google Scholar] [CrossRef]
- Hosein, I.D.; Liddell, C.M. Convectively assembled nonspherical mushroom cap-based colloidal crystals. Langmuir 2007, 23, 8810–8814. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Stoianov, S.V.; Bangerter, J.; Robinson, H.D. Restricted meniscus convective self-assembly. J. Colloid Interface. Sci. 2010, 344, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Im, S.; Park, O. Rapid fabrication of two- and three-dimensional colloidal crystal films via confined convective assembly. Adv. Funct. Mater. 2005, 15, 1329–1335. [Google Scholar] [CrossRef]
- Dimitrov, A.; Nagayama, K. Continuous convective assembling of fine particles into two-dimensional arrays on solid surfaces. Langmuir 1996, 12, 1303–1311. [Google Scholar] [CrossRef]
- Denkov, N.; Velev, O.; Kralchevsky, P.; Ivanov, I.; Yoshimura, H.; Nagayama, K. Mechanism of formation of two-dimensional crystals from latex particles on substrates. Langmuir 1992, 8, 3183–3190. [Google Scholar] [CrossRef]
- Denkov, N.; Velev, O.; Kralchevsky, P.; Ivanov, I.; Yoshimura, H.; Nagayama, K. Two-dimensional crystallization. Nature 1993, 361, 26. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, A; Nagayama, K. Steady-state unidirectional convective assembling of fine particles into two-dimensional arrays. Chem. Phys. Lett. 1995, 243, 462–468. [Google Scholar] [CrossRef]
- Jenkins, J.S. Engineering Multifunctional Living Paints: Thin, Convectively-Assembled Biocomposite Coatings of Live Cells and Colloidal Latex Particles Deposited by Continuous Convective-Sedimentation Assembly. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2013. [Google Scholar]
- Prevo, B.; Hwang, Y.; Velev, O. Convective assembly of antireflective silica coatings with controlled thickness and refractive index. Chem. Mater. 2005, 17, 3642–3651. [Google Scholar] [CrossRef]
- Prevo, B.; Fuller, J.; Velev, O. Rapid deposition of gold nanoparticle films with controlled thickness and structure by convective assembly. Chem. Mater. 2005, 17, 28–35. [Google Scholar] [CrossRef]
- Kuncicky, D.; Christesen, S.; Velev, O. Role of the micro- and nanostructure in the performance of SERS substrates assembled from gold nanoparticles. Appl. Spectrosc. 2005, 59, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Lawton, C.W.; Klei, H.E.; Sundstrom, D.W.; Voronoko, P.J. Immobilization of whole cells using polymeric coatings. Biotechnol. Bioeng. Symp. 1986, 17, 507–517. [Google Scholar]
- Lawton, C.W. Saccharomyces Cerevisiae Immobilization Using Latex Polymers. M.S. Thesis, University of Connecticut, Storrs, CT, USA, 2001. [Google Scholar]
- Bunning, T.J. Physical Property Improvements of A Biocatalyst. M.S. Thesis, University of Connecticut, Storrs, CT, USA, 1988. [Google Scholar]
- Schaffer, J.R.; Burdick, B.A.; Abrams, C.T. Thin-film biocatalysts. Chemtech 1988, 9, 546–550. [Google Scholar]
- Flanagan, W.P.; Klei, H.E.; Sundstrom, D.W.; Lawton, C.W. Optimization of a pellicular biocatalyst for penicillin G production by Penicillium chrysogenum. Biotechnol. Bioeng. 1990, 36, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, J.B.; Mills, P.D.A.; Jones, E.; Stewart, R.F. Immobilized cells. Eur. Pat. 0288203, 26 October 1988. [Google Scholar]
- Martens, N.; Hall, E.A.H. Immobilisation of photosynthetic cells based on film-forming emulsion polymers. Anal. Chim. Acta 1994, 292, 49–63. [Google Scholar] [CrossRef]
- Pansu, B.; Pieranski, P.; Pieranski, P. Structures of thin layers of hard spheres: High pressure limit. J. Phys. 1984, 45, 331–339. [Google Scholar] [CrossRef]
- Pansu, B.; Pieranski, P.; Strzelecki, L. Thin colloidal crystals: A series of structural transitions. J. Phys. 1983, 44, 531–536. [Google Scholar] [CrossRef]
- Tessier, P.; Velev, O.; Kalambur, A.; Rabolt, J.; Lenhoff, A.; Kaler, E. Assembly of gold nanostructured films templated by colloidal crystals and use in surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2000, 122, 9554–9555. [Google Scholar] [CrossRef]
- Gupta, S.; Alargova, R.G.; Kilpatrick, P.K.; Velev, O.D. On-chip dielectrophoretic co-assembly of live cells and particles into responsive biomaterials. Langmuir 2010, 26, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Prevo, B.G.; Kuncicky, D.M.; Velev, O.D. Engineered deposition of coatings from nano- and micro-particles: A brief review of convective assembly at high volume fraction. Colloid Surf. A Physicochem. Eng. Asp. 2007, 311, 2–10. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; Brandy, M.; Cayre, O.J.; Velev, O.D.; Paunov, V.N. Live celloidosome structures based on the assembly of individual cells by colloid interactions. Phys. Chem. Chem. Phys. 2010, 12, 11912–11922. [Google Scholar] [CrossRef] [PubMed]
- Brandy, M.; Cayre, O.J.; Fakhrullin, R.F.; Velev, O.D.; Paunov, V.N. Directed assembly of yeast cells into living yeastosomes by microbubble templating. Soft Matter 2010, 6, 3494–3498. [Google Scholar] [CrossRef]
- Kosourov, S.N.; Seibert, M. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol. Bioeng. 2009, 102, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tredici, M.R. Bioreactors. In The Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis, and Bioseparation; Flickinger, M.C., Drew, S.W., Eds.; John Wiley: New York, NY, USA, 1999; p. 395. [Google Scholar]
- Melo, L.F.; Bott, T.R. Biofilms: Science and Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Bryers, J.D. Biofilms II: Process Analysis and Applications; Wiley-Liss, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Characklis, W.G.; Marshall, K.C. Biofilms; John Wiley: New York, NY, USA, 1990. [Google Scholar]
- Evans, L.V. Biofilms: Recent Advances in Their Study and Control; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Donlan, R. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Boyd, K.; Armstrong, E.; Jiang, Z.; Yan, L.; Berggren, M.; May, U.; Pisacane, T.; Granmo, Å.; Adams, D. The development of a marine natural product-based antifouling paint. Biofouling 2003, 19, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.S.; Wu, A.H.F.; Zhang, H.; Coffey, J.; Huddle, T.; Lafountaine, J.S.; Lim, Z.-J.; White, E.A.; Tuong, N.T.; Lamb, R.N. Self-cleaning surfaces: A third-year undergraduate research project. J. Chem. Educ. 2009, 86, 365–367. [Google Scholar] [CrossRef]
- Stamm, L.B.J. Meniscus-Directed Assembly of Biologically Active Coatings of Cells, Microparticles, and Nanoparticles. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2009. [Google Scholar]
- Zhou, H.; Fan, T.; Zhang, D. An insight into artificial leaves for sustainable energy inspired by natural photosynthesis. ChemCatChem 2011, 3, 513–528. [Google Scholar] [CrossRef]
- Bensaid, S.; Centi, G.; Garrone, E.; Perathoner, S.; Saracco, G. Towards artificial leaves for solar hydrogen and fuels from carbon dioxide. ChemSusChem 2012, 5, 500–521. [Google Scholar] [CrossRef] [PubMed]
- Nocera, D.G. The artificial leaf. Acc. Chem. Res. 2010, 45, 767–776. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, J.H.; Lee, S.H.; Park, C.B. Biomimetic artificial photosynthesis by light-harvesting synthetic wood. ChemSusChem. 2011, 4, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Rooke, J.C.; Meunier, C.; Leonard, A.; Su, B. Energy from photobioreactors: Bioencapsulation of photosynthetically active molecules, organelles, and whole cells within biologically inert matrices. Pure Appl. Chem. 2008, 80, 2345–2376. [Google Scholar] [CrossRef]
- Jain, S.L.; Joseph, J.K.; Kuehn, F.E.; Reiser, O. Retraction: An efficient synthesis of poly(ethylene glycol)-supported iron(II) porphyrin using a click reaction and its application for the catalytic olefination of aldehydes. Adv. Synth. Catal. 2009, 351, 230–234. [Google Scholar] [CrossRef]
- Frolov, L.; Wilner, O.; Carmeli, C.; Carmeli, I. Fabrication of oriented multilayers of photosystem I proteins on solid surfaces by auto-metallization. Adv. Mater. 2008, 20, 263–266. [Google Scholar] [CrossRef]
- Krassen, H.; Schwarze, A.; Friedrich, B.; Ataka, K.; Lenz, O.; Heberle, J. Photosynthetic hydrogen production by a hybrid complex of photosystem I and [NiFe]-hydrogenase. ACS Nano. 2009, 3, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Branyik, T.; Kuncova, G.; Paca, J.; Demnerova, K. Encapsulation of microbial cells into silica gel. J. Sol. Gel. Sci. Technol. 1998, 13, 283–287. [Google Scholar] [CrossRef]
- Finnie, K.; Bartlett, J.; Woolfrey, J. Encapsulation of sulfate-reducing bacteria in a silica host. J. Mat. Chem. 2000, 10, 1099–1101. [Google Scholar] [CrossRef]
- Livage, J.; Coradin, T.; Roux, C. Encapsulation of biomolecules in silica gels. J. Phys. Condens. Matter. 2001, 13, R673–R691. [Google Scholar] [CrossRef]
- Fennouh, S.; Guyon, S.; Livage, J.; Roux, C. Sol-gel entrapment of Escherichia coli. J. Sol. Gel. Sci. Technol. 2000, 19, 647–649. [Google Scholar] [CrossRef]
- Ferrer, M.; Yuste, L.; Rojo, F.; del Monte, F. Biocompatible sol-gel route for encapsulation of living bacteria in organically modified silica matrixes. Chem. Mater. 2003, 15, 3614–3618. [Google Scholar] [CrossRef]
- Bottcher, H.; Soltmann, U.; Mertig, M.; Pompe, W. Biocers: Ceramics with incorporated microorganisms for biocatalytic, biosorptive and functional materials development. J. Mat. Chem. 2004, 14, 2176–2178. [Google Scholar] [CrossRef]
- Fiedler, D.; Thron, A.; Soltmann, U.; Bottcher, H. New packing materials for bioreactors based on coated and fiber-reinforced biocers. Chem. Mater. 2004, 16, 3040–3044. [Google Scholar] [CrossRef]
- Rooke, J.C.; Leonard, A.; Sarmento, H.; Meunier, C.F.; Descy, J.; Su, B. Novel photosynthetic CO2 bioconvertor based on green algae entrapped in low-sodium silica gels. J. Mat. Chem. 2011, 21, 951–959. [Google Scholar] [CrossRef]
- Meunier, C.F.; Rooke, J.C.; Leonard, A.; van Cutsem, P.; Su, B. Design of photochemical materials for carbohydrate production via the immobilisation of whole plant cells into a porous silica matrix. J. Mater. Chem. 2010, 20, 929–936. [Google Scholar] [CrossRef]
- Leino, H.; Kosourov, S.N.; Saari, L.; Sivonen, K.; Tsygankov, A.A.; Aro, E.; Allahverdiyeva, Y. Extended H2 photoproduction by N2-fixing cyanobacteria immobilized in thin alginate films. Int. J. Hydrog. Energy 2012, 37, 151–161. [Google Scholar] [CrossRef]
- Buchholz, K.; Kasche, V.; Bornscheuer, U.T. Biocatalysis and Enzyme Technology; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005. [Google Scholar]
- Webb, C.; Dervakos, G.A. Studies in Viable Cell Immobilization; Academic Press, RG Landes Company: Austin, TX, USA, 1996. [Google Scholar]
- Karel, S.; Robertson, C. Autoradiographic determination of mass-transfer limitations in immobilized cell reactors. Biotechnol. Bioeng. 1989, 34, 320–336. [Google Scholar] [CrossRef] [PubMed]
- Karel, S.; Robertson, C. Cell mass synthesis and degradation by immobilized Escherichia coli. Biotechnol. Bioeng. 1989, 34, 337–356. [Google Scholar] [CrossRef] [PubMed]
- De Bont, J.A.M.; Visser, J.; Mattiassen, B.; Tramper, J. Physiology of Immobilized Cells; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Willert, R.G.; Baron, G.V.; de Backer, L. Immobilized Living Cells: Modeling and Experimental Methods; John Wiley: Chichester, UK, 1996. [Google Scholar]
- Guisan, J.M. Immobilization of Enzymes and Cells; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Wijffels, E.H. Immobilized Cells; Springer-Verlag: Berlin, Germany, 2001. [Google Scholar]
- Kosourov, S.N.; Ghirardi, M.L.; Seibert, M. A truncated antenna mutant of Chlamydomonas reinhardtii can produce more hydrogen than the parental strain. Int. J. Hydrog. Energy 2011, 36, 2044–2048. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jenkins, J.S.; Flickinger, M.C.; Velev, O.D. Engineering Cellular Photocomposite Materials Using Convective Assembly. Materials 2013, 6, 1803-1825. https://doi.org/10.3390/ma6051803
Jenkins JS, Flickinger MC, Velev OD. Engineering Cellular Photocomposite Materials Using Convective Assembly. Materials. 2013; 6(5):1803-1825. https://doi.org/10.3390/ma6051803
Chicago/Turabian StyleJenkins, Jessica S., Michael C. Flickinger, and Orlin D. Velev. 2013. "Engineering Cellular Photocomposite Materials Using Convective Assembly" Materials 6, no. 5: 1803-1825. https://doi.org/10.3390/ma6051803
APA StyleJenkins, J. S., Flickinger, M. C., & Velev, O. D. (2013). Engineering Cellular Photocomposite Materials Using Convective Assembly. Materials, 6(5), 1803-1825. https://doi.org/10.3390/ma6051803




