A Review of Aspects of Oxidative Hair Dye Chemistry with Special Reference to N-Nitrosamine Formation
Abstract
:1. Introduction
2. Discussion
- 2.1.
- The Chemistry of N-nitrosamines
- 2.2.
- The Toxicology of N-nitrosamines
- 2.3.
- Nitrosation of Secondary Amines in Polluted Air
- 2.4.
- Secondary Amines in Oxidative Hair Dye Products
- 2.5.
- Systemic Activity of Hair Dye Products
2.1. The Chemistry of N-Nitrosamines
- Primary alkylamines react, decompose, and yield nitrogen and alcohols. Primary arylamines form the relatively stable diazo compounds which may react further or decompose, depending on the surrounding environment;
- Secondary amines react and form stable N-nitrosamines;
- Tertiary amines usually form unstable salts that decompose upon neutralization, or nitrosate away from the nitrogen atom.
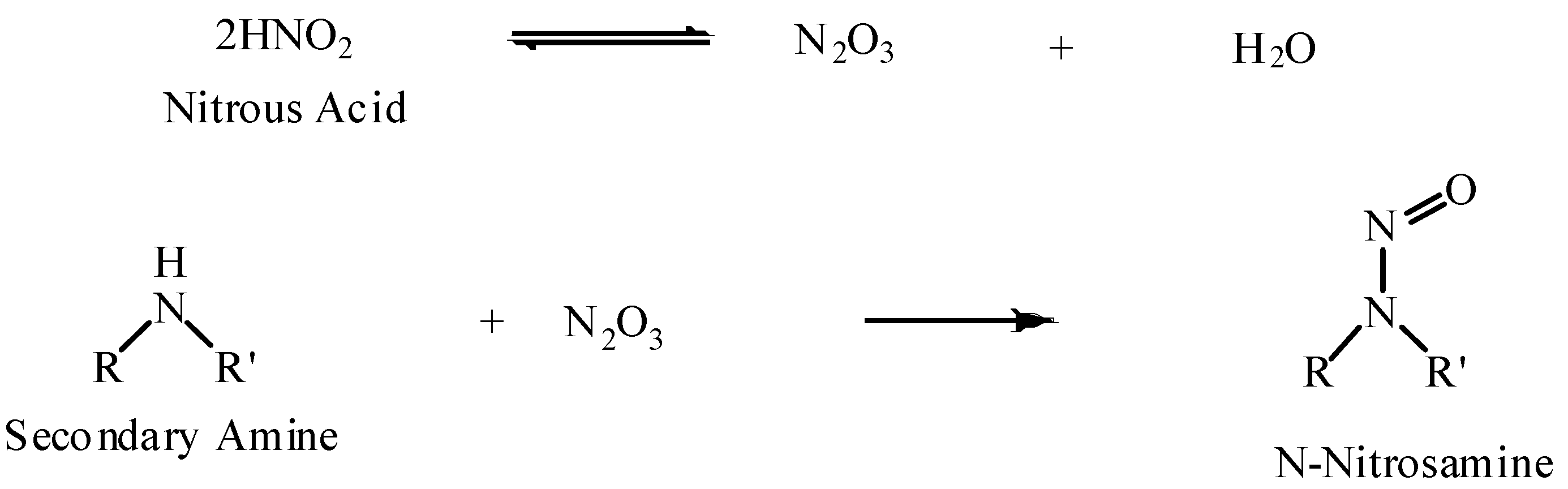
2.2. The Toxicology of N-Nitrosamines
2.3. Nitrosation of Secondary Amines in Polluted Air
2.4. Secondary Amines in Oxidative Hair Dye Products
- 2.4.1.
- Oxidative Hair Dye Precursors
- 2.4.2.
- Semi-Permanent Hair Color (HC) Dyes (“Direct” Dyes)
- 2.4.3.
- Oxidative Reaction Products
- 2.4.4.
- Degradation of Oxidative Hair Dyes
2.4.1. Oxidative Hair Dye Precursors
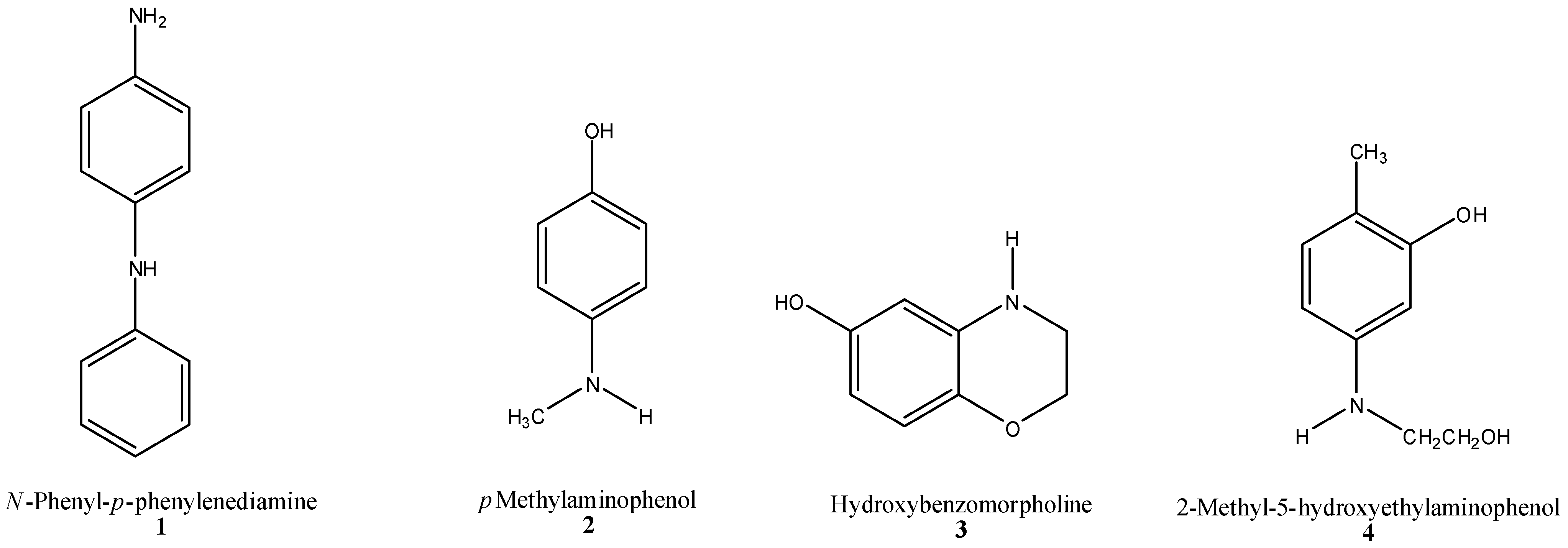
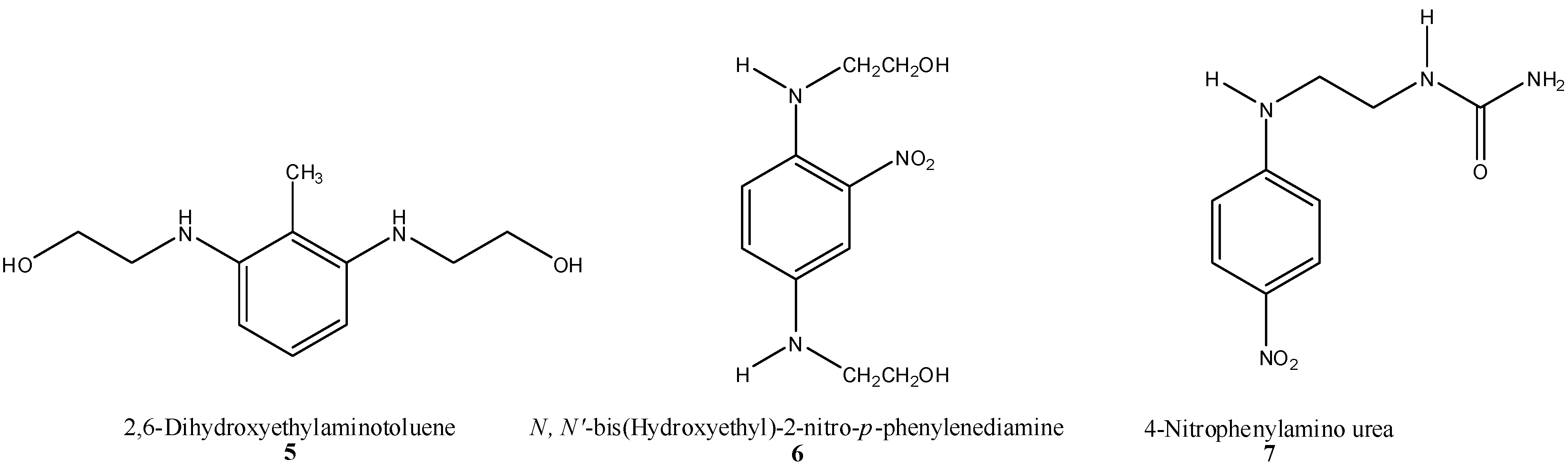
2.4.2. Semi-Permanent Hair Color (HC) Dyes (“Direct” Dyes)
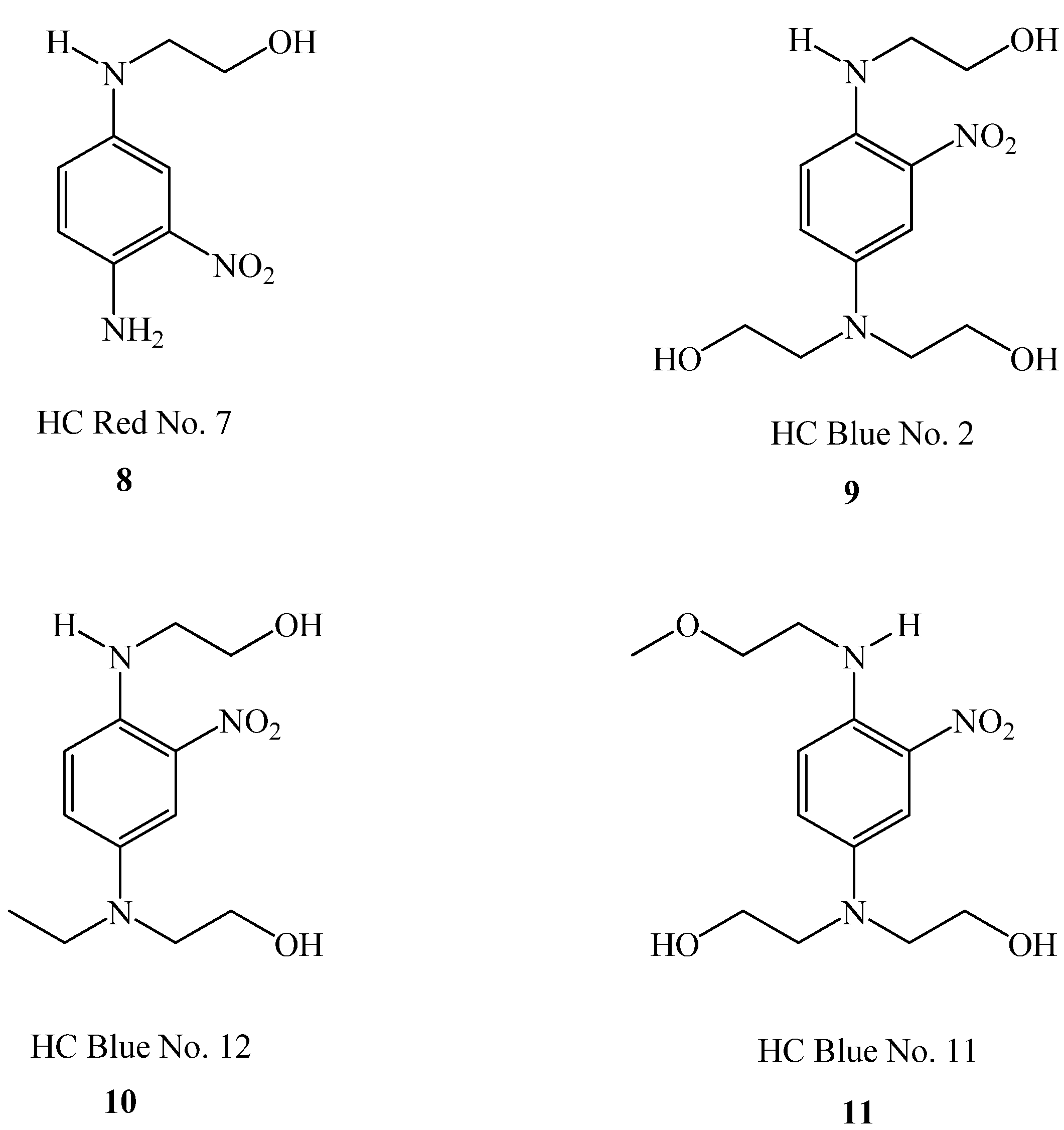
| Dye | Contains a secondary amine group? | Maximum Permitted Concentration allowed in the final product (%) |
|---|---|---|
| HC Blue No. 2 | √ | 2.8 |
| HC Blue No. 7 | √ | 0.68 |
| HC Blue No. 11 | √ | 2.0 |
| HC Blue No. 12 | √ | 1.5 |
| HC Blue No. 13 | √ | No limit |
| HC Blue No. 14 | √ | 0.3 |
| HC Blue No. 15 | – | NA |
| HC Blue No. 16 | √ | No limit |
| HC Green No. 1 | √ | Banned |
| HC Orange No. 1 | √ | 1.0 |
| HC Orange No. 2 | √ | 1.0 |
| HC Orange No. 3 | √ | Banned |
| HC Orange No. 5 | √ | No limit |
| HC Red No. 1 | √ | 1.0 |
| HC Red No. 3 | √ | 3.0 |
| HC Red No. 7 | √ | 1.0 |
| HC Red No. 8 and its salts | √ | Banned |
| HC Red No. 10 | √ | 1.0 |
| HC Red No. 11 | √ | 1.0 |
| HC Red No. 13 | √ | 2.5 |
| HC Red No. 14 | – | NA |
| HC Red No. 15 | √ | No limit |
| HC Red No. 16 | √ | 0.75 |
| HC Violet No. 1 | √ | 0.28 |
| HC Violet No. 2 | √ | 2.0 |
| HC Yellow No. 2 | √ | 0.75 |
| HC Yellow No. 4 | √ | 1.5 |
| HC Yellow No. 7 | – | NA |
| HC Yellow No. 9 | √ | 0.5 |
| HC Yellow No. 10 | √ | 0.1 |
| HC Yellow No. 11 | √ | Banned |
| HC Yellow No. 13 | √ | 2.5 |
| HC Yellow No. 14 | √ | No limit |
| HC Yellow No. 15 | √ | No limit |
2.4.3. Oxidative Reaction Products
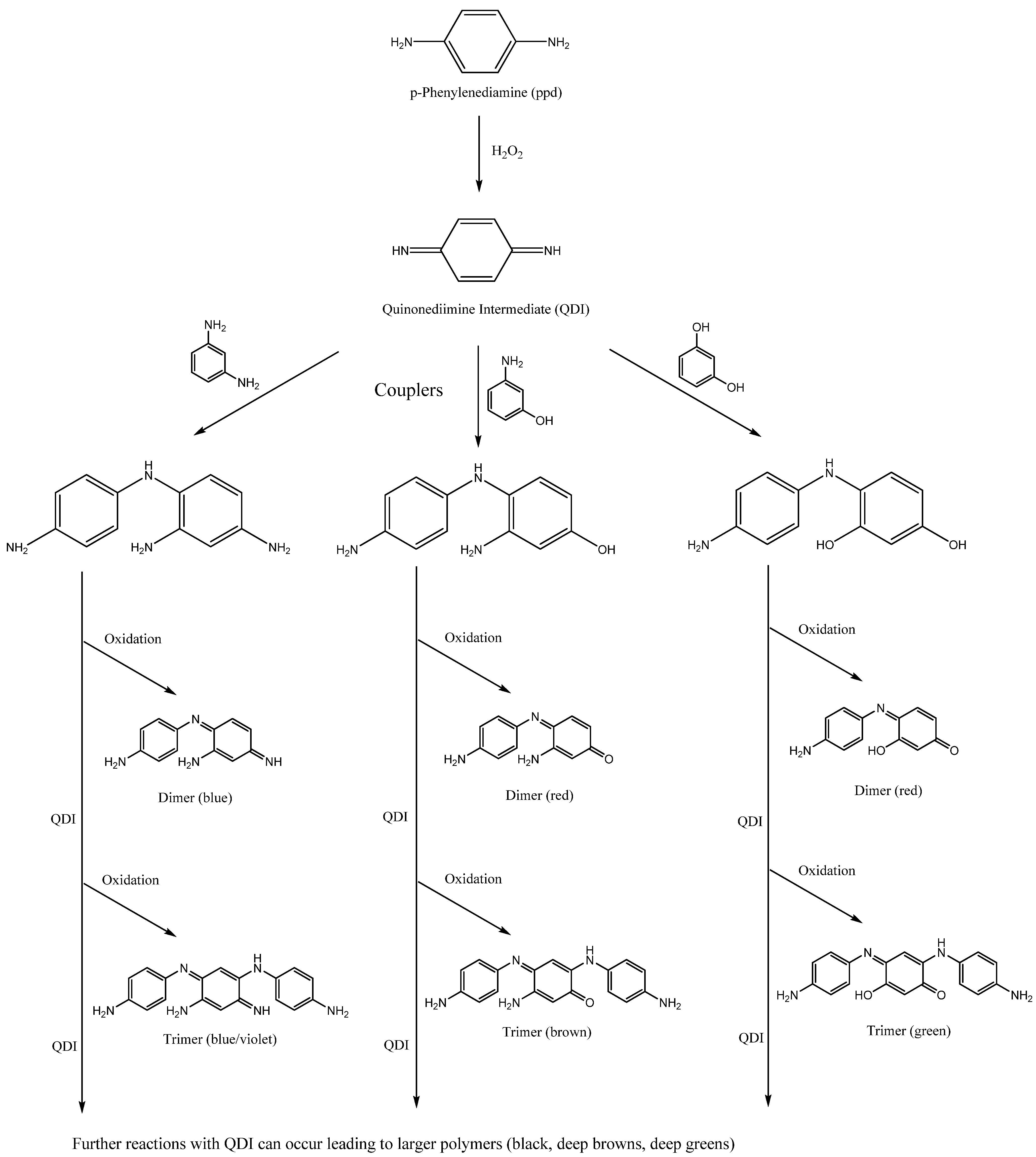
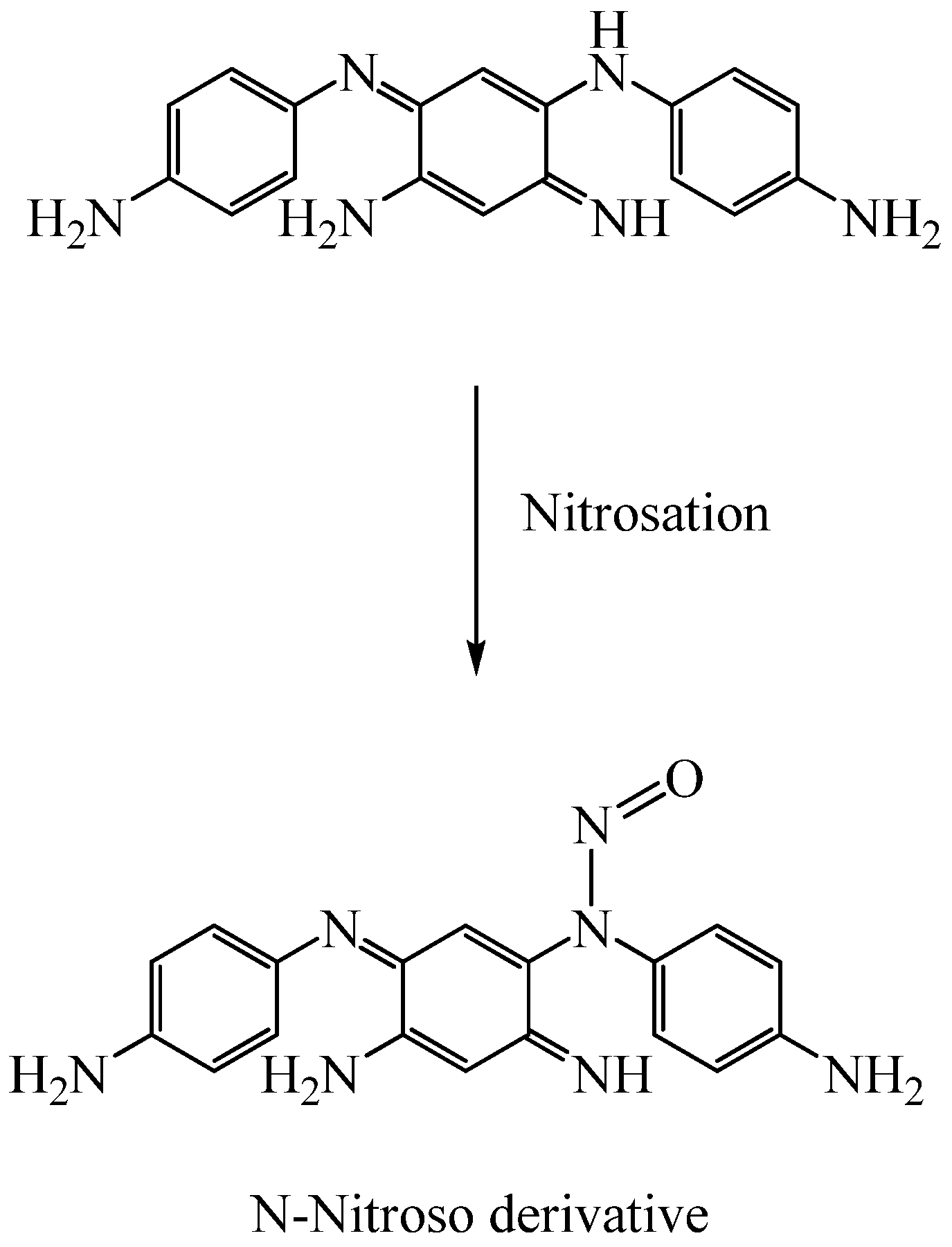
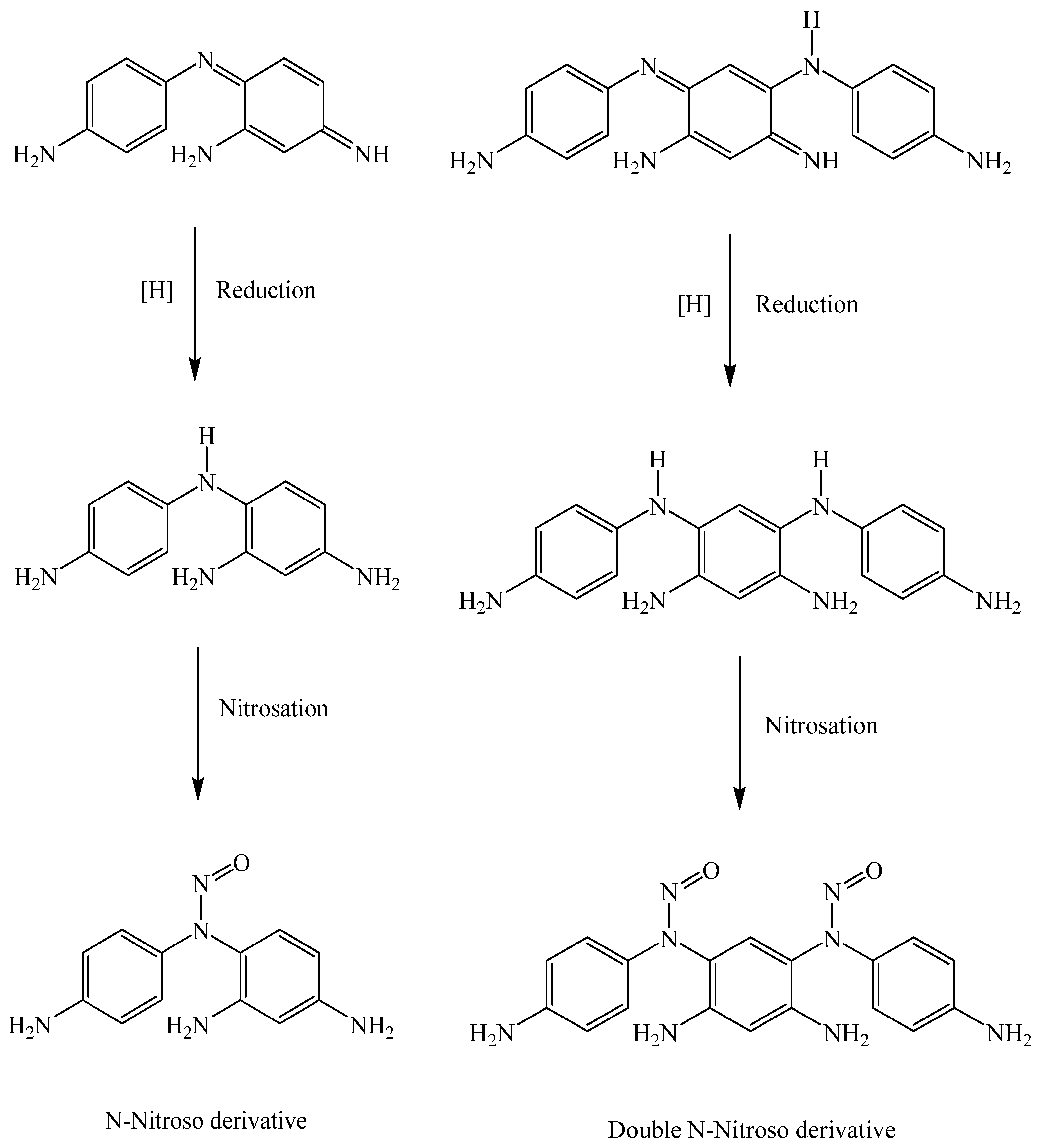
2.4.4. Degradation of Oxidative Hair Dyes
2.5. Systemic Activity of Hair Dye Products
3. Conclusions
- Secondary amines are produced and are a potential source of NOC’s.
- NOC’s are carcinogenic and even those that have not been tested should be assumed to be just as genotoxic until proven otherwise [51].
- N-nitrosation of secondary amines can, and does occur in the atmosphere due to the presence of nitrogen oxides.
- There are a number of sources for secondary amines in oxidative hair products as well as any product using HC dyes, and that these penetrate the skin to become biologically available.
References
- Charle, R.; Sag, G. Early synthetic organic hair dyes. Manuf. Chem. Aerosol News 1967, 33–37. [Google Scholar]
- Green, A. Landmarks in the evolution of the dyestuff industry during the past half-century. J. Soc. Dyers Colour. 1934, 49–64. [Google Scholar]
- European Commission Scientific Committee on Consumer Products (SCCP). Memorandum on Hair Dye Substances and Their Skin Sensitising Properties. Available online: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_s_05.pdf (accessed on 28 January 2013).
- European Commission Scientific Committee on Consumer Safety (SCCS). Opinion on Toluene-2,5-diamine and Its Sulphate. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_093.pdf (accessed on 28 January 2013).
- McFadden, J.P.; White, I.R.; Frosch, P.J.; Sosted, H.; Johansen, J.D; Menne, T. Allergy to hair dye. Brit. Med. J. 2007, 334, 220. [Google Scholar]
- Hair dye allergy left woman looking like “Elephant Woman”. Available online: http://www.telegraph.co.uk/health/5558616/Hair-dye-allergy-left-woman-looking-like-Elephant-Woman.html (accessed on 22 June 2012).
- Teenage girl dies 20 minutes after dyeing her hair. Available online: http://www.metro.co.uk/news/878424-teenage-girl-dies-20-minutes-after-dyeing-her-hair (accessed on 22 June 2012).
- Pictured: Woman suffers horrendous burns after reaction to Boots hair dye. Available online: http://www.dailymail.co.uk/news/article-1223746/Woman-suffers-horrendous-burns-reaction-Boots-hair-dye.html (accessed on 22 June 2012).
- Mother left in coma after using hair dye. Sky News. 22 November 2011. Available online: http://news.sky.com/home/uk-news/article/16114630 (accessed on 22 June 2012).
- Call for ban on hair dye chemical over allergy fears. The Guardian. 14 October 2011. Available online: http://www.guardian.co.uk/fashion/2011/oct/14/hair-dye-chemical-allergy-fears?INTCMP=SRCH (accessed on 22 June 2012).
- Tell cosmetics companies to stop using dangerous PPD. Available online: http://news.change.org/stories/tell-cosmetics-companies-to-stop-using-dangerous-ppd (accessed on 22 June 2012).
- Does your hair dye contain the chemical feared to have killed this woman? The Daily Mail. 19 October 2011. Available online: http://www.dailymail.co.uk/femail/article-2051098/Does-YOUR-hair-dye-contain-chemical-feared-killed-woman.html (accessed on 22 June 2012).
- Ames, B.N.; Kammen, H.O.; Yamasaki, E. Hair dyes are mutagenic: Identification of a variety of mutagenic ingredients. Proc. Natl. Acad. Sci. USA 1975, 72, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Ghissassi, E.F.; Bouvard, V.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of some aromatic amines, organic dyes, and related exposures. Lancet Oncol. 2008, 9, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Schned, A.R.; Heaney, J.A.; Karagas, M.R. Bladder cancer risk and personal hair dye use. Int. J. Cancer 2004, 109, 571–586. [Google Scholar] [CrossRef]
- Gago-Dominguez, M.; Castelao, J.E.; Yuan, J.-M.; Yu, M.C.; Ross, R.K. Use of permanent hair dyes and bladder-cancer risk. Int. J. Cancer 2001, 91, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Gago-Dominguez, M.; Bell, D.A.; Watson, M.A.; Yuan, J.-M.; Castelao, J.E.; Hein, D.W.; Chan, K.K.; Coetzee, G.A.; Ross, R.K.; Yu, M.C. Permanent hair dyes and bladder cancer: Risk modification by cytochrome P4501A2 and N-acetyltransferases 1 and 2. Carcinogenesis 2003, 24, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hunchareck, M.; Kupelnick, B. Personal use of hair dyes and the risk of bladder cancer: Results of a meta-analysis. Public Health Rep. 2005, 120, 31–38. [Google Scholar] [PubMed]
- Kelsey, K.T.; Hirao, T.; Hirao, S.; Devi-Ashok, T.; Nelson, H.H.; Andrew, A.; Colt, J.; Baris, D.; Morris, J.S.; Schned, A.; Karagas, M. TP53 alterations and patterns of carcinogen exposure in a U.S. population-based study of bladder cancer. Int. J. Cancer 2005, 117, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Takkouche, B.; Regueira-Méndez, C.; Montes-Martínez, A. Risk of cancer among hairdressers and related workers: A meta-analysis. Int. J. Epidemiol. 2009, 38, 1512–1531. [Google Scholar] [CrossRef] [PubMed]
- Zahm, S.H.; Weisenburger, D.D.; Babbitt, P.A.; Saal, R.C.; Vaught, J.B.; Blair, A. Use of hair colouring products and the risk of lymphoma, multiple myeloma, and chronic lymphocytic leukaemia. Am. J. Public Health 1992, 82, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; de Sanjose, S.; Bracci, P.M.; Morton, L.M.; Wang, R.; Brennan, P.; Hartge, P.; Boffetta, P.; Becker, N.; Maynadie, M.; et al. Personal use of hair dye and the risk of certain subtypes of non-hodgkin lymphoma. Am. J. Epidemiol. 2008, 167, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Cantor, K.P.; Blair, A.; Everett, G.; VanLier, S.; Burmeister, L.; Dick, F.R.; Gibson, R.W.; Gibson, R.W.; Schuman, L. Hair dye use and risk of leukemia and lymphoma. Am. J. Public Health 1988, 78, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Holford, T.R.; Leaderer, B.; Boyle, P.; Zahm, S.H.; Flynn, S.; Tallini, G.; Owens, P.H.; Zheng, T. Hair-coloring product use and risk of non-Hodgkin’s lymphoma: A population-based case-control study in Connecticut. Am. J. Epidemiol. 2004, 159, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, G.H.; Shore, D.; Sandler, D.P. Hair dye use and risk of adult acute leukemia. Am. J. Epidemiol. 2004, 160, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Petro-Nustas, W.; Norton, M.E.; Al-Masarweh, I. Risk factors for breast cancer in Jordanian women. J. Nurs. Scholarsh. 2002, 34, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Shafer, N.; Shafer, R.W. Potential of carcinogenic effects of hair dyes. N. Y. State J. Med. 1976, 76, 394–396. [Google Scholar] [PubMed]
- Hennekens, C.H.; Speizer, F.E.; Rosner, B.; Bain, C.J.; Belanger, C.; Peto, R. Use of permanent hair dyes and cancer among registered nurses. Lancet 1979, 1, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Shore, R.E.; Pasternack, B.S.; Thiessen, E.V.; Sadow, M.; Forbes, R.; Albert, R.E. A case-control study of hair dye use and breast cancer. J. Natl. Cancer Inst. 1979, 62, 277–283. [Google Scholar] [PubMed]
- Stavraky, K.M.; Clarke, E.A.; Donner, A. Case-control study of hair dye use by patients with breast cancer and endometrial cancer. J. Natl. Cancer Inst. 1979, 63, 941–943. [Google Scholar] [PubMed]
- Brown, L.M.; Everett, G.D.; Burmeister, L.F.; Blair, A. Hair dye use and multiple myeloma in white men. Am. J. Public Health 1992, 82, 1673–1674. [Google Scholar] [CrossRef] [PubMed]
- Takkouche, B.; Etminan, M.; Montes-Martinez, A. Personal use of hair dyes and risk of cancer: A meta-analysis. JAMA 2005, 293, 2516–2525. [Google Scholar] [PubMed]
- Ahlbom, A.; Navier, I.L.; Norell, S.; Olin, R.; Spännare, B. Non-occupational risk indicators for astrocytomas in adults. Am. J. Epidemiol. 1986, 124, 334–337. [Google Scholar] [PubMed]
- Burch, J.D.; Craib, K.J.; Choi, B.C.; Miller, A.B.; Risch, H.A.; Howe, G.R. An exploratory case-control study of brain tumours in adults. J. Natl. Cancer Inst. 1987, 78, 601–609. [Google Scholar] [PubMed]
- European Commission Scientific Committee on Consumer Products (SCCP). Opinion on Personal Use of Hair Dyes and Cancer Risk. Available online: http://ec.europa.eu/health/archive/ph_risk/committees/04_sccp/docs/sccp_o_00l.pdf (accessed on 30 January 2013).
- European Commission Scientific Committee on Consumer Products (SCCP). Opinion on Intermediates and Reaction Products of Oxidative Hair Dye Ingredients Formed during Hair Dyeing. Available online: http://ec.europa.eu/health/archive/ph_risk/committees/04_sccp/docs/sccp_o_162.pdf (accessed on 30 January 2013).
- European Commission Scientific Committee on Consumer Safety (SCCS). Opinion on Reaction Products of Oxidative Hair Dye Ingredients Formed during Hair Dyeing. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_037.pdf (accessed on 30 January 2013).
- Rowe, F.M.; Chamberlain, K.A.J. The “fading” of dyeings on cellulose acetate rayon the action of “burnt gas fumes” (oxides of nitrogen, etc., in the atmosphere) on cellulose acetate rayon dyes. J. Soc. Dyers Colour. 1937, 53, 268–278. [Google Scholar]
- Feuer, H. The Chemistry of the Nitro and Nitroso Groups; Interscience Publishers: New York, NY, USA, 1969. [Google Scholar]
- Fieser, L.F.; Fieser, M. Organic Chemistry, 3rd ed; Reinhold: New York, NY, USA, 1956. [Google Scholar]
- Mirvish, S.S. Formation of N-nitroso compounds—Chemistry, kinetics and in vivo occurrence. Toxicol. Appl. Pharmacol. 1975, 31, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Mirvish, S.S. Studies on N-nitrosation reactions: Kinetics of nitrosation, correlation with mouse feeding experiments, and natural occurrence of nitrosatable compounds (ureides and guanidines). In Topics in Chemical Carcinogenesis; Nakahara, W., Takayama, S., Sugimura, S., Odashima, S., Eds.; University of Tokyo Press: Tokyo, Japan, 1972; pp. 279–295. [Google Scholar]
- U.S. Environmental Protection Agency. Scientific and Technical Assessment Report on Nitrosamines; U.S. Environmental Protection Agency: Washington, DC, USA, 1978.
- Chow, Y.L.; Lau, M.P.; Perry, R.A.; Tam, J.N.S. Photochemistry of nitroso compounds in solution. XX. Photoreduction, photoelimination, and photoaddition of nitroamines. Can. J. Chem. 1972, 50, 1044–1050. [Google Scholar] [CrossRef]
- Magee, P.N.; Barnes, J.M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br. J. Cancer 1956, 10, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bogovski, P.; Bogovski, S. Animal species in which N-nitroso compounds induce cancer. Int. J. Cancer 1981, 27, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Rounbehler, D.P.; Fajen, J.M. N-Nitroso Compounds in the Factory Environment; US Department of Health and Human Services: Cincinnati, OH, USA, 1983.
- International Agency for Research on Cancer (IARC). Some N-nitroso compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1978; Volume 17, pp. 83–175. [Google Scholar]
- Peto, R.; Gray, R.; Brantom, P.; Grasso, P. Effects on 4080 rats of chronic ingestion of N-nitrosoethylamine or N-nitrosodimethylamine: A detailed dose-response study. Cancer Res. 1991, 51, 6415–6451. [Google Scholar] [PubMed]
- Peto, R.; Gray, R.; Brantom, P.; Grasso, P. Dose and time relationship for tumor induction in the live rand esophagus of 4080 inbred rats by chronic ingestion of N-nitrosoethylamine or N-nitrosodimethylamine. Cancer Res. 1991, 51, 6452–6469. [Google Scholar] [PubMed]
- European Commission Scientific Committee on Consumer Safety (SCCS). Opinion on Nitrosamines and Secondary Amines in Cosmetic Products. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_090.pdf (accessed on 30 January 2013).
- European Commission Cosmetics Directive 76/768/EEC. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1976L0768:20110603:EN:PDF (accessed on 30 January 2013).
- Neurath, G.B. N-Nitroso Compound Analysis and Formation; Bogovski, P., Preussmann, R., Walker, E.A., Eds.; International Agency for Research on Cancer: Lyon, France, 1972; pp. 134–136. [Google Scholar]
- Fine, D.H.; Rufeh, F.; Lieb, D.; Epstein, S.S. A possible nitrogen oxide-nitrosamine-cancer link. Bull. Environ. Contam. Toxicol. 1974, 11, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Challis, B.C.; Kyrtopoulos, S.A. Rapid formation of carcinogenic N-nitrosamines in aqueous alkaline solutions. Br. J. Cancer 1977, 35, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Challis, B.C.; Edwards, A.; Hunma, R.R.; Kyrtopoulos, S.A.; Outram, J.R. Rapid formation of N-nitrosamines from nitrogen oxides under neutral and alkaline conditions. IARC Sci. Publ. 1978, 19, 127–142. [Google Scholar] [PubMed]
- Challis, B.C.; Kyrtopoulos, S.A. The chemistry of nitroso compounds. Part 12. The mechanism of nitrosation and nitration of aqueous piperidine by gaseous dinitrogen tetraoxide and dinitrogen trioxide in aqueous alkaline solutions. Evidence for the existence of molecular isomers of dinitrogen tetraoxide and dinitrogen trioxide. J. Chem. Soc. Perkin Trans. 2 1978, 12, 1296–1302. [Google Scholar] [CrossRef]
- Challis, B.C.; Shuker, D.E.G. Rapid nitrosation of amines in aqueous alkaline solutions by beta-substituted alkyl nitrites. J. Chem. Soc. Chem. Commun. 1979, 7, 315–316. [Google Scholar] [CrossRef]
- Challis, B.C.; Outram, J.R. The chemistry of nitroso compounds. Part 15. Formation of N-nitrosamines in solution from gaseous nitric oxide in the presence of iodine. J. Chem. Soc. Perkin Trans. 1 1979, 11, 2768–2775. [Google Scholar] [CrossRef]
- Challis, B.C.; Outram, J.R. The chemistry of nitroso-compounds. Part 11. Nitrosation of amines by the two-phase interaction of amines in solution with gaseous oxides of nitrogen. J. Chem. Soc. Perkin Trans. 1 1979, 2, 299–304. [Google Scholar] [CrossRef]
- Challis, B.C.; Outram, J.R.; Shuker, D.E. New pathways for the rapid formation of N-nitrosamines under neutral and alkaline conditions. IARC Sci. Publ. 1980, 31, 43–58. [Google Scholar] [PubMed]
- Challis, B.C.; Li, B.F. Formation of N-nitrosamines and N-nitramines by photolysis. IARC Sci. Publ. 1982, 41, 31–40. [Google Scholar] [PubMed]
- Challis, B.C.; Shuker, D.E.; Fine, D.H.; Goff, E.U.; Hoffman, G.A. Formation of N-nitrosamines and N-nitramines by gaseous nitrogen dioxide. Acta Cient. Compostelana 1982, 19, 153–166. [Google Scholar]
- Eisenbrand, G.; Blankart, M.; Sommer, H.; Weber, B. N-nitrosoalkanolamines in cosmetics. IARC Sci. Publ. 1991, 105, 238–241. [Google Scholar] [PubMed]
- Williams, D.L.H. Nitrosation Reactions in the Chemistry of Nitric Oxide; Elsevier: Amsterdam, the Netherland, 2004. [Google Scholar]
- Iqbal, Z.M.; Dahl, K.; Epstein, S.S. Role of nitrogen dioxide in the biosynthesis of nitrosamines in mice. Science 1980, 207, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Mirvish, S.S.; Sams, J.P.; Issenberg, P. The nitrosating agent in mice exposed to nitrogen dioxide: Improved extraction method and localisation in skin. Cancer Res. 1983, 43, 2550–2554. [Google Scholar] [PubMed]
- Mirvish, S.S.; Ramm, M.D.; Sams, J.P. Nitrosamine formation from amines applied to the skin of mice after and before exposure to nitrogen dioxide. Cancer Res. 1988, 48, 1095–1099. [Google Scholar] [PubMed]
- Miyanishi, K.; Kinouchi, T.; Kataoka, K.; Kanoh, T.; Ohnishi, Y. In vivo formation of mutagens by intraperitoneal administration of polycyclic aromatic hydrocarbons in animals during exposure to nitrogen dioxide. Carcinogenesis 1996, 17, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, M.; Gundel, L.A.; Pankow, J.F.; Jacob, P.; Singer, B.C.; Destaillats, H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential third hand smoke hazards. Available online: http://www.pnas.org/content/early/2010/02/04/0912820107 (accessed on 28 January 2013).
- European Commission Scientific Committee on Consumer Products (SCCP). Skin Penetration of Oxidative Hair Dyes Formed by the Coupling of Precursors and Couplers under Simulated Conditions of Hair Dyeing. Available online: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_067.pdf (accessed on 28 January 2013).
- Griffiths, J. Colour and Constitution of organic molecules; Academic Press: London, UK, 1976. [Google Scholar]
- Jarocol® Direct Dyes. Available online: http://www.vivimedlabs.com/vivimed-products/hair-care/jarocol-hair-dyes/jarocol-direct-dyes (accessed on 21 January 2013).
- Tucker, H.H. The formulation of oxidation hair dyes. Am. Perf. Cosm. 1968, 83, 59–62. [Google Scholar]
- Corbett, J.F. Benzoquinone imines. Part IX. Mechanism and kinetics of the reaction of p-benzoquinone di-imines with m-aminophenols. J. Chem. Soc., Perkin Trans. 2 1972, 5, 539–548. [Google Scholar] [CrossRef]
- Corbett, J.F. p-Benzoquinonediimine—A vital intermediate in oxidative hair dyeing. J. Soc. Cosmet. Chem. 1969, 20, 253–263. [Google Scholar]
- Corbett, J.F. Benzoquinone imines. Part VI. Mechanism and kinetics of the reaction of p-benzoquinone di-imines with m-phenylenediamines. J. Chem. Soc. B 1969, 827–835. [Google Scholar]
- Corbett, J.F. Benzoquinone imines. Part V. Mechanism and kinetics of the reaction of p-benzoquinone monoimines with m-phenylenediamines. J. Chem. Soc. B 1969, 823–826. [Google Scholar]
- Corbett, J.F. Benzoquinone imines. Part VII. The mechanism and kinetics of the reaction of p-benzoquinone di-imines with monohydric phenols and the ultraviolet, infrared, and nuclear magnetic resonance spectra of the resulting indoanilines. J. Chem. Soc. B 1970, 1418–1427. [Google Scholar]
- Corbett, J.F. Benzoquinone imines. Part VIII. Mechanism and kinetics of the reaction of p-benzoquinone monoimines with monohydric phenols. J. Chem. Soc. B 1970, 1502–1509. [Google Scholar]
- Corbett, J.F. Benzoquinone imines. Part X. The mechanism and kinetics of the reactions of p-benzoquinone di-imine and p-benzoquinone monoimine with C-methoxy-m-diamines and p-methoxy- and p-chloro-phenols. J. Chem. Soc. Perkin Trans. 2 1972, 8, 999–1005. [Google Scholar] [CrossRef]
- European Commission Scientific Committee on Consumer Products (SCCP). Opinion on Exposure to Reactants and Reaction Products of Oxidative Hair Dye Formulations. Available online: http://ec.europa.eu/health/archive/ph_risk/committees/04_sccp/docs/sccp_o_032.pdf (accessed on 30 January 2013).
- Cumming, J.; Giles, C.H.; McEachran, A.E. A study of the photochemistry of dyes on proteins and other substrates. J. Soc. Dyers Colour. 1956, 72, 373–381. [Google Scholar] [CrossRef]
- Green, A.G. The Analysis of Dyestuffs, 3rd ed.; Charles Griffin & Company: London, UK, 1949. [Google Scholar]
- European Commission Scientific Committee on Consumer Products (SCCP). Updated Recommended Strategy for Testing Oxidative Hair Dye Substances for Their Potential Mutagenicity/Genotoxicity. Available online: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_s_02.pdf (accessed on 30 January 2013).
- European Commission Scientific Committee on Consumer Products (SCCP). Opinion on the Presence and Release of Nitrosamines and Nitrosatable Compounds from Rubber Balloons. Available online: http://ec.europa.eu/health/archive/ph_risk/committees/04_sccp/docs/sccp_o_121.pdf (accessed on 30 January 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lewis, D.; Mama, J.; Hawkes, J. A Review of Aspects of Oxidative Hair Dye Chemistry with Special Reference to N-Nitrosamine Formation. Materials 2013, 6, 517-534. https://doi.org/10.3390/ma6020517
Lewis D, Mama J, Hawkes J. A Review of Aspects of Oxidative Hair Dye Chemistry with Special Reference to N-Nitrosamine Formation. Materials. 2013; 6(2):517-534. https://doi.org/10.3390/ma6020517
Chicago/Turabian StyleLewis, David, John Mama, and Jamie Hawkes. 2013. "A Review of Aspects of Oxidative Hair Dye Chemistry with Special Reference to N-Nitrosamine Formation" Materials 6, no. 2: 517-534. https://doi.org/10.3390/ma6020517
APA StyleLewis, D., Mama, J., & Hawkes, J. (2013). A Review of Aspects of Oxidative Hair Dye Chemistry with Special Reference to N-Nitrosamine Formation. Materials, 6(2), 517-534. https://doi.org/10.3390/ma6020517




