Potential Applications of Zeolite Membranes in Reaction Coupling Separation Processes
Abstract
:1. Introduction
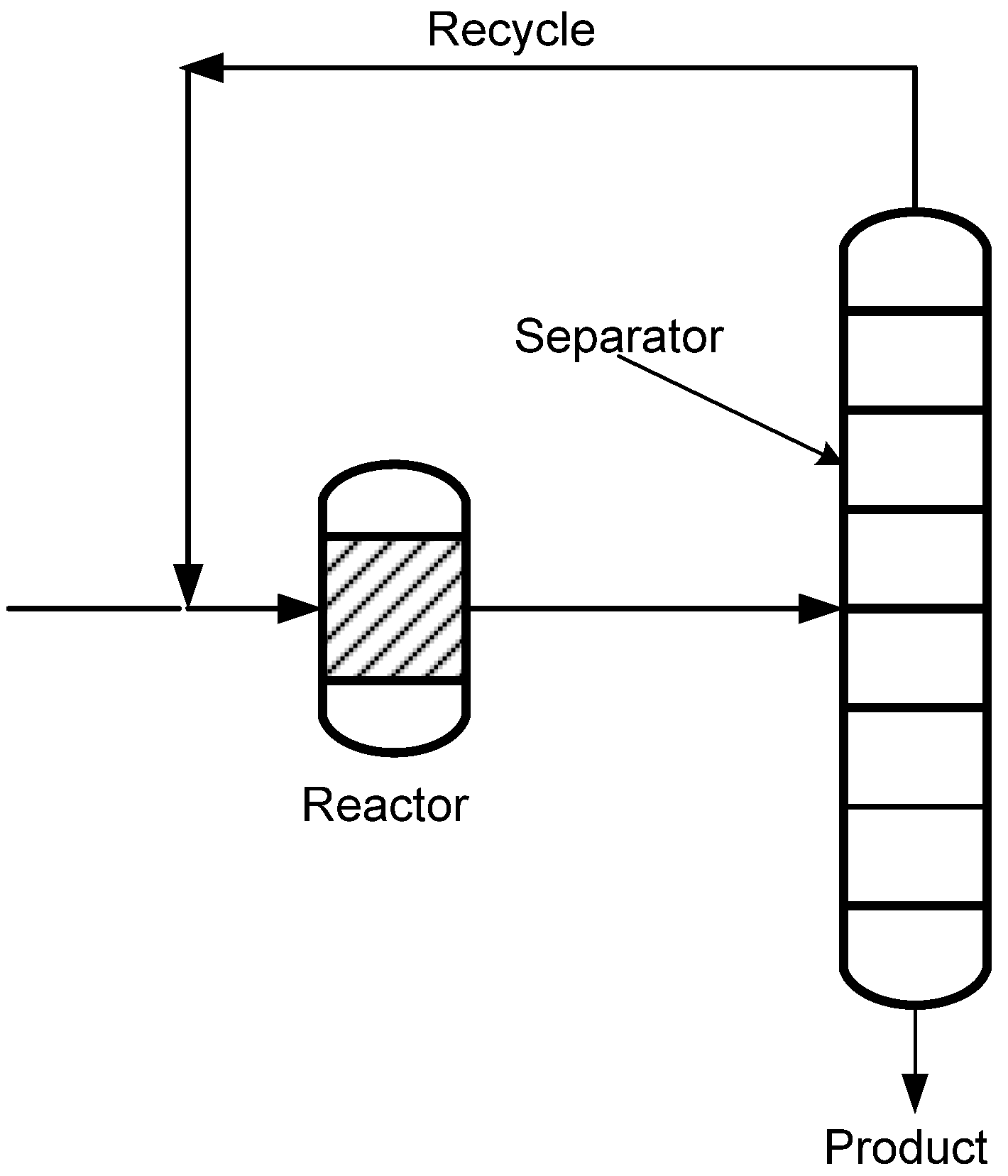
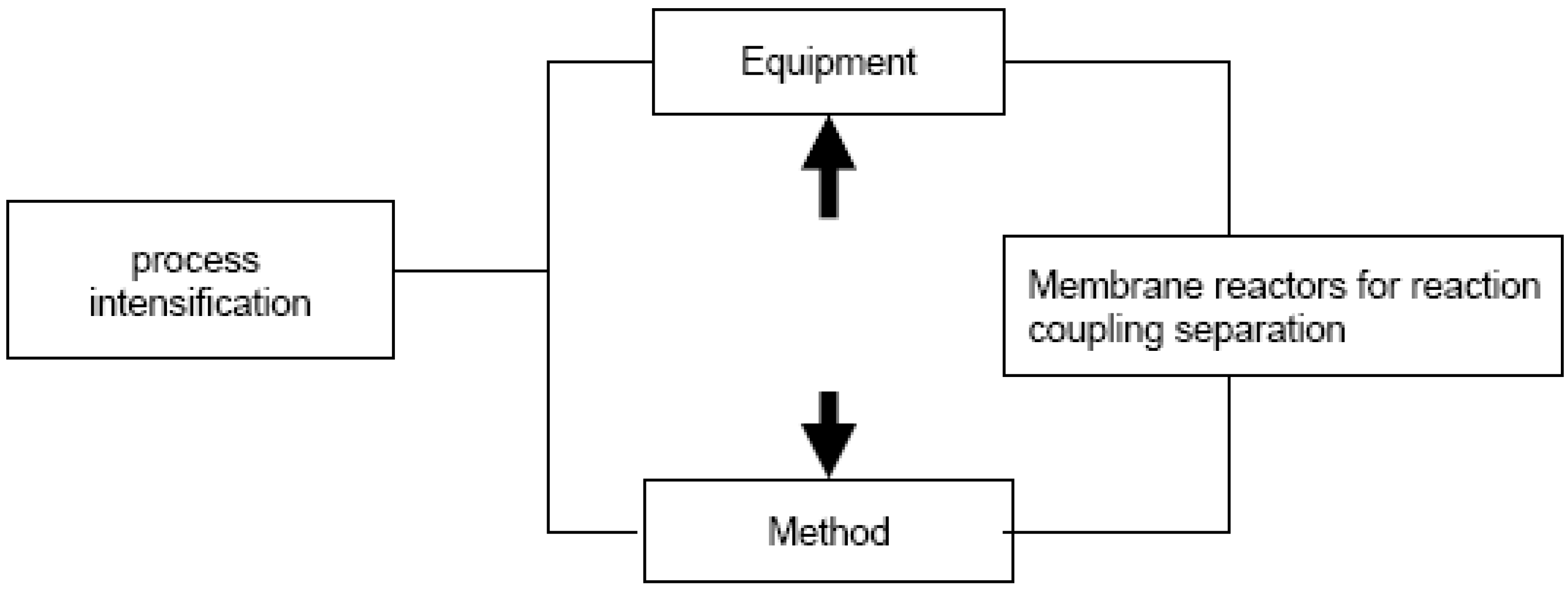
1.1. Process Intensification, Membranes and Membrane Reactors
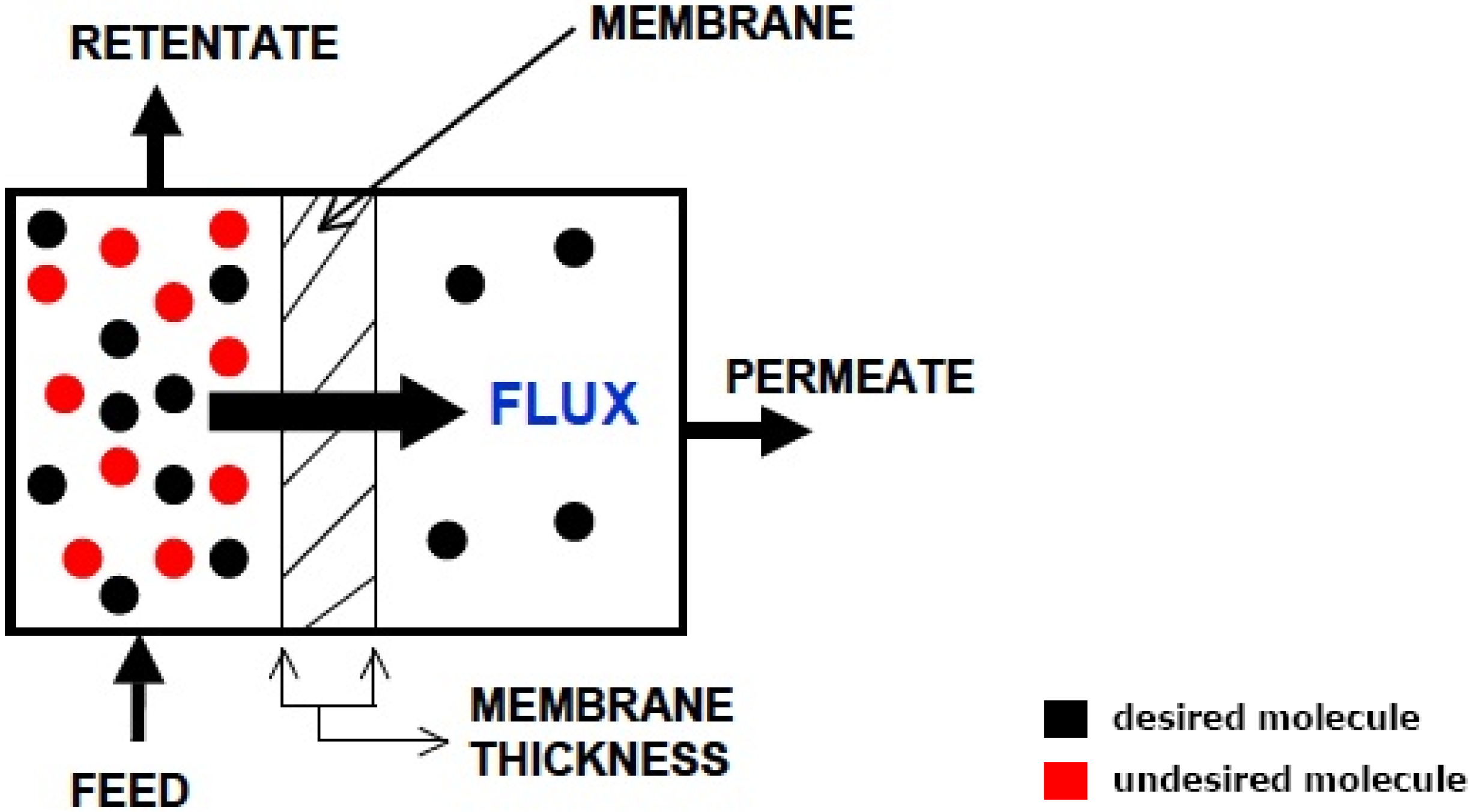
- adsorption of the molecule onto the interface of the high-pressure side of the membrane;
- dissolution of the molecule into the membrane at the interface and diffusion of the molecule through;
- the elution of the molecule from the membrane at the interface;
- desorption of the molecule from the low pressure side of the membrane.
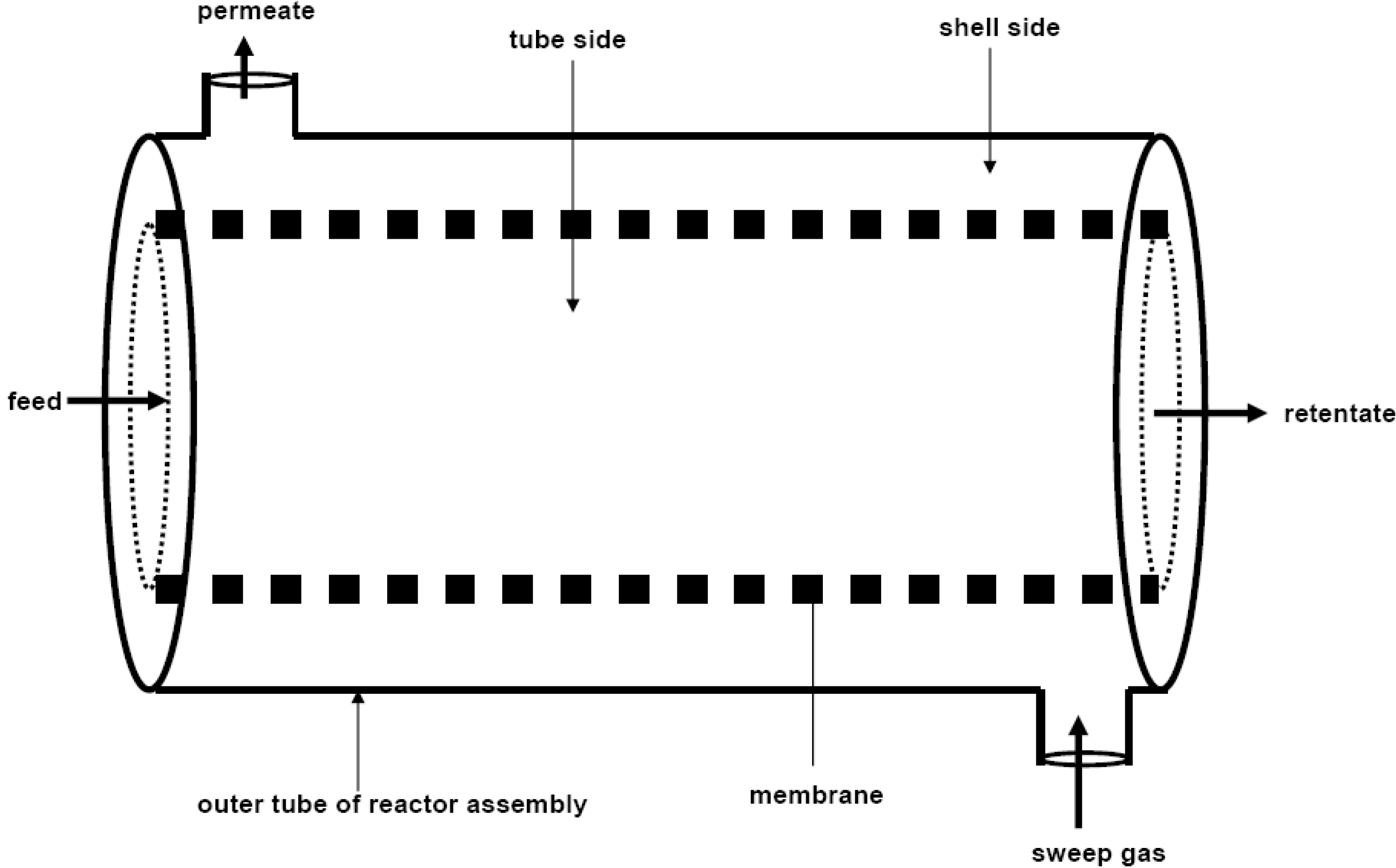
| Membrane system | Reaction | Reference |
|---|---|---|
| Pd-Alloy membrane reactor | Dehydrogenation of hydrocarbons | [6] |
| Pd-Rh foil membrane | Dehydrogenation of cyclohexanediol to pyrocatechol | [7] |
| Pd-Ru-Ni Alloy membrane | Dehydrogenation of isopropanol | [8] |
| Pt/Al2O3-Pd membrane | Dehydrogenation of cyclohexane to benzene | [9] |
| Ceramic membranes | Polymeric membranes |
|---|---|
| Do not swell | Do swell |
| Possibility of uniform, molecular sized pores allowing for molecular sieving | Do not have uniform molecular sized pores |
| Chemically resistant to solvents and low pH | Not chemically stable. Denatured at low pH |
| Thermally stable | Not thermally stable, denatured at high temperature |
| High cost of production | Lower cost of production |
| More brittle | Less brittle |
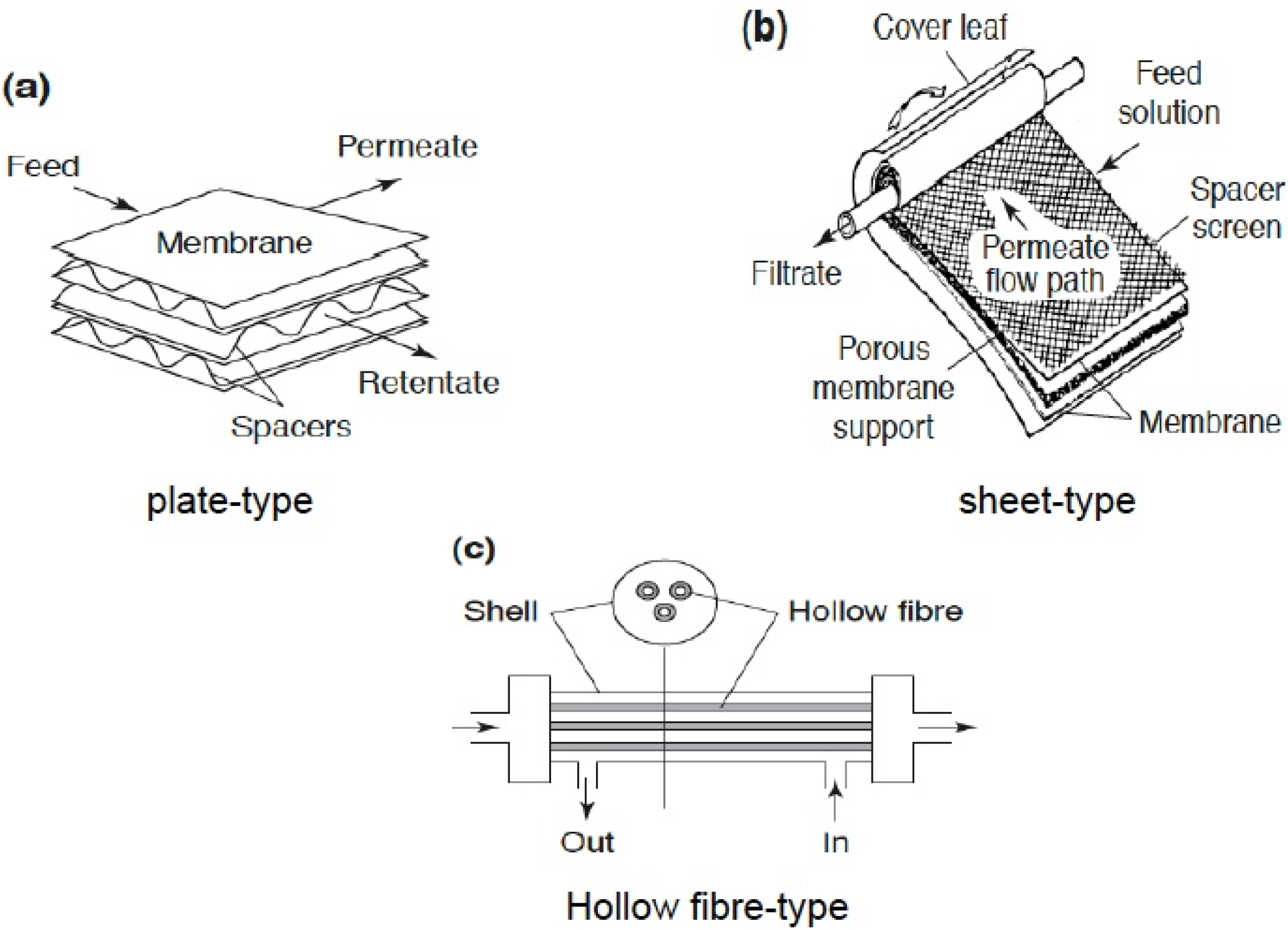
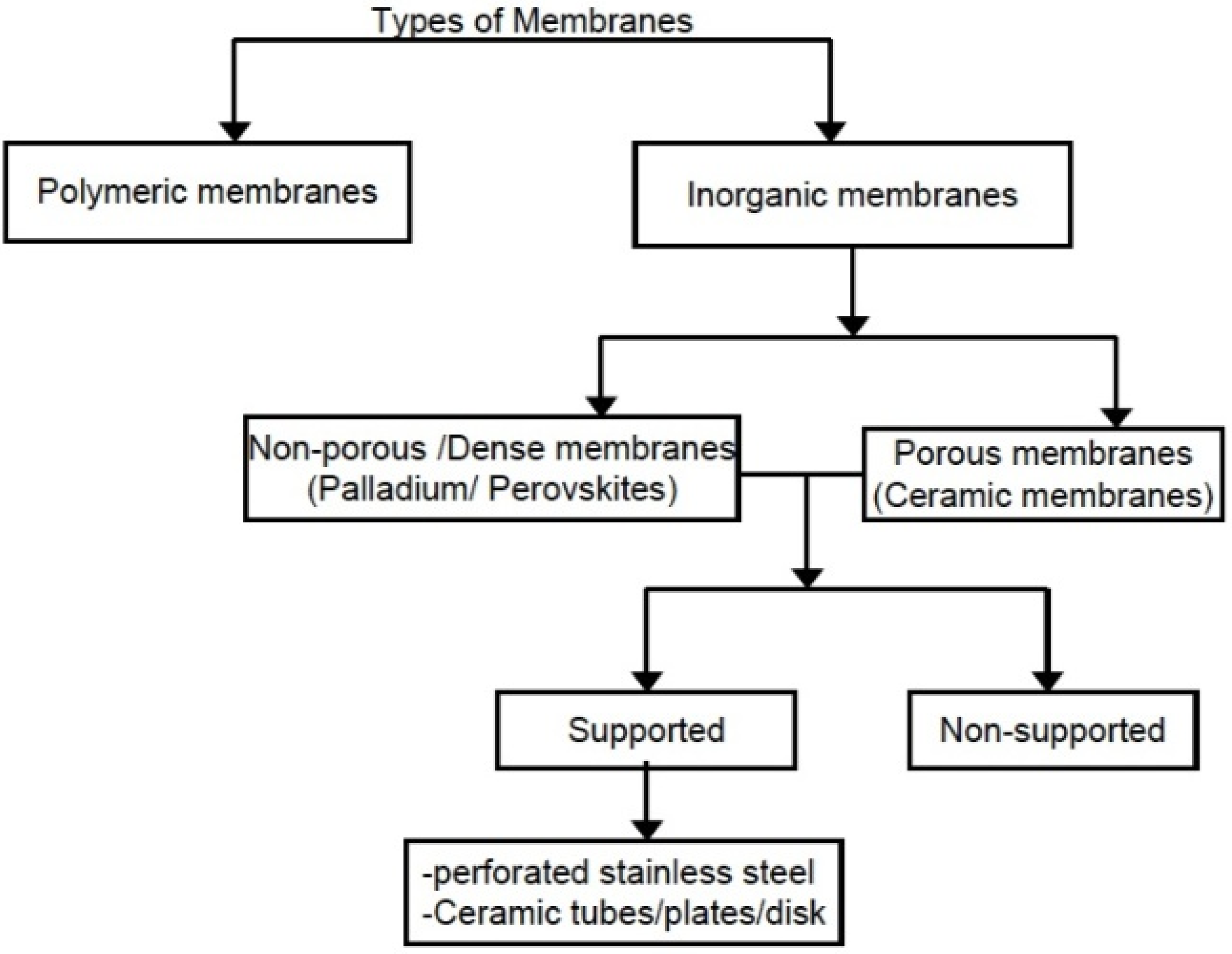
1.2. Zeolite, Zeolite Membranes and Zeolite Membrane Reactors
1.2.1. Zeolites
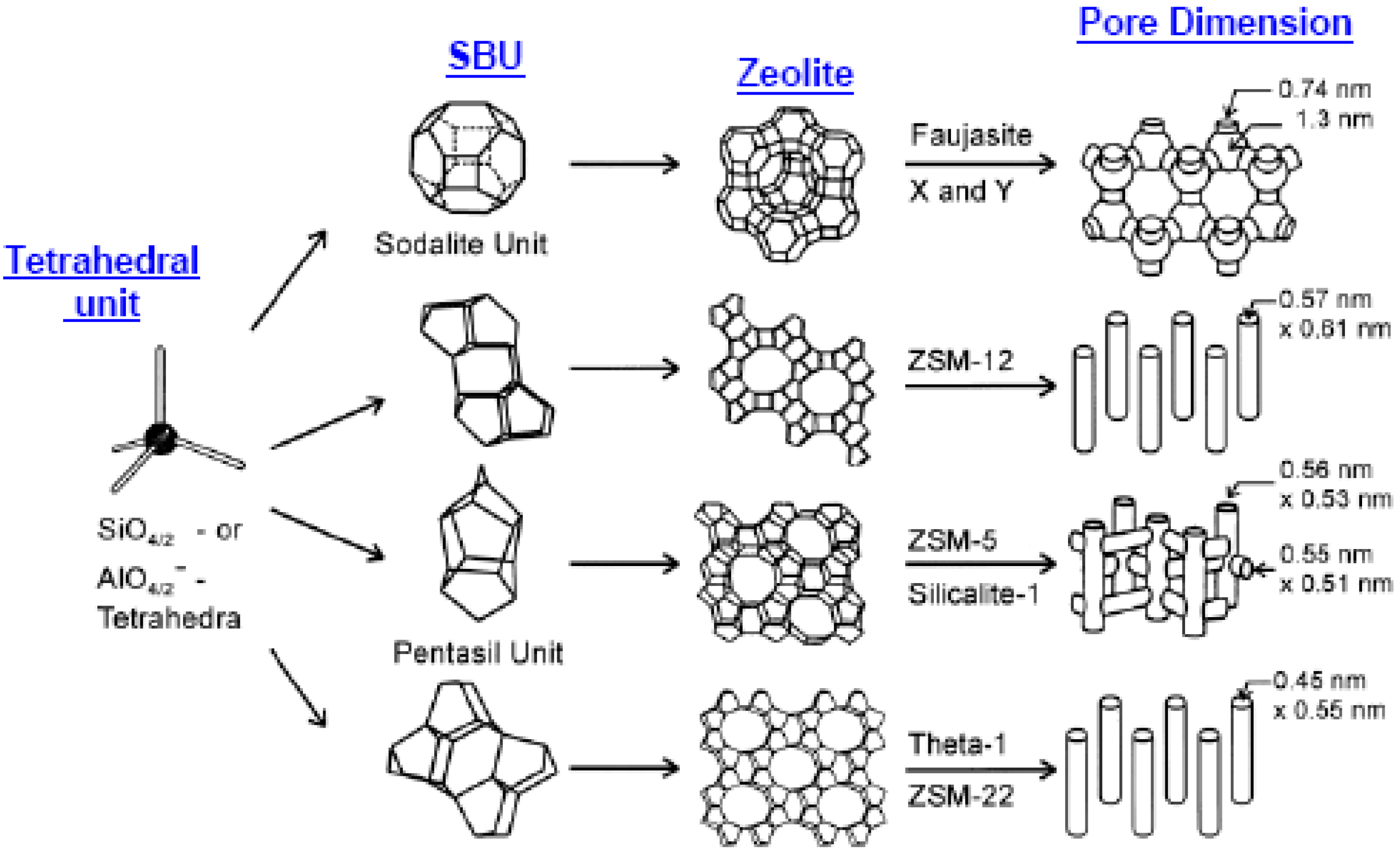

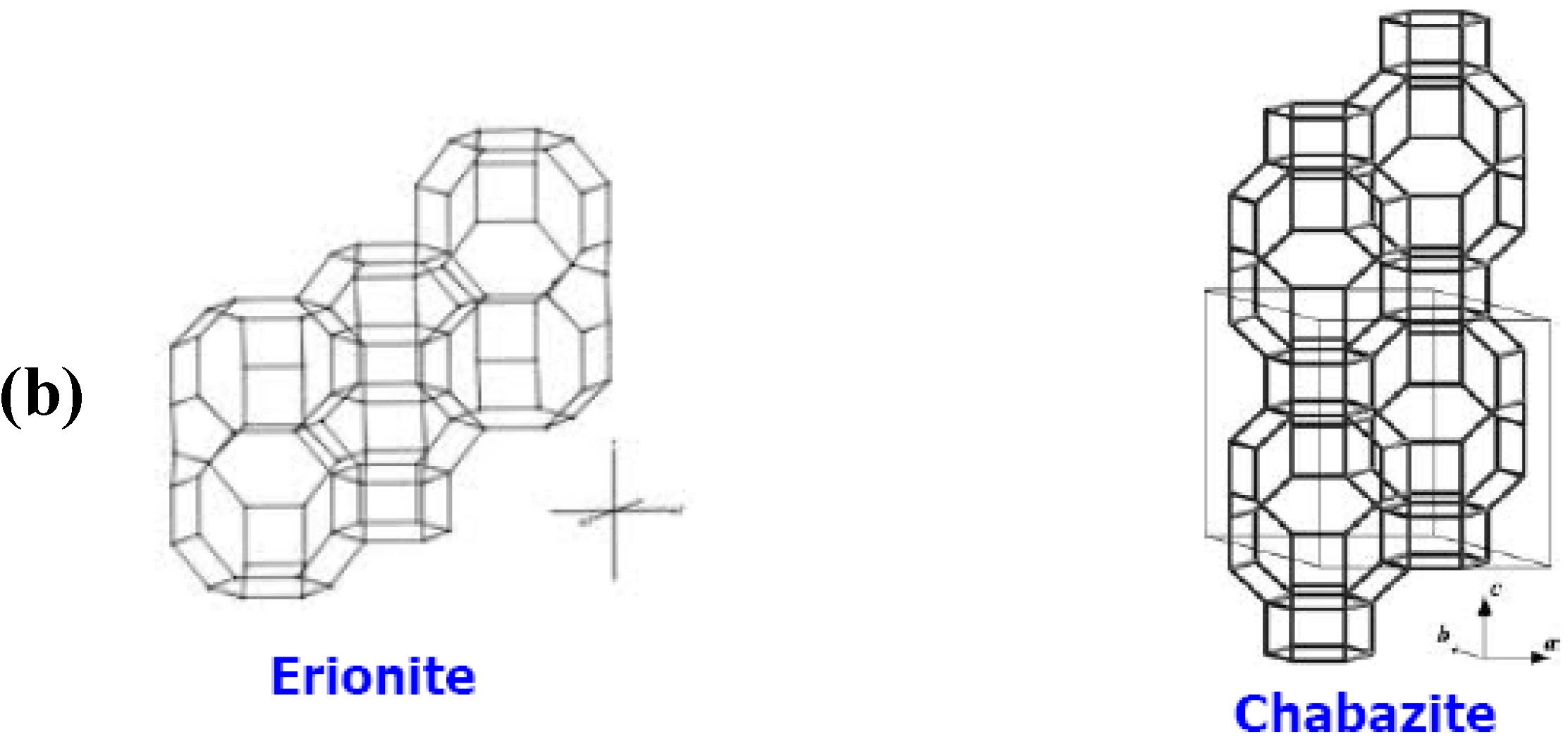
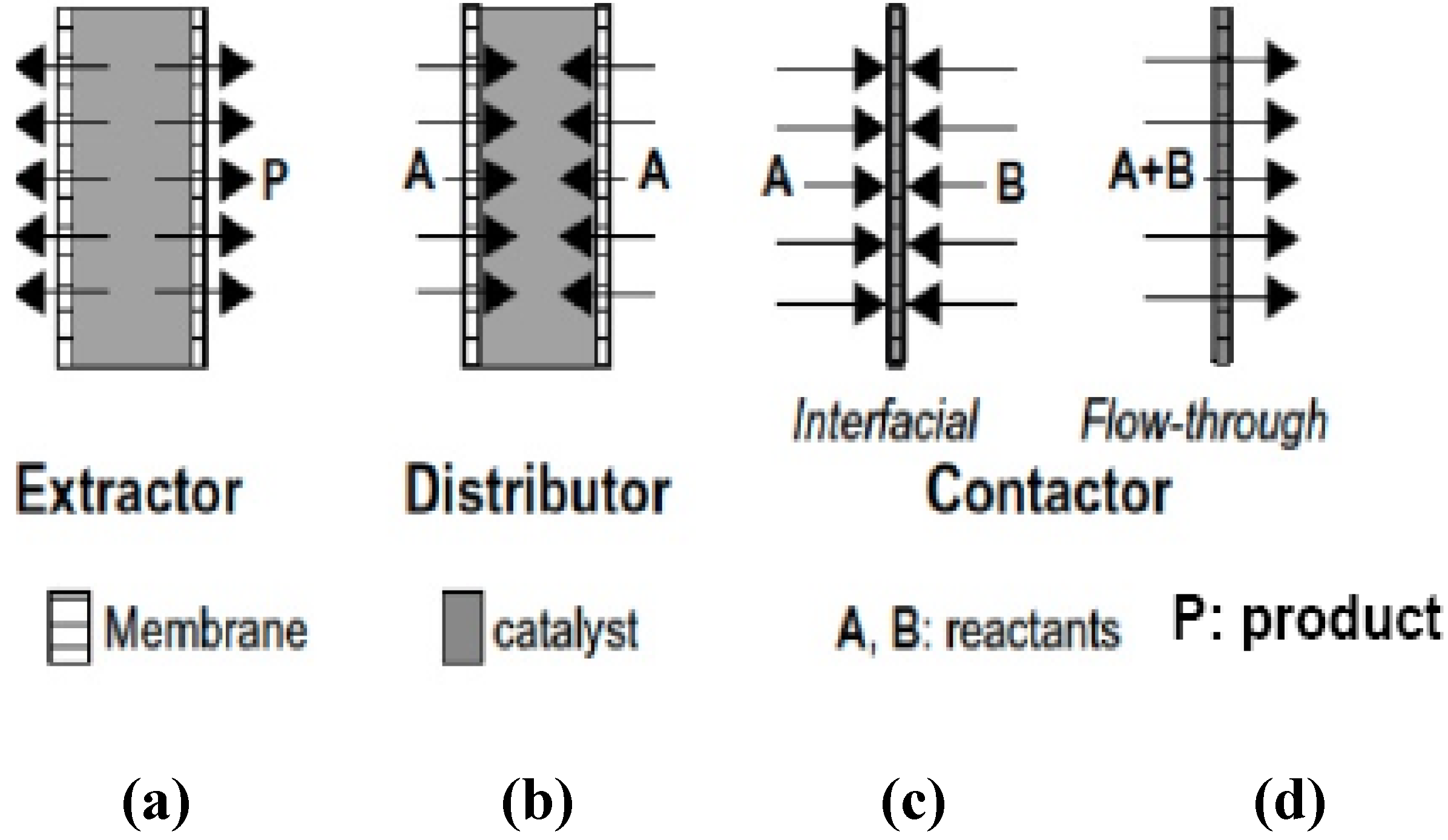
1.2.2. Zeolite Membranes
| Synthesis technique | Description | Reference |
|---|---|---|
| Liquid-phase hydrothermal (LH) synthesis technique (in situ hydrothermal synthesis) (LH) | One-step deposition of a layer containing the Si and Al precursor as a dry amorphous aluminosilicate gel onto a support using sol-gel technique followed by zeolitization under vapor | [75,76,77] |
| Vapor phase transport (VPT) technique | Two-step technique involving coating a support with amorphous gel containing Si and Al , followed by crystallization | [70,71] |
| Secondary seeded growth (SSG) technique | Two-step technique involving initial ex-situ seeding of a support by previously synthesized zeolite crystals followed by hydrothermal crystallization | [78,79,80,81,82] |
| Pore-plugging hydrothermal (PH) synthesis technique | One-stage technique involving growing zeolite crystals within pores of a support until the pores are completely blocked the zeolite materials | [53,54,55,66,67,68] |
- Elimination of seeding technique because the zeolite crystals embedded within the matrix of polymer-zeolite composite, used as supports, serve as seeds;
- Easy formation of uniform crystal distribution, enhancing reproducibility;
- Possibility of obtaining membranes at low temperatures, reducing energy cost;
- Desirable mechanical properties, economical processability of the polymers;
- Unique structure of the dispersed inorganic phase possesses unique structure, good surface chemistry and mechanical strength.
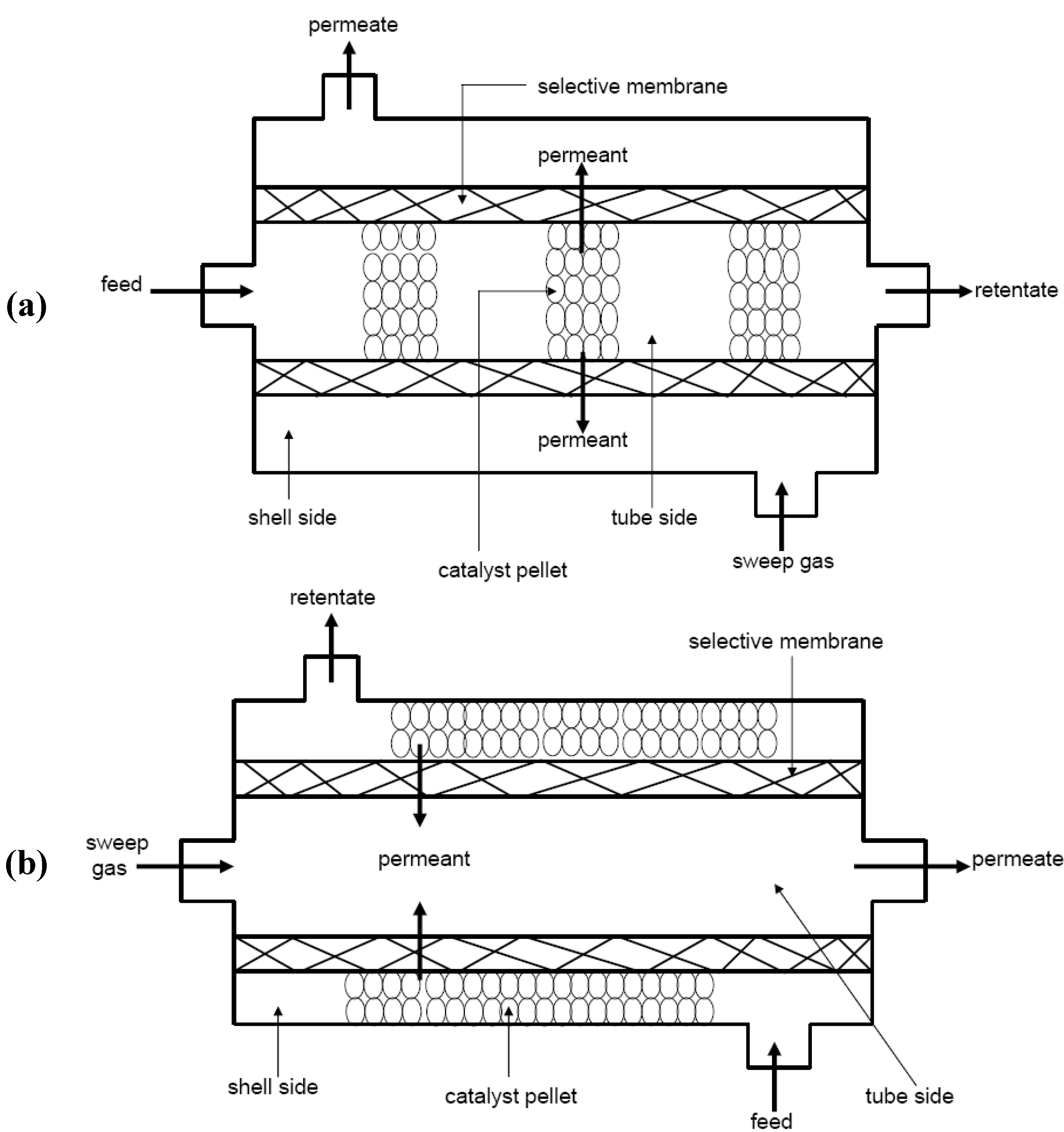
1.2.3. Zeolite Membrane Reactors
- Mole balance in the catalytic bed : Material in – Material out + Generation = Accumulation.
- Rate law that accounts for disappearance of reactant: . Where , the reaction rate; , reaction rate constant; , reactant concentration and , the reaction order.
- Transport law accounting for the transport or flux of product through the membrane: . Where , is the flux of the product through the membrane; , the mass transfer coefficient; and , the concentration gradient across the membrane. It is noteworthy to mention that the transport law takes into account the adsorption-diffusion mechanism that governs the transport of molecule through zeolite membranes.
2. Potential Applications of Zeolite Membrane Reactors in Reactive-Separation
2.1. Synthesis of Chemicals in the Chemical and Petrochemical Industry
- Presence of cheap and high quality membrane supports. In some cases, cheap supports are modified before synthesis or membrane defects healed for enhanced membrane selectivities.
- Optimized membrane synthesis conditions and membrane configuration that could result in very reasonable membrane fluxes. In this regard, the use of a hollow fiber membrane configuration is a promising option [56].
2.2. Potential Applications in the Fuel Cell System
2.3. Application in Selective Removal of Water from Industrial Processes
| Process | Reaction | Reference |
|---|---|---|
| Production of N-Methylpyrrolidone (NMP) from γ-butyrolactone | 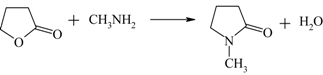 | [122,123] |
| Tetrahydrofuran from 1,4-butanediol | 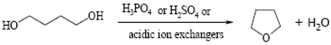 | [124,125,126] |
| Conversion of methanol to a mixture of hydrocarbons in the Mobil process | 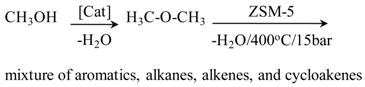 | [127] |
| Dioctylphthalate, (DOP), from phthalic anhydride and 2-ethylhexanol | 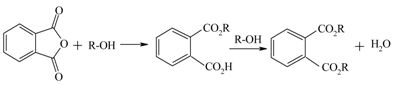 | [128] |
| Glyoxal from ethyleneglycol |  | [129,130,131,132,133] |
| 1,4-Dioxane from diglycol |  | [134,135] |
| Morpholine from diethanolamine | 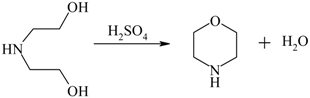 | [136,137,138] |
| Ethylene diamine from monoethanol amine | 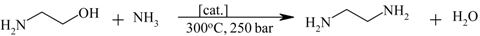 | [139,140,141,142] |
| Esters of ethylene glycol monoalkyl ethers | 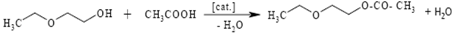 | [143] |
| 2-Vinyl picoline from 2-picoline | 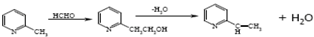 | [144] |
| 2- and 4-Picoline from acetaldehyde and ammonia | 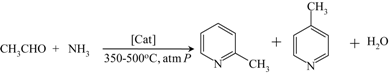 | [145] |
| Anthraquinone from anthracene | 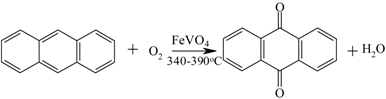 | [146,147] |
| Benzoic acid from toluene | 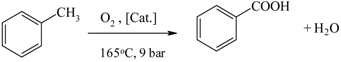 | [148,149] |
| Butene oxidation to maleic anhydride |  | [150,151,152] |
| Sorbitans (monolaurate, monopalmitate, monostearate, etc.) from D- sorbitol and fatty acids | 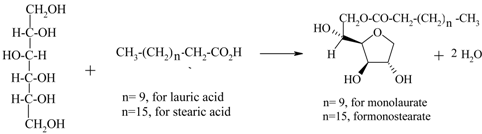 | [153,154,155] |
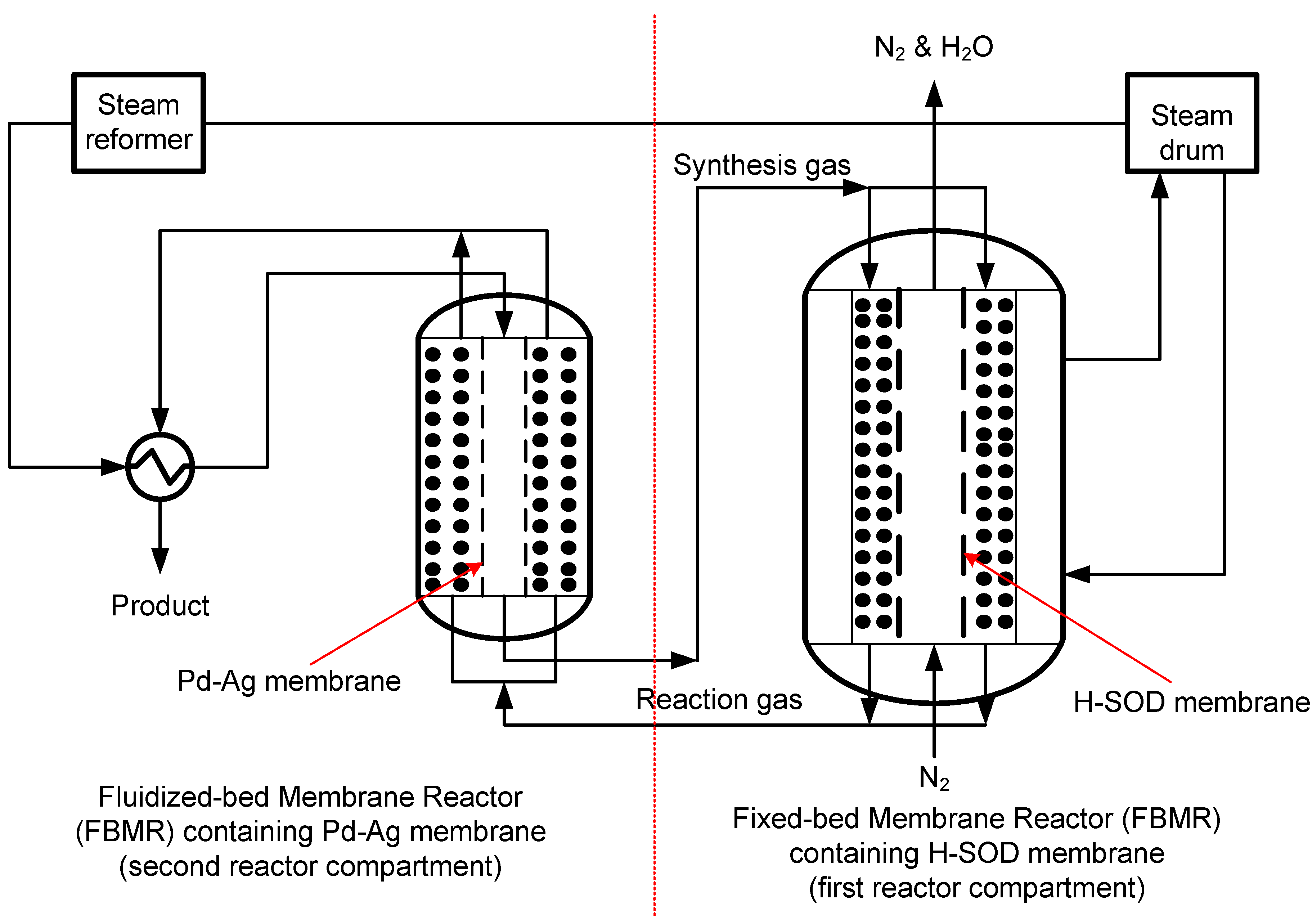
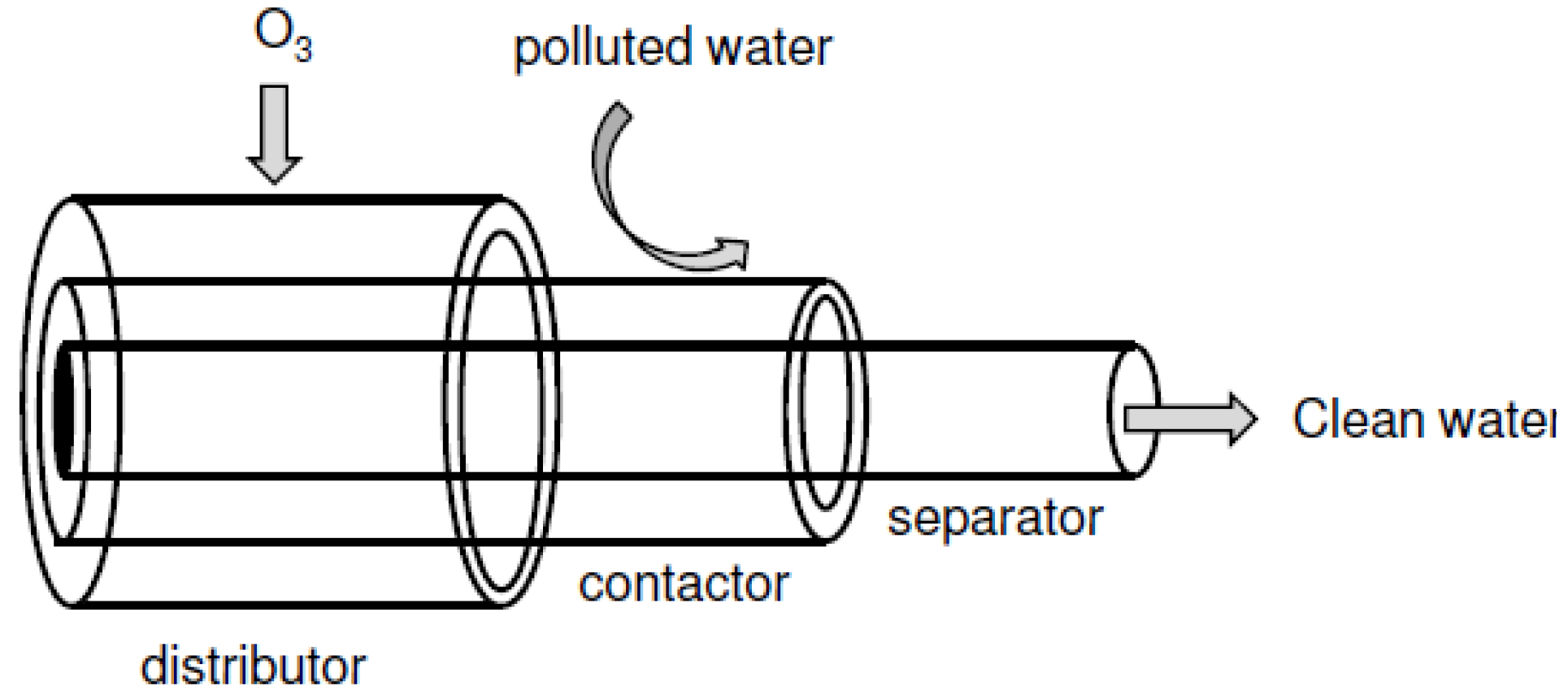
2.4. Application in Water Treatment and Purification Industry
2.5. Application in the Bio-Refinery Industry

3. Conclusions and Future Outlook

- Zeolite membrane synthesis and optimization. Reaction coupling separation using ZCMRs requires highly-selective and defect- free zeolite membrane prepared through a robust and scalable reproducible technique. Also the zeolite membranes should display reasonable membrane flux for commercialization. However to enhance separation and catalytic performance of ZCMRs, factors like geometry and operational conditions, have to be optimized. Recently, further advances in catalytic membrane reactors and reactions have resulted in development of selective zeolite membranes with hollow fiber configurations. These membrane configurations offer great advantages as the hollow structure can be packed with catalysts for catalytic processes with separation occurring simultaneously [183]. In comparison with conventional membranes, hollow fibers have a larger surface area-to-volume ratios >3000 m2/m3 and a thinner membrane wall, resulting in about 30% increase in membrane flux , when compared with membrane tubes fabricated using the same synthesis technique [56]. In addition, several hollow fibers can be made into fiber bundles, thereby reducing both the size and cost of the permeating modules for selective water removal from industrial processes. Therefore, improvement is required in this line to make the incorporation of the catalytic centre into the membranes possible without unnecessarily increasing the thickness of the inorganic supports, promoting permeability without forming pinholes or cracks. At the same time, limitations in terms of uniform temperature control and heat transfer may be overcome.
- Zeolite membrane reactor configuration and reactor analysis. To avoid formation of undesired products in IZCMRs and thus enhance the yield, conversion and overall reactor performance, the reaction rate to membrane flux ratio should approach one. For example in PX isomerization, if the reaction rate > membrane flux (in the case of packed-bed ZCMRs), PX is isomerized to undesirable products like o-xylene and m-xylene. On the other hand, if the membrane flux > reaction rate, isomerization of m-xylene to p-xylene is affected. Therefore a suitable reactor configuration is essential to strike a balance between membrane performance and catalyst performance. In general terms, ZCMRs could be about 10 times more active than IZCMRs provided that the membrane thickness and porous texture, as well as the quantity and location of the catalyst in the membranes are adapted to the reaction kinetics [120,121]. Research efforts are still limited in the development and application of ZCMRs due to challenges in ensuring homogenous distribution of catalytic particles/layer on the membranes.
- Zeolite membrane and zeolite membrane stability. Although zeolite membranes and zeolite membrane reactors can be employed at high temperature and chemically harsh conditions, their long-term thermal stability and operational stability under real operating conditions require significant improvement to attract industrial acceptance. Most of the fabricated zeolite membranes are thermally stable up to 400–500 oC. However, some industrial applications occur at higher temperatures, requiring high thermally stable membranes. Efforts are required to produce such membranes to extend future applications of ZCMRs.
- Techno-economical feasibility and scale-up studies. Techno-economical feasibility studies of ZCMRs are essential. The studies will lucidly elucidate the comparative advantages of the technology over existing conventional technologies. Virtually, all research efforts reported on the development and application of ZCMRs are still limited to laboratory scale studies. In view of this, scale-up studies of the technology are necessary to evaluate the competitiveness of the technology with existing processes to fast-track commercialization of the technology.
Supplementary Files
Supplementary File 1References
- Energy Information Administration. International Energy Outlook 2009; DOE/EIA-0484; Energy Information Administration: Washington, DC, USA, 2009.
- Siirola, J.J. An industrial perspective on process synthesis. AIChE Symp. Ser. 1995, 91, 222–233. [Google Scholar]
- Stankiewicz, A. Reactive separations for process intensification: An industrial perspective. Chem. Eng. Process. 2003, 42, 137–144. [Google Scholar] [CrossRef]
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for membranes and membrane processes. Pure Appl. Chem. 1996, 68, 479–1489. [Google Scholar] [CrossRef]
- Cao, P.; Tremblay, A.Y.; Dubé, M.A.; Morse, K. Effect of membrane pore size on the performance of a membrane reactor for biodiesel production. Ind. Eng. Chem. Res. 2007, 46, 52–58. [Google Scholar] [CrossRef]
- Pfefferle, W.C. Process for Dehydrogenation. US Patent 3,290,406, 6 December 1966. [Google Scholar]
- Sarylova, M.E.; Mischenko, A.P.; Gryaznov, V.M.; Smirnov, V.S. The influence of binary palladium alloys on the route of cyclohexanediol-1,2 transformations. Izv. Akad. Nauk SSSR. Ser. Khim. 1977, 190, 430–432. [Google Scholar]
- Mikahlenko, N.N.; Khrapova, E.V.; Gryaznov, V.M. Dehydrogenation of isopropanol on the membrane catalysts of binary alloys of palladium with ruthenium and nickel. Neftekhimia 1978, 18, 189–192. [Google Scholar]
- Itoh, N. A membrane reactor using palladium. AIChE J. 1987, 33, 1576–1578. [Google Scholar]
- Armor, J.N. Catalysis with permselective inorganic membranes. Appl. Catal. 1989, 49, 1–25. [Google Scholar] [CrossRef]
- Burkhanov, G.S.; Gorina, N.B.; Kolchugina, N.B.; Roshan, N.R. Palladium-based alloy membranes for separation of high-purity hydrogen from hydrogen-containing gas mixtures. Platinum Metals Rev. 2011, 55, 3–12. [Google Scholar] [CrossRef]
- Hershman, A.; Gross, D.E.; Friedman, R.M. Oxidation with Coated Catalyst. US Patent 4,579,689, 1 April 1986. [Google Scholar]
- Friedman, R.M.; Gross, D.E.; Hershman, A.; Jakse, F.P. Permselective catalysis. Appl. Catal. 1984, 11, 147–150. [Google Scholar] [CrossRef]
- Demertzis, M.; Evmiridis, N.P.; Demertzis, M.A.; Vlessidis, A.G. Effect of treatment of synthetic zeolite-polymer membranes on their electrochemical potential response characteristics. Fress. J. Anal. Chem. 1991, 340, 145–152. [Google Scholar] [CrossRef]
- Te Hennepe, H.J.C.; Bargeman, D.; Mulder, M.H.V.; Smolders, C.A. Zeolite-filled silicone rubber membranes: Part 1. Membrane preparation and pervaporation results. J. Membr. Sci. 1987, 35, 39–55. [Google Scholar] [CrossRef]
- Breslau, B.R. Catalytic Process Utilizing Hollow Fiber Membranes. US Patent 4,266,026, 4 May 1981. [Google Scholar]
- Chee, Y.C.; Ihm, S.K. The influence of acid site distribution on the catalytic deactivation of sulfonated poly (styrene-divinylbenzene) embrane catalyst. J. Catal. 1986, 102, 180–189. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes-recent developments and progress. Micropor. Mesopor. Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Julbe, A. Zeolite membranes-synthesis, characterization and application. In Introduction to Zeolite Science and Practice; Cejka, J., van Bekkum, H., Corma, A., Schuth, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 181–219. [Google Scholar]
- Bowen, T.C.; Noble, R.D.; Falconer, J.L. Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci. 2004, 245, 1–33. [Google Scholar] [CrossRef]
- Daramola, M.O. Characterisation and optimisation of an extractor-type catalytic membrane reactor for meta-xylene isomerisation over Pt-HZSM-5 catalyst. PhD Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2010. [Google Scholar]
- Burton, A. Zeolites: Porous architectures. Nat. Mater. 2003, 2, 438–400. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Rey, F.; Valencia, S.; Jorda, J.L.; Rus, J. A zeolite with interconnected 8-, 10- and 12-ring pores and its unique catalytic selectivity. Nature 2003, 2, 493–497. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolite and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Jobic, H.; Karger, J.; Bee, M. Simultaneous measurement of self-and transport diffusivities in zeolites. Phys. Rev. Lett. 1999, 82, 4260–4263. [Google Scholar] [CrossRef]
- Krishna, R. Problems and pitfalls in the use of the Fick formulation for intraparticle diffusion. Chem. Eng. Sci. 1993, 48, 845–861. [Google Scholar] [CrossRef]
- Skoulidas, A.I.; Sholl, D.S. Transport diffusivities of CH4, CF4, He, Ne, Ar, Xe, and SF6 in silicalite from atomistic simulations. J. Phys. Chem. B 2002, 106, 5058–5067. [Google Scholar] [CrossRef]
- Ruthven, D.M. Diffusion of aromatic hydrocarbons in silicalite/HZSM-5. Adsorption 2007, 13, 225–230. [Google Scholar] [CrossRef]
- Muller, G.; Narbeshuber, T.; Mirth, G.; Lercher, J.A. Infrared microscopic study of sorption and diffusion of toluene in ZSM-5. J. Phys. Chem. 1994, 98, 7436–7439. [Google Scholar] [CrossRef]
- Song, L.; Sun, Z.L.; Ban, H.Y.; Dai, M.; Rees, L.V.C. Studies of unusual adsorption and diffusion behaviour of benzene in silicalite-1. Phys. Chem. Chem. Phys. 2004, 6, 4722–4731. [Google Scholar] [CrossRef]
- Krishna, R. Multicomponent surface diffusion of adsorbed species: A description based on the generalized Maxwell-Stefan equations. Chem. Eng. Sci. 1990, 45, 1779–1791. [Google Scholar] [CrossRef]
- Krishna, R.; Wesselingh, J.A. The Maxwell-Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Paschek, D.; Krishna, R. Monte Carlo simulations of sorption and diffusion of isobutane in silicalite. Chem. Phys. Lett. 2001, 342, 148–154. [Google Scholar]
- Reed, D.A.; Ehrlich, G. Surface diffusion, atomic jump rates and thermodynamics. Surf. Sci. 1981, 102, 588–609. [Google Scholar] [CrossRef]
- Shelekhin, A.B.; Dixon, A.G.; Ma, Y.H. Theory of gas diffusion and permeation in inorganic molecular-sieve membranes. AIChE J. 1995, 41, 58–67. [Google Scholar] [CrossRef]
- Krishna, R.; Vlugt, T.J.H.; Smit, B. Influence of isotherm inflection on diffusion in silicalite. Chem. Eng. Sci. 1999, 54, 1751–1959. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Lombardo, E.A.; Avila, A.M. Xylene permeation transport through composite Ba-ZSM-5/SS tubular membranes: Modelling the steady-state permeation. Ind. Eng. Chem. Res. 2008, 47, 2377–2385. [Google Scholar] [CrossRef]
- Giroir-Fendler, A.; Peureux, J.; Mozzanega, H.; Dalmon, J-A. Characterization of a zeolite membrane for catalytic membrane reactor application. Stud. Surf. Sci. Catal. 1996, 101, 127–136. [Google Scholar]
- Zhu, W.; Kapteijn, F.; Moulijn, J.A. Adsorption of light alkanes on silicalite-1: Reconciliation of experimental data and molecular simulations. Phys. Chem. Chem. Phys. 2000, 2, 1989–1995. [Google Scholar] [CrossRef]
- Krishna, R.; Vlugt, T.J.H.; Smit, B. Influence of isotherm inflection on diffusion in silicalite. Chem. Eng. Sci. 1999, 54, 1751–1757. [Google Scholar] [CrossRef]
- Yu, M.; Falconer, J.L.; Noble, R.D.; Krishna, R. Modeling transient permeation of polar organic mixtures through a MFI zeolite membrane using the Maxwell-Stefan equations. J. Membr. Sci. 2007, 293, 167–173. [Google Scholar] [CrossRef]
- Krishna, R. Diffusivity of binary mixtures in zeolites: MD simulations vs. Maxwell-Stefan theory. Chem. Phys. Lett. 2000, 326, 477–484. [Google Scholar] [CrossRef]
- Krishna, R.; Paschek, D. Verification of the Maxwell-Stefan theory for diffusion of three-component mixtures in zeolites. Chem. Eng. J. 2002, 87, 1–9. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A.; Krishna, R. The Generalized Maxwell-Stefan model for diffusion in zeolites: Sorbate molecules with different saturation loadings. Chem. Eng. Sci. 2000, 55, 2923–2930. [Google Scholar] [CrossRef]
- Van de Graaf, J.M.; Kapteijn, F.; Moulijn, J.A. Diffusivities of light alkanes in a silicalite-1 membrane layer. Micropor. Mesopor. Mater. 2000, 35–36, 267–281. [Google Scholar] [CrossRef]
- Avila, A.M.; Bidabehere, C.M.; Sedran, U. Assessment and modelling of adsorption selectivities in the transport of mixtures of hydrocarbons in FCC catalysts. Ind. Eng. Chem. Res. 2007, 46, 7927–7935. [Google Scholar] [CrossRef]
- Mohanty, S.; Davis, H.T.; McCormick, A.V. Shape selective adsorption in atomistic nanopores—A study of xylene isomers in silicalite. Chem. Eng. Sci. 2000, 55, 2779–2792. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Lai, Z.; Tsapatsis, M. Separation of xylene isomer vapors with oriented MFI membranes made by seeded growth. Ind. Eng. Chem. Res. 2001, 40, 544–552. [Google Scholar] [CrossRef]
- Lai, Z.; Tsapatsis, M. Gas and organic vapor permeation through b-oriented MFI membranes. Ind. Eng. Chem. Res. 2004, 43, 3000–3007. [Google Scholar] [CrossRef]
- Gu, X.; Dong, J.; Nenoff, T.M.; Ozokwelu, D.E. Separation of p-Xylene from multicomponent vapor mixtures using tubular MFI zeolite membranes. J. Membr. Sci. 2006, 280, 624–633. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Irusta, S.; Lombardo, E.A. Xylene isomerization in a membrane reactor: Part I: The synthesis of MFI membranes for the p-xylene separation. Chem. Eng. J. 2006, 122, 167–174. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Horowitz, G.I.; Lombardo, E.A. A durable ZSM-5/SS composite tubular membrane for the selective separation of p-Xylene from its isomers. J. Membr. Sci. 2006, 281, 692–699. [Google Scholar] [CrossRef]
- Alshebani, A.; Pera-Titus, M.; Landrivon, E.; Schiestel, T.; Miachon, S.; Dalmon, J.-A. Nanocomposite MFI-ceramic hollow fibres: Prospects for CO2 separation. Micropor. Mesopor. Mater. 2008, 115, 197–205. [Google Scholar] [CrossRef]
- Daramola, M.O.; Burger, A.J.; Pera-Titus, M.; Giroir-Fendler, A.; Lorenzen, L.; Dalmon, J.-A. Xylene vapour mixture separator in nanocomposite MFI-alumina tubular membranes: Influence of operating variables. Sep. Sci. Technol. 2010, 45, 21–27. [Google Scholar] [CrossRef]
- Deng, Z.; Nicolas, C.-H.; Daramola, M.O.; Sublet, J.; Schiestel, Th.; Burger, A.J.; Guo, Y.; Giroir-Fendler, A.; Pera-Titus, M. Nanocomposite MFI-alumina hollow fibre membranes prepared via pore-plugging synthesis: Influence of the porous structure of hollow fibres on the gas/vapour separation performance. J. Membr. Sci. 2010, 364, 1–8. [Google Scholar] [CrossRef]
- Daramola, M.O.; Burger, A.J.; Pera-Titus, M.; Giroir-Fendler, A.; Lorenzen, L.; Miachon, S.; Dalmon, J.-A. Nanocomposite MFI hollow-fibre membranes via pore-plugging synthesis: Prospects for xylene isomer separation. J. Membr. Sci. 2009, 337, 106–112. [Google Scholar] [CrossRef]
- Vlugt, T.J.H.; Krishna, R.; Smit, B. Molecular simulations of adsorption isotherms for linear and branched alkanes and their mixtures in silicalite. J. Phys. Chem. 1999, 103, 1102–1118. [Google Scholar] [CrossRef]
- Calero, S.; Smit, B.; Krishna, R. Configurational entropy effects during sorption of hexane isomers in silicalite. J. Catal. 2001, 202, 395–401. [Google Scholar] [CrossRef]
- Krishna, R.; van Baten, J.M. Influence of isotherm inflection on the diffusivities of C5-C8 linear alkanes in MFI zeolite. Chem. Phy. Lett. 2005, 407, 159–165. [Google Scholar] [CrossRef]
- Krishna, R.; Paschek, D. Separation of hydrocarbon mixtures using zeolite membranes: A modelling approach combining molecular simulations with the Maxwell-Stefan theory. Sep. Puri. Technol. 2001, 21, 111–136. [Google Scholar] [CrossRef]
- Krishna, R.; Paschek, D. Molecular simulations of adsorption and siting of light alkanes in silicalite-1. Phys. Chem. Chem. Phys. 2001, 3, 453–462. [Google Scholar] [CrossRef]
- Krishna, R. Diffusion of binary mixtures across zeolite membranes: Entropy effects on permeation selectivity. Int. Comm. Heat Mass Transfer. 2001, 28, 337–346. [Google Scholar] [CrossRef]
- Marshall, C.E. The use of zeolitic membrane electrodes. J. Phys. Chem. 1939, 43, 1155–1164. [Google Scholar] [CrossRef]
- Marshall, C.E.; Bergman, W.E. The Electrochemical properties of mineral membranes I. The estimation of potassium ion activities. J. Am. Chem. Soc. 1941, 63, 1911–1915. [Google Scholar] [CrossRef]
- Marshall, C.E.; Bergman, W.E. The Electrochemical properties of mineral membranes I. The estimation of potassium ion activities in colloidal clays. J. Phys. Chem. 1942, 46, 52–61. [Google Scholar] [CrossRef]
- Miachon, S.; Landrivon, E.; Aouine, M.; Sun, Y.; Kumakiri, I.; Li, Y.; Pachtova, P.O.; Guilhaume, N.; Giroir-Fendler, A.; Mozzanega, H.; Dalmon, J.-A. Nanocomposite MFI-alumina membranes via pore-plugging synthesis: Preparation and morphological characterization. J. Membr. Sci. 2006, 281, 228–238. [Google Scholar] [CrossRef]
- Miachon, S.; Ciavarella, P.; van Dyk, L.; Kumakiri, I.; Fiaty, K.; Schuurman, Y.; Dalmon, J.-A. Nanocomposite MFI-alumina membranes via pore-plugging synthesis: Specific transport and separation properties. J. Membr. Sci. 2007, 298, 71–79. [Google Scholar] [CrossRef]
- Li, Y.; Pera-Titus, M.; Xiong, G.; Yang, W.; Landrivon, E.; Miachon, S.; Dalmon, J.-A. Nanocomposite MFI zeolite-alumina membranes via pore-plugging synthesis: Genesis of the material. J. Membr. Sci. 2008, 325, 973–981. [Google Scholar] [CrossRef]
- Xu, W.; Dong, J.; Li, J.; Wu, F. A novel method for the preparation of zeolite ZSM-5. J. Chem. Soc. Chem. Commun. 1990, 10, 755–756. [Google Scholar] [CrossRef]
- Matsufuji, T.; Nishiyama, N.; Matsukata, M.; Ueyama, K. Separation of butane and xylene isomers with MFI-type zeolitic membrane synthesized by vapour phase transport method. J. Membr. Sci. 2000, 178, 25–34. [Google Scholar] [CrossRef]
- Bonilla, G.; Vlachos, D.G.; Tsapatsis, M. Simulations and experiments on the growth and microstructure of zeolite MFI films and membranes made by secondary growth. Micropor. Mesopor. Mater. 2001, 42, 191–203. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Production of ultra pure water by desalination of seawater using a hydroxy sodalite membrane. J. Membr. Sci. 2010, 356, 52–57. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Performance of hydroxy sodalite membranes as absolute water selective materials under acidic and basic conditions. J. Membr. Sci. 2010, 356, 1–6. [Google Scholar] [CrossRef]
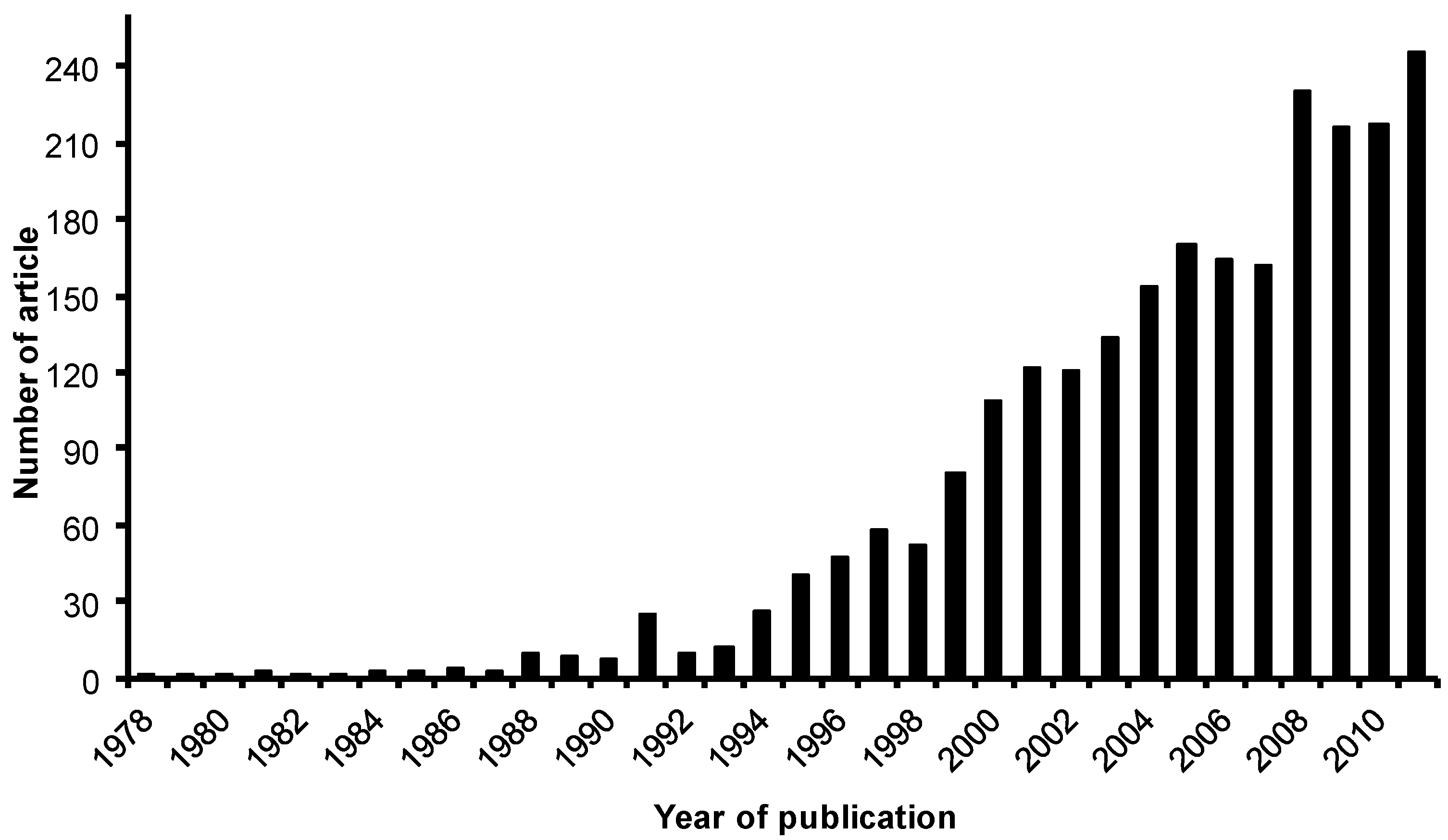
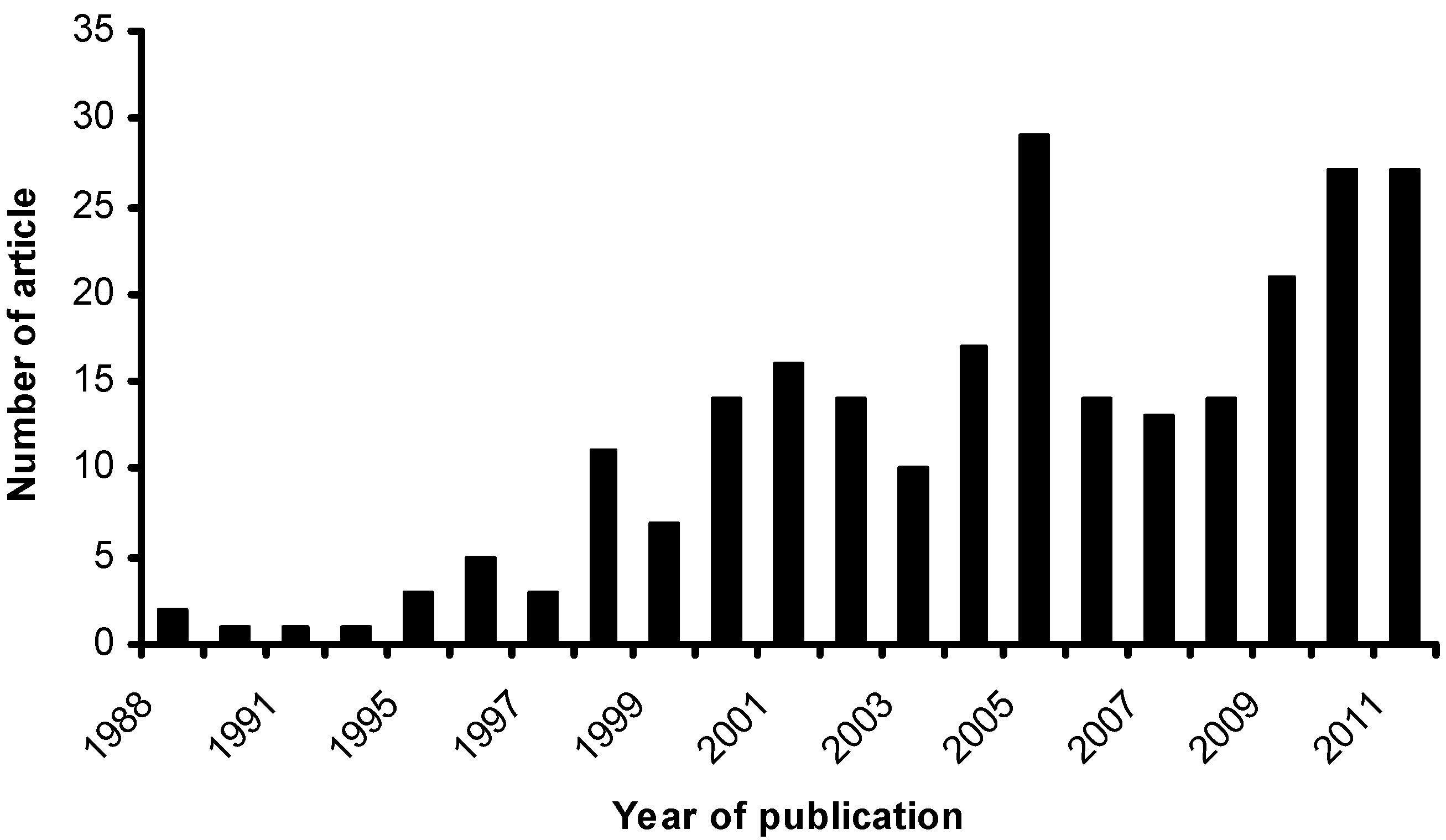
- Scopus Homepgae. Available online: www.scopus.com (accessed 30 June 2012).
- Matsukata, M.; Nishiyama, N.; Ueyama, K. Zeolitic membrane synthesized on a porous alumina support. Chem. Commun. 1994, 3, 339–340. [Google Scholar] [CrossRef]
- Matsukata, M.; Nishiyama, N.; Ueyama, K. Synthesis of zeolites under vapour atmosphere. Effect of synthetic conditions on zeolite structure. Micropor. Mater. 1993, 1, 219–222. [Google Scholar] [CrossRef]
- Davis, B.H.; Singh, K.S.W. Handbook of Porous Solids; Schüth, F., Singh, K.S.W., Weitkamp, J., Eds.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Boudreau, L.C.; Kuck, J.A.; Tsapatsis, M. Deposition of oriented zeolite a films: In situ and secondary growth. J. Membr. Sci. 1999, 152, 41–59. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Tellez, C.; Yeung, K.L.; Ho, K. The role of surface chemistry in zeolite membrane formation. J. Membr. Sci. 2000, 164, 257–275. [Google Scholar] [CrossRef]
- Gouzinis, A.; Tsapatsis, M. On the preferred orientation and microstructural manipulation of molecular sieve films prepared by secondary growth. Chem. Mater. 1998, 10, 2497–2504. [Google Scholar] [CrossRef]
- Hedlund, J.; Noack, M.; Kolsch, P.; Creaser, D.; Caro, J.; Sterte, J. ZSM-5 membranes synthesized without organic organic templates using a seeding technique. J. Membr. Sci. 1999, 159, 263–273. [Google Scholar] [CrossRef]
- Lai, Z.; Bonilla, G.; Diaz, I.; Nery, J.G.; Sujaoti, K.; Amat, M.A.; Kokkoli, E.; Terasak, O.; Thompson, R.W.; Tsapatsis, M.; Vlachos, D.G. Microstructural optimization of a zeolite membrane for organic vapour separation. Science 2003, 300, 456–460. [Google Scholar] [PubMed]
- Daramola, M.O; Burger, A.J.; Miachon, S.; Giroir-Fendler, A.; Lorenzen, L. Extractor-type catalytic membrane reactors having nanocomposite MFI-alumina membrane tube as separation unit: Prospect for ultra-pure p-Xylene production from m-Xylene isomerization over Pt-HZSM-5 catalyst. Appl. Catal. A Gen. 2010, 386, 109–115. [Google Scholar] [CrossRef]
- Daramola, M.O.; Deng, Z.; Pera-Titus, M.; Giroir-Fendler, A.; Burger, A.J.; Lorenzen, L.; Guo, Y. Nanocomposite MFI-alumina membrane prepared via pore-plugging synthesis: Application as packed-bed membrane reactors for m-Xylene isomerization over a Pt-HZSM-5 catalyst. Catal. Today 2010, 156, 261–267. [Google Scholar] [CrossRef]
- Gascon, J.; Kapteijn, F.; Zornoza, B.; Sebastian, V.; Casado, C.; Coronas, J. Practical approach to zeolitic membranes and coatings: state of the art, opportunities, barriers and future perspectives. Chem. Mater. 2012, 24, 2829–2844. [Google Scholar] [CrossRef]
- Maloncy, M.L.; Maschmeyer, T.; Jansen, J.C. Technical and economical evaluation of a zeolite membrane based heptane hydroisomerization process. Chem. Eng. J. 2005, 106, 187–195. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Chemistry of hydrogen separation membranes. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Morigami, Y.; Kondo, M.; Abe, J.; Kita, H.; Okamoto, K. The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane. Sep. Purif. Technol. 2001, 25, 251–260. [Google Scholar] [CrossRef]
- Tennison, S. Current hurdles in the commercial development of inorganic membrane reactors. Membr. Technol. 2000, 128, 4–9. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, Z.; Yan, Y. High performance zeolite NaA membranes on polymer-zeolite composite hollow fibre supports. J. Am. Chem. Soc. 2009, 13, 17056–17057. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Memb. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Fogler, H. Scott. Elements of Chemical Reaction Engineering, 4th ed; Prentice-Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Miachon, S.; Dalmon, J.-A. Catalysis in membrane reactors: What about the catalyst? Top. Catal. 2004, 29, 59–65. [Google Scholar] [CrossRef]
- Hsieh, H.P. Inorganic Membranes for Separation and Reaction; Elsevier: Amsterdam, The Netherland, 1996. [Google Scholar]
- Reif, M.; Dittmeyer, R. Porous, catalytically active ceramic membranes for gas-liquid reactions: A comparison between catalytic diffuser and forced through flow concept. Catal. Today 2003, 82, 3–14. [Google Scholar] [CrossRef]
- Armor, J.N. Applications of catalytic inorganic membrane reactors to refinery products. J. Membr. Sci. 1998, 147, 217–233. [Google Scholar] [CrossRef]
- Deshayes, A.L.; Miro, E.E.; Horowitz, G.I. Xylene isomerization in a membrane reactor: Part II. Simulation of an industrial reactor. Chem. Eng. J. 2006, 122, 149–157. [Google Scholar] [CrossRef]
- Li, Y.; Chang, X.; Zeng, Z. Kinetics study of the isomerization of xylene on HZSM5 zeolite. 1. Kinetics model and reaction mechanism. Ind. Eng. Chem. Res. 1992, 31, 187–192. [Google Scholar] [CrossRef]
- Yeong, Y.F.; Abdullah, A.Z.; Ahmad, A.L.; Bhatia, S. Xylene isomerization kinetic over acid-functionalized silicalite-1 catalytic membranes: Experimental and modelling studies. Chem. Eng. J. 2010, 157, 579–589. [Google Scholar]
- Kumar, S.; Shankar, S.; Shah, P.R.; Kumar, S. A comprehensive model for catalytic membrane reactor. Int. J. Chem. React. Eng. 2006, A5, 1–26. [Google Scholar]
- Daramola, M.O.; Burger, A.J.; Giroir-Fendler, A. Modelling and sensitivity analysis of a nanocomposite MFI-alumina membrane based extractor-type catalytic membrane reactor for m-Xylene isomerization over Pt-HZSM-5 catalyst. Chem. Eng. J. 2011, 171, 618–627. [Google Scholar] [CrossRef]
- Wu, S.; Gallot, J.E.; Bousmina, M.; Bouchard, C.; Kalaguine, S. Zeolite containing catalytic membranes as interphase contactors. Catal. Today 2000, 56, 113–129. [Google Scholar] [CrossRef]
- Kong, C.; Lu, J.; Yang, J.; Wang, J. Catalytic dehydrogenation of ethylbenzene to styrene in a zeolite silicalite-1 membrane reactor. J. Membr. Sci. 2007, 306, 29–35. [Google Scholar] [CrossRef]
- Van de Graaf, J.M.; Zwiep, M.; Kapteijn, F.; .Moulijn, J.A. Application of a silicalite-1 membrane reactor in metathesis reactions. Appl. Catal. A Gen. 1999, 178, 225–241. [Google Scholar] [CrossRef]
- Casanave, D.; Ciavarella, P.; Fiatty, K.; Dalmon, J.-A. Zeolite membrane reactor for isobutane dehydrogenation: Experimental results and theoretical modeling. Chem. Eng. Sci. 1999, 54, 2807–2815. [Google Scholar] [CrossRef]
- Van den Bergh, J.; Gucuyener, C.; Gascon, J.; Kapteijn, F. Isobutane dehydrogenation in DD3R zeolite membrane reactors. Chem. Eng. J. 2011, 166, 368–377. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. 2004, 43, 1029–1036. [Google Scholar] [CrossRef]
- Rahmani, F.; Haghighi, M.; Estifaee, P.; Rahimpour, M.R. A comparative study of two different membranes applied for auto-thermal methanol synthesis process. J. Nat. Gas Sci. Eng. 2012, 7, 60–74. [Google Scholar] [CrossRef]
- Fong, Y.Y.; Abdullah, A.Z.; Ahmad, A.L.; Bhatia, S. Development of functionalized zeolite membrane and its potential role as reactor combined separator for para-xylene production from xylene isomers. Chem. Eng. J. 2008, 139, 172–193. [Google Scholar] [CrossRef]
- Haag, S.; Hanebuth, S.M.; Mabande, G.T.P.; Avhale, A.; Schwieger, W.; Dittmeyer, R. On the use of a catalytic H-ZSM-5 membrane for xylene isomerization. Micropor. Mesopor. Mater. 2006, 96, 168–176. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Horowitz, G.I.; Lombardo, E.A. Xylene isomerization in a ZSM-5/SS membrane reactor. Catal. Lett. 2008, 123, 7–15. [Google Scholar] [CrossRef]
- Van Dyk, L.; Lorenzen, L.; Miachon, S.; Dalmon, J-A. Xylene isomerization in an extractor type catalytic membrane reactor. Catal. Today 2005, 104, 274–280. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, Z.; Gu, X.; Zhong, Z.; Jin, W.; Xu, N. Silicalite-1 zeolite membrane reactor packed with HZSM-5 catalyst for meta-xylene isomerization. Ind. Eng. Chem. Res. 2009, 48, 4293–4299. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, Z.; Chen, J.; Gu, X.; Jin, W.; Xu, N. Catalytic MFI zeolite membranes supported on α-Al2O3 substrates for m-Xylene isomerization. J. Membr. Sci. 2012, 389, 451–458. [Google Scholar] [CrossRef]
- Tweedle, G. The impact of Asian PX/PTA developments on Europe. In Proceedings of the 6th European Aromatics & Derivatives Conference, Antwerp, Belgium, 14–15 November 2007.
- Igarashi, H.; Fajino, T.; Watanabe, M. Hydrogen electrode oxidation on platinum catalysis in the presence of trace CO. J. Electroanal. Chem. 1995, 391, 119–123. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kusakabe, K.; Morooka, S. Selective oxidation of carbon monoxide in hydrogen-rich mixture by permeation through a platinum-loaded Y-type zeolite membrane. J. Membr. Sci. 2001, 190, 1–8. [Google Scholar] [CrossRef]
- Bernardo, P.; Algieri, C.; Barbieri, G.; Drioli, E. Hydrogen purification from carbon monoxide by means of selective oxidation using zeolite catalytic membranes. Sep. Purif. Technol. 2008, 62, 629–635. [Google Scholar] [CrossRef]
- Bosko, M.L.; Munera, J.F.; Lombardo, E.A.; Cornaglia, L.M. Dry reforming of methane in membrane reactors using Pd and Pd-Ag composite membranes on a NaA zeolite modified porous stainless steel support. J. Membr. Sci. 2010, 364, 17–26. [Google Scholar] [CrossRef]
- Harold, M.P.; Lee, C.; Burggraaf, A.J.; Keizer, K.; Zaspalis, V.T.; de Lange, R.S.A. Catalysts with inorganic membranes. MRS Bull. 1994, 19, 34–39. [Google Scholar]
- Zaspalis, V.T.; van Praag, W.; Keizer, K.; van Ommen, J.G.; Ross, J.R.H.; Burggraaf, A.J. Reaction of methanol over catalytically active alumina membranes. Appl. Catal. 1991, 4, 205–222. [Google Scholar]
- Otake, M.; Fukushima, J. Process for Producing Lactams. US Patent 4,731,454, 9 June 1986. [Google Scholar]
- Bertola, A. Process for the production of N-methylpyrrolidone (NMP). US Patent 6,248,902B1, 17 January 2001. [Google Scholar]
- Copenhaver, J.W.; Bigelow, M.H. Acetylene and Carbon Monoxide Chemistry; Reinhold Publishing Co.: New York, NY, USA, 1949. [Google Scholar]
- Reppe, W.; Trieschanann, H.-G. Production of tetrahydrofurane from 1,4-butylene glycol. US Patent 2,251,835, 5 August 1941. [Google Scholar]
- Müller, H.; Palm, C. Preparation of tetrahydrofuran. US Patent 4,588,827, 13 May 1986. [Google Scholar]
- Keil, F.J. Methanol-to-hydrocarbons: process technology. Micropor. Mesopor. Mater. 1999, 29, 49–66. [Google Scholar] [CrossRef]
- Sears, J.K.; Darby, J.R. The Technology of Plasticizers; Wiley and Sons: New York, NY, USA, 1982. [Google Scholar]
- Debus, H. Ueber Einige Oxydationsproducts Des Alcohols (In German). Ann. Chem. Pharm. 1857, 102, 20–24. [Google Scholar] [CrossRef]
- Pincellt, U.; Cadioli, B.; David, D.J. A theoretical study of the electronic structure and conformation of glyoxal. J. Mol. Struct. 1971, 9, 173–176. [Google Scholar] [CrossRef]
- Whipple, E.B. Structure of glyoxal in water. J. Am. Chem. Soc. 1970, 92, 7183–7186. [Google Scholar] [CrossRef]
- Chastrette, F.; Bracoud, C.; Chastrette, M.; Mattioda, G.; Christidis, Y. Study of glyoxal aqeous-solutions by C-13-NMR. Bull. Soc. Chim. Fr. 1983, 1–2, 33–40. [Google Scholar]
- Kliegman, J.M.; Barnes, R.K. Glyoxal derivatives. 5. Reaction of alcohols with glyoxal. J. Org. Chem. 1973, 38, 556–560. [Google Scholar] [CrossRef]
- Sainsbury, M. Rodd’s Chemistry of Carbon Compounds, 2nd ed.; Elsevier: Amsterdam, The Netherland, 1978. [Google Scholar]
- Schecker, H.; Koehler, W.; Sander, B. Production of Anhydrous 1,4-Dioxane. US Patent 3,825 568, 23 July 1974. [Google Scholar]
- Cook, G. Enamines; Marcel Dekker: New York, NY, USA, 1969. [Google Scholar]
- Hampton, B.L.; Pollard, C.B. A new synthesis of morpholine. J. Am. Chem. Soc. 1936, 58, 2338–2339. [Google Scholar] [CrossRef]
- Schroeder, W.; Lengsfeld, W.; Heilen, G.; Hertel, O.; Boettger, G. Preparation of Morpholine. US Patent 4,739,051, 19 April 1988. [Google Scholar]
- Boettger, G.; Hammer, H.-I.; Hertel, O.; Jeschek, G.; Mueller, H.; Scharf, E.; Schoenleben, W. Process for the Production or Reaction of Alkanol Amines. Eur. Pat. 0,036,152B1, 18 May 1983. [Google Scholar]
- Bosche, H.G.; Hammer, H.; Jeschek, G. Process for the Preparation of Colourless Technical Ethanol Amines. Eur. Pat. 0,004,015, 7 January 1981. [Google Scholar]
- Neville, G.D.; Matheson, Y.D. Process for Reducing the Colour of Discoloured Alkanolamines. US Patent 3,207,790, 21 September 1965. [Google Scholar]
- Deeba, M.; Ford, M.E.; Johnson, T.A. Production of Ethylenediamine from Monoethanolamine and Ammonia. US Patent 4,918,233, 17 April 1990. [Google Scholar]
- Carothers, W.H.; Hill, J.W. Studies of polymerization and ring formation. XV. Artificial fibres from synthetic linear condensation superpolymers. J. Am. Chem. Soc. 1932, 54, 1579–1587. [Google Scholar] [CrossRef]
- Katritzky, R.; Boulton, A.J. Advances in Heterocyclic Chemistry; Elsevier: New York, NY, USA, 1968. [Google Scholar]
- Shimizu, S.; Watanable, N.; Kataoka, T.; Shoji, T.; Abe, N.; Morishita, S.; Ichimura, H. Pyridine and pyridine derivatives. In Ullmann’s Encylopedia of Industrial Chemistry; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Vegel, A. Anthraquinone, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Ebke, K.; Ohm, J.; Schroeder, J. Process for the Preparation of Anthraquinone And Anthraquinone-2 Sulphonic Acid from Mother Liquors Obtained in the Alpha,Alpha-Disulphonation of Anthraquinone. DE 3,106,933, 9 September 1982. [Google Scholar]
- Sidi, H.; Hughes, W.G. Process for the Production of Benzoic Acid From Process Residues That Contain Benzyl Benzoate. US Patent 4,281,178, 28 July 1981. [Google Scholar]
- Gizli, A.; Aytimur, G.; Alpay, E.; Atalay, S. Catalytic liquid phase oxidation of toluene to benzoic acid. Chem. Eng. Technol. 2008, 31, 409–416. [Google Scholar] [CrossRef]
- Wrobleski, J.T. Process for the Manufacture of Maleic Anhydride. US Patent 4,456,764, 26 June 1984. [Google Scholar]
- Bosch, H.; Bruggink, A.A.; Ross, J.R.H. Selective oxidation of n-butane to maleic anhydride under oxygen-defficient conditions over V-P-O mixed oxides. Appl. Catal. 1987, 31, 323–337. [Google Scholar]
- Centi, G.; Trifiro, F.; Ebner, J.R.; Franchetti, V.M. Mechanistic aspects of maleic anhydride synthesis from C4 hydrocarbons over phosphorus vanadium oxide. Chem. Rev. 1988, 88, 55–80. [Google Scholar] [CrossRef]
- Albert, R.; Strätz, A.; Vollheim, G. Die katalytische Herstellung von Zuckeralkoholen und deren Verwendung (In German). Chem. Ing. Tech. 1980, 52, 582–587. [Google Scholar] [CrossRef]
- Paterson, S.L.; Fane, A.G.; Fell, C.J.D.; Chun, U.H.; Rogers, P.L. Sorbitol and Gluconate production in a hollow fibre membrane reactor by immobilized. Zymomonas Mobilis Biocatal. Biotransformation 1988, 1, 217–229. [Google Scholar] [CrossRef]
- Kim, D.M.; Kim, H.S. Continuous production of gluconic acid and sorbitol from Jerusalem artchoke and glucose using an oxidoreductase of Zymomonas mobilis and inulinase. Biotechnol. Bioeng. 1992, 39, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Yamanis, J. Production of Anhydrous 1,4-Dioxane. US Patent 4,365,071, 21 December 1982. [Google Scholar]
- Szmuszkovicz, J. Stork enamine reaction: Review. Adv. Org. Chem. 1963, 4, 1. [Google Scholar]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. From Zeolite to Porous MOF Materials; Xu, R., Gao, Z., Chen, J., Yan, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of hydroxy sodalite films as novel water selective. J. Membr. Sci. 2009, 326, 153–160. [Google Scholar] [CrossRef]
- Fernandes, F.A.N. Modeling of fischer-tropsch synthesis in a slurry reactor with water permeable membrane. J. Nat. Gas Chem. 2007, 16, 107–114. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of a sodalite membrane reactor in esterification coupling reaction and separation. Catal. Today 2010, 156, 132–139. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mirvakili, A.; Paymooni, K. A novel water perm-selective membrane dual-type reactor concept for Fischer-Tropsch synthesis of GTL (gas to liquid) technology. Energy 2011, 36, 1223–1235. [Google Scholar] [CrossRef]
- Rohde, M.; Schaub, G.; Khajavi, S.; Jansen, J.C.; Kapteijn, F. Fischer-Tropsch synthesis with in situ H2O removal and directions of membrane development. Micropor. Mesopor. Mater. 2008, 115, 123–136. [Google Scholar] [CrossRef]
- Moteki, T.; Chaittisilp, W.; Sakamoto, Y.; Shimojima, A.; Okubo, T. Role of acidic pretreatment of layered silicate RUB-15 in Its topotactic conversion into pure silica sodalite. Chem. Mater. 2011, 23, 3564–3570. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef] [PubMed]
- Camel, V.; Bermond, A. The use of ozone and associated oxidation processes in drinking water treatment. Water Res. 1998, 32, 3208–3222. [Google Scholar] [CrossRef]
- Huber, M.C.; Canonica, S.; Park, G.Y.; Gunten, U.V. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Ermawati, R.; Morimura, S.; Tang, Y.Q.; Liu, K.; Kida, K. Degradation and behavior of natural steroid hormones in cow manure waste during biological treatments and ozone oxidation. J. Biosci. Bioeng. 2007, 103, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Yeung, K.L.; Julbe, A.; Ayral, A.; Schrotter, J.-C. Preparation of composite zeolite membrane separator/contactor for ozone water treatment. Micropor. Mesopor. Mater. 2008, 115, 139–146. [Google Scholar] [CrossRef]
- Chan, W.K.; Jouet, J.; Heng, S.; Yeung, K.L.; Schrotter, J.-C. Membrane contactor/separator for an advanced ozone membrane reactor for treatment of recalcitrant organic pollutants in water. J. Solid State Chem. 2012, 189, 96–100. [Google Scholar] [CrossRef]
- Ho, H.L.; Chan, W.K.; Blondy, A.; Yeung, K.L.; Schrotter, J.-C. Experimental and modeling of advanced ozone membrane reactor for treatment of organic endocrine disrupting pollutants in water. Catal. Today 2012, in press. [Google Scholar]
- Ghaleb, E. Biorefinery of industrial potato wastes to ethanol by solid state fermentation. Res. J. Agric. Biol. Sci. 2011, 7, 126–134. [Google Scholar]
- Amidon, T.E.; Liu, S. Water-based woody biorefinery. Biotechnol. Adv. 2009, 27, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.P.M.; Clark, J.H.; Harmsen, G.J.; Heeres, H.J.; Heijnen, J.J.; Kersten, S.R.A.; van Swaaij, W.P.M.; Moulijn, J.A. Process intensification in the future production of base chemicals from biomass. Chem. Eng. Proc. 2012, 51, 117–136. [Google Scholar] [CrossRef]
- Sanchez Marcano, J.G.; Tsotsis, T.T. Catalytic Membranes and Membrane Reactors; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Atadashi, I.; Aroua, M.; Abdul Aziz, A.; Sulaiman, N. Membrane biodiesel production and refining technology: A critical review. Renew. Sustain. Energy Rev. 2011, 88, 4239–4251. [Google Scholar]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Roldán, L.; Mallada, R.; Fraile, J.M.; Mayoral, J.A.; Menéndez, M. Glycerol upgrading by ketalization in a zeolite membrane reactor. Asia Pac. J. Chem. Eng. 2009, 4, 279–284. [Google Scholar] [CrossRef]
- De la Iglesia, Ó.; Mallada, R.; Menéndez, M.; Coronas, J. Continuous zeolite membrane reactor for esterification of ethanol and acetic acid. Chem. Eng. J. 2007, 131, 35–39. [Google Scholar] [CrossRef]
- Babajide, O.; Musyoka, N.; Petrik, L.; Ameer, F. Novel zeolite Na-X synthesized from fly ash as a heterogeneous catalyst in biodiesel production. Catal. Today 2012, 190, 54–60. [Google Scholar] [CrossRef]
- Lammens, T.M.; Franssen, M.C.R.; Scott, E.L.; Sanders, J.P.M. Synthesis of biobased N-methylpyrrolidone by one-pot cyclization and methylation of γ-aminobutyric acid. Green Chem. 2010, 12, 1430–1436. [Google Scholar] [CrossRef]
- Pantazidis, A.; Dalmon, J-A.; Mirodatos, C. Oxidative dehydrogenation of propane on catalytic membrane reactors. Catal. Today 1995, 25, 403–408. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual high throughput screening confirmed experimentally: porous coordination polymer hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef] [PubMed]
- Couck, S.; Denayer, J.F.M.; Baron, G.V.; Remy, T.; Gascon, J.; Kapteijn, F. An amine-functionalized MIL-53 metal organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 2009, 131, 6326–6327. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Daramola, M.O.; Aransiola, E.F.; Ojumu, T.V. Potential Applications of Zeolite Membranes in Reaction Coupling Separation Processes. Materials 2012, 5, 2101-2136. https://doi.org/10.3390/ma5112101
Daramola MO, Aransiola EF, Ojumu TV. Potential Applications of Zeolite Membranes in Reaction Coupling Separation Processes. Materials. 2012; 5(11):2101-2136. https://doi.org/10.3390/ma5112101
Chicago/Turabian StyleDaramola, Michael O., Elizabeth F. Aransiola, and Tunde V. Ojumu. 2012. "Potential Applications of Zeolite Membranes in Reaction Coupling Separation Processes" Materials 5, no. 11: 2101-2136. https://doi.org/10.3390/ma5112101
APA StyleDaramola, M. O., Aransiola, E. F., & Ojumu, T. V. (2012). Potential Applications of Zeolite Membranes in Reaction Coupling Separation Processes. Materials, 5(11), 2101-2136. https://doi.org/10.3390/ma5112101





