Surface Engineering and Patterning Using Parylene for Biological Applications
Abstract
:1. Introduction
2. Background
2.1. History and Chemical Vapour Deposition of Parylene

2.2. Types of Parylene
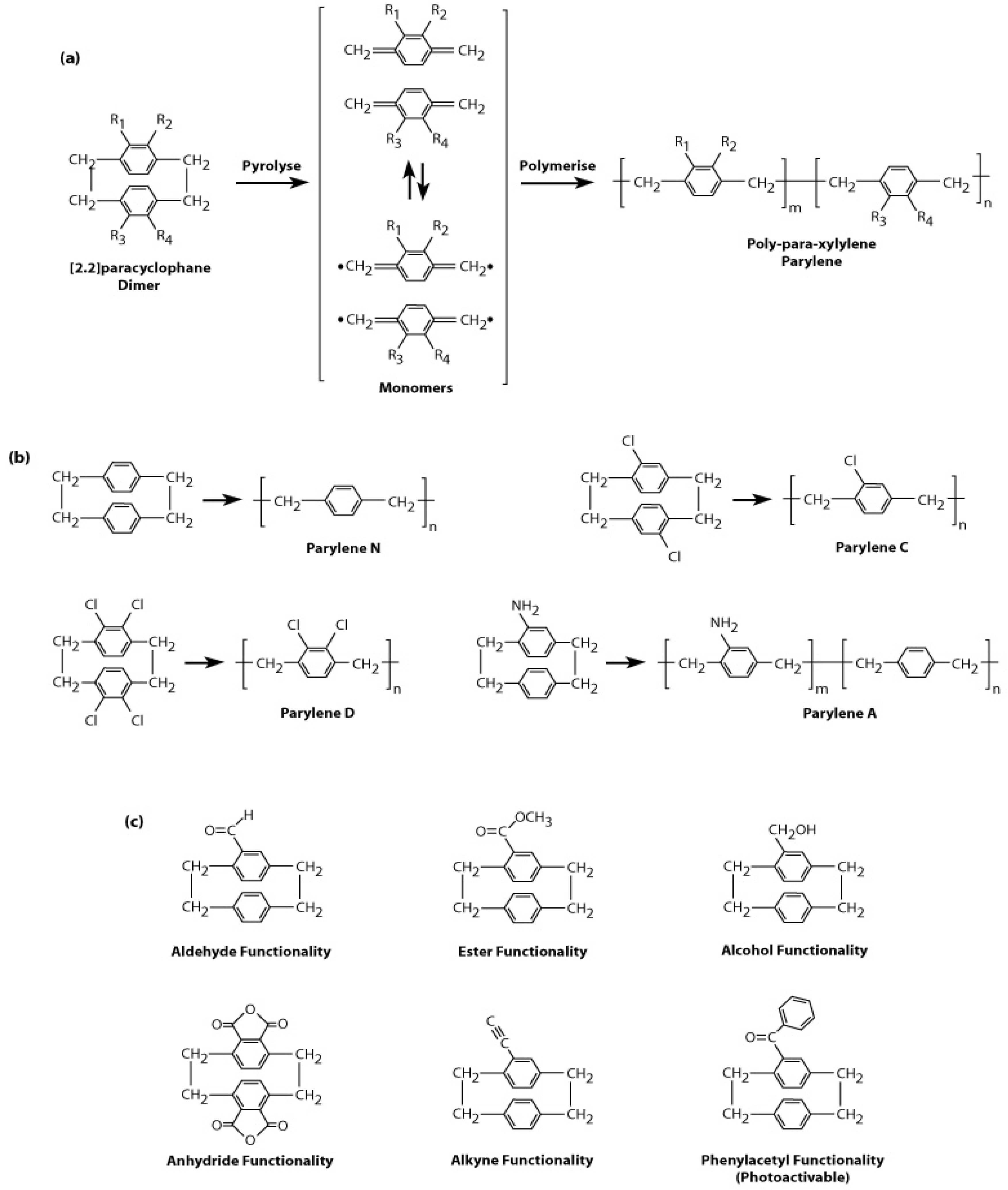
2.3. Material Properties and Applications of Parylene
| Parylene-N | Parylene-C | Parylene-D | |
|---|---|---|---|
| Density (g/cm3) | 1.11 | 1.289 | 1.418 |
| Refractive Index | 1.661 | 1.639 | 1.669 |
| Melting Point (°C) | 420 | 290 | 380 |
| Dielectric Constant (60Hz) | 2.65 | 3.15 | 2.84 |
| Tensile Modulus (GPa) | 2.4 | 3.2 | 2.84 |
| Elongation to Break (%) | 30 | 200 | 10 |
| Static Coefficient of Friction | 0.25 | 0.29 | 0.31 |
| Dynamic Coefficient of Friction | 0.25 | 0.29 | 0.31 |
| Water Absorption (% in 24h) | <0.1 | <0.1 | <0.1 |
| Oxygen Gas Permeability (cc.mm)/(m2.day) | 15.4 | 2.8 | 12.6 |
| Water Vapour Transmission Rate (g.mm)/(m2.day) | 0.59 | 0.08 | 0.09 |
| Water Contact Angle | 79° | 87° | 97° |
2.4. Post-Coating Surface Modification of Parylene

3. Micropatterning Using Parylene “Peel-Off” Stencils
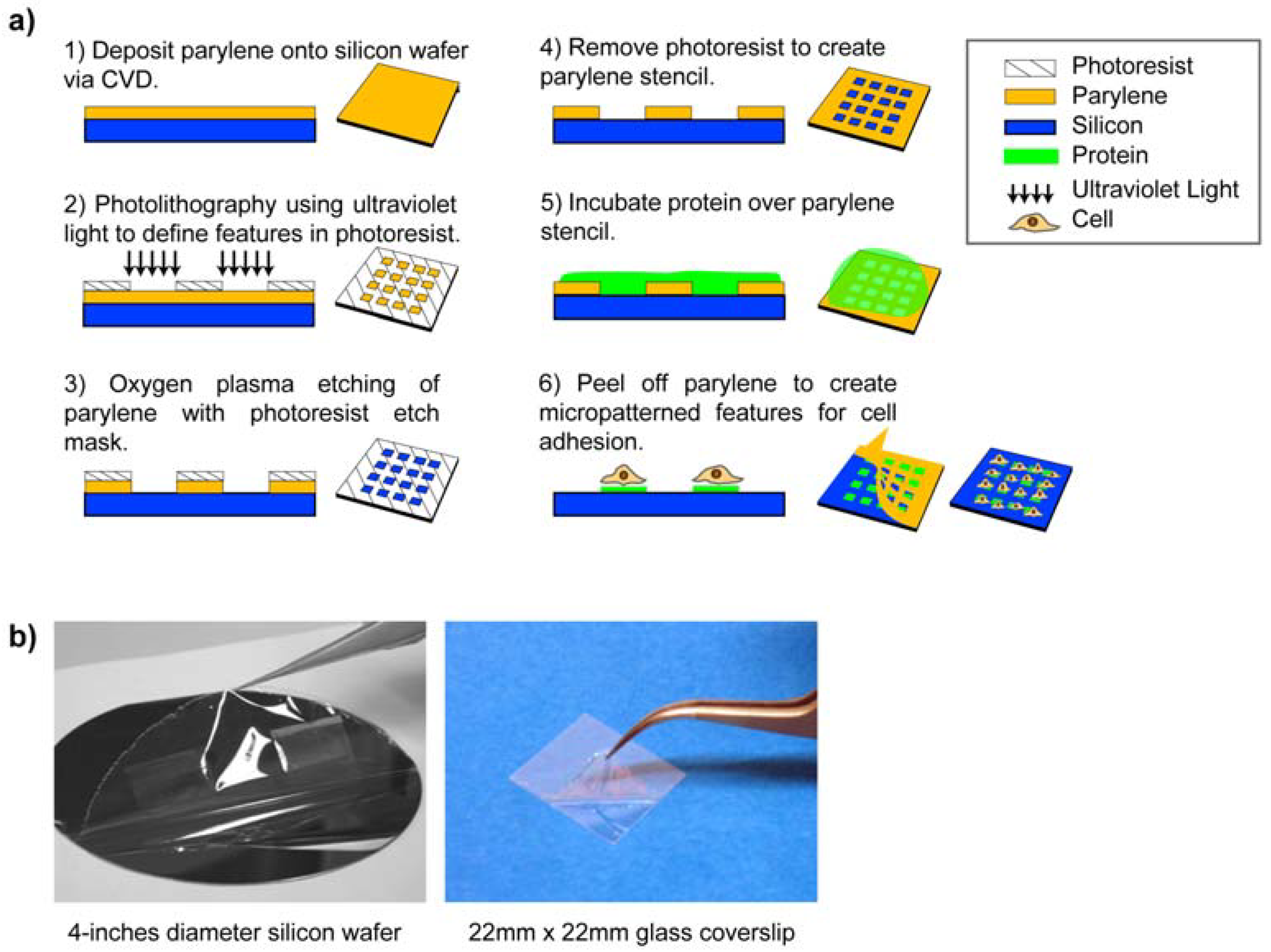
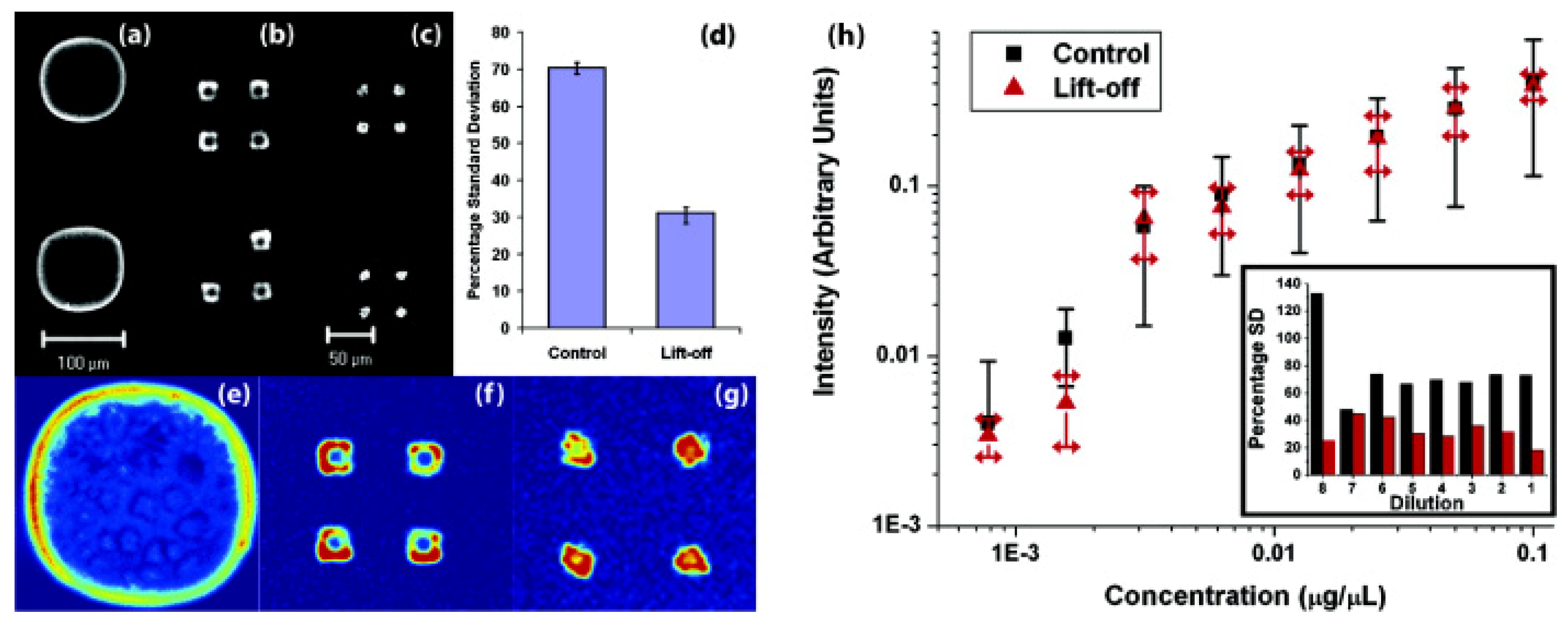
3.1. Multi-Component Protein Arrays with Nanoscale Resolution


3.2. Controlling Deoxyribonucleic Acid (DNA) Microarray Spot Morphology
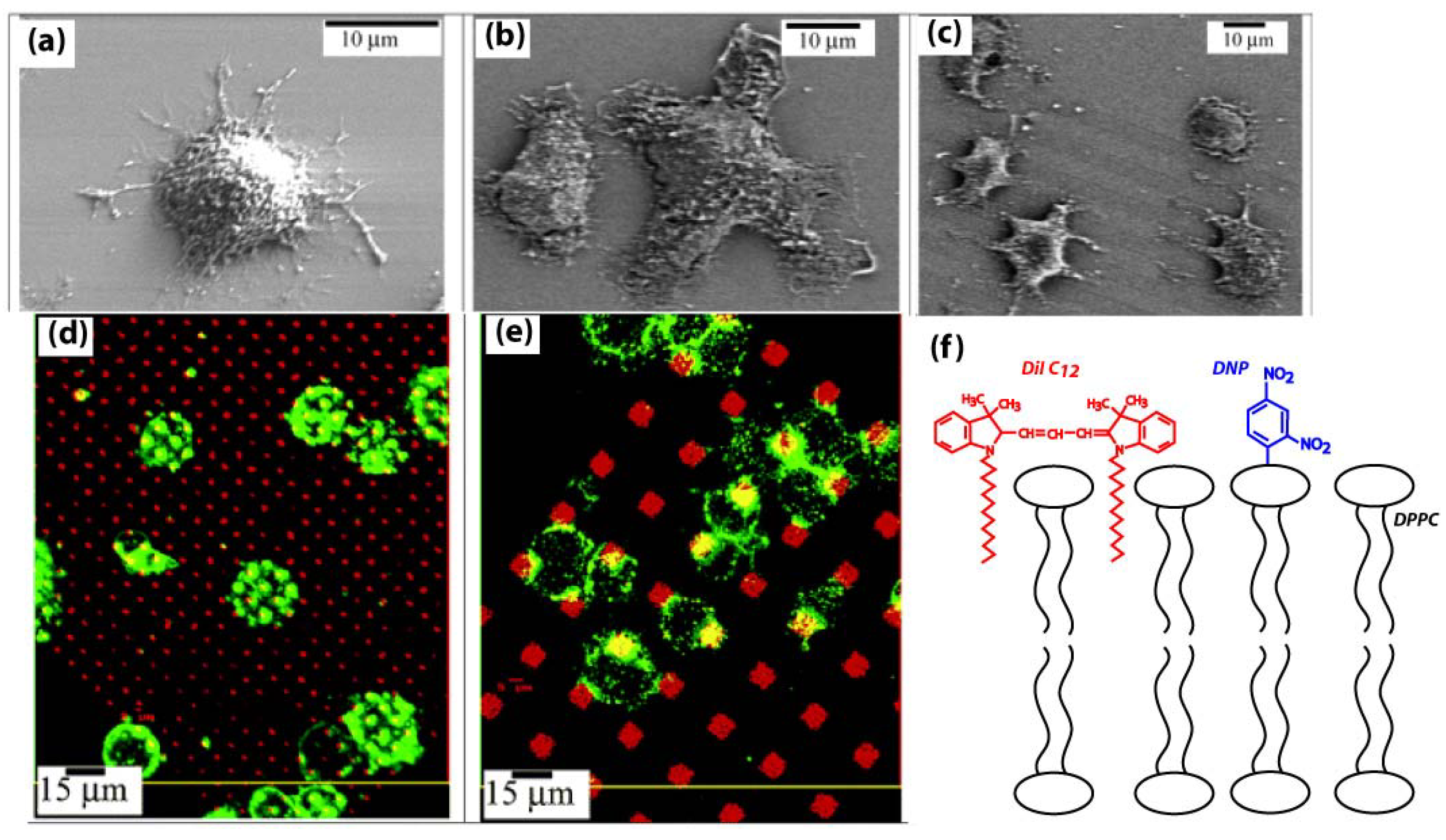
3.3. Patterned Lipid Bilayers for Immune Cell Activation
3.4. Patterning Biomolecules Inside Microfludic Channels

3.5. Cell Culture Arrays
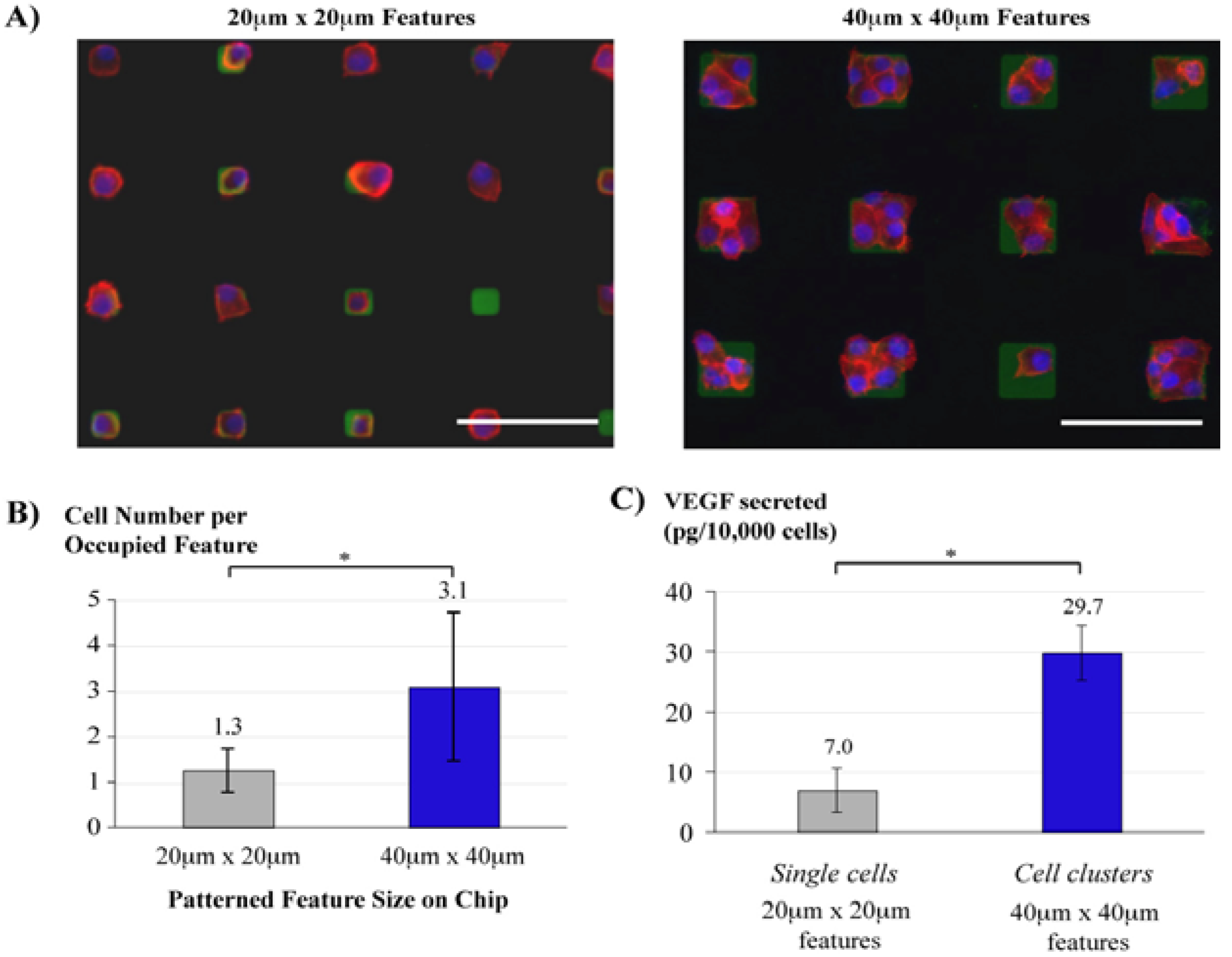
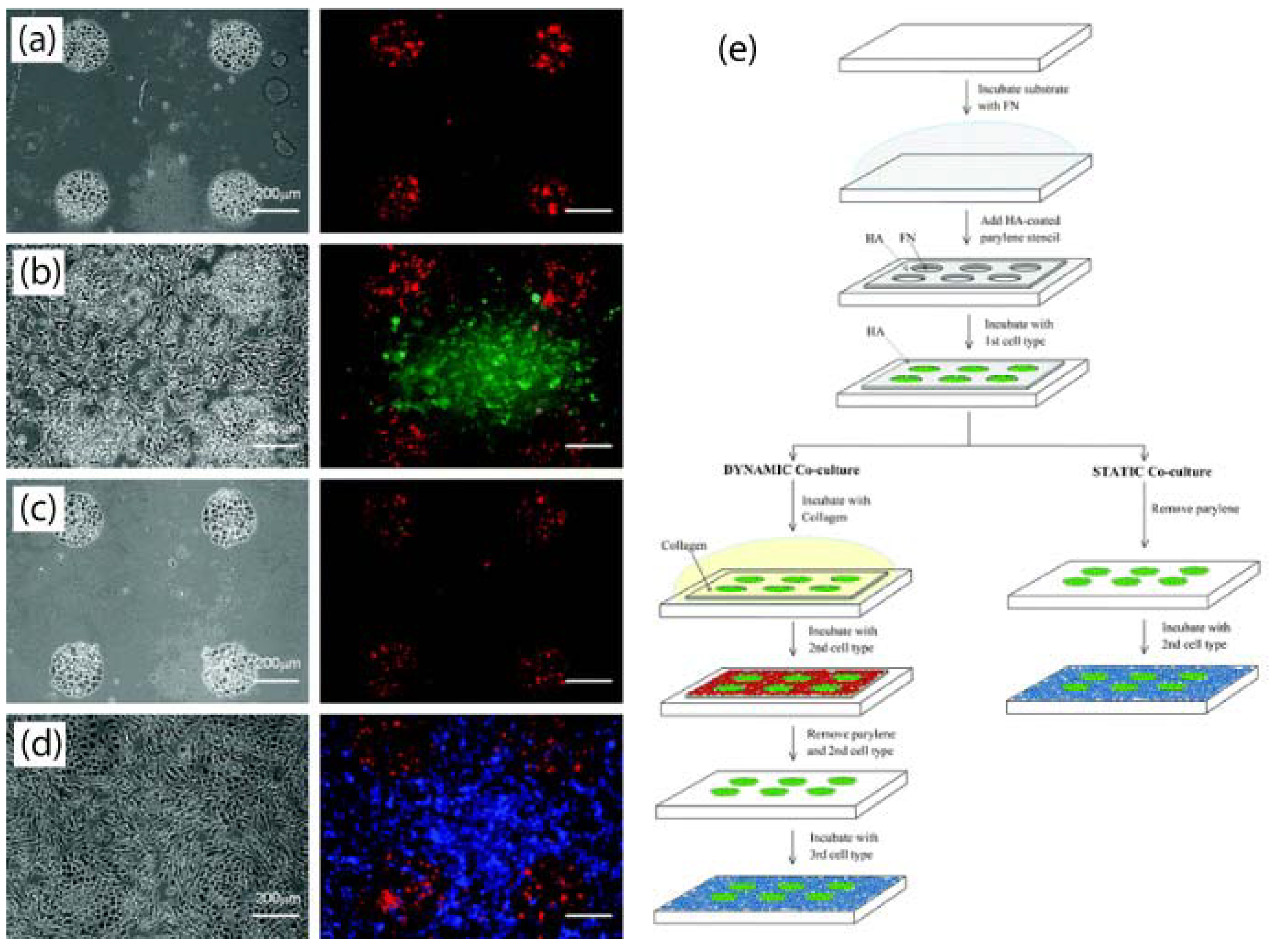
3.6. Multilayer Parylene Stencils

3.7. Biophysical Studies and High-Resolution Optical Imaging
4. Reactive Parylene Coatings
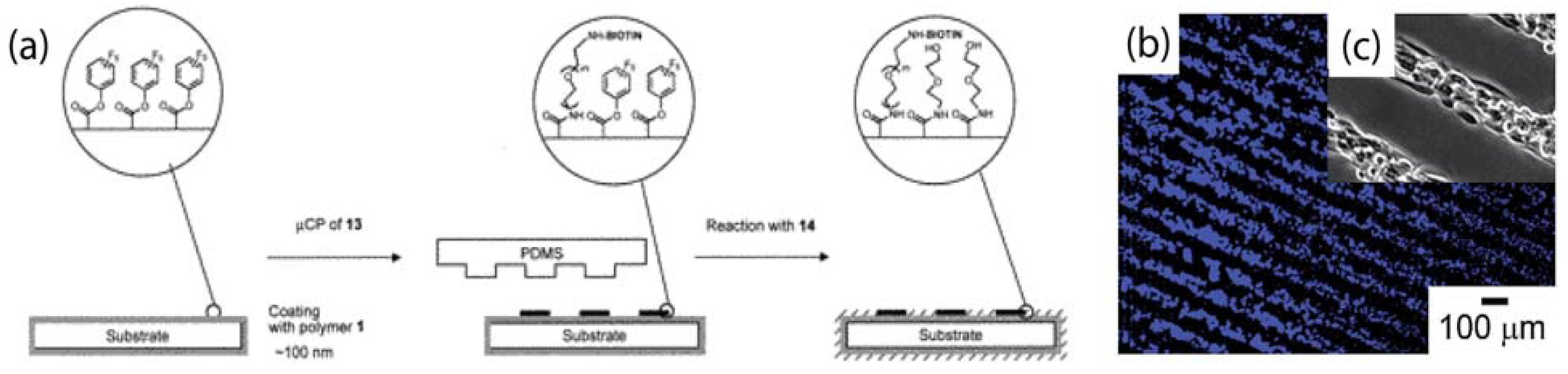
4.1. Functionalised Microfluidic Channels

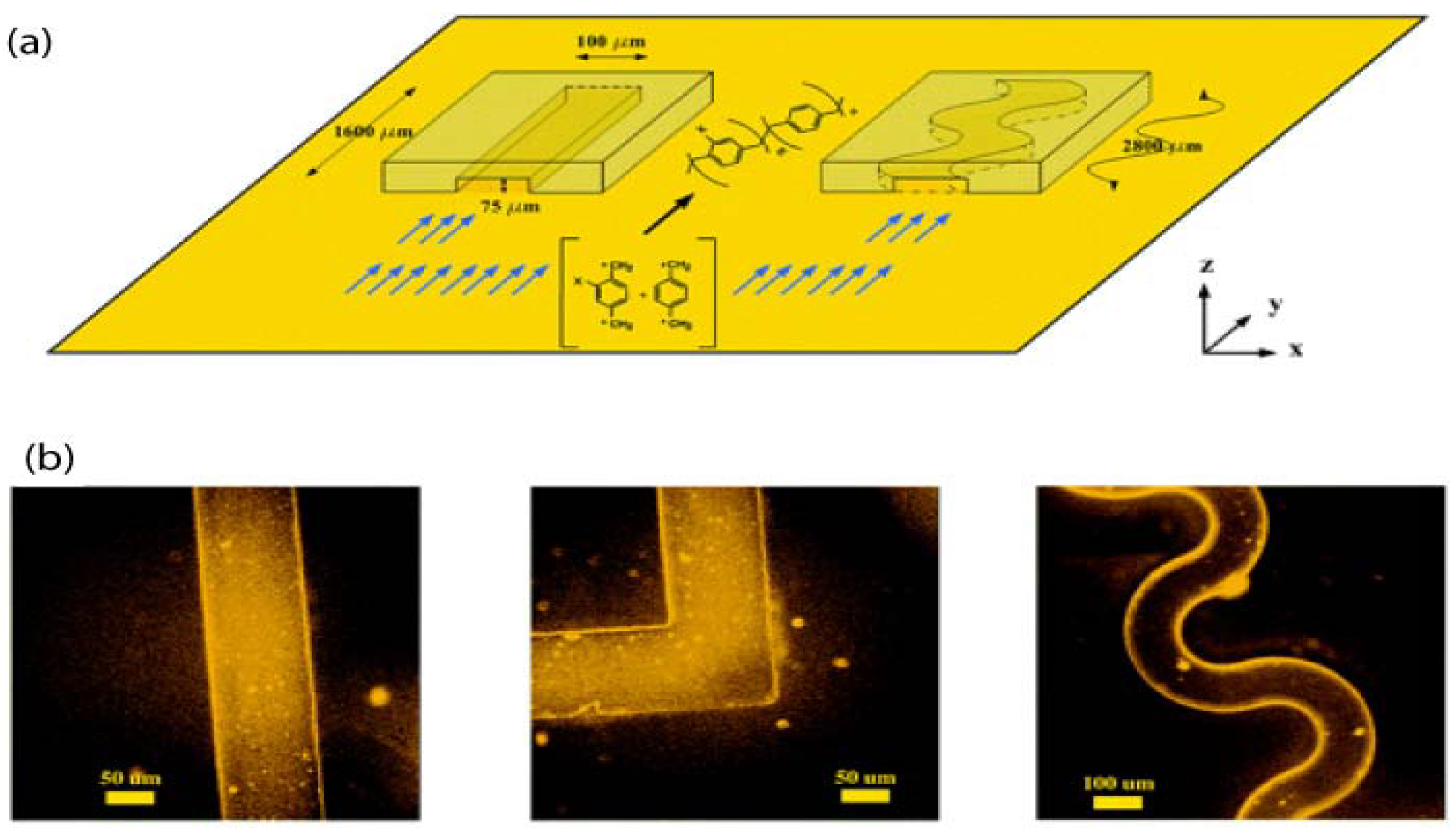
4.2. Vapour Assisted Microstructures Using Replicas and Templates
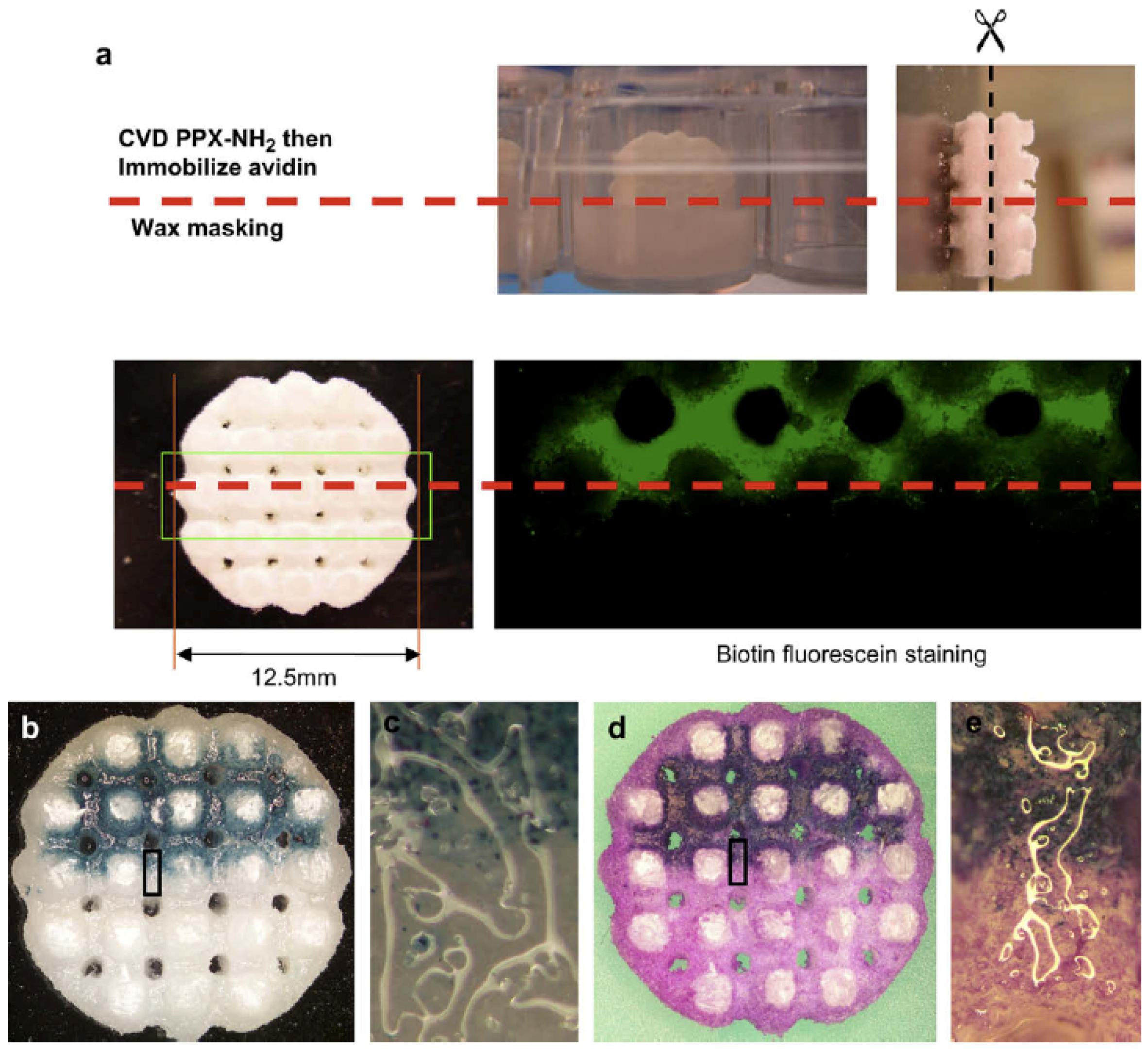
4.3. Three-Dimensional Scaffolds for Adenovirus Gene Delivery
5. Parylene Microstructures
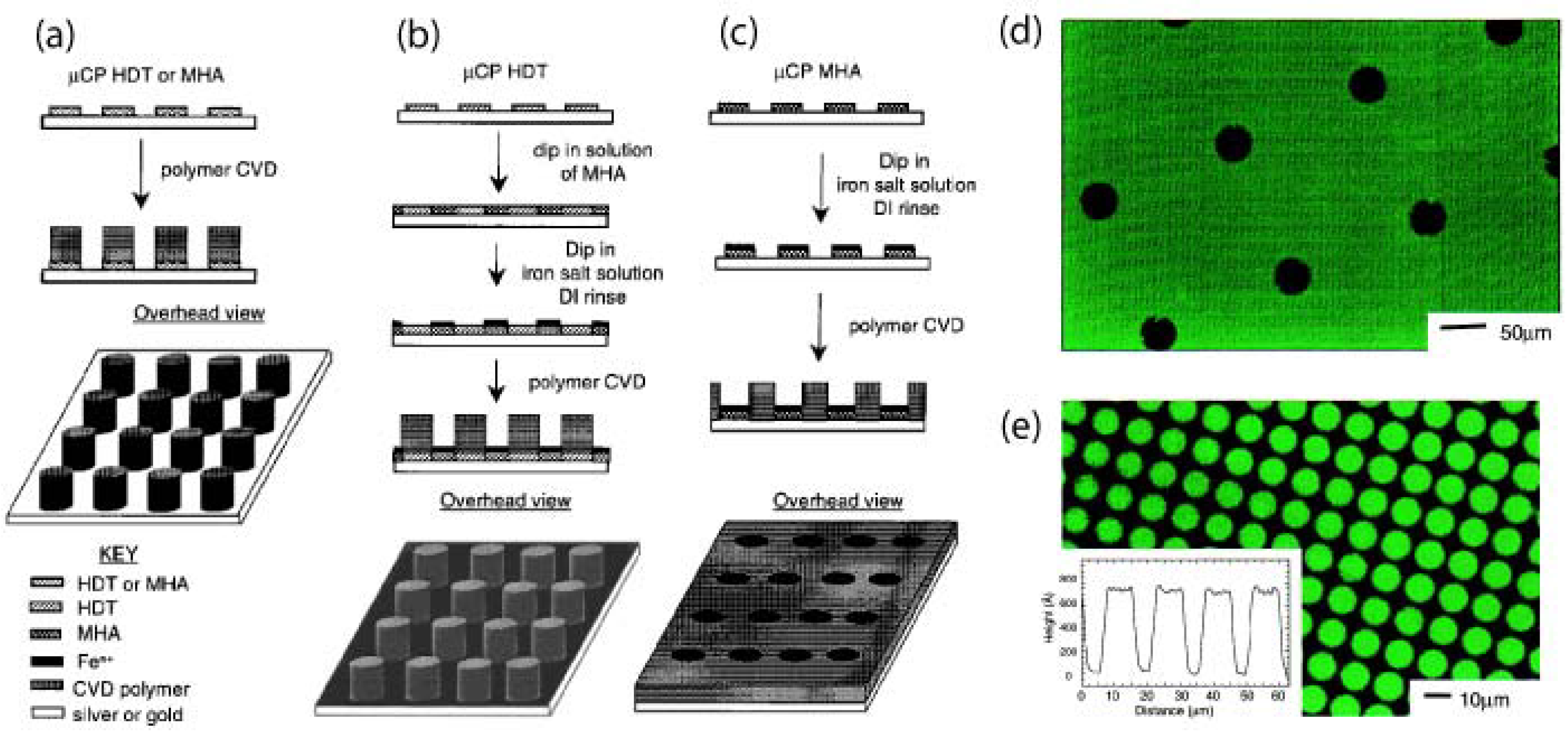
5.1. Substrate-Selective Vapour Deposition
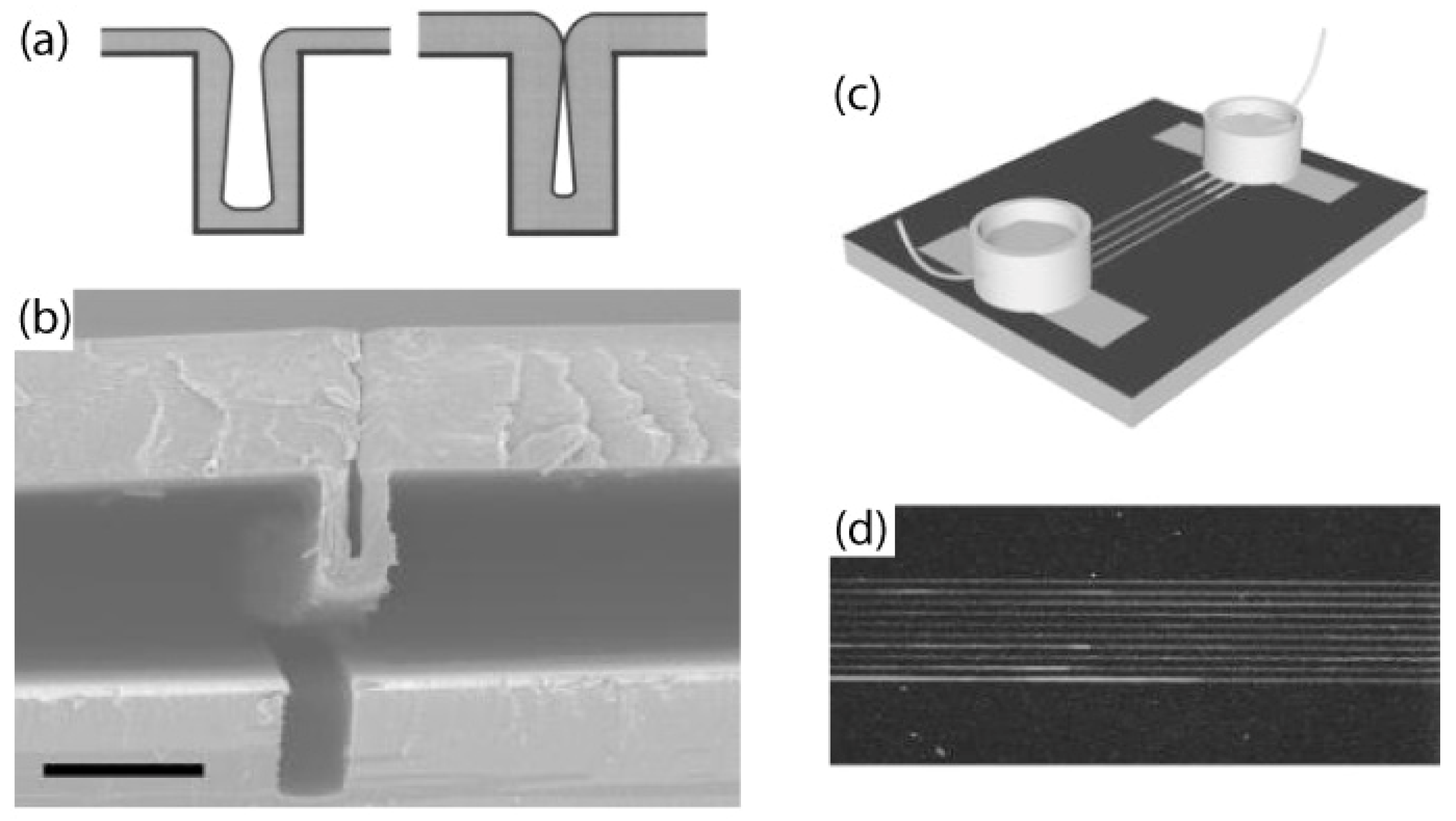
5.2. Parylene Tubes and Microfluidics


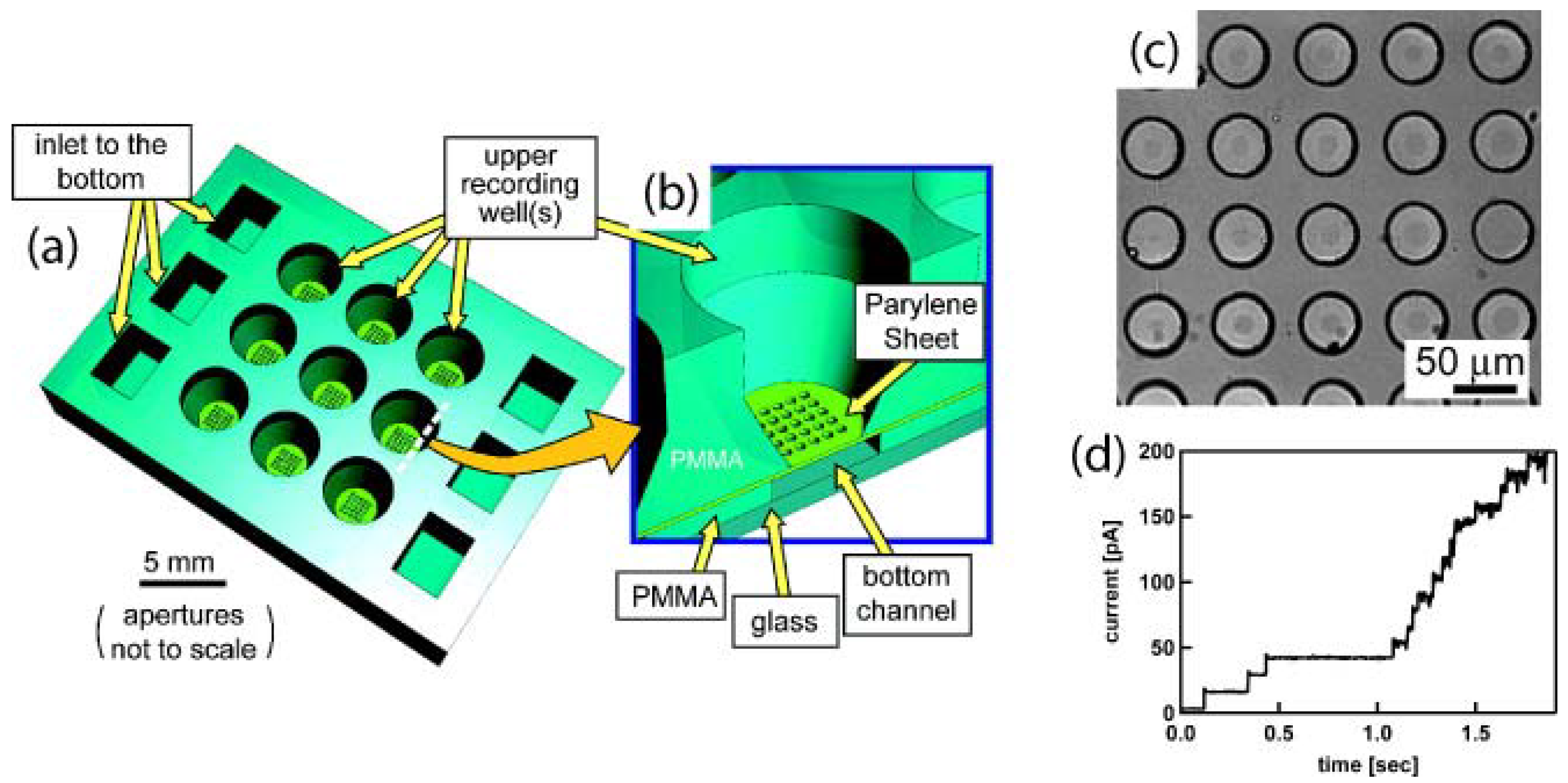
5.3. Micromachined Structures and Membranes

6. Future Outlook and Conclusions
Acknowledgements
References and Notes
- Christman, K.L.; Schopf, E.; Broyer, R.M.; Li, R.C.; Chen, Y.; Maynard, H.D. Positioning multiple proteins at the nanoscale with electron beam cross-linked functional polymers. J. Am. Chem. Soc. 2009, 131, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Coyer, S.R.; Garcia, A.J.; Delamarche, E. Facile preparation of complex protein architectures with sub-100-nm resolution on surfaces. Angew. Chem. Int. Ed. 2007, 46, 6837–6840. [Google Scholar] [CrossRef]
- Lee, K.B.; Park, S.J.; Mirkin, C.A.; Smith, J.C.; Mrksich, M. Protein nanoarrays generated by dip-pen nanolithography. Science 2002, 295, 1702–1705. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blummel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of integrin function by nanopatterned adhesive interfaces. Chem. Phys. Chem. 2004, 5, 383–388. [Google Scholar] [PubMed]
- Takayama, S.; Ostuni, E.; LeDuc, P.; Naruse, K.; Ingber, D.E.; Whitesides, G.M. Laminar flows - subcellular positioning of small molecules. Nature 2001, 411, 1016–1016. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Cipriany, B.R.; Lin, D.M.; Craighead, H.G. Nanoscale resolution, multi-component biomolecular arrays generated by aligned printing with parylene peel-off. Nano Lett. 2010. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 2006, 442, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A. Micro and nanoengineering of the cell microenvironment. In Micro and Nanoengineering of the Cell Microenvironment; Artech House: Boston, MA, USA, 2008. [Google Scholar]
- Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J.P. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA 2006, 103, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Nandivada, H.; Chen, H.Y.; Elkasabi, Y.; Lahann, J. Reactive polymer coatings for biological applications. In Polymers for biomedical applications; American Chemical Society: Washington, DC, USA, 2008; pp. 283–298. [Google Scholar]
- Szwarc, M. New monomers of the quinoid type and their polymers. J. Polym. Sci. 1951, 6, 319–329. [Google Scholar] [CrossRef]
- Gorham, W.F. A new general synthetic method for preparation of linear poly-p-xylylenes. J. Polym. Sci. A 1966, 4, 3027–3039. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.M. Deposition kinetics for polymerization via the Gorham route. In Chemical Vapor Deposition Polymerization—The Growth and Properties of Parylene Thin Films; Kluwer Academic Publishers: Norwell, MA, USA, 2004; pp. 41–55. [Google Scholar]
- Vaeth, K.M.; Jackman, R.J.; Black, A.J.; Whitesides, G.M.; Jensen, K.F. Use of microcontact printing for generating selectively grown films of poly(p-phenylene vinylene) and parylenes prepared by chemical vapor deposition. Langmuir 2000, 16, 8495–8500. [Google Scholar] [CrossRef]
- Vaeth, K.M.; Jensen, K.F. Transition metals for selective chemical vapor deposition of parylene-based polymers. Chem. Mater. 2000, 12, 1305–1313. [Google Scholar] [CrossRef]
- Vaeth, K.M.; Jensen, K.F. Selective growth of poly(p-phenylene vinylene) prepared by chemical vapor deposition. Adv. Mater. 1999, 11, 814–820. [Google Scholar] [CrossRef]
- Xu, Y.; Tai, Y.C. Selective Deposition of Parylene C for Underwater Shear-Stress Sensors. In Proceedings of TRANSDUCERS 2003, The 12th International Conference on Solid-State Sensors, Actuators and Microsystems, Boston, MA, USA, 8–12 June 2003; pp. 1307–1310.
- Charlson, E.M.; Charlson, E.J.; Sabeti, R. Temperature selective deposition of parylene-C. IEEE Trans. Biome. Eng. 1992, 39, 202–206. [Google Scholar] [CrossRef]
- Gazicki, M.; Surendran, G.; James, W.; Yasuda, H. Polymerization of para-xylylene derivatives (parylene polymerization). 3. Heat-effects during deposition of parylene-N at different temperatures. J. Polym. Sci. A 1986, 24, 215–240. [Google Scholar] [CrossRef]
- Gazicki, M.; Surendran, G.; James, W.; Yasuda, H. Polymerization of para-xylylene derivatives (parylene polymerization). 2. Heat-effects during deposition of parylene-C at different temperatures. J. Polym. Sci. A 1985, 23, 2255–2277. [Google Scholar]
- Ilic, B.; Czaplewski, D.; Zalalutdinov, M.; Schmidt, B.; Craighead, H.G. Fabrication of flexible polymer tubes for micro and nanofluidic applications. J. Vac. Sci. Technol. B. 2002, 20, 2459–2465. [Google Scholar] [CrossRef]
- Chen, P.J.; Shih, C.Y.; Tai, Y.C. Design, fabrication and characterization of monolithic embedded parylene microchannels in silicon substrate. Lab. Chip. 2006, 6, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Lahann, J.; Langer, R. Novel poly(p-xylylenes): Thin films with tailored chemical and optical properties. Macromolecules 2002, 35, 4380–4386. [Google Scholar] [CrossRef]
- Wahjudi, P.N.; Oh, J.H.; Salman, S.O.; Seabold, J.A.; Rodger, D.C.; Tai, Y.C.; Thompson, M.E. Improvement of metal and tissue adhesion on surface-modified parylene C. J. Biomed. Mater. Res. 2009, 89A, 206–214. [Google Scholar]
- Lahann, J.; Hocker, H.; Langer, R. Synthesis of amino[2.2]paracyclophanes - beneficial monomers for bioactive coating of medical implant materials. Angew. Chem., Int. Ed. 2001, 40, 726–728. [Google Scholar] [CrossRef]
- Nandivada, H.; Chen, H.Y.; Lahann, J. Vapor-based synthesis of poly [(4-formyl-p-xylylene)-co-(p-xylylene)] and its use for biomimetic surface modifications. Macromol. Rapid Commun. 2005, 26, 1794–1799. [Google Scholar] [CrossRef]
- Lahann, J.; Balcells, M.; Rodon, T.; Lee, J.; Choi, I.S.; Jensen, K.F.; Langer, R. Reactive polymer coatings: A platform for patterning proteins and mammalian cells onto a broad range of materials. Langmuir 2002, 18, 3632–3638. [Google Scholar] [CrossRef]
- Nandivada, H.; Chen, H.Y.; Bondarenko, L.; Lahann, J. Reactive polymer coatings that "Click". Angew. Chem. Int. Ed. 2006, 45, 3360–3363. [Google Scholar] [CrossRef]
- Lahann, J.; Langer, R. Surface-initiated ring-opening polymerization of epsilon-caprolactone from a patterned poly(hydroxymethyl-p-xylylene). Macromol. Rapid Commun. 2001, 22, 968–971. [Google Scholar] [CrossRef]
- Suh, K.Y.; Langer, R.; Lahann, J. A novel photodefinable reactive polymer coating and its use for microfabrication of hydrogel elements. Adv. Mater. 2004, 16, 1401–1405. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lahann, J. Fabrication of discontinuous surface patterns within microfluidic channels using photodefinable vapor-based polymer coatings. Anal. Chem. 2005, 77, 6909–6914. [Google Scholar] [CrossRef] [PubMed]
- Lahann, J.; Klee, D.; Pluester, W.; Hoecker, H. Bioactive immobilization of r-hirudin on cvd-coated metallic implant devices. Biomaterials 2001, 22, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Lahann, J.; Balcells, M.; Lu, H.; Rodon, T.; Jensen, K.F.; Langer, R. Reactive polymer coatings: A first step toward surface engineering of microfluidic devices. Anal. Chem. 2003, 75, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Elkasabi, Y.; Chen, H.Y.; Lahann, J. Multipotent polymer coatings based on chemical vapor deposition copolymerization. Adv. Mater. 2006, 18, 1521–1526. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.M. Introduction: Types of parylene and its applications. In Chemical Vapor Deposition Polymerization—The Growth and Properties of Parylene Thin Films; Kluwer Academic Publishers: Norwell, MA, USA, 2004; p. 7. [Google Scholar]
- Fortin, J.B.; Lu, T.M. Film properties: Chemical properties and biocompatibility. In Chemical Vapor Deposition Polymerization—The Growth and Properties of Parylene Thin Films; Kluwer Academic Publishers: Norwell, MA, USA, 2004; p. 62. [Google Scholar]
- Ilic, B.; Craighead, H.G. Topographical patterning of chemically sensitive biological materials using a polymer-based dry lift off. Biomed. Microdevices 2000, 2, 317–322. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.M. Film properties. In Chemical Vapor Deposition Polymerization—The Growth and Properties of Parylene Thin Films; Kluwer Academic Publishers: Norwell, MA, USA, 2004; p. 58. [Google Scholar]
- Hwang, K.S.; Park, J.H.; Lee, J.H.; Yoon, D.S.; Kim, T.S.; Han, I.; Noh, J.H. Effect of atmospheric-plasma treatments for enhancing adhesion of Au on parylene-c-coated protein chips. J. Korean Phys. Soc. 2004, 44, 1168–1172. [Google Scholar]
- Chang, T.Y.; Yadav, V.G.; De Leo, S.; Mohedas, A.; Rajalingam, B.; Chen, C.L.; Selvarasah, S.; Dokmeci, M.R.; Khademhosseini, A. Cell and protein compatibility of parylene-c surfaces. Langmuir 2007, 23, 11718–11725. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Moran-Mirabal, J.M.; Brooks, D.J.; Ilic, B.R.; Fischbach, C.; Craighead, H.G. Surface Modification and Patterning of Biomolecules and Cells Using a Polymer Lift-off Template. In Proceedings of MicroTAS 2008, The Twelfth International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Diego, CA, USA, 12–16 October 2008; pp. 1552–1554.
- Lange, K.; Grimm, S.; Rapp, M. Chemical modification of parylene C coatings for saw biosensors. Sens. Actuators A Phys. 2007, 125, 441–446. [Google Scholar] [CrossRef]
- Wright, D.; Rajalingam, B.; Selvarasah, S.; Dokmeci, M.R.; Khademhosseini, A. Generation of static and dynamic patterned co-cultures using microfabricated parylene-c stencils. Lab Chip 2007, 7, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, K.; Hiratsuka, Y.; Yamamura, T.; Takeuchi, S. Sequential Parylene Lift-off Process for Selective Patterning of Biological Materials. In Proceedings of IEEE MEMS 2007, The 20th International Conference on Micro Electro Mechanical Systems, Kobe, Japan, 21–25 January 2007; pp. 501–504.
- Jinno, S.; Moeller, H.C.; Chen, C.L.; Rajalingam, B.; Chung, B.G.; Dokmeci, M.R.; Khademhosseini, A. Microfabricated multilayer parylene-c stencils for the generation of patterned dynamic co-cultures. J. Biomed. Mater. Res. 2008, 86A, 278–288. [Google Scholar] [CrossRef]
- Wright, D.; Rajalingam, B.; Karp, J.M.; Selvarasah, S.; Ling, Y.B.; Yeh, J.; Langer, R.; Dokmeci, M.R.; Khademhosseini, A. Reusable, reversibly sealable parylene membranes for cell and protein patterning. J. Biomed. Mater. Res. 2008, 85A, 530–538. [Google Scholar] [CrossRef]
- Tan, C.P.; Seo, B.R.; Brooks, D.J.; Chandler, E.M.; Craighead, H.G.; Fischbach, C. Parylene peel-off arrays to probe the role of cell-cell interactions in tumour angiogenesis. Integr. Biol. 2009, 1, 587–594. [Google Scholar] [CrossRef]
- Pollack, M.G.; Fair, R.B.; Shenderov, A.D. Electrowetting-based actuation of liquid droplets for microfluidic applications. Appl. Phys. Lett. 2000, 77, 1725–1726. [Google Scholar] [CrossRef]
- Cho, S.K.; Moon, H.J.; Kim, C.J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar] [CrossRef]
- Moran-Mirabal, J.M.; Tan, C.P.; Orth, R.N.; Williams, E.O.; Craighead, H.G.; Lin, D.M. Controlling microarray spot morphology with polymer liftoff arrays. Anal. Chem. 2007, 79, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.N.; Kameoka, J.; Zipfel, W.R.; Ilic, B.; Webb, W.W.; Clark, T.G.; Craighead, H.G. Creating biological membranes on the micron scale: Forming patterned lipid bilayers using a polymer lift-off technique. Biophys. J. 2003, 85, 3066–3073. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.N.; Wu, M.; Holowka, D.A.; Craighead, H.G.; Baird, B.A. Mast cell activation on patterned lipid bilayers of subcellular dimensions. Langmuir 2003, 19, 1599–1605. [Google Scholar] [CrossRef]
- Morigaki, K.; Baumgart, T.; Jonas, U.; Offenhausser, A.; Knoll, W. Photopolymerization of diacetylene lipid bilayers and its application to the construction of micropatterned biomimetic membranes. Langmuir 2002, 18, 4082–4089. [Google Scholar] [CrossRef]
- Groves, J.T.; Boxer, S.G. Micropattern formation in supported lipid membranes. Acc. Chem. Res. 2002, 35, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Dusseiller, M.R.; Niederberger, B.; Stadler, B.; Falconnet, D.; Textor, M.; Voros, J. A novel crossed microfluidic device for the precise positioning of proteins and vesicles. Lab Chip 2005, 5, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, K.; Suzuki, H.; Takeuchi, S. A parylene lift-off process with microfluidic channels for selective protein patterning. J. Micromech. Microeng. 2007, 17, 496–500. [Google Scholar] [CrossRef]
- Moran-Mirabal, J.M.; Edel, J.B.; Meyer, G.D.; Throckmorton, D.; Singh, A.K.; Craighead, H.G. Micrometer-sized supported lipid bilayer arrays for bacterial toxin binding studies through total internal reflection fluorescence microscopy. Biophys. J. 2005, 89, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Delivopoulos, E.; Murray, A.F.; MacLeod, N.K.; Curtis, J.C. Guided growth of neurons and glia using microfabricated patterns of parylene-c on a sio2 background. Biomaterials 2009, 30, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Moran-Mirabal, J.M.; Santhanam, N.; Corgie, S.C.; Craighead, H.G.; Walker, L.P. Immobilization of cellulose fibrils on solid substrates for cellulase-binding studies through quantitative fluorescence microscopy. Biotechnol. Bioeng. 2008, 101, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yokokawa, R.; Suzuki, H.; Atsuta, K.; Fujita, H.; Takeuchi, S. Biomolecular linear motors confined to move upon micro-patterns on glass. J. Micromech. Microeng. 2006, 16, 1550–1554. [Google Scholar] [CrossRef]
- Torres, A.J.; Vasudevan, L.; Holowka, D.; Baird, B.A. Focal adhesion proteins connect ige receptors to the cytoskeleton as revealed by micropatterned ligand arrays. Proc. Natl. Acad. Sci. USA 2008, 105, 17238–17244. [Google Scholar] [CrossRef] [PubMed]
- Klee, D.; Ademovic, Z.; Bosserhoff, A.; Hoecker, H.; Maziolis, G.; Erli, H.J. Surface modification of poly(vinylidenefluoride) to improve the osteoblast adhesion. Biomaterials 2003, 24, 3663–3670. [Google Scholar] [CrossRef] [PubMed]
- Miwa, J.; Suzuki, Y.; Kasagi, N. Adhesion-based cell sorter with antibody-coated amino-functionalized-parylene surface. J. Microelectromech. Syst. 2008, 17, 611–622. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lahann, J. Vapor-assisted micropatterning in replica structures: A solventless approach towards topologically and chemically designable surfaces. Adv. Mater. 2007, 19, 3801–3808. [Google Scholar] [CrossRef]
- Chen, H.Y.; Elkasabi, Y.; Lahann, J. Surface modification of confined microgeometries via vapor-deposited polymer coatings. J. Am. Chem. Soc. 2006, 128, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Elkasabi, Y.; Chen, H.Y.; Zhang, Y.; Lahann, J.; Hollister, S.J.; Krebsbach, P.H. The use of reactive polymer coatings to facilitate gene delivery from poly (epsilon-caprolactone) scaffolds. Biomaterials 2009, 30, 5785–5792. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lai, J.H.; Jiang, X.W.; Lahann, J. Substrate-selective chemical vapor deposition of reactive polymer coatings. Adv. Mater. 2008, 20, 3474–3480. [Google Scholar] [CrossRef]
- Liu, M.C.; Ho, D.; Tai, Y.C. Monolithic fabrication of three-dimensional microfluidic networks for constructing cell culture array with an integrated combinatorial mixer. Sens. Actuators B 2008, 129, 826–833. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ziegler, D.; Yoshida, Y.; Mabuchi, K.; Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip 2005, 5, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.; Tooker, A.; Tai, Y.C.; Pine, J. Caged neuron MEA: A system for long-term investigation of cultured neural network connectivity. J. Neurosci. Methods 2008, 175, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.; Tai, Y.C.; Erickson, J.; Pine, J. Parylene Technology for Mechanically Robust Neurocages. In Proceedings of MicroTAS 2003, The Seventh International Conference on Micro Total Analysis Systems, Squaw Valley, CA, USA, 5–9 October 2003; pp. 1109–1112.
- Tooker, A.; Meng, E.; Erickson, J.; Tai, Y.C.; Pine, J. Biocompatible parylene neurocages. IEEE Eng. Med. Biol. Mag. 2005, 24, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Le Pioufle, B.; Suzuki, H.; Tabata, K.V.; Noji, H.; Takeuchi, S. Lipid bilayer microarray for parallel recording of transmembrane ion currents. Anal. Chem. 2008, 80, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Le Pioufle, B.; Takeuchi, S. Ninety-six-well planar lipid bilayer chip for ion channel recording fabricated by hybrid stereolithography. Biomed. Microdevices 2009, 11, 17–22. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, C.P.; Craighead, H.G. Surface Engineering and Patterning Using Parylene for Biological Applications. Materials 2010, 3, 1803-1832. https://doi.org/10.3390/ma3031803
Tan CP, Craighead HG. Surface Engineering and Patterning Using Parylene for Biological Applications. Materials. 2010; 3(3):1803-1832. https://doi.org/10.3390/ma3031803
Chicago/Turabian StyleTan, Christine P., and Harold G. Craighead. 2010. "Surface Engineering and Patterning Using Parylene for Biological Applications" Materials 3, no. 3: 1803-1832. https://doi.org/10.3390/ma3031803
APA StyleTan, C. P., & Craighead, H. G. (2010). Surface Engineering and Patterning Using Parylene for Biological Applications. Materials, 3(3), 1803-1832. https://doi.org/10.3390/ma3031803





