Polymer-Nanoparticle Composites: From Synthesis to Modern Applications
Abstract
:1. Introduction
- optical and magnetic properties
- microelectronic devices
- piezoelectric actuators and sensors
- electrolytes, anodes in lithium-ion-batteries and supercapacitors
- organic solar cells and intrinsic conductive polymers
- photoresists used in microelectronics and microsystems technologies
- biomedical sciences.
- electrical and thermal conductivity
- polymer phase behavior and thermal stability
- mechanical properties like stiffness, Young’s modulus, wear, fatigue, and others
- flame retardancy [9]
- density
- physical properties such as magnetic, optic, or dielectric properties.
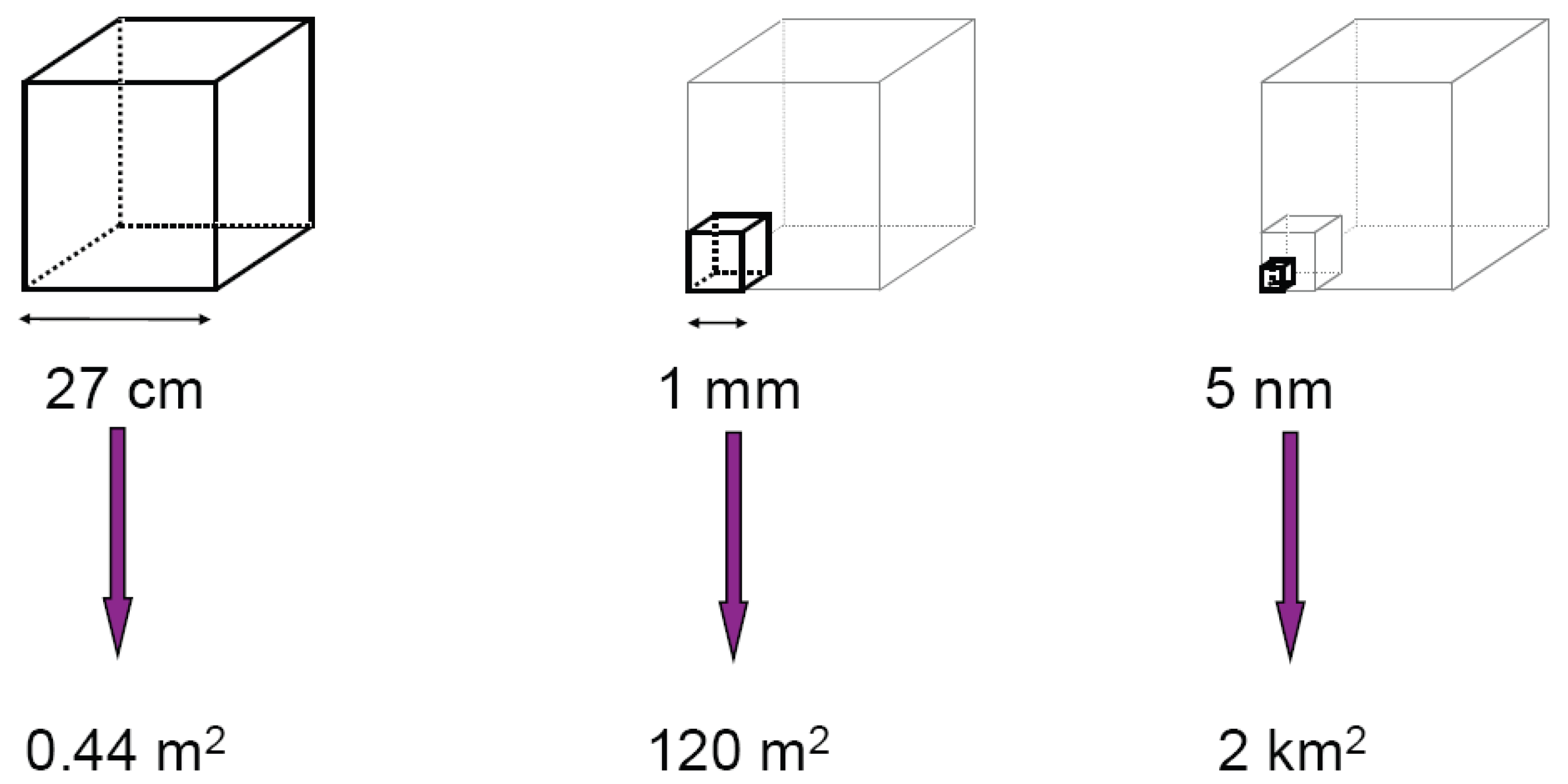
2. Special Features of Nanoparticles
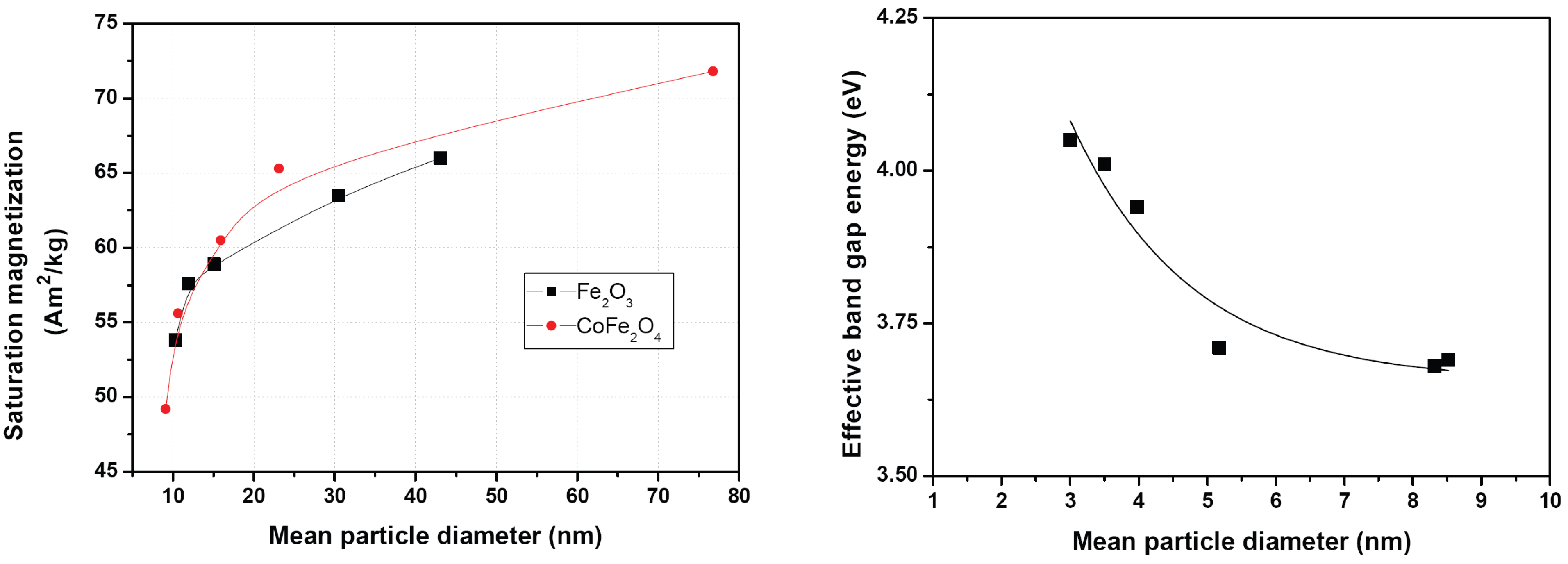
2.1. Particle size dependent properties of inorganic nanoparticles
2.2. Polymer-nanoparticle interface
3. Composite Types
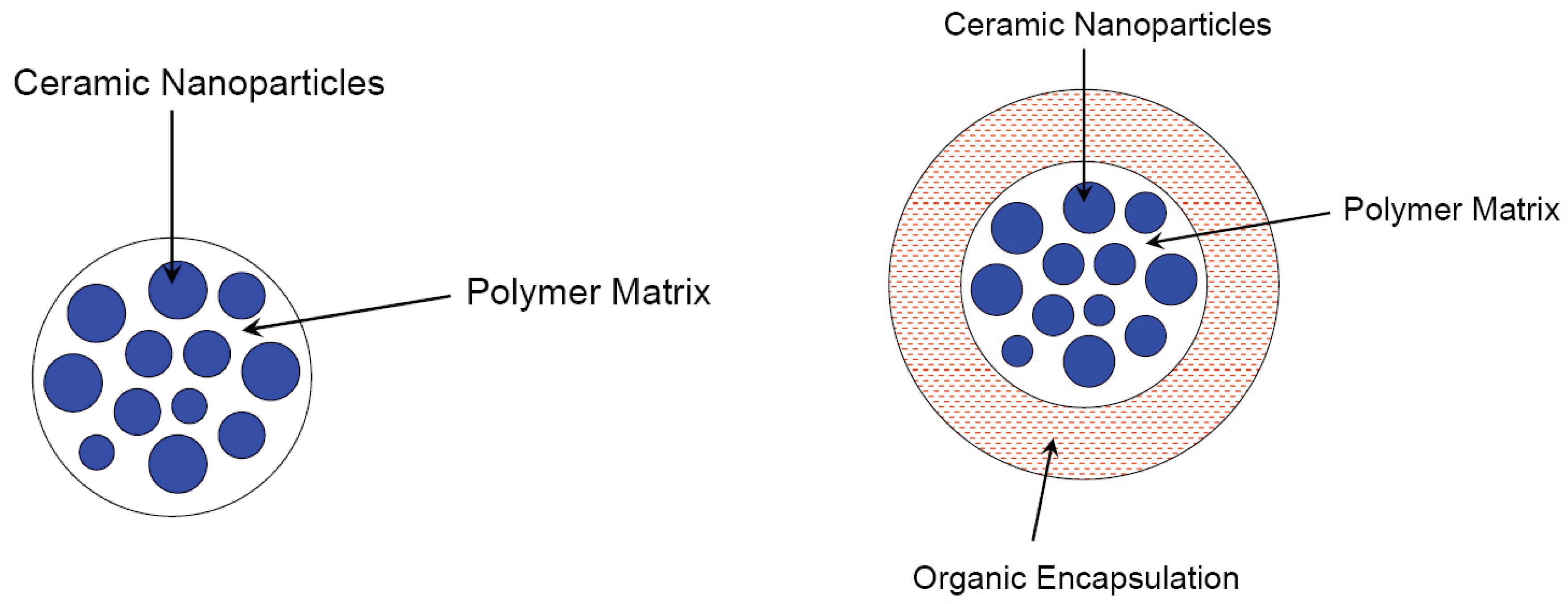
3.1. Polymer-matrix composites
3.2. Composite nanoparticles
| Core | Shell | Synthesis Method | Ref. |
|---|---|---|---|
| Metal-oxides | Polymerizable | Microwave Plasma plus in situ coating | [39] |
| HfO2, ZrO2, ZnO, Fe2O3, TiO2, Al2O3 | MMA; Fluoropolymers | Microwave Plasma plus in situ coating | [52] |
| Fe2O3 | Modified PMMA | Microwave Plasma plus in situ coating | [40] |
| Fe2O3 | Initiator plus styrene | Complex | [55] |
| Al2O3 | Polyacrylic acid (PAA) | Commercial nanoparticles, layer by layer deposition with controlled polymer adsorption | [42] |
| Al2O3 | Polyethylene (PE) | In situ Chemical Vapor Synthesis | [50] |
| Al2O3 | Pyrrole | Ex situ deposition using plasma polymerization | [49] |
| ZrO2 | PE | Ex situ by inductively coupled plasma polymerization | [51] |
| TiO2 | PMMA | Ex situ deposition on commercial, nanoparticles by mixing with MMA solution and irradiation with electron beam | [47] |
| TiO2 | PMMA | Ex situ by plasma polymerization | [46] |
| TiO2 | Polystyrene (PS) | Ex situ by radical polymerization | [44] |
| SiO2 | PS | SiO2 by Stöber synthesis; surface modification with coupling reagent; polymerization | [43] |
| SiO2 | Acrylate based polymers | In situ Chemical Vapor Synthesis | [48] |
| ZnO | Acrylic acid | Ex situ deposition using plasma polymerization | [45] |
| Fe3O4 | ε-Caprolactone | Fe3O4 by alkaline hydrolysis, followed by surface functionalization with ultrasound; surface initiated ring opening polymerization | [53] |
| Fe3O4 | ε-Caprolactone | Fe3O4 by alkaline hydrolysis, followed by surface functionalization; graft polymerization using microwaves | [54] |
| Core | Shell | Synthesis Method | Ref. |
|---|---|---|---|
| TiO2 | C | Emulsion polymerization plus heat treatment | [56] |
| C (micro-sized) | SnO2 | Sol-gel, using commercial graphite | [57] |
| SnO2 | C | Thermal evaporation | [58] |
| SnO2 | C | One-pot solvothermal synthesis and subsequent calcination | [59] |
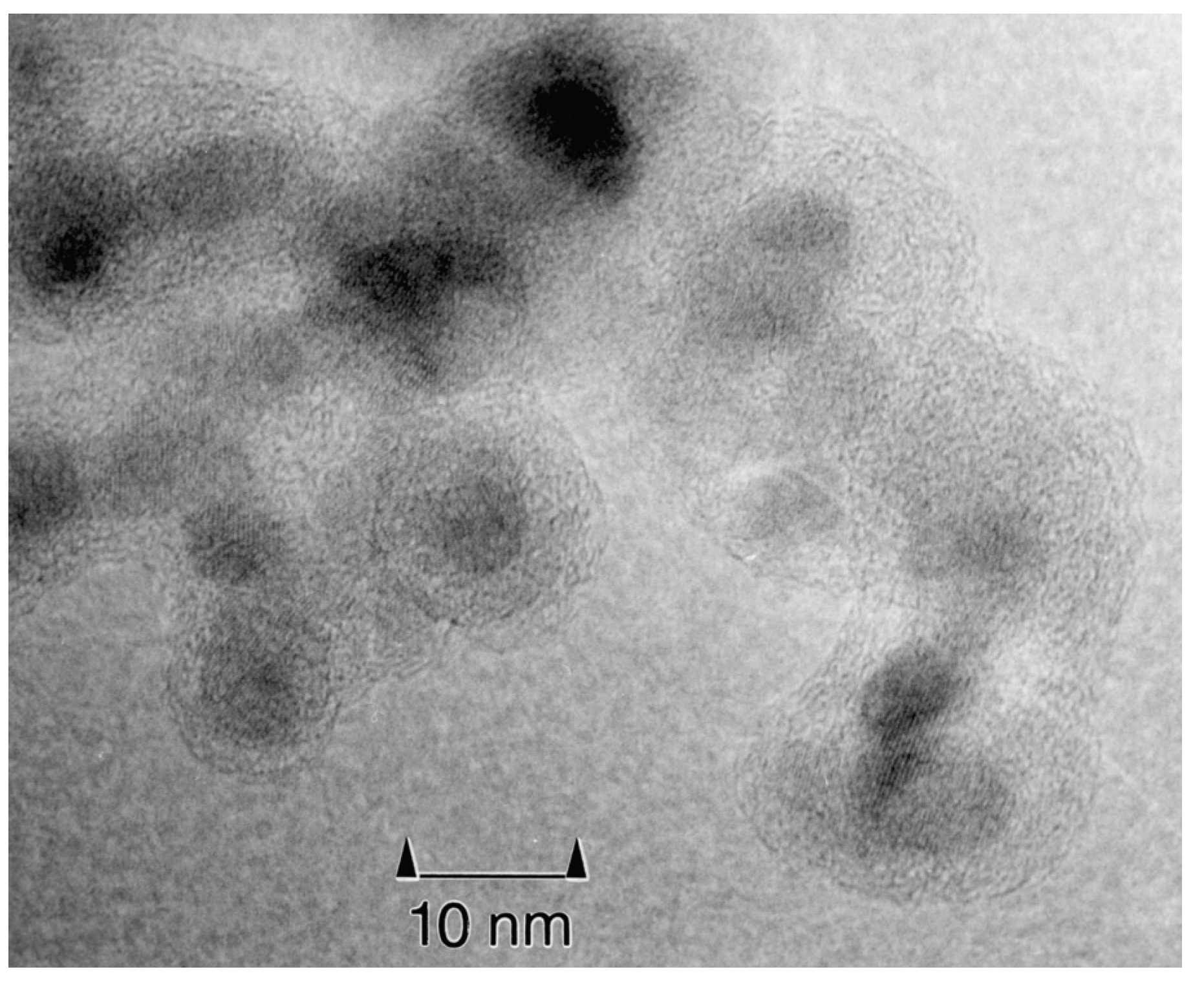
3.3. Microsphere composite nanoparticles
4. Composite Formation Techniques
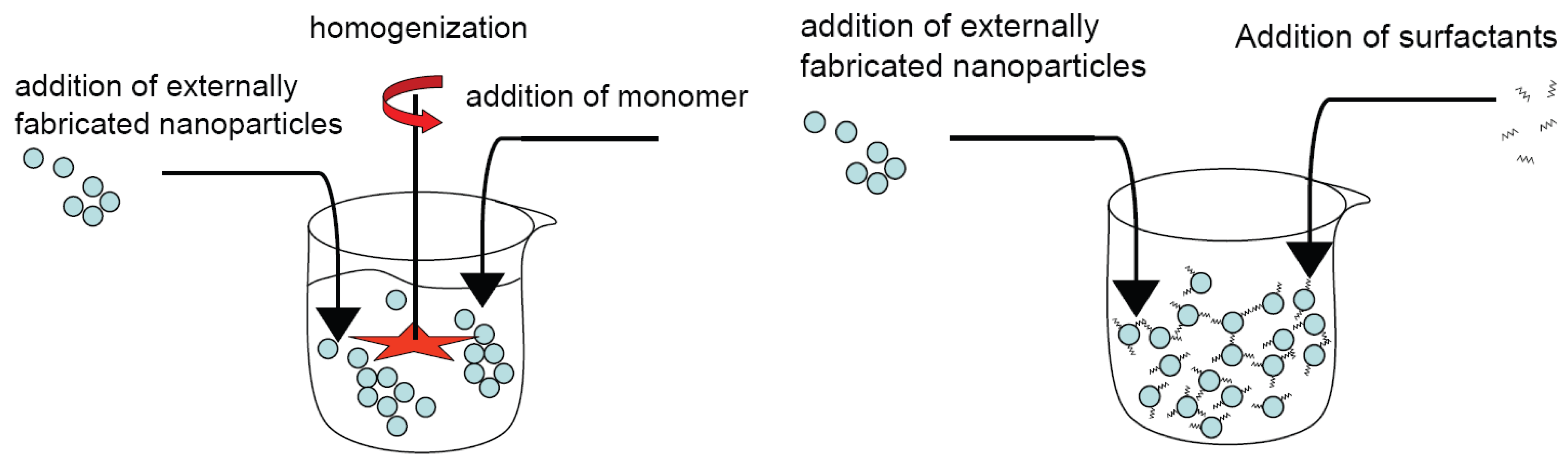
4.1. Ex situ processes
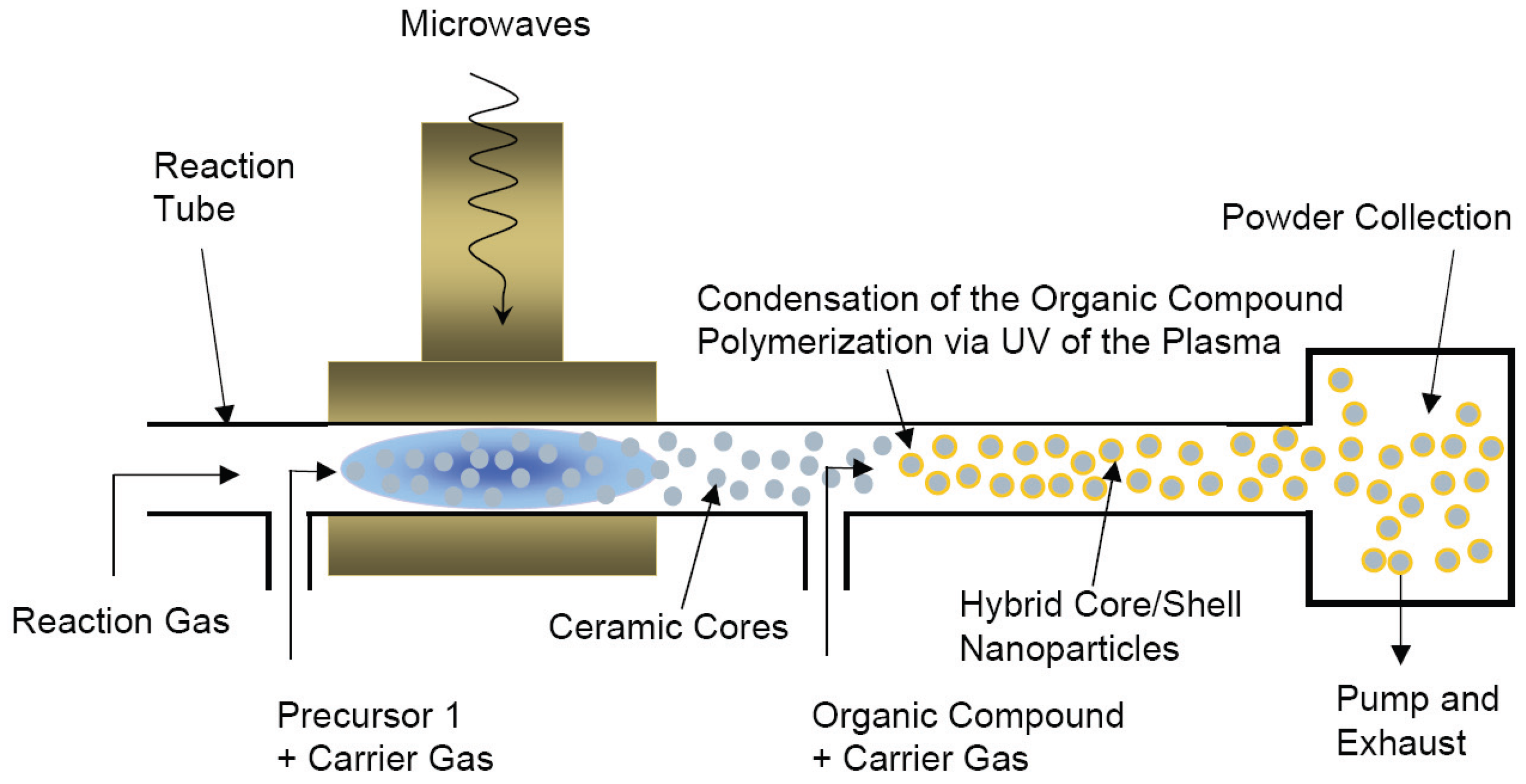
4.2. Chemical in situ methods
4.3. Physical in situ methods
4.4. Drawbacks in composite formation
- shear forces during compounding
- particle surface chemistry and polarity
- interaction between bulk polymer and interfacial-polymer layer as well as interaction between interfacial-polymer layer and ceramic nanoparticles.
5. Thermomechanical Composite Properties
- particle shape, agglomeration, and size distribution
- particle specific surface area and related surface chemistry
- particle-polymer matrix interface and interaction
- compounding method and related shear forces.
| Item | Polymer-filler interaction | Impact |
|---|---|---|
| Elastic modulus | Attractive/repulsive | Increase with volume fraction |
| Attractive/repulsive | Increase with size decrease | |
| Density/volume | Attractive | Increased volume as size decreases |
| Repulsive | n.a. | |
| Glass transition temperature | Attractive | Increase with size decrease |
| Repulsive | Level until 0.5%, drops off level from 1–10% |
| Item | Polymer-filler interaction | Impact |
|---|---|---|
| Elastic modulus | Attractive/repulsive | Increase with volume fraction |
| Attractive/repulsive | Increase with size decrease | |
| Density/volume | Attractive | Increased volume as size decreases |
| Repulsive | n.a. | |
| Glass transition temperature | Attractive | Decrease with addition of particles |
| Repulsive | n.a. | |
| Crystallinity | Attractive/repulsive | No major effect |
5.1. Glass transition temperature and coefficient of thermal expansion
- the addition of ceramic fillers lowers the CTE
- an increase of TG can be observed if an attractive interaction of the nanofiller with the polymer matrix by physic- or chemisorption is given
- a decrease of TG occurs if the nanoparticle has a repulsive interaction with the matrix.
5.2. Elastic modulus, tensile strength, flexural strength and impact performance
5.3. Scratch resistance, wear and creep properties
6. Functional Properties and Applications of Nanocomposites
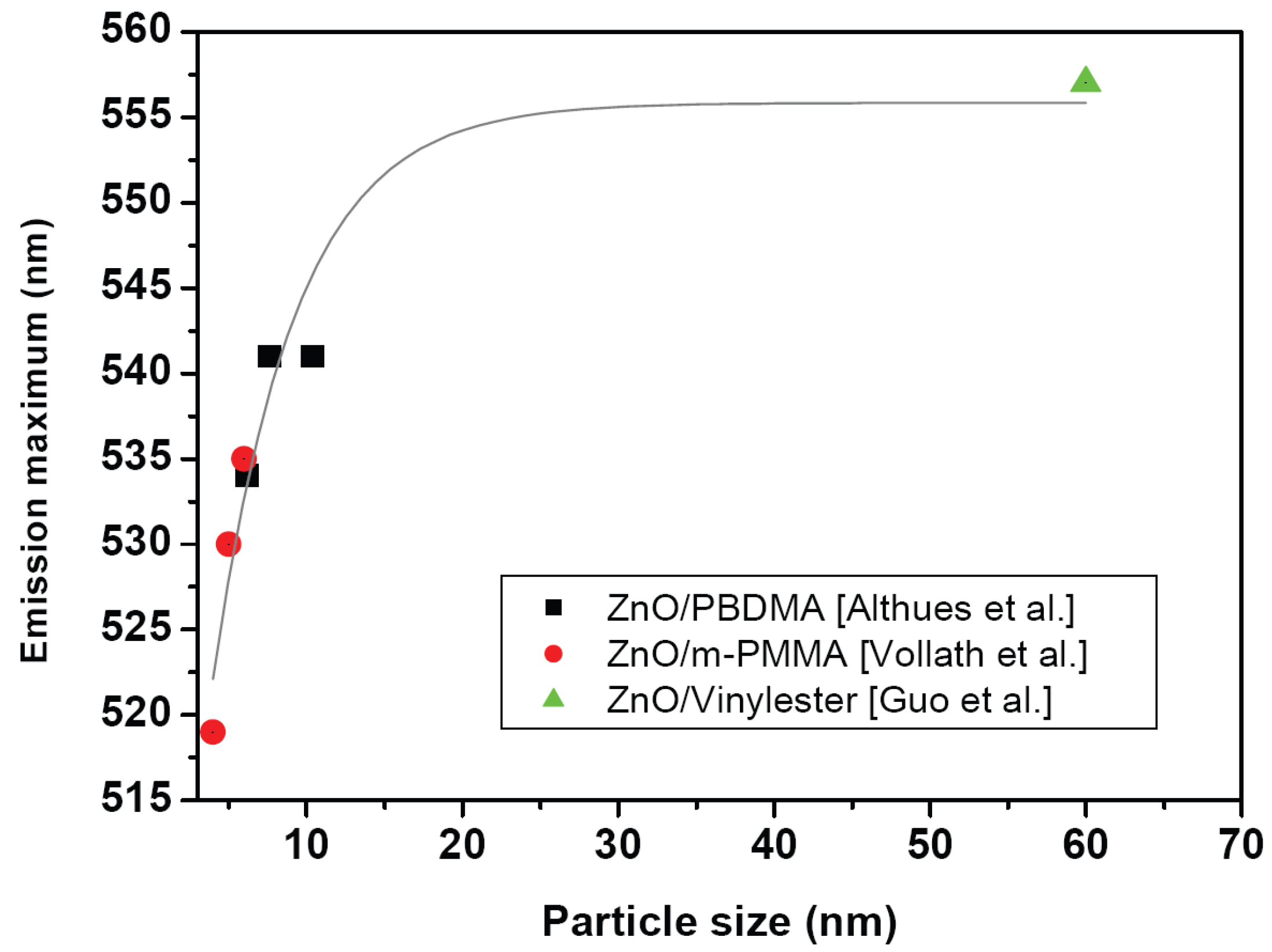
6.1. Optical properties
- especially in the case of TiO2 not all authors indicate the phase they use
- different units as wt % or vol % are used for the filler
- surface modified nanoparticles as well as pristine nanoparticles are used
- different particle sizes are used
- different processes for the synthesis of the composites are used
- the influence of remaining precursor residuals is unclear.
| Nano-Filler | Diameter [nm] | Matrix | Δn, Refractive index increase | Reference |
|---|---|---|---|---|
| ZnS/PMAA + acetic acid; 50 vol % | ZnS: 3 nm | DMAA/St/DVB | 0.023 | [78] |
| ZrO2 50 wt % TiO2 42 wt % | 5 nm 7 nm | PC PC | 0.067 (at 589 nm) 0.135 (at 589 nm) | [142] |
| TiO2, 60 wt % | Amorphous | Epoxy | 0.221 | [143] |
| ME-capped ZnS 30 wt % | ZnS: 3 nm | DMAA/St/DVB | 0.048 | [87] |
| TiO2 27.3 vol % TiO2 90 vol % | <10 nm | PS | 0.22 0.41 | [144] |
| TiO2 acetic acid mod. 10 wt % 30 wt % | ~ 15 +/- 10 nm | Epoxy | 0.71 (at 633 nm) 0.9 (at 633 nm) | [145] |
| TiO2, 50 wt % | Anatase: 4 nm | Organic silica sol | 0.163 (at 633 nm) | [146] |
| ZrO2, 5 wt % | 5 – 25 nm | TMP-TGE | 0.1 (at 631 nm) | [147] |
| TiO2 surface modified 80 wt % 80 wt % | TiO2: 3 – 6 nm | PHE PSTMA | 0.23 (at 589 nm) 0.19 (at 633 nm) | [148] |
| TiO2, 65 wt % | amorphous | Epoxy | 0.187 (at 633 nm) | [149] |
| PbS, 41.8 wt % | <10 nm | Polythiourethane | 0.481 (at 633 nm) | [150] |
| Al2O3-C®, 1 wt % ZrO2 VP®, 0.2 wt % | 13 nm 30 nm | PMMA | 0.0016 (at 633 nm) 0.0014 (at 633 nm) | [95] |
| TiO2, 35 wt % (=10.5 vol %) | Rutile: 2.5 nm | PVAL | 0.088 (at 589 nm) | [151] |
| Al2O3-C®, 1 wt % | 13 nm | High temperature stable PC | 0.0043 (at 633 nm) 0.0031 (at 1550 nm | [152] |
| SiO2, 10 wt % Al2O3-C®, 1 wt % Al2O3, 0.5 wt % | 12 nm 13 nm 38 nm | PMMA PMMA PMMA | -0.007 (at 633 nm) 0.007 (at 633 nm) 0.004 (at 633 nm) | [94,96] |
| ZnO, 7.76 vol % | 22 nm | PMMA | 0.02 (at 633 nm) | [153] |
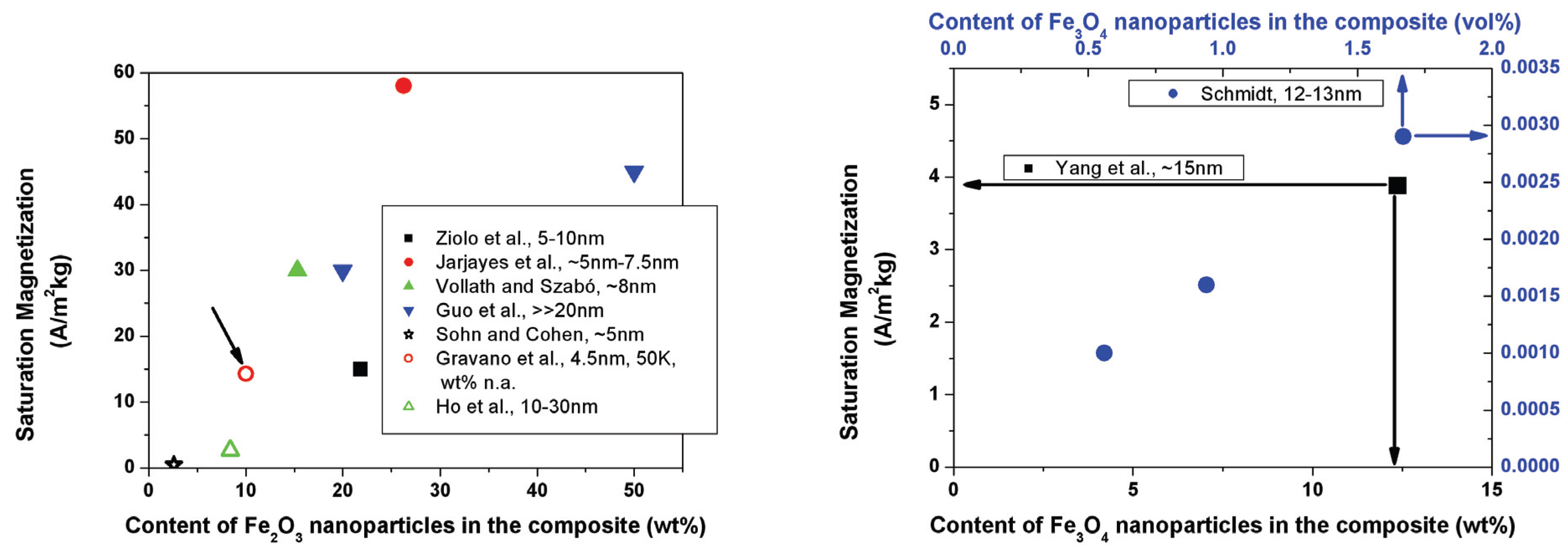
6.2. Magnetic properties
6.3. Microelectronic devices
- huge functionality like large capacitance values in case of integrated capacitors
- process compatibility to industrial PCB-fabrication
- abandonment of lead-containing materials
- low overall costs
- high reliability and extended life cycle.
6.4. Piezoelectric actuators and sensors
6.5. Lithium-ion batteries
- mechanical and chemical stability of the used electrode and electrolyte materials
- huge energy storage capability
- wide temperature range of operation (-40–85 °C)
- negligible self-discharge
- flat shape of the discharge curve
- short charge time
- long cycle life time with almost unchanged capacity
- low costs
- enhanced safety especially inflammability.
- anode: pure lithium metal or more common graphite
- cathode: spinel-type lithium-metal oxides like LiCoO2 or LiMn2O4
- electrolyte: highly polar, aprotic low-viscous organic solvents mixtures containing a conducting salt like LiClO4, LiPF6 or LiBF4
- separator: physical barrier between the electrodes avoiding short-circuit and supporting a mechanical stability, consisting of a porous inert material filled with e.g., a polymer-gel.
6.5.1. Polymer-nanocomposite electrolytes applying passive ceramic nanofillers
6.5.2. Polymer-nanocomposite electrolytes applying active ceramic nanofillers
6.5.3. Nanocomposites as electrodes and supercapacitors
- a barrier to suppress the aggregation of active particles
- a buffering matrix to relax the volume expansion during the lithiation/delithiation process
- an improvement of the conductance of the electronic material.
6.6. Organic solar cells and intrinsic conductive polymer nanocomposites
6.7. Polymer-nanocomposite-photoresists
- improved sensitivity to electromagnetic radiation of a certain wavelength region
- improved resolution
- improved chemical resist stability
- improved mechanical stability during processing
- tailoring of the coefficient of thermal expansion
- introduction of new functionalities like electrical conductivity or magnetic properties
- direct fabrication of microstructured ceramic or metal components via microstereo-lithography (rapid prototyping).
| Material | Initial specific capacity [mAh/g] | # cycles/capacity retention | # cycles/capacity retention | # cycles/capacity retention | Ref. |
|---|---|---|---|---|---|
| Pure graphite (C) | 300 | 10/100 % | 30/99.1 % | 50/97.8 % | [228] |
| Bulk SnO2 | 652 | 10/63 % | 30/49.8 % | 50/31.7 % | [228] |
| 4 wt % SnO2 in C | 342 | 10/99.6 % | 30/96.6 % | 50/88 % | [228] |
| 9.8 wt % SnO2 in C | 384 | 10/99.4 % | 30/96.3 % | 50/88.3 % | [228] |
| 16.5 wt % SnO2 in C | 428 | 10/99.2 % | 30/90.1 % | 50/72.4 % | [228] |
| 14.2 wt % SnO2 in C | 465 | 40/90 % | 60/80 % | [229] | |
| 14.9 wt % SnO2 in C | 472 | 40/89 % | 60/75 % | [229] | |
| 14.4 wt % SnO2 in C | 460 | 40/74 % | 60/56 % | [229] | |
| TiO2 | n.a. | 10/67.5 % | [56] | ||
| TiO2/C (87/13) | 122 | 10/96.7 % | [56] | ||
| SnO2/PPy (81.75/18.25) | 562 | 20/70 % | [230] | ||
| SnO2 | 570 | 20/40 % | [230] | ||
| SnO2/graphite | 633 | 30/57 % | [57] | ||
| SnO2 at C | 667 | 18/55 % | 30/55 % | 40/55 % | [59] |
| TNHCs | 831 | 10/>96 % | >100/66.2 % | [65] | |
| SnO2/C (75/25) | 993 | 50/62 % | 200/62 % | [231] | |
| SnO2/graphene (40/60) | 765 | 100/66.9 % | [232] |
6.8. Biomedical sciences
- bone fracture repair: Epoxide-carbon fibers-composite for external fixators
- bone plates and screws: Epoxide, PMMA, polypropylene, polyethylene, PS, Nylon, polybutylterephthalate, PEEK, reinforced with carbon fibers
- joints replacement: Ultrahigh molecular weight polyethylene or PEEK-carbon fibers composites for total hip replacement
- bone cement: PMMA-glass powder
- dental applications: Acrylates, filled with surface modified nanosized SiO2 or ZrO2
- catheters: Urethanes or silicone rubber, reinforced with nanosized SiO2
- prosthetic limbs: Thermosets, reinforced with glass, carbon, or Kevlar fibers.
7. Summary
- Size dependent physical properties of the nanoparticles used
- Particle agglomeration
- Maximum accessible shear forces during compounding affects composite properties
- Reproducibility and comparability of composite formation techniques
- Influence of additives like surfactants, plasticizers, and others, on the composite properties
- Beyond target property: side effects on the flow behavior, thermal stability, and others
- Low cost device fabrication by e.g., suitable shaping methods possible?
Acknowledgements
References and Notes
- Krummenacker, M.; Lewis, J. (Eds.) Prospects in Nanotechnology toward Molecular Manufacturing; John Wiley & Sons: New York, NY, USA, 1995.
- Bhushan, B. (Ed.) Springer Handbook of Nanotechnology; Springer: Berlin, Germany, 2004.
- NASA Science, Science News: Audacious & Outrageous: Space elevators. http://science.nasa.gov/headlines/y2000/ast07sep_1.htm (accessed December 2009).
- Luther, W.; Bachmann, G. (Eds.) Nanoparticles-Small Thing, Big Effects; German Federal Ministry of Education and Research, Division Nanomaterials, New Materials, 2008. http://www.bmbf.de/pub/nanoparticles_small_things_big_effects.pdf (access December 2009).
- Fendler, J.H. (Ed.) Nanoparticles and Nanostructured Films: Preparation, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 1998.
- Thrower, P.; Mason, T.W. Materials in Today’s World, 3rd ed.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Talreja, R.; Manson, J.A.E. (Eds.) Polymer Matrix Composites; Elsevier Science Ltd: Oxford, UK, 2002.
- Gerard, J.F. (Ed.) Fillers and Filled Polymers; Wiley-VCH: Weinheim, Germany, 2001; Volume 169.
- Mittal, V. Polymer layered silicate nanocomposites: a review. Materials 2009, 2, 992–1057. [Google Scholar] [CrossRef]
- Pinnavaia, T.J.; Beall, G.W. (Eds.) Polymer-clay Nanocomposites; John Wiley & Sons: Chichester, UK, 2000.
- Ajayan, P.M.; Schadler, L.S.; Braun, P.V. Nanocomposite Science and Technology; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Krishnamoorti, R. Strategies for dispersing nanoparticles in polymers. Mater. Res. Bull. 2007, 32, 341–347. [Google Scholar] [CrossRef]
- Tang, Z.X.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Size-dependent magnetic properties of manganese ferrite fine particles. J. Appl. Phys. 1991, 69, 5279–5281. [Google Scholar] [CrossRef]
- Tang, Z.X.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Size-dependent Curie-temperature in nanoscaled MnFe2O4 particles. Phys. Rev. Lett. 1991, 67, 3602–3605. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Wang, J.P.; Luo, H.L. Crystallite Size Effect on Saturation Magnetization of Fine Ferrimagnetic Particles. J. Magn. Magn. Mater. 1994, 136, 176–182. [Google Scholar] [CrossRef]
- Kyprianidou-Leodidou, T.; Caseri, W.; Suter, U.W. Size variation of PbS Particles in high-refractive-index nanocomposites. J. Phys. Chem. 1994, 98, 8992–8997. [Google Scholar] [CrossRef]
- Trwoga, P.F.; Kenyon, A.J.; Pitt, C.W. Modelling the contribution of quantum confinement to luminescence from silicon nanoclusters. J. Appl. Phys. 1998, 83, 3789–3794. [Google Scholar] [CrossRef]
- Sharma, A.C. Size-dependent energy band gap and dielectric constant within the generalized Penn model applied to a semiconductor nanocrystallite. J. Appl. Phys. 2006, 100, 084301:1–084301:8. [Google Scholar]
- Lee, E.J.H.; Ribeiro, C.; Giraldi, T.R.; Longo, E.; Leite, E.R.; Varela, J.A. Photoluminescence in quantum-confined SnO2 nanocrystals: evidence of free exciton decay. Appl. Phys. Lett. 2004, 84, 1745–1747. [Google Scholar] [CrossRef]
- Nienhaus, H.; Kravets, V.; Koutouzov, S.; Meier, C.; Lorke, A.; Wiggers, H.; Kennedy, M.K.; Kruis, F E. Quantum size effect of valence band plasmon energies in Si and SnOx nanoparticles. J. Vac. Sci. Technol. B 2006, 24, 1156–1161. [Google Scholar] [CrossRef]
- Szabó, D.V.; Schlabach, S.; Ochs, R. Analytical TEM investigations of size effects in SnO2 nanoparticles produced by microwave plasma synthesis. Microsc. Microanal 2007, 13 (Suppl. 3), 430–431. [Google Scholar] [CrossRef]
- Suresh, A.; Mayo, M.J.; Porter, W.D. Thermodynamics of the tetragonal-to-monoclinic phase transformation in fine and nanocrystalline yttria-stabilized zirconia powders. J. Mater. Res. 2003, 18, 2912–2921. [Google Scholar] [CrossRef]
- Li, S.; Zheng, W.T.; Jiang, Q. Size and pressure effects on solid transition temperatures of ZrO2. Scr. Mater. 2006, 54, 2091–2094. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Schlabach, S.; Szabó, D.V.; Vollath, D.; de la Presa, P.; Forker, M. Structure and grain growth of TiO2 nanoparticles investigated by electron and X-ray diffractions and Ta-181 perturbed angular correlations. J. Appl. Phys. 2006, 100, 024305:1–024305:9. [Google Scholar] [CrossRef]
- Schlabach, S.; Szabó, D.V.; Vollath, D.; de la Presa, P.; Forker, M. Zirconia and titania nanoparticles studied by electric hyperfine interactions, XRD and TEM. J. Alloy. Compd. 2007, 434, 590–593. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, G.; Dong, C.; Wen, L. Amorphous TiO2 films with high refractive index deposited by pulsed bias arc ion plating. Surf. Coat. Tech. 2007, 201, 7252–7258. [Google Scholar] [CrossRef]
- Jiang, C.; Wei, M.; Qi, Z.; Kudo, T.; Honma, I.; Zhou, H. Particle size dependence of the lithium storage capability and high rate performance of nanocrystalline anatase TiO2 electrode. J. Power Sources 2007, 166, 239–243. [Google Scholar] [CrossRef]
- Jiang, C.H.; Honma, I.; Kudo, T.; Zhou, H.S. Nanocrystalline rutile TiO2 electrode for high-capacity and high-rate lithium storage. Electrochem. Solid-State Lett. 2007, 10, A127–A129. [Google Scholar] [CrossRef]
- Deng, D.; Kim, M.G.; Lee, J.Y.; Cho, J. Green energy storage materials: Nanostructured TiO2 and Sn-based anodes for lithium-ion batteries. Energy Environ. Sci. 2009, 2, 818–837. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Ayyub, P.; Palkar, V.R.; Gurjar, A.V.; Wankar, R.M.; Multani, M. Finite-size effects in antiferroelectric PbZrO3 nanoparticles. J. Phys-Condens. Mat. 1997, 9, 8135–8145. [Google Scholar] [CrossRef]
- Yan, T.; Shen, Z.G.; Zhang, W.W.; Chen, J.F. Size dependence on the ferroelectric transition of nanosized BaTiO3 particles. Mater. Chem. Phys. 2006, 98, 450–455. [Google Scholar] [CrossRef]
- Wada, S.; Hoshina, T.; Yasuno, H.; Ohishi, M.M.; Kakemoto, H.; Tsurumi, T.; Yashima, M. Size effect of dielectric properties for barium titanate particles and its model. Key Eng. Mat. 2003, 301, 27–30. [Google Scholar] [CrossRef]
- Caseri, W.R. Nanocomposites of polymers and inorganic particles: preparation, structure and properties. Mater. Sci. Tech. 2006, 22, 807–817. [Google Scholar] [CrossRef]
- Schadler, L.S.; Brinson, L.C.; Sawyer, W.G. Polymer nanocomposites: A small part of the story. J. Miner. Met. Mater. Soc. 2007, 59, 53–60. [Google Scholar] [CrossRef]
- Schadler, L.S.; Kumar, S.K.; Benicewicz, B.C.; Lewis, S.L.; Harton, S.E. Designed interfaces in polymer nanocomposites: A fundamental viewpoint. Mater. Res. Bull. 2007, 32, 335–340. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; VCH Publisher: New York, NY, USA, 1994. [Google Scholar]
- Mezger, T.R. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 2nd ed.; Vincentz Network: Hannover, Germany, 2006. [Google Scholar]
- Vollath, D.; Szabó, D.V.; Seith, B. Verfahren zur Herstellung von Partikeln mit einem Kern und einer Hülle. German Patent DE 196 38 601 C1, 1996. [Google Scholar]
- Vollath, D.; Szabó, D.V.; Fuchs, J. Synthesis and properties of ceramic-polymer composites. Nanostruct. Mater. 1999, 12, 433–438. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. Coated Nanoparticles: A new way to improved nanocomposites. J. Nanopart. Res. 1999, 1, 235–242. [Google Scholar] [CrossRef]
- Chen, T.Y.; Somasundaran, P. Preparation of novel core-shell nanocomposite particles by controlled polymer bridging. J. Am. Ceram. Soc. 1998, 1, 140–144. [Google Scholar]
- Gu, S.; Kondo, T.; Konno, M. Preparation of silica-polystyrene core-shell particles up to micron sizes. J. Colloid Interf. Sci. 2004, 272, 314–320. [Google Scholar] [CrossRef]
- Rong, Y.; Chen, H.Z.; Wu, G.; Wang, M. Preparation and characterization of titanium dioxide nanoparticle/polystyrene composites via radical polymerization. Mater. Chem. Phys. 2005, 91, 370–374. [Google Scholar] [CrossRef]
- Shi, D.; He, P.; Lian, J.; Wang, L.; van Ooij, W. Plasma deposition and characterization of acrylic acid thin film on ZnO nanoparticles. J. Mater. Res. 2002, 17, 2555–2560. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, J.; Yang, Z.; Guo, Y.; Li, H.; Zhang, Y. The dispersion study of TiO2 nanoparticles surface modification through plasma polymerization. Physica E 2005, 27, 457–461. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zu, X.T.; Xiang, X.; Lian, J.; Wang, L.M. Preparation and characterization of polymer/inorganic nanoparticle composites through electron irradiation. J. Mater. Sci. 2006, 41, 1973–1978. [Google Scholar]
- Suffner, J.; Schechner, G.; Sieger, H.; Hahn, H. In situ coating of silica nanoparticles with acrylate-based polymers. Chem. Vap. Depos. 2007, 12, 459–464. [Google Scholar] [CrossRef]
- Shi, D.; He, P.; Wang, S.X.; van Ooij, W.; Wang, L.M.; Zhao, J.; Yu, Z. Interfacial particle bonding via an ultrathin polymer film on Al2O3 nanoparticles via plasma polymerization. J. Mater. Res. 2002, 17, 981–990. [Google Scholar] [CrossRef]
- Schallehn, M.; Winterer, M.; Weirich, T.E.; Hahn, H. In situ preparation of polymer coated alumina nanopowders by chemical vapour synthesis. Chem. Vap. Depos. 2003, 9, 40–44. [Google Scholar] [CrossRef]
- He, W.; Guo, Z.; Pu, Y.; Yan, L.; Si, W. Polymer coating on the surface of zirconia nanoparticles by inductively coupled plasma polymerization. Appl. Phys. Lett. 2004, 85, 896–898. [Google Scholar] [CrossRef]
- Lamparth, I.; Szabó, D.V.; Vollath, D. Ceramic nanoparticles coated with oligomers based on acrylic derivatives. Macromol. Symp. 2002, 181, 107–112. [Google Scholar] [CrossRef]
- Schmidt, A. The synthesis of magnetic core-shell nanoparticles by surface-initiated ring-opening polymerization of ε-caprolactone. Macromol. Rapid Comm. 2005, 26, 93–97. [Google Scholar] [CrossRef]
- Nan, A.; Turcu, R.; Craciunescu, I.; Pana, O.; Schaft, H.; Liebscher, J. Microwave assisted graft polymerization of ε-caprolactone onto magnetite. J. Polym. Sci. Pol. Chem. 2009, 47, 5379–5386. [Google Scholar] [CrossRef]
- Gravano, S.M.; Dumas, R.; Liu, K.; Patten, T.E. Methods for the surface functionalization of γ-Fe2O3 nanoparticles with initiators for atom transfer radical polymerization and the formation of core-shell inorganic polymer structures. J. Polym. Sci. Pol. Chem. 2005, 43, 3675–3688. [Google Scholar]
- Fu, L.J.; Liu, H.; Zhang, H.P.; Li, C.; Zhang, T.; Wu, Y.P.; Wu, H.Q. Novel TiO2/C nanocomposites made for anode materials of lithium ion batteries. J. Power Sources 2006, 159, 219–222. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, Y.M; Huang, Y.H.; Lee, Y.R.; Shi, H.C. Size effects of tin oxide nanoparticles on high capacity lithium battery anode materials. Surf. Coat. Tech. 2007, 202, 1313–1318. [Google Scholar] [CrossRef]
- Park, M.S.; Kang, Y.M.; Kim, J.H.; Wang, G.X.; Dou, S.X.; Liu, H.K. Effects of low-temperature carbon encapsulation on the electrochemical performance of SnO2 nanopowders. Carbon 2008, 46, 35–40. [Google Scholar] [CrossRef]
- Qiao, H.; Zheng, Z.; Zhang, L.; Xiao, L. SnO2@C core-shell spheres: synthesis, characterization and performance in reversible Li-ion storage. J. Mater. Sci. 2008, 43, 2778–2784. [Google Scholar] [CrossRef]
- Mangeney, C.; Fertani, M.; Bousalem, S.; Zhicai, M.; Ammar, S.; Herbst, F.; Beaunier, P.; Elaissari, A.; Chehimi, M.M. Magnetic Fe2O3-polysyrene/PPy core/shell particles: Bioreactivity and self-assembly. Langmuir 2007, 23, 10940–10949. [Google Scholar]
- Ho, K.M.; Li, P. Design and synthesis of novel magnetic core-shell polymeric particles. Langmuir 2008, 24, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Ho, K.S.; Bi, X.; Han, Y.K.; Chen, Z.L.; Hsu, C.H.; Chang, Y.C. Synthesis and electromagnetic properties of polyaniline-coated silica/maghemite nanoparticles. Eur. Polym. J. 2009, 45, 613–620. [Google Scholar] [CrossRef]
- Jeon, B.S.; Cho, E.J.; Yang, H.M.; Suh, J.S.; Huh, Y.M.; Kim, J.D. Controlled Aggregates of magnetic nanoparticles for highly sensitive MR contrast agent. J. Nanosci. Nanotechnol. 2009, 9, 7118–7112. [Google Scholar] [PubMed]
- Yang, C.; Li, H.; Xiong, D.; Cao, Z. Hollow polyaniline/Fe3O4 microsphere composites: Preparation, characterization, and applications in microwave absorption. React. Funct. Polym. 2009, 69, 137–144. [Google Scholar] [CrossRef]
- Zhang, W.-M.; Hu, J.-S.; Guo, Y.-G.; Zheng, S.-F.; Zhong, L.-S.; Song, W.-G.; Wan, L.-J. Tin nanoparticles encapsulated in elastic hollow carbon spheres for high-performance anode material in lithium-ion batteries. Adv. Mater. 2008, 20, 1160–1165. [Google Scholar]
- Musikhin, S.; Bakueva, L.; Sargent, E.H.; Shik, A. Luminescent properties and electronic structure of conjugated polymer-dielectric nanocrystal composites. J. Appl. Phys. 2002, 91, 6679–6683. [Google Scholar] [CrossRef]
- Sangermano, M.; Priola, A.; Kortabierra, G.; Jimeno, A.; Garcia, I.; Mondragon, I.; Rizza, G. Photopolymerization of epoxy coatings containing iron-oxide nanoparticles. Macromol. Mater. Eng. 2007, 292, 956–961. [Google Scholar] [CrossRef]
- Tang, E.J.; Dong, S.Y. Preparation of styrene polymer/ZnO nanocomposite latex via miniemulsion polymerization and its antibacterial property. Colloid Polym. Sci. 2009, 287, 1025–1032. [Google Scholar] [CrossRef]
- Mahdavian, A.R.; Sarrafi, Y.; Shabankareh, M. Nanocomposite particles with core-shell morphology III: preparation and characterization of nano Al2O3-poly(styrene-methyl methacrylate) particles via miniemulsion polymerization. Polym. Bull. 2009, 63, 329–340. [Google Scholar] [CrossRef]
- Chen, C.H.; Jin, J.Y.; Yen, F.S. Preparation and characterization of epoxy/γ-aluminium oxide nanocomposites. Compos. Pt. A-Appl. Sci. Manuf. 2009, 40, 463–468. [Google Scholar] [CrossRef]
- Omrani, A.; Simon, L.C.; Rostami, A.A. The effects of alumina nanoparticle on the properties of an epoxy resin system. Mater. Chem. Phys. 2009, 114, 145–150. [Google Scholar] [CrossRef]
- Canillo, V.; Bondioli, F.; Lusvarghi, L.; Montorsi, M.; Avella, M.; Errico, M.E.; Malinconico, M. Modeling of ceramic particles filled polymer-matrix nanocomposites. Compos. Sci. Technol. 2006, 66, 1030–1037. [Google Scholar] [CrossRef]
- Pan, G.; Guo, Q.; Tian, A.; He, Z. Mechanical behaviors of Al2O3 nanoparticles reinforced polyetheretherketone. Mater. Sci. Eng. A 2008, 492, 383–391. [Google Scholar] [CrossRef]
- Althues, H.; Henle, J.; Kaskel, S. Functional inorganic nanofillers for transparent polymers. Chem. Soc. Rev. 2007, 36, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Ziolo, R.F.; Giannelis, E.P.; Weinstein, B.A.; Ohoro, M.P.; Ganguly, B.N.; Mehrotra, V.; Russell, M.W.; Huffman, D.R. Matrix-mediated synthesis of nanocrystalline gamma-Fe2O3-a new optically transparent magnetic material. Science 1992, 257, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Ziolo, R.F.; Giannelis, E.P.; Shull, R.D. Matrix-Mediated Synthesis and Properties of Nanostructured Materials. Nanostruct. Mater. 1993, 3, 85–92. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, W.Q.; Ye, X.Z.; Gong, X.L. Preparation of superparamagnetic Fe3O4/PMMA nano composites and their magnetorheological characteristics. J. Magn. Magn. Mater. 2008, 320, 1499–1502. [Google Scholar] [CrossRef]
- Guan, C.; Lu, C.L.; Cheng, Y.R.; Song, S.Y.; Yang, B. A facile one-pot route to transparent polymer nanocomposites with high ZnS nanophase contents via in situ bulk polymerization. J. Mater. Chem. 2009, 19, 617–621. [Google Scholar] [CrossRef]
- Gonsalves, K.E.; Chen, X.H.; Baraton, M.I. Mechanistic investigation of the preparation of polymer/ceramic nanocomposites. Nanostruct. Mater. 1997, 9, 181–184. [Google Scholar] [CrossRef]
- Yang, Y.; Leppert, V.J.; Risbud, S.H.; Twamley, B.; Power, P.P.; Lee, H.W.H. Blue luminescence from amorphous GaN nanoparticles synthesized in situ in a polymer. Appl. Phys. Lett. 1999, 74, 2262–2264. [Google Scholar] [CrossRef]
- Gangopadhyay, R.; De, A. Polypyrrole-ferric oxide conducting nanocomposites: I. Synthesis and characterization. Eur. Polym. J. 1999, 35, 1985–1992. [Google Scholar] [CrossRef]
- Xiong, M.; Zhou, S.; Wu, L.; Wang, B.; Yang, L. Sol-gel derived organic-inorganic hybrid from trialkoxysilane-capped acrylic resin and titania: effects of preparation conditions on the structure and properties. Polymer 2004, 45, 8127–8138. [Google Scholar] [CrossRef]
- Peres, M.; Costa, L.C.; Neves, A.; Soares, M.J.; Monteiro, T.; Esteves, A.C.; Barros-Timmons, A.; Trindade, T.; Kholkin, A.; Alves, E. A green-emitting CdSe/poly(butyl acrylate) nanocomposite. Nanotechnology 2005, 9, 1969–1973. [Google Scholar] [CrossRef]
- Esteves, A.C.C.; Barros-Timmons, A.; Monteiro, T.; Trindade, T. Polymer encapsulation of CdE (E=S, Se) quantum dot ensembles via in situ radical polymerization in miniemulsion. J. Nanosci. Nanotechnol. 2005, 5, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Althues, H.; Simon, P.; Philipp, F.; Kaskel, S. Integration of zinc oxide nanoparticles into transparent poly(butanediolmonoacrylate) via photopolymerisation. J. Nanosci. Nanotechnol. 2006, 6, 409–413. [Google Scholar] [PubMed]
- Jiang, J. Ultrasonic-assisted synthesis of PMMA/Ni0.5Zn0.5Fe2O4 nanocomposite in mixed surfactant system. Eur. Polym. J. 2007, 43, 1724–1728. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Lu, C.; Lin, Z.; Liu, Y.F.; Guan, C.; Lu, H.; Yang, B. Preparation and properties of transparent bulk polymer nanocomposites with high nanophase contents. J. Mater. Chem. 2008, 18, 4062–4068. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Zhao, J.; Zhao, D.; Fan, Q. Effect of inorganic phase on polymeric relaxation dynamics in PMMA/silica hybrids studied by dielectric analysis. Eur. Polym. J. 2004, 40, 1807–1814. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. Synthesis and properties of nanocomposites. Adv. Eng. Mater. 2004, 6, 117–127. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. The Microwave plasma process - a versatile process to synthesise nanoparticulate materials. J. Nanopart. Res. 2006, 8, 417–428. [Google Scholar] [CrossRef]
- Srikanth, H.; Hajndl, R.; Chirinos, C.; Sanders, J.; Sampath, A.; Sudarshan, T.S. Magnetic studies of polymer-coated Fe nanoparticles synthesized by microwave plasma polymerization. Appl. Phys. Lett. 2001, 79, 3503–3505. [Google Scholar] [CrossRef]
- Qin, C.; Coulombe, S. Synthesis of organic layer-coated copper nanoparticles in a dual plasma process. Mater. Lett. 2006, 60, 1973–1976. [Google Scholar] [CrossRef]
- Winey, K.I.; Vaia, R.A. Polymer nanocomposites. Mater. Res. Bull. 2007, 32, 314–318. [Google Scholar] [CrossRef]
- Ritzhaupt-Kleissl, E.; Böhm, J.; Haußelt, J.; Hanemann, T. Thermoplastic polymer nanocomposites for applications in optical devices. Mater. Sci. Eng. C 2006, 26, 1067–1071. [Google Scholar] [CrossRef]
- Böhm, J.; Haußelt, J.; Henzi, P.; Litfin, K.; Hanemann, T. Tuning the refractive index of polymers for polymer waveguides using nanoscaled ceramics or organic dyes. Adv. Eng. Mater. 2004, 6, 52–57. [Google Scholar] [CrossRef]
- Ritzhaupt-Kleissl, E.; Böhm, J.; Haußelt, J.; Hanemann, T. Process chain for tailoring the refractive index of thermoplastic optical materials using ceramic nanoparticles. Adv. Eng. Mater. 2005, 7, 540–545. [Google Scholar] [CrossRef]
- Smits, V.; Chevalier, P.; Deheunynck, D.; Miller, S. A new filler dispersion technology. Reinf. Plast. 2008, 12, 37–73. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Ruan, W.H. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: a review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Karsa, D.R. Surfactants in Polymers, Coatings, Inks and Adhesives. Blackwell Publishing: Oxford, UK, 2003. [Google Scholar]
- Cheng, D.C.H.; Kruszewski, A.P.; Senior, J.R.; Roberts, T.A. The effect of particle size distribution on the rheology of an industrial suspension. J. Mater. Sci. 1990, 25, 353–373. [Google Scholar] [CrossRef]
- Moreno, R. The role of slip additives in tape-casting technology: Part I-solvents and dispersants. Am. Ceram. Soc. Bull. 1992, 71, 1521–1531. [Google Scholar]
- Moreno, R. The role of slip additives in tape-casting technology: Part II-Binders and plasticisers. Am. Ceram. Soc. Bull. 1992, 71, 1647–1657. [Google Scholar]
- Song, J.H.; Evans, J.R.G. Ultrafine ceramic powder injection moulding: The role of dispersants. J. Rheol. 1996, 40, 131–152. [Google Scholar] [CrossRef]
- Leyland, N.S.; Evans, J.R.G.; Harrison, D.J. Lithographic printing of ceramics. J. Eur. Ceram. Soc. 2002, 22, 1–13. [Google Scholar] [CrossRef]
- Hanemann, T. Influence of particle properties on the viscosity of polymer-alumina composites. Ceram. Int. 2008, 34, 2099–2105. [Google Scholar] [CrossRef]
- Hanemann, T.; Heldele, R.; Haußelt, J. Particle size dependent viscosity of polymer-silica-composites. In Proceedings of the 4M 2006 Conference (Multi-Material-Micro-Manufacture); Menz, W., Fillon, B., Dimov, S., Eds.; Elsevier Publisher: Oxford, UK, 2006; pp. 191–194. [Google Scholar]
- Hanemann, T. Polymer nano-TiO2 composites: Influence of the nanoparticle surface chemistry on rheological properties. In Proceedings of the 4M/ICOMM 2009 Conference (Multi-Material-Micro-Manufacture); Saile, V., Ehmann, K., Dimov, S., Eds.; Cardiff University: Cardiff, UK, 2009; pp. 199–202. [Google Scholar]
- Jordan, J.; Jacob, K.I.; Tannenbaum, R.; Sharaf, M.A.; Jasiuk, I. Experimental trends in polymer nanocomposites - a review. Mater. Sci. Eng. A 2005, 393, 1–11. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Ash, B.J.; Schadler, L.S.; Siegel, R.W. Glass transition behavior of alumina/ polymethylmethacrylate nanocomposites. Mater. Lett. 2002, 55, 83–87. [Google Scholar] [CrossRef]
- Ash, B.J.; Schadler, L.S.; Siegel, R.W. Glass transition behavior of alumina/ polymethylmethacrylate (PMMA) nanocomposites. Polym. Prepr. 2003, 44, 2445–2446. [Google Scholar]
- Hu, Y.H.; Chen, C.Y.; Wang, C.C. Viscoelastic properties and thermal degradation kinetics of silica/PMMA nanocomposites. Polym. Degrad. Stab. 2004, 84, 545–553. [Google Scholar] [CrossRef]
- Ritzhaupt-Kleissl, E.; Haußelt, J.; Hanemann, T. Thermo-mechanical properties of thermoplastic polymer-nanofiller composites. In Proceedings of the 4M 2005 conference (Multi-Material-Micro-Manufacture); Menz, W., Dimov, S., Eds.; Elsevier Publisher: Oxford, UK, 2005; pp. 87–90. [Google Scholar]
- Bansal, A.; Li, C.; Yang, H.; Benicewicz, B.C.; Kumar, S.K.; Schadler, L.S. Glass transition behavior of polystyrene filled with surface modified silica nanoparticles. PMSE Prepr. 2005, 92, 260–261. [Google Scholar]
- Goyal, R.K.; Tiwari, A.N.; Mulik, U.P.; Negi, Y.S. Novel high performance Al2O3/ poly(etherether ketone) nanocomposites for electronics applications. Compos. Sci. Technol. 2007, 67, 1802–1812. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.I.; Choe, C.R.; Park, M.; Rim, S.; Kim, J. Preparation and characterization of epoxy composites filled with functionalized nanosilica particles obtained via sol-gel process. Polymer 2001, 42, 879–887. [Google Scholar] [CrossRef]
- Siegel, R.W.; Chang, S.K.; Ash, B.J.; Stone, J.; Ajayan, P.M.; Doremus, R.W.; Schadler, L.S. Mechanical behavior of polymer and ceramic matrix nanocomposites. Scr. Mater. 2001, 44, 2061–2064. [Google Scholar] [CrossRef]
- Garcia, M.; Garica-Turiel, J.; Norder, B.; Chavez, F.; Kooi, B.J.; van Zyl, W.E.; Verweij, H.; Blank, D.H.A. Polyamide-6/silica nanocomposites. Adv. Eng. Mater. 2004, 6, 724–729. [Google Scholar] [CrossRef]
- Cho, J.; Joshi, M.S.; Sun, C.T. Effect of inclusion size on mechanical properties of polymeric composites with micro and nano particles. Compos. Sci. Technol. 2006, 66, 1941–1952. [Google Scholar] [CrossRef]
- Sangermann, M.; Priola, A.; Kortaberria; Jimeno, A.; Garcia, I.; Mondragon, I.; Rizza, G. Photopolymerization of epoxy coatings containing iron-oxide nanoparticles. Macromol. Mater. Eng. 2007, 292, 956–961. [Google Scholar] [CrossRef]
- Oberdisse, J. Aggregation of colloidal nanoparticles in polymer matrices. Soft Matter 2006, 2, 29–36. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Justice, R.S. How nano are nanocomposites. Macromolecules 2007, 40, 8501–8517. [Google Scholar] [CrossRef]
- Ng, C.B.; Schadler, L.S.; Siegel, R.W. Synthesis and mechanical properties of TiO2-epoxy nanocomposites. Nanostruct. Mater. 1999, 12, 507–510. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Rong, M.Z.; Yu, S.L.; Wetzel, B.; Friedrich, K. Effect of particle surface treatment on the tribologcal performance of epoxy based nanocomposites. Wear 2002, 253, 1086–1093. [Google Scholar] [CrossRef]
- Bauer, F.; Gläsel, H.J.; Decker, U.; Ernst, H.; Freyer, A.; Hartmann, E.; Sauerland, V.; Mehnert, R. Trialkoxysilane grafting onto nanoparticles fort he preparation of clear coat polyacrylate system with excellent scratch performance. Prog. Org. Coat. 2003, 47, 147–153. [Google Scholar] [CrossRef]
- Bauer, F.; Mehnert, R. UV curable acrylate nanocomposites: properties and applications. J. Polym. Res. 2005, 12, 483–491. [Google Scholar] [CrossRef]
- Bhimaraj, P.; Burris, D.L.; Action, J.; Sawyer, W.G.; Toney, C.G.; Siegel, R.W.; Schadler, L.S. Effect of matrix morphology on the wear and friction behavior of alumina nanoparticle/poly(ethylene) terephthalate composites. Wear 2005, 258, 1437–1443. [Google Scholar] [CrossRef]
- Daseri, A.; Yu, Z.Z.; Nai, Y.W. Fundamental aspects and recent progress on wear/scratch damage in polymer nanocomposites. Mater. Sci. Eng. R 2009, 63, 31–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.L.; Friedrich, K. Creep resistant polymeric nanocomposites. Polymer 2004, 45, 3481–3485. [Google Scholar] [CrossRef]
- Hanemann, T.; Böhm, J.; Honnef, K.; Ritzhaupt-Kleissl, E.; Haußelt, J. Polymer/phenanthrene-derivative host-guest systems: Rheological, optical and thermal properties. Macromol. Mater. Eng. 2007, 292, 285–294. [Google Scholar] [CrossRef]
- Guo, Z.H.; Wei, S.Y.; Shedd, B.; Scaffaro, R.; Pereira, T.; Hahn, H.T. Particle surface engineering effect on the mechanical, optical and photoluminescent properties of ZnO/vinyl-ester resin nanocomposites. J. Mater. Chem. 2007, 17, 806–813. [Google Scholar] [CrossRef]
- Althues, H.; Pötschke, P.; Kim, G.M.; Kaskel, S. Structure and Mechanical Properties of Transparent ZnO/PBDMA Nanocomposites. J. Nanosci. Nanotechnol. 2009, 9, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Z.; Sue, H.J. Tunable ultraviolet emission of ZnO quantum dots in transparent poly(methyl methacrylate). Appl. Phys. Lett. 2009, 94, 253106:1–253106:3. [Google Scholar]
- Du, X.W.; Fu, Y.S.; Sun, J.; Han, X.; Liu, J. Complete UV emission of ZnO nanoparticles in a PMMA matrix. Semicond. Sci. Technol. 2006, 21, 1202–1206. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zu, X.T.; Xiang, X.; Yu, H.J. Photoluminescence from TiO2/PMMA nanocomposite prepared by gamma radiation. J. Nanopart. Res. 2006, 8, 137–139. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V.; Schlabach, S. Oxide/polymer nanocomposites as new luminescent materials. J. Nanopart. Res. 2004, 6, 181–191. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zu, X.T.; Zhu, S.; Xiang, X.; Fang, L.M.; Wang, L.M. Origin of luminescence from PMMA functionalized nanoparticles. Phys. Lett. B 2006, 350, 252–257. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.Q.; Fu, S.Y.; Xiao, H.M. Transparent and Light-Emitting Epoxy Nanocomposites Containing ZnO Quantum Dots as Encapsulating Materials for Solid State Lighting. J. Phys. Chem. C 2008, 112, 10553–10558. [Google Scholar] [CrossRef]
- Xiong, H.M.; Xu, Y.; Ren, Q.G.; Xia, Y.Y. Stable Aqueous ZnO at Polymer Core/Shell Nanoparticles with Tunable Photoluminescence and Their Application in Cell Imaging. J. Am. Chem. Soc. 2008, 130, 7522–7523. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liang, H.; Ming, H.; Zhang, Q.; Yang, J.; Zheng, Z.; Ma, H.; Zhang, Y.; Zhang, J.; Xie, J.; Cao, L.; Zhang, Z. The investigation on Eu3+ high-doped PMMA planar optical waveguide by using scanning near-field optical microscopy. Opt. Commun. 2004, 240, 75–80. [Google Scholar] [CrossRef]
- Li, X.F.; Lau, K.T.; An, Y.; Yin, Y.S.; Wong, T.T. Luminescent and mechanical properties of the epoxy composites doped with europium complex. Mater. Lett. 2008, 62, 4434–4436. [Google Scholar] [CrossRef]
- Imai, Y.; Terahara, A.; Hakuta, Y.; Matsui, K.; Hayashi, H.; Ueno, N. Transparent poly(bisphenol A carbonate)-based nanocomposites with high refractive index nanoparticles. Eur. Polym J. 2009, 45, 630–638. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Tung, C.T.; Lin, Y.M.; Li, A.K. Preparation and optical properties of titania/epoxy nanocomposite coatings. Mater. Lett. 2008, 62, 3416–3418. [Google Scholar] [CrossRef]
- Rao, Y.Q.; Chen, S. Molecular composites comprising TiO2 and their optical properties. Macromolecules 2008, 41, 4838–4844. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Lin, Y.M.; Li, A.K.; Su, W.F.; Chang, K.S.; Hsu, S.L.C.; Li, T.L. Transparent high refractive index nanocomposite thin films. Mater Lett. 2007, 61, 2908–2910. [Google Scholar] [CrossRef]
- Liu, Y.F.; Lu, C.L.; Li, M.J.; Zhang, L.; Yang, B. High refractive index organic-inorganic hybrid coatings with TiO2 nanocrystals. Colloids Surf. A 2008, 328, 67–72. [Google Scholar] [CrossRef]
- Sangermano, M.; Voit, B.; Sordo, F.; Eichhorn, K. J.; Rizza, G. High refractive index transparent coatings obtained via UV/thermal dual-cure process. Polymer 2008, 49, 2018–2022. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation and characterization of TiO2 and polymer nanocomposite films with high refractive index. J. Appl. Polym. Sci. 2007, 105, 3662–3672. [Google Scholar] [CrossRef]
- Guan, C.; Lu, C.L.; Liu, Y.F.; Yang, B. Preparation and characterization of high refractive index thin films of TiO2/epoxy resin nanocomposites. J. Appl. Polym. Sci. 2006, 102, 1631–1636. [Google Scholar] [CrossRef]
- Lu, C.L.; Guan, C.; Liu, Y.F.; Cheng, Y.R.; Yang, B. PbS/polymer nanocomposite optical materials with high refractive index. Chem. Mater. 2005, 17, 2448–2454. [Google Scholar] [CrossRef]
- Nussbaumer, R.J.; Caseri, W.R.; Smith, P.; Tervoort, T. Polymer-TiO2 nanocomposites: A route towards visually transparent broadband UV filters and high refractive index materials. Macromol. Mater. Eng. 2003, 288, 44–49. [Google Scholar] [CrossRef]
- Hanemann, T.; Haußelt, J.; Ritzhaupt-Kleissl, E. Compounding, micro injection molding and characterization of polycarbonate-nanosized alumina-composites for application in microoptics. Microsystem Technol. 2009, 15, 421–427. [Google Scholar] [CrossRef]
- Demir, M. M.; Koynov, K.; Akbey, U.; Bubeck, C.; Park, I.; Lieberwirth, I.; Wegner, G. Optical properties of composites of PMMA and surface-modified zincite nanoparticles. Macromolecules 2007, 40, 1089–1100. [Google Scholar] [CrossRef]
- Kataby, G.; Ulman, A.; Prozorov, R.; Gedanken, A. Coating of amorphous iron nanoparticles by long-chain alcohols. Langmuir 1998, 14, 1512. [Google Scholar] [CrossRef]
- Burke, N.A.D.; Stöver, H.D.H.; Dawson, F.P. Magnetic nanocomposites: preparation and characterization of polymer-coated iron nanoparticles. Chem. Mater. 2002, 14, 4752–4761. [Google Scholar] [CrossRef]
- Jarjayes, O.; Fries, P.H.; Bidan, G. Magnetic properties of fine maghemite particles in an electroconducting polymer matrix. J. Magn. Magn. Mater. 1994, 137, 205–218. [Google Scholar] [CrossRef]
- Guo, Z.; Shin, K.; Karki, A.; Young, D.; Kaner, R.; Hahn, H.T. Fabrication and characterization of iron oxide nanoparticles filled polypyrrole nanocomposites. J. Nanopart. Res. 2009, 11, 1441–1452. [Google Scholar] [CrossRef]
- Sohn, B.H.; Cohen, R.E. Processible optically transparent block copolymer films containing superparamagnetic iron oxide nanoclusters. Chem. Mater. 1997, 9, 264–269. [Google Scholar] [CrossRef]
- Roca, A.G.; Costo, R.; Rebolledo, A.F.; Veintemillas-Verdaguer, S.; Tartaj, P.; Gonzalez-Carreno, T.; Morales, M.P.; Serna, C.J. Progress in the preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D: Appl. Phys. 2009, 42, 224002. [Google Scholar] [CrossRef]
- Frimpong, R.A.; Fraser, S.; Hilt, J. Z. Synthesis and temperature response analysis of magnetic-hydrogel nanocomposites. J. Biomed. Mater. Res. Part A 2007, 80, 1–6. [Google Scholar] [CrossRef]
- Morales, M.A.; Finotelli, P.V.; Coaquira, J.A.H.; Rocha-Leão, M.H.M.; Diaz-Aguila, C.; Baggio-Saitovitch, E.M.; Rossi, A.M. In situ synthesis and magnetic studies of iron oxide nanoparticles in calcium-alginate matrix for biomedical applications. Mater. Sci. Eng. C 2008, 28, 253–257. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Gupta, R. Evaluation of water sorption behavior and in vitro blood compatibility of Polyvinyl Alcohol based magnetic bionanocomposites. J. Appl. Polym. Sci. 2009, 114, 3548–3560. [Google Scholar] [CrossRef]
- Masotti, A.; Pitta, A.; Ortaggi, G.; Corti, M.; Innocenti, C.; Lascialfari, A.; Marinone, M.; Marzola, P.; Daducci, A.; Sbarbati, A.; Micotti, E.; Orsini, F.; Poletti, G.; Sangregorio, C. Synthesis and characterization of polyethylenimine-based iron oxide composites as novel contrast agents for MRI. Magn. Reson. Mat. Phys. Biol. Med. 2009, 22, 77–87. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, G.; Sun, J.; Wu, B.Y.; Gong, Q.Y.; Song, B.; Ai, H.; Gu, Z.W. Self-Assembly of Magnetite Nanocrystals with Amphiphilic Polyethylenimine: Structures and Applications in Magnetic Resonance Imaging. J. Nanosci. Nanotechnol. 2009, 9, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Samantha, A.M.; Anderson, A.A.; Mehul, S.; Kimberly, W.A.; Hilt, J.Z. Biocompatibility analysis of magnetic hydrogel nanocomposites based on poly(N-isopropylacrylamide) and iron oxide. J. Biomed. Mater. Res. Part A 2009, 91, 903–909. [Google Scholar]
- van Landeghem, F.K.H.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.T.; Gneveckow, U.; Scholz, R.; Thiesen, B.; Brück, W.; von Deimling, A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperthermia 2008, 24, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; López-Viota, M.; López-Viota, J.; Delgado, Á.V. Development of iron/ethylcellulose (core/shell) nanoparticles loaded with diclofenac sodium for arthritis treatment. Inter. J. Pharm. 2009, 382, 270–276. [Google Scholar] [CrossRef]
- Thierry, B.; Al-Ejeh, F.; Khatri, A.; Yuan, Z.; Russell, P.J.; Ping, S.; Brown, M.P.; Majewski, P. Multifunctional core-shell magnetic cisplatin nanocarriers. Chem. Commun. 2009, 47, 7348–7350. [Google Scholar] [CrossRef]
- Zhu, Y.; Kaskel, S.; Ikoma, T.; Hanagata, N. Magnetic SBA-15/poly(N-isopropylacrylamide) composite: Preparation, characterization and temperature-responsive drug release property. Micropor. Mesopor. Mater. 2009, 123, 107–112. [Google Scholar] [CrossRef]
- Albornoz, C.; Jacobo, S.E. Preparation of a biocompatible magnetic film from an aqueous ferrofluid. J. Magn. Magn. Mater. 2006, 305, 12–15. [Google Scholar] [CrossRef]
- Hanemann, T.; Böhm, J.; Honnef, K.; Heldele, R.; Schumacher, B. Properties and application of polymer-ceramic-composites in microsystem technologies. In Ceramics Processing in Microtechnology; Ritzhaupt-Kleissl, H.J., Johander, P., Eds.; Whittles Publishing: Dunbeath, Scotland, UK, 2009; pp. 120–133. [Google Scholar]
- Bhattacharya, S.K.; Tummala, R.R. Next generation integral passives: materials, processes, and integration of resistors and capacitors on PWB substrates. J. Mater. Sci.: Mater. Electron. 2000, 11, 253–268. [Google Scholar] [CrossRef]
- Dougherty, J.P. Integrated passives technology and economics. Circuits Assembly 2003, 09, 18–23. [Google Scholar]
- AT&S, Technology. Key Topics: Embedded capacitors. http://www.ats.net/en/index.php/-Technology/Key+Topics/Embedded+Technologies/c-12848-Embedded+Capacitors.html/ (access December 2009).
- Bhattacharya, S.K.; Tummala, R.R. Integral passives for next generation of electronic packaging: application of epoxy/ceramic nanocomposites as integral capacitors. Microelectron. J. 2001, 32, 11–19. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, Z.Y.; Olson, D.; Mai, T.; Zhang, Q.M.; Kavarnos, G. Ferroelectric and electromechanical properties of poly(vinylidenefluoride-trifluoroethylene-chlorotrifluoroethy-lene) terpolymer. Appl. Phys. Lett. 2001, 78, 2360–2362. [Google Scholar] [CrossRef]
- Moulson, A.J.; Herbert, J.M. Electroceramics: Materials, Properties, Applications, 2nd ed.; J. Wiley & Sons: Chichester, West Sussex, England, 2003; pp. 71–82. [Google Scholar]
- Kinoshita, K.; Yamaji, A. Grain-size effects on dielectric properties in barium titanate ceramics. J. Appl. Phys. 1976, 47, 371–373. [Google Scholar] [CrossRef]
- Buscaglia, V.; Buscaglia, M.T.; Viviani, M.; Mitoseriu, L.; Nanni, P.; Trefiletti, V.; Piaggio, P.; Gregora, I.; Ostapchuk, T.; Pokorny, J.; Petzelt, J. Grain size and grain boundary-related effects on the properties of nanocrystalline barium titanate ceramics. J. Eur. Ceram. Soc. 2006, 26, 2889–2898. [Google Scholar] [CrossRef]
- Schumacher, B.; Geßwein, H.; Hanemann, T.; Haußelt, J. Influence of the crystallite size of BaTiO3 on the dielectric properties of polyester reactive resin composite materials. Proc. NSTI Nanotech. 2008, 1, 385–388. [Google Scholar]
- Schumacher, B.; Geßwein, H.; Haußelt, J.; Hanemann, T. Temperature treatment of nano scaled barium titanate filler to improve the dielectric properties of high-k-polymer based composites. Microelectron. Eng. 2010. [Google Scholar] [CrossRef]
- Schumacher, B.; Geßwein, H.; Hanemann, T.; Haußelt, J. Influence of the crystallite and particle size of BaTiO3 and SrTiO3 on the dielectric properties of polyester reactive-resin composite materials. In Proceedings Smart Systems Integration, Brussels, Belgium, March 2009; pp. 134–139.
- Schumacher, B.; Geßwein, H.; Haußelt, J.; Hanemann, T. Permittivity of BaTiO3 polymer composite with different particle size distribution. In Proceedings NSTI Nanotech, Houston, TX, USA, May 2009; Volume 2, pp. 546–549.
- Motamedi, M.E. (Ed.) MOEMS Micro-Opto-Electro-Mechnical Systems; SPIE Press: Bellingham; Washington, DC, USA, 2005.
- Menz, W.; Mohr, J.; Paul, O. Microsystem Technologies; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Ploss, B.; Ng, W.Y.; Chan, H.L.W.; Ploss, B.; Choy, C.L. Poling study of PZT/P(VDF/TrFE) composites. Compos. Sci. Technol. 2001, 61, 957–962. [Google Scholar] [CrossRef]
- Glushanin, S.V.; Topolov, V.Y.; Krivoruchko, A.V. Features of piezoelectric properties of 0-3 PbTiO3-type ceramic-polymer composites. Mater. Chem. Phys. 2006, 97, 357–364. [Google Scholar] [CrossRef]
- Brodd, R.J.; Bullock, K.R.; Leising, R.A.; Middaugh, R.L.; Miller, J.R.; Takeuchi, E. Batteries, 1977 to 2002. J. Electrochem. Soc. 2004, 151, K1–K11. [Google Scholar] [CrossRef]
- Kazunori, O. (Ed.) Lithium Ion Rechargeable Batteries; Wiley-VCH: Weinheim, Germany, 2008.
- Winter, M.; Brod, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245. [Google Scholar] [CrossRef] [PubMed]
- Van Schalkwijk, W.A.; Scrosati, B. Advances in Lithium-Ion Batteries; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Liu, H.K.; Wang, G.X.; Guo, Z.P.; Wang, J.Z.; Konstantinov, K. The impact of nanomaterials on Li-ion rechargeable batteries. J. New Mat. Electrochem. Syst. 2007, 10, 101–104. [Google Scholar]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethyleneoxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Wieczorek, W.; Such, K.; Wycislik, H.; Plocharski, J. Modification of crystalline structure of PEO polymer electrolytes with ceramic additives. Solid State Ionics 1989, 36, 255–257. [Google Scholar] [CrossRef]
- Przyluski, J.; Wieczorek, W. Increasing the conductivity of polymer solid electrolytes: A review. Solid State Ionics 1989, 36, 165–169. [Google Scholar] [CrossRef]
- Wieczorek, W.; Florjanczyk, Z.; Stevens, J.R. Composite polyether based solid electrolytes. Electrochim. Acta 1995, 40, 2251–2258. [Google Scholar] [CrossRef]
- Przyluski, J.; Siekerski, M.; Wieczorek, W. Effective medium theory in studies of conductivity of composite polymeric electrolytes. Electrochim. Acta 1995, 40, 2101–2108. [Google Scholar] [CrossRef]
- Krawiec, W.; Scranlon, L.G.Jr.; Fellner, J.P.; Vaia, R.A.; Vasudevan, S.; Giannelis, E.P. Polymer nanocomposites: a new strategy for synthesizing solid electrolytes for recharchable lithium batteries. J. Power Sources 1995, 54, 310–315. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Best, A.S.; Ferry, A.; MacFarlane, D.R.; Forsyth, M. Conductivity in amorphous polyether nanocomposite materials. Solid State Ionics 1999, 126, 269–276. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, W.; Choi, B.K. Relation between glass transition and melting PEO-salt complexes. Electrochim. Acta 2000, 45, 1473–1477. [Google Scholar] [CrossRef]
- Croce, F.; Persi, L.; Ronci, F.; Scrosati, B. Nanocomposite polymer electrolytes and their impact on the lithium battery technology. Solid State Ionics 2000, 135, 47–52. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Persi, L.; Ronci, F.; Scrosati, B. Transport and interfacial properties of composite polymer electrolytes. Electrochim. Acta 2000, 45, 1481–1490. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Hassoun, J.; Scrosati, B.; Salomon, M.; Cassel, F. Hot-pressed, dry, composite, PEO-based electrolyte membranes. I. Ionic conductivity characterization. J. Power Sources 2003, 114, 105–112. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Hassoun, J.; Scrosati, B.; Croce, F.; Cassel, F.; Salomon, M. Hot-pressed, dry, composite, PEO-based electrolyte membranes. II. All solid-state Li/LiFePO4 polymer batteries. J. Power Sources 2003, 124, 246–253. [Google Scholar] [CrossRef]
- Wachtler, M.; Ostrovskii, D.; Jacobsson, P.; Scrosati, B. A study of PVdF-based SiO2-containing composite gel-type polymer electrolytes for lithium batteries. Electrochim. Acta 2004, 50, 357–361. [Google Scholar] [CrossRef]
- Song, I.C.; Oh, J.S.; Kim, S.H.; Ko, J.M.; Kim, D.W. Effect of an inorganic additive on cycling performance of Li/V2O5 polymer cells prepared with gel polymer electrolyte. J. Power Sources 2005, 150, 202–207. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Sutto, T.E.; Ollinger, M.; Kim, H.; Arnold, C.B.; Pique, A. Laser transferable polymer-ionic liquid separator/electrolytes for solid-state recharchable lithium-ion microbatteries. Electrochem. Solid-State Lett. 2006, 9, A69–A71. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; Volume 1, pp. 57–174. [Google Scholar]
- Li, Y.; Fedkiw, P.S. Effect of gel electrolytes containing silica nanoparticles on aluminum corrosion. Electrochim. Acta 2007, 52, 2471–2477. [Google Scholar] [CrossRef]
- Krause, L.; Lamanna, W.; Summerfield, J.; Engle, M.; Korba, G.; Loch, R.; Atanasoki, R. Corrosion of aluminum at high voltages in non-aqueous electrolytes containing perfluoroalkylsulfonyl imides; new lithium salts for lithium-ion cells. J. Power Sources 1997, 68, 320–325. [Google Scholar] [CrossRef]
- Walkowiak, M.; Zalewska, A.; Jesionowski, T.; Pokora, M. Stability of poly(vinylidene fluoride-co-hexafluoropropylene)-based composite gel electrolytes with functionalized silicas. J. Power Sources 2007, 173, 721–728. [Google Scholar] [CrossRef]
- Krejza, O.; Velická, J.; Sedlariková, M.; Vondrák, J. The presence of nanostructured Al2O3 in PMMA-based gel electrolytes. J. Power Sources 2008, 178, 774–778. [Google Scholar] [CrossRef]
- Jog, J.P. Crystallisation in polymer nanocomposites. Mater. Sci. Technol. 2006, 22, 797–806. [Google Scholar] [CrossRef]
- Capuano, F.; Croce, F.; Scrosati, B. Composite polymer electrolytes. J. Electrochem. Soc. 1991, 138, 1918–1922. [Google Scholar] [CrossRef]
- Borghini, M.C.; Mastragostini, A.; Passerini, S.; Scrosati, B. Electrochemical properties of polyethylene oxide-Li[(CF3SO2)2N]-γ-LiAlO2-composite polymer electrolytes. J. Electrochem. Soc. 1995, 142, 2118–2121. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Dautzenberg, G.; Mastragostino, M.; Ronci, F.; Scrosati, B.; Soavi, F.; Zanelli, A.; Alessandrini, F.; Prosini, P.P. Composite polymer electrolytes with improved lithium metal electrode interfacial properties I. Electrochemical properties of dry PEO-LiX systems. J. Electrochem. Soc. 1998, 145, 4126–4132. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Mastragostino, S.B.; Soavi, F.; Zanelli, A. Composite polymer electrolytes with improved lithium metal electrode interfacial properties I. Application in recharchable batteries. J. Electrochem. Soc. 1998, 145, 4133–4135. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Scaccia, S.; Passerini, S. Investigation on the stability of the lithium-polymer-electrolyte interface. J. Electrochem. Soc. 2000, 147, 4448–4452. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Alessandrini, F.; Duan, R.G.; Arzu, A.; Passerini, S. Electrochemical testing of industrially produced PEO-based polymer electrolytes. J. Power Sources 2001, 101, 46–46. [Google Scholar] [CrossRef]
- Kum, K.S.; Song, M.K.; Kim, Y.T.; Kim, H.S.; Cho, B.W.; Rhee, H.W. The effect of mixed salts in gel-coated polymer electrolyte for advanced lithium battery. Electrochim. Acta 2004, 50, 285–288. [Google Scholar] [CrossRef]
- Sundaram, N.T.K.; Subramania, A. Nano-size LiAlO2 ceramic filler incorporated porous PVDF-co-HFP electrolyte for lithium-ion battery applications. Electrochim. Acta 2007, 52, 4987–4993. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem., Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J. Y.; Chen, B. H. Microemulsion syntheses of Sn and SnO2-graphite nanocomposite anodes for Li-ion batteries. J. Electrochem. Soc. 2004, 151, A563–A570. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J. Y. Microwave-assisted synthesis of SnO2-graphite nanocomposites for Li-ion battery applications. J. Power Sources 2005, 144, 220–225. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Chew, S.Y.; Chen, J.; Guo, Z.P.; Zhao, L.; Konstantinov, K.; Liu, H.K. Synthesis and characterization of SnO2-polypyrrole composite for lithium-ion battery. J. Power Sources 2007, 174, 1183–1187. [Google Scholar] [CrossRef]
- Liu, H.P.; Long, D.H.; Liu, X.J.; Qiao, W.M.; Zhan, L.; Ling, L.C. Facile synthesis and superior anodic performance of ultrafine SnO2-containing nanocomposites. Electrochim. Acta 2009, 54, 5782–5788. [Google Scholar] [CrossRef]
- Yao, J.; Shen, X.; Wang, B.; Liu, H.; Wang, G. In situ chemical synthesis of SnO2-graphene nanocomposite as anode materials for lithium-ion batteries. Electrochem. Commun. 2009, 11, 1849–1852. [Google Scholar] [CrossRef]
- Li, F.H.; Song, J.F.; Yang, H.F.; Gan, S.Y.; Zhang, Q.X.; Han, D.X.; Ivaska, A.; Niu, L. One-step synthesis of graphene/SnO2 nanocomposites and its application in electrochemical supercapacitors. Nanotechnology 2009, 20, 455602:1–455602:6. [Google Scholar]
- Hu, Z.A.; Xie, Y.L.; Wang, Y.X.; Mo, L.P.; Yang, Y.Y.; Zhang, Z.Y. Polyaniline/SnO2 nanocomposite for supercapacitor applications. Mater. Chem. Phys. 2009, 114, 990–995. [Google Scholar] [CrossRef]
- O’Regan, B.; Graetzel, M. A low-cost, high efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kim, M.R.; Jin, S.H.; Park, S.H.; Lee, H.J.; Kang, E.H.; Lee, J.K. Photovoltaic properties and preparations of dye-sensitized solar cells using solid-state polymer electrolytes. Mol. Cryst. Liq. Cryst. 2006, 444, 233–239. [Google Scholar] [CrossRef]
- Anandan, S.; Sivakumar, R. Effect of loaded TiO2 nanofiller on heropolyacid-impregnated PVDF polymer electrolyte for the performance of dye-sensitized solar cells. Phys. Status Solidi A 2009, 206, 343–350. [Google Scholar] [CrossRef]
- Ballav, N.; Biswas, M. Preparation and evaluation of a nanocomposite of polythiophen with Al2O3. Polym. Int. 2003, 52, 179–184. [Google Scholar] [CrossRef]
- Yang, B.D.; Yoon, K.H.; Chung, K.W. Effect of TiO2 and SiO2 nanoparticles on the stability of poly(p-phenylene vinylene) precursor. Synth. Met. 2004, 143, 25–29. [Google Scholar] [CrossRef]
- Yang, S.H.; Nguyen, T.P.; Le Rendu, P.; Hsu, C.S. Optical properties of PPV/SiO2 and PPV/TiO2 composite materials. Compos. Pt. A-Appl. Sci. Manuf. 2005, 36, 509–519. [Google Scholar] [CrossRef]
- Xu, J.C; Liu, W.M.; Li, H.L. Titanium dioxide doped polyaniline. Mater. Sci. Eng. C 2005, 25, 444–447. [Google Scholar] [CrossRef]
- Gangopadhyay, R.; De, A. Conducting polymer nanocomposites: A brief overview. Chem. Mater. 2000, 12, 608–622. [Google Scholar] [CrossRef]
- Merhari, L.; Gonsalves, K.E.; Hu, Y.; He, W.; Huang, W.S.; Angelopoulus, M.; Bruenger, W.H.; Dzionk, C.; Torkler, M. Nanocomposite resist systems for next generation lithography. Microelectron. Eng. 2002, 63, 391–403. [Google Scholar] [CrossRef]
- Wang, Y.W.; Yen, C.T.; Chen, W.C. Photosensitive polyimide/silica hybrid optical materials: Synthesis, properties, and patterning. Polymer 2005, 46, 6959–6967. [Google Scholar] [CrossRef]
- Mueller, C.; Hanemann, T.; Wiche, G.; Kumar, C.; Goettert, J. Fabrication of ceramic microcomponents using deep X-ray lithography. Microsyst. Technol. 2005, 11, 271–277. [Google Scholar]
- Jiguet, S.; Bertsch, A.; Hofmann, H.; Renaud, P. SU8-silver photosensitive nanocomposite. Adv. Eng. Mater. 2004, 6, 719–724. [Google Scholar] [CrossRef]
- Hanemann, T.; Mueller, C.; Schulz, M. Filled Resist Systems. In Advanced Micro & Nanosystems. LIGA and its Applications; Saile, V., Wallrabe, U., Tabata, O., Korvink, J.G., Eds.; Wiley-VCH: Weinheim, Germany, 2009; Volume 7, pp. 415–441. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: a review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Schmitt, L.; Lurtz, C.; Sternberg, K.; Haubold, A.; Schmitz, K.P.; Behrendt, D. Correlation of ultrastructure with mechanical properties of nano-hybrid dental composites. Adv. Eng. Mater.-Adv. Biomater. 2009, 11, B137–B143. [Google Scholar] [CrossRef]
- Dang, Z.M.; Tian, C.Y.; Zha, J.W.; Yao, S.H.; Xia, Y.J.; Li, J.Y.; Shi, C.Y.; Bai, J. Potential bioelectroactive bone regeneration polymer nanocomposite with high dielectric permittivity. Adv. Eng. Mater.-Adv. Biomater. 2009, 11, B144–B147. [Google Scholar] [CrossRef]
- Rhee, S. H.; Choi, J. Y. Preparation of a bioactive poly(methyl methacrylate)/silica nanocomposite. J. Am. Ceram. Soc. 2002, 85, 1318–1320. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468-3517. https://doi.org/10.3390/ma3063468
Hanemann T, Szabó DV. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials. 2010; 3(6):3468-3517. https://doi.org/10.3390/ma3063468
Chicago/Turabian StyleHanemann, Thomas, and Dorothée Vinga Szabó. 2010. "Polymer-Nanoparticle Composites: From Synthesis to Modern Applications" Materials 3, no. 6: 3468-3517. https://doi.org/10.3390/ma3063468
APA StyleHanemann, T., & Szabó, D. V. (2010). Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials, 3(6), 3468-3517. https://doi.org/10.3390/ma3063468