Controlled Aloin Release from Crosslinked Polyacrylamide Hydrogels: Effects of Mesh Size, Electric Field Strength and a Conductive Polymer
Abstract
:1. Introduction
2. Methodology
2.1. Materials
2.2. Synthesis of Poly(p-phenylene vinylene)-PPV
2.3. Preparation of Aloin Doped Poly(p-phenylene vinylene), Aloin Doped PPV
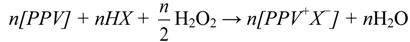
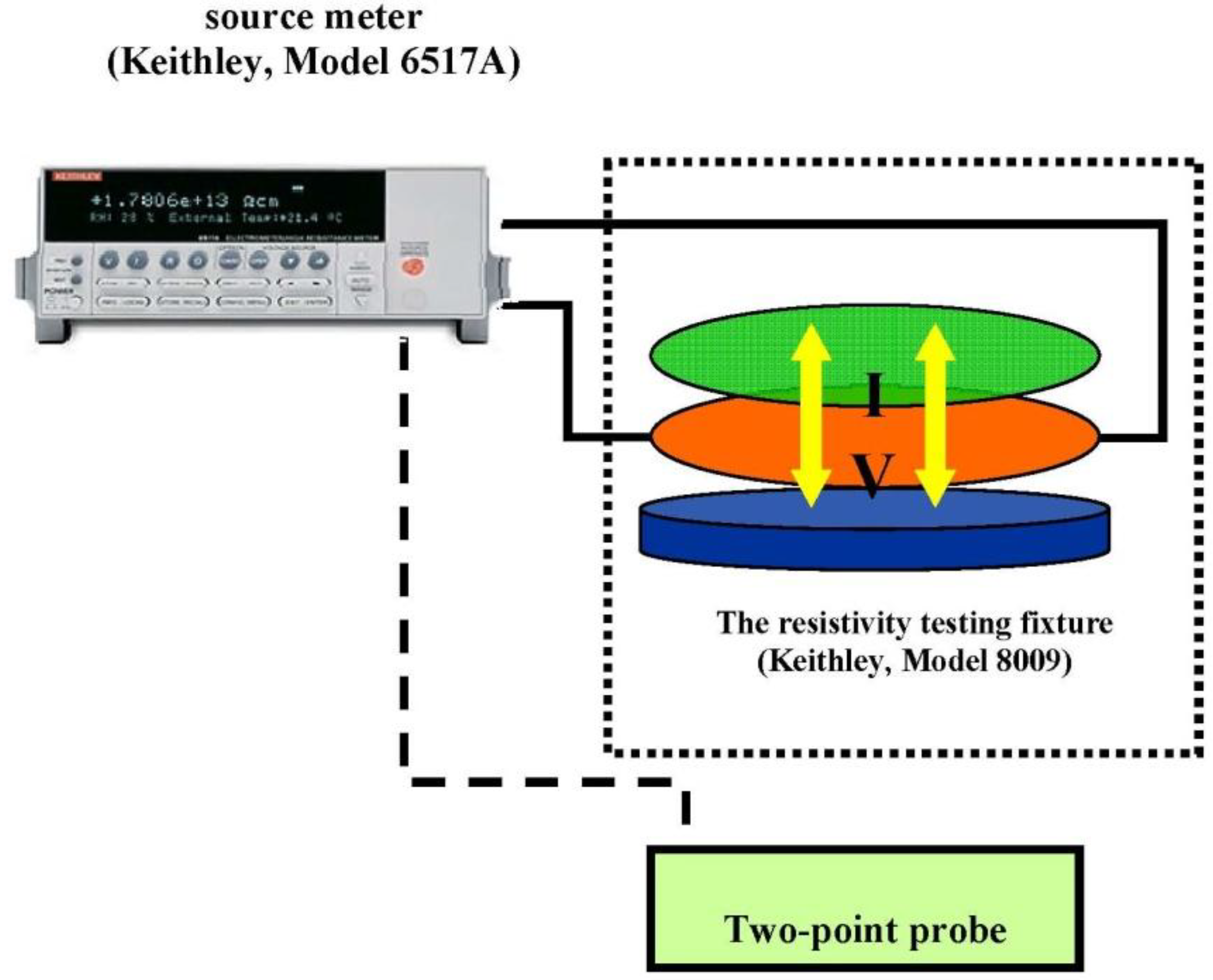
2.4. Electrical Conductivity Measurements
2.5. Preparation of Aloin-Loaded Polyacrylamide Hydrogel (Aloin/PAAM)
2.6. Preparation of Aloin-Doped PPV/PAAM Hydrogel
2.7. Characterization of PAAM Hydrogel, Aloin-Loaded PAAM Hydrogel and Aloin-Doped PPV/PAAM Hydrogel
2.8. Drug Release Studies
3. Results and Discussion
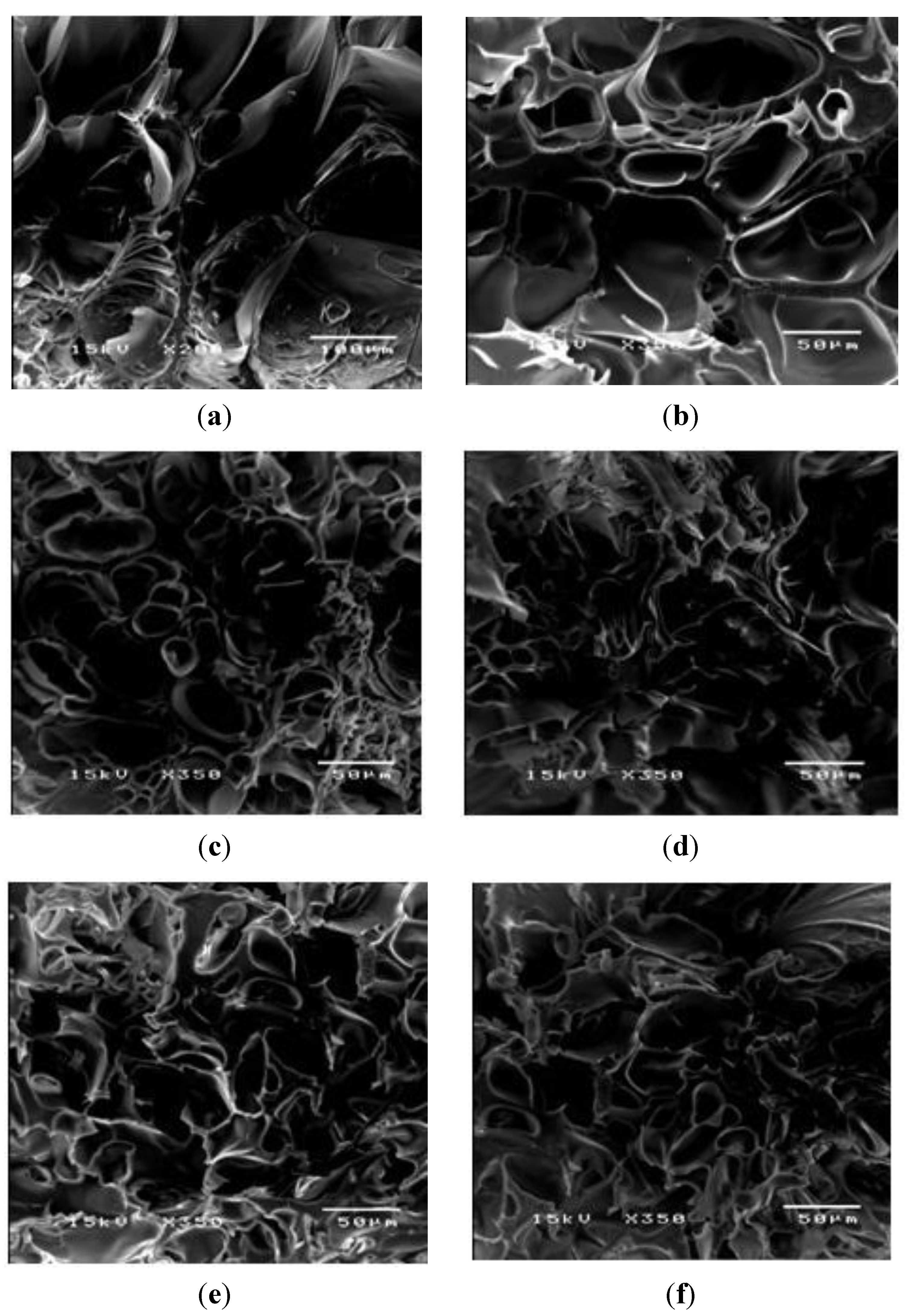
3.1. Characterization PAAM Hydrogel, Aloin-Loaded PAAM Hydrogel and Aloin-Doped PPV/PAAM Hydrogel
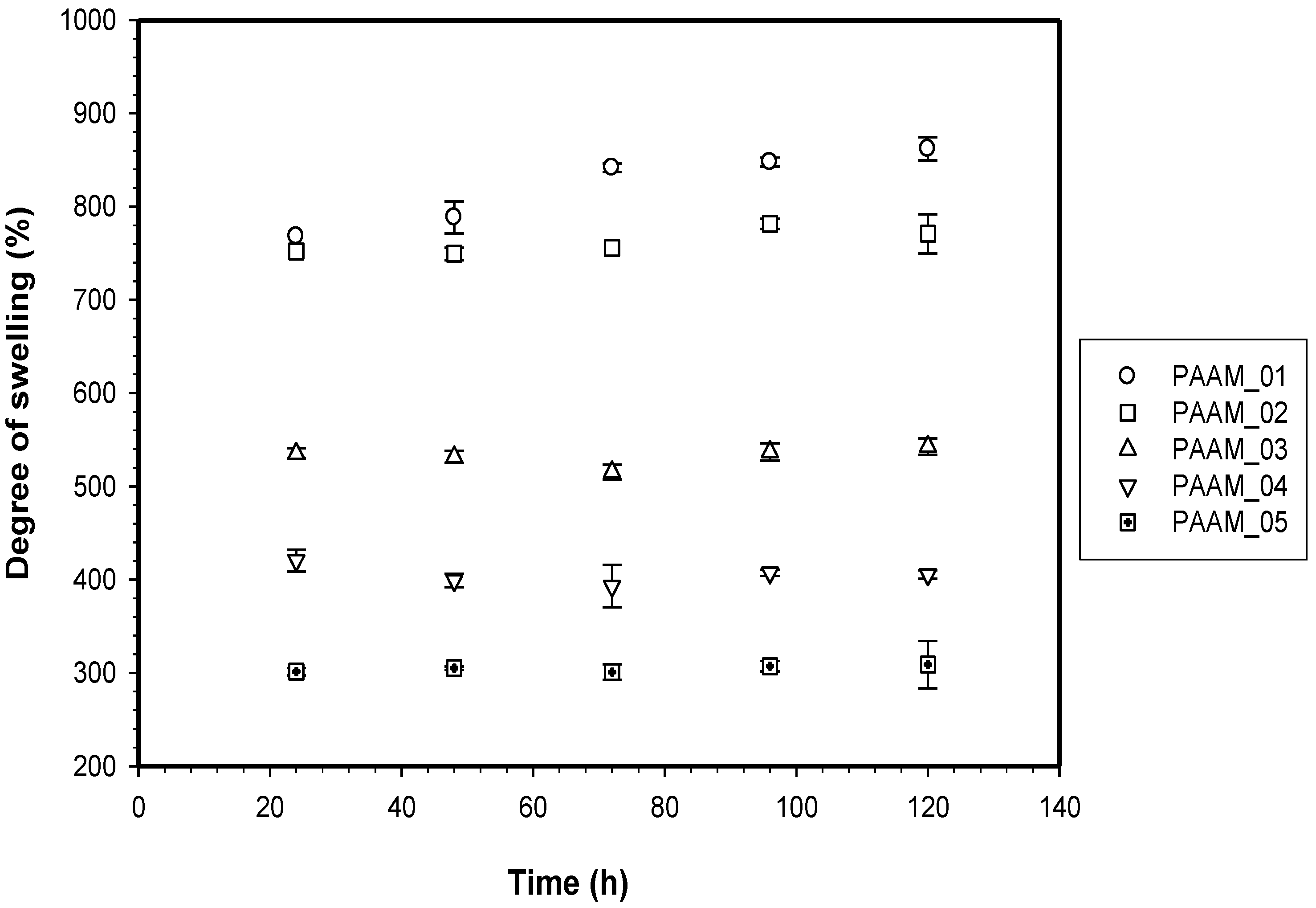
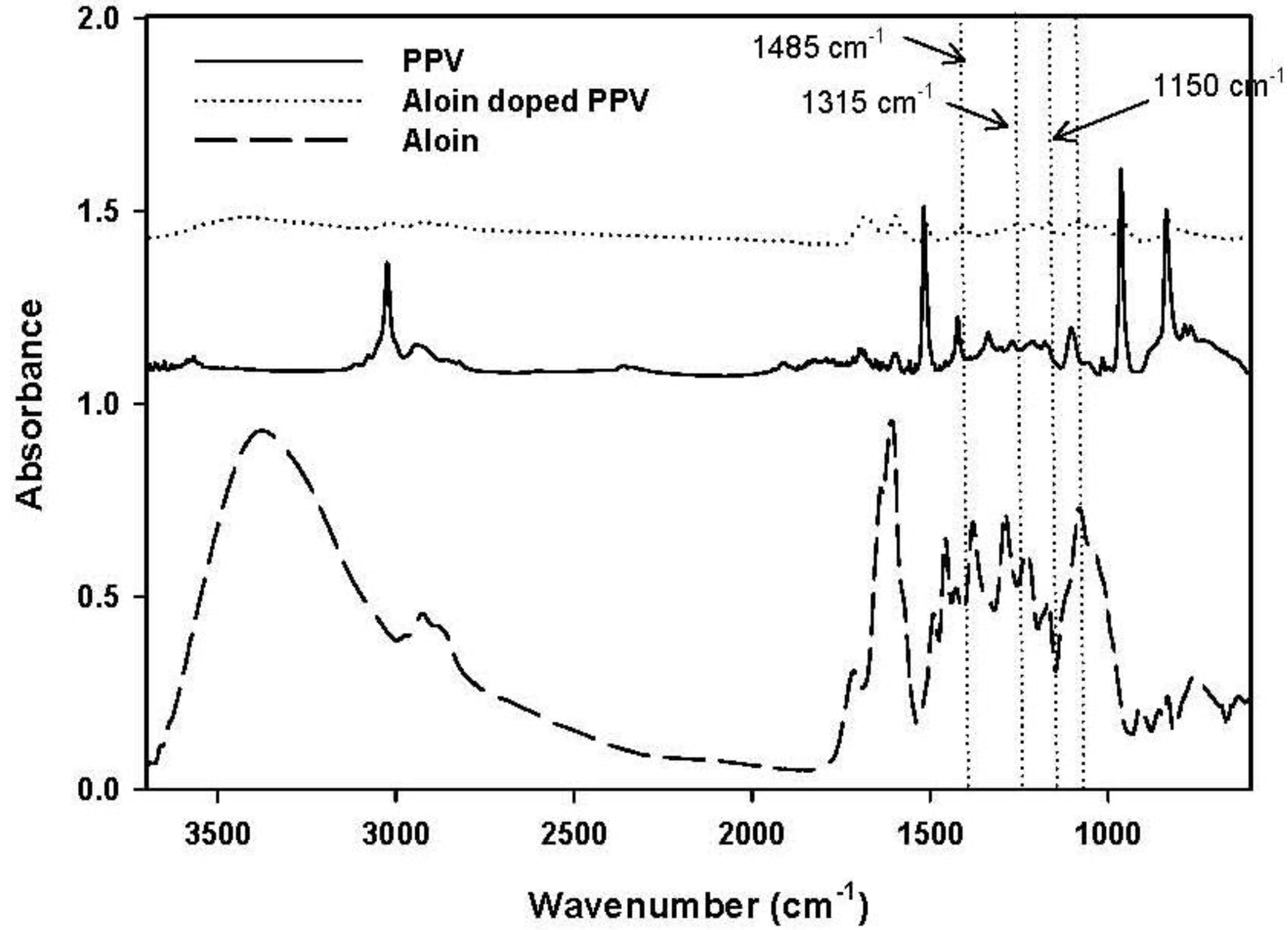
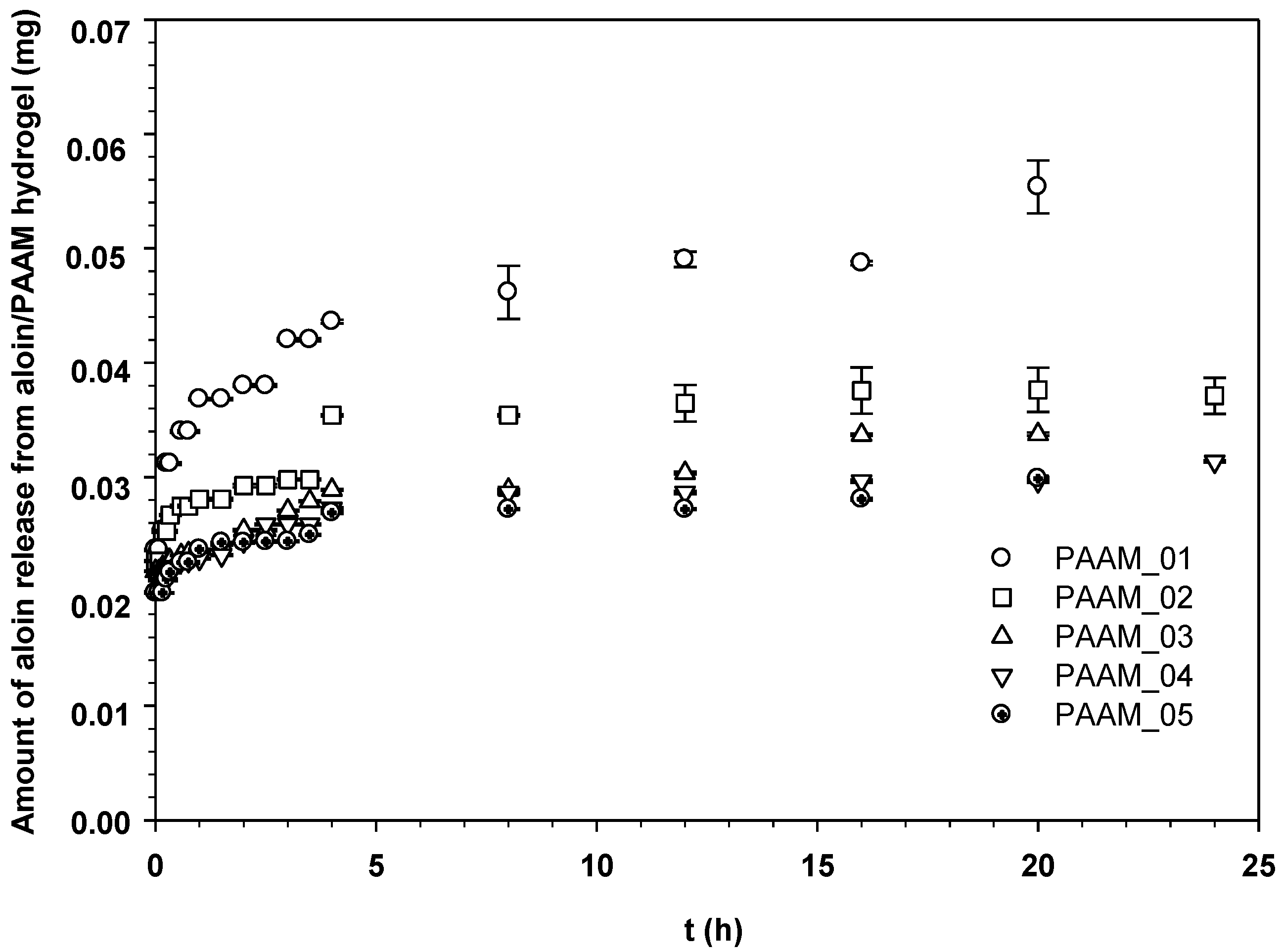
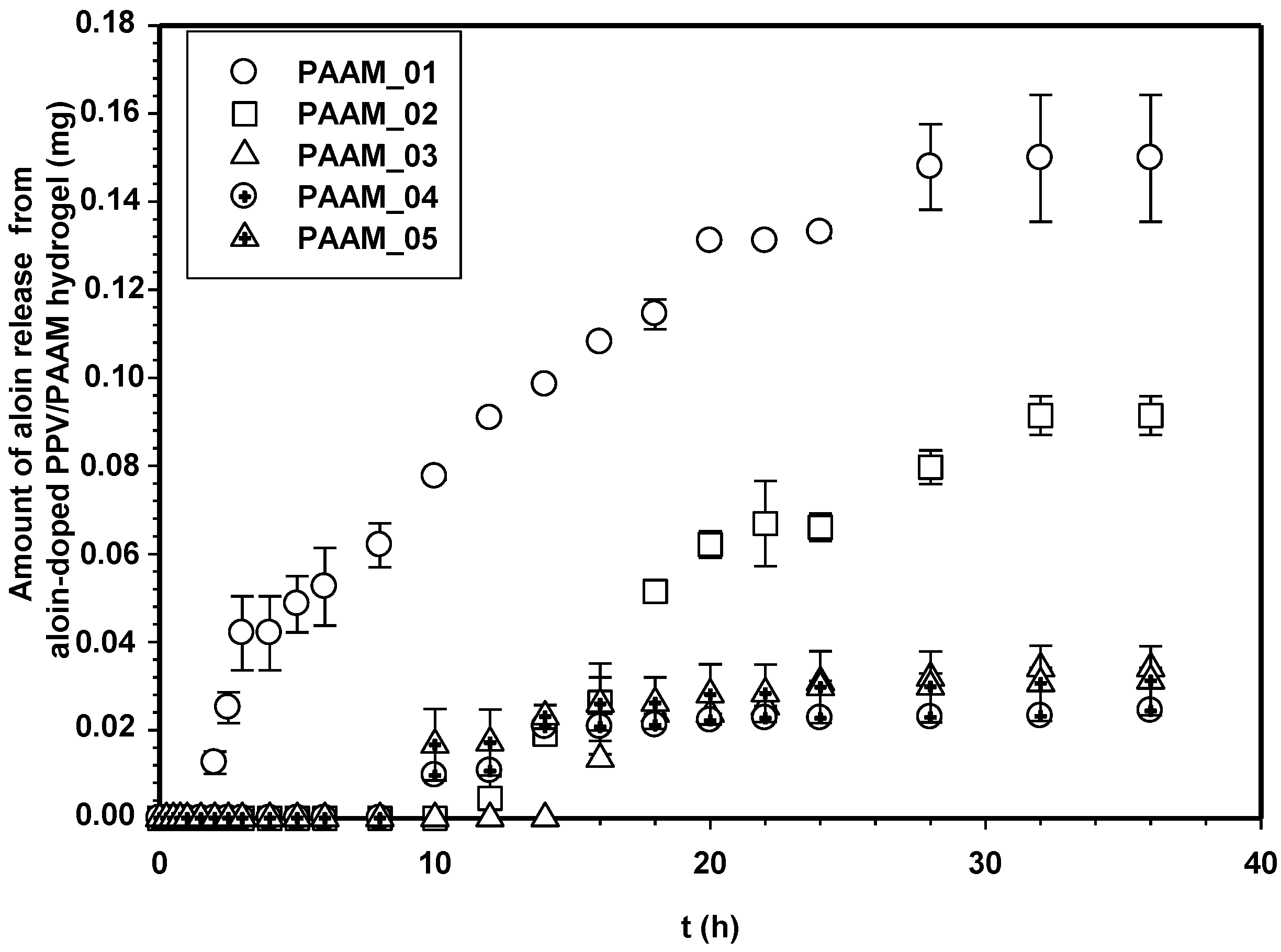
3.2. Release Characteristics
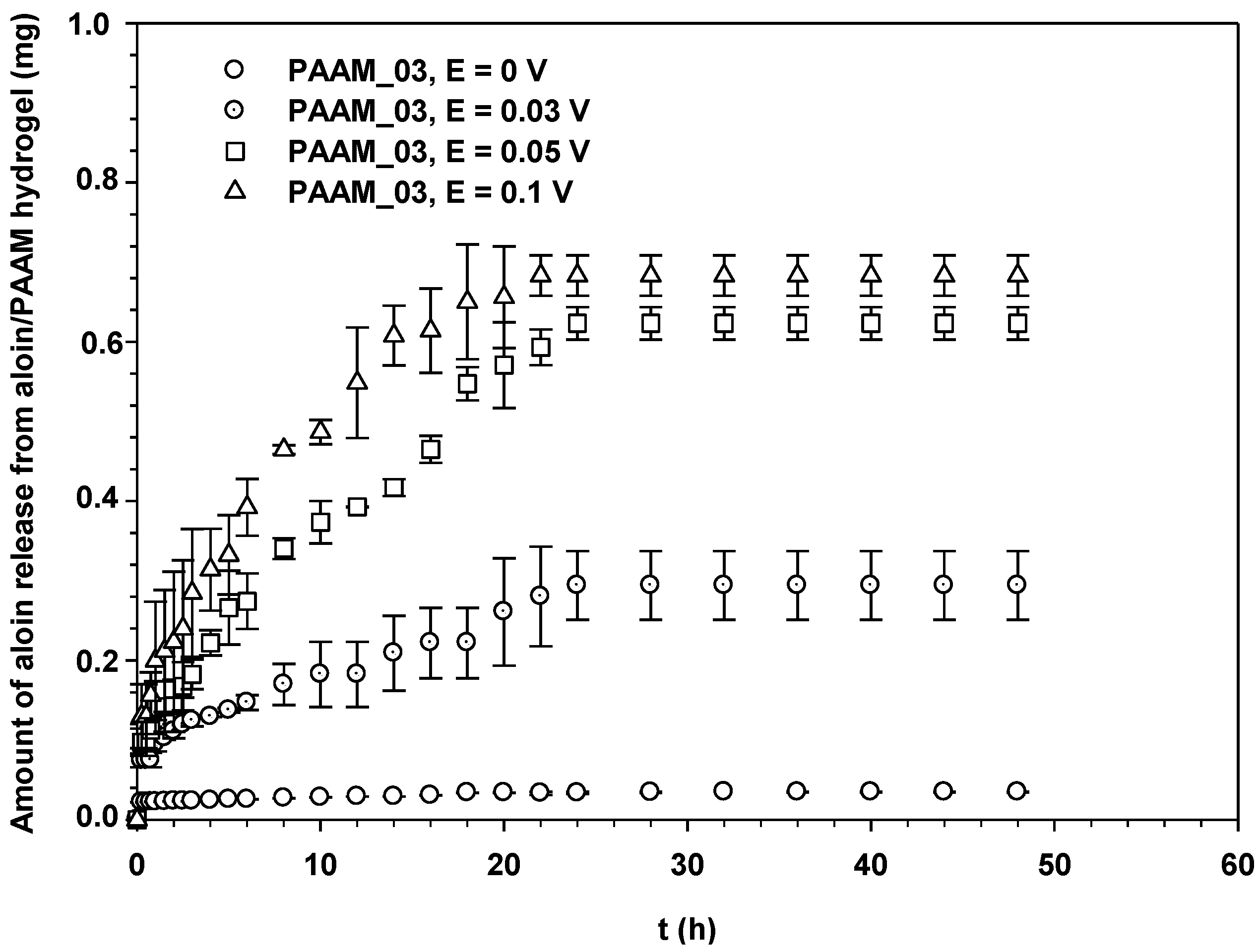
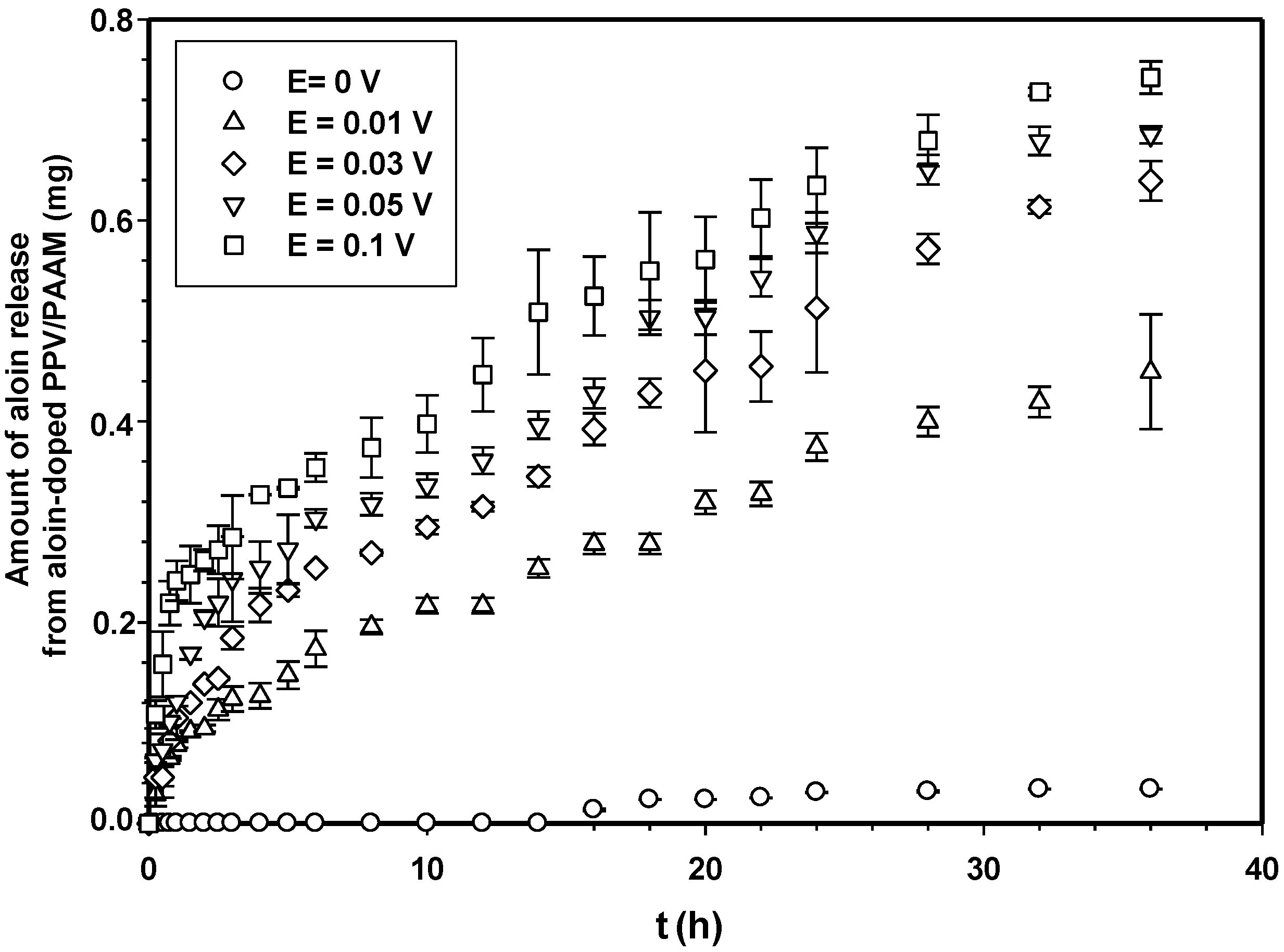
3.3. Drug Release Kinetics
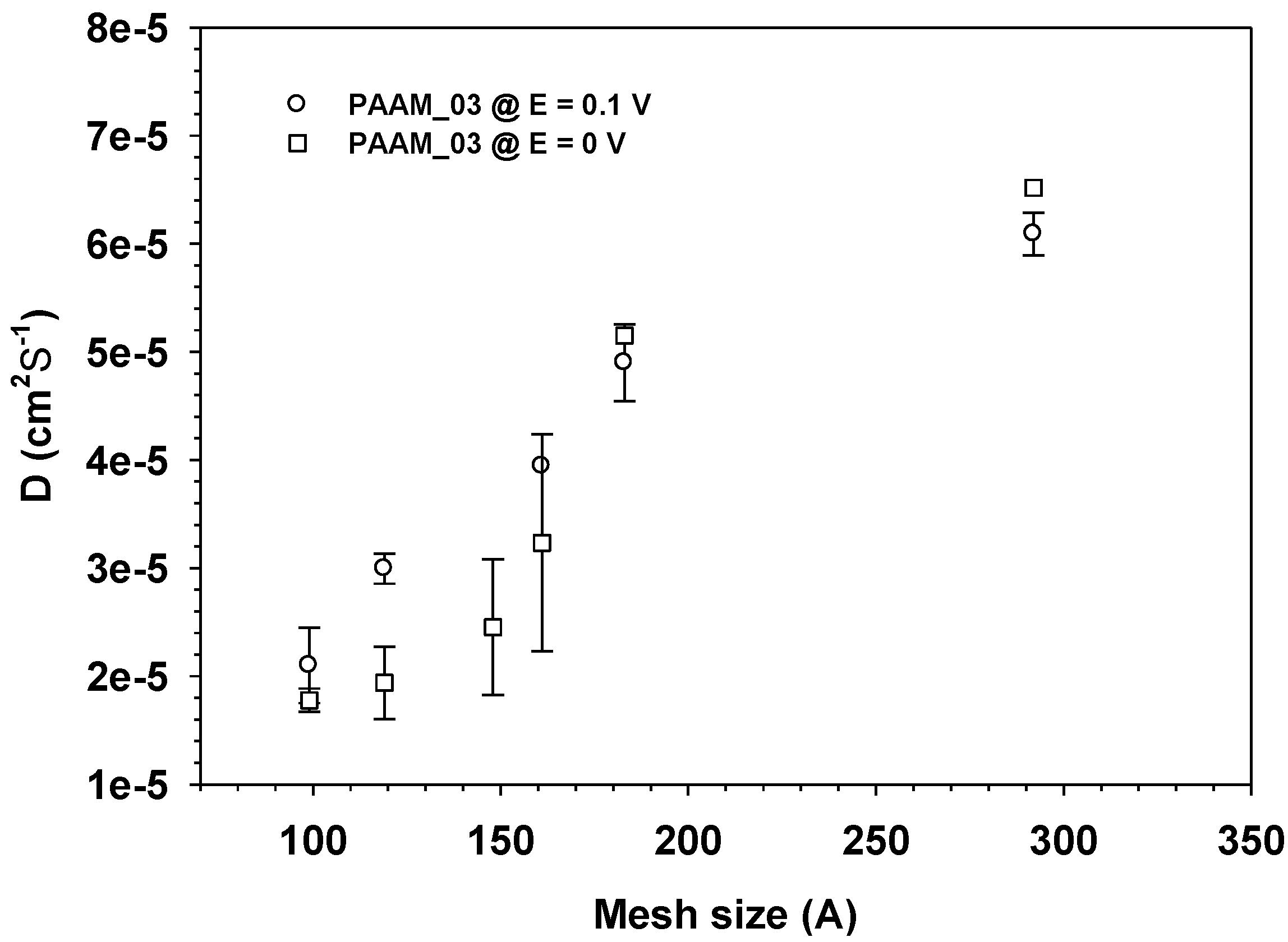
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hamman, J.H. Compositions of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Scheindlin, S. Transdermal drug delivery: Pass, present, future. Mol. Interv. 2004, 4, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.H. Current Status and Future Prospects of Transdermal Drug Delivery. Pharm. Res. 1996, 13, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Kantaria, S.; Rees, G.D.; Lawrence, M.J. Gelatin stabilized microemultion-based organogels: Rheology and application in iontophoretic transdermal drug delivery. J. Cotrol. Release 1999, 60, 355–365. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- George, P.M.; Lavan, D.A.; Burdick, J.A.; Chen, C.Y.; Liang, E.; Langer, R. Electrically controlled drug delivery from biotin doped conductive polypyrole. Adv. Mater. 2006, 18, 577–581. [Google Scholar] [CrossRef]
- Lira, L.M.; Torresi, C. Conducting polymer-hydrogel composites for electrochemical release devices: Systhesis and characterization of semi-interpenetrating polyaniline-polyacrylamide network. Electrochem. Commun. 2005, 7, 717–723. [Google Scholar] [CrossRef]
- Burn, B.P.; Bradley, D.D.C.; Friend, R.H.; Halliday, D.A. Precusor route chemistry and electronic properties of poly(p-phenylene vinylene), Poly[(2,5-dimethyl-p-phenylene)vinylene] and poly[(2,5-dimethoxy-p-phenylene)vinylene]. Chem. Soc. Perkin Trans. 1992, 1, 3225–3231. [Google Scholar] [CrossRef]
- Sanden, M.C.M. Counter-ion induced processibility of conducting Polymers: 1. Acid-assisted oxidative doping and solubilization. Synth. Met. 1997, 87, 137–140. [Google Scholar] [CrossRef]
- Kamonsawas, J.; Sirivat, A.; Niamlang, S.; Hormnirun, P.; Prossanaroon-Ouajai, W. Electrical conductivity response of poly(p-phenylene vinylene)/zeolite composites exposed to ammonium nitrate. Sensors 2010, 10, 5590–5603. [Google Scholar] [CrossRef] [PubMed]
- Niamlang, S.; Sirivat, A. Electric field assisted trandermal drug delivery from salicylic acid-loaded polyacrylamide hydrogels. Drug Deliv. 2009, 16, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Juntanon, K.; Niamlang, S.; Rujiravanit, R.; Sirivat, A. Electrically controlled release of sulfosalicylic acid from crosslinked poly(vinyl alcohol) hydrogel. Int. J. Pharm. 2008, 356, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.S.; Peppas, N.A. Solute diffusion in poly(vinyl alcohol)/(polyacrylic acid) composite membranes prepared by freezing/thawing techniques. Polymer 1997, 38, 5931–5936. [Google Scholar] [CrossRef]
- Másson, M.; Loftsson, T.; Másson, G.; Stefánsson, E. Stef Cyclodextrins as permeation enhancers: Some theoretical evaluations and in vitro testing. J. Cotrol. Release 1999, 59, 107–118. [Google Scholar] [CrossRef]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sairam, M.; Babu, R.; Naidu, V.; Aminabhavi, T. Encapsulation efficiency and controlled release characteristics of crosslinked polyacrylamide particles. Int. J. Pharm. 2006, 320, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Thongchai, N.; Kunanuruksapong, R.; Niamlang, S.; Wannatong, L.; Sirivat, A.; Wongkasemjit, S. Interactions between CO and Poly(p-phenylene vinylene) as Induced by Ion-Exchanged Zeolites. Materials 2009, 2, 2259–2275. [Google Scholar] [CrossRef]
- Valento, A.; Burrows, H.; Miguel, M.; Lobo, V. Diffusion coefficients of sodium dodecyl sulfate in water swollen crosslinked polyacrylamide membranes. Eur. Polym. J. 2000, 38, 2187–2196. [Google Scholar] [CrossRef]
- Li, Y.; Neoh, K.G.; Kang, E.T. Controlled release of heparin from polypyrrole-poly(vinyl alcohol) assembly by electrical stimulation. J. Biomed. Mater. Res. 2005, 73A, 171–181. [Google Scholar] [CrossRef]
- Niamlang, S.; Sirivat, A. Electrically controlled release of salicylic acid from poly(p-phenylene vinylene)/polyacrylamide hydrogels. Int. J. Pharm. 2010, 356, 1–11. [Google Scholar]
- Reichling, J.; Landvatter, U.; Wagner, H.; Kostka, K.; Schaefer, U.F. In vitro studies on release and human skin permeation of Australian tea tree oil (TTO) from topical formulations. Eur. J. Pharm. Niopharm. 2006, 64, 222–228. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Li, W.; Hao, X. A common profile for polymer-based controlled releases and its logical interpretation to general release process. J. Pharm. Pharm. Sci. 2006, 9, 238–244. [Google Scholar] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Niamlang, S.; Buranut, T.; Niansiri, A.; Sirivat, A. Controlled Aloin Release from Crosslinked Polyacrylamide Hydrogels: Effects of Mesh Size, Electric Field Strength and a Conductive Polymer. Materials 2013, 6, 4787-4800. https://doi.org/10.3390/ma6104787
Niamlang S, Buranut T, Niansiri A, Sirivat A. Controlled Aloin Release from Crosslinked Polyacrylamide Hydrogels: Effects of Mesh Size, Electric Field Strength and a Conductive Polymer. Materials. 2013; 6(10):4787-4800. https://doi.org/10.3390/ma6104787
Chicago/Turabian StyleNiamlang, Sumonman, Tawansorn Buranut, Amornrat Niansiri, and Anuvat Sirivat. 2013. "Controlled Aloin Release from Crosslinked Polyacrylamide Hydrogels: Effects of Mesh Size, Electric Field Strength and a Conductive Polymer" Materials 6, no. 10: 4787-4800. https://doi.org/10.3390/ma6104787
APA StyleNiamlang, S., Buranut, T., Niansiri, A., & Sirivat, A. (2013). Controlled Aloin Release from Crosslinked Polyacrylamide Hydrogels: Effects of Mesh Size, Electric Field Strength and a Conductive Polymer. Materials, 6(10), 4787-4800. https://doi.org/10.3390/ma6104787



