Antimicrobial Materials Used in Coating Dental Implant Surfaces: State of the Art and Future Prospectives
Abstract
1. Introduction
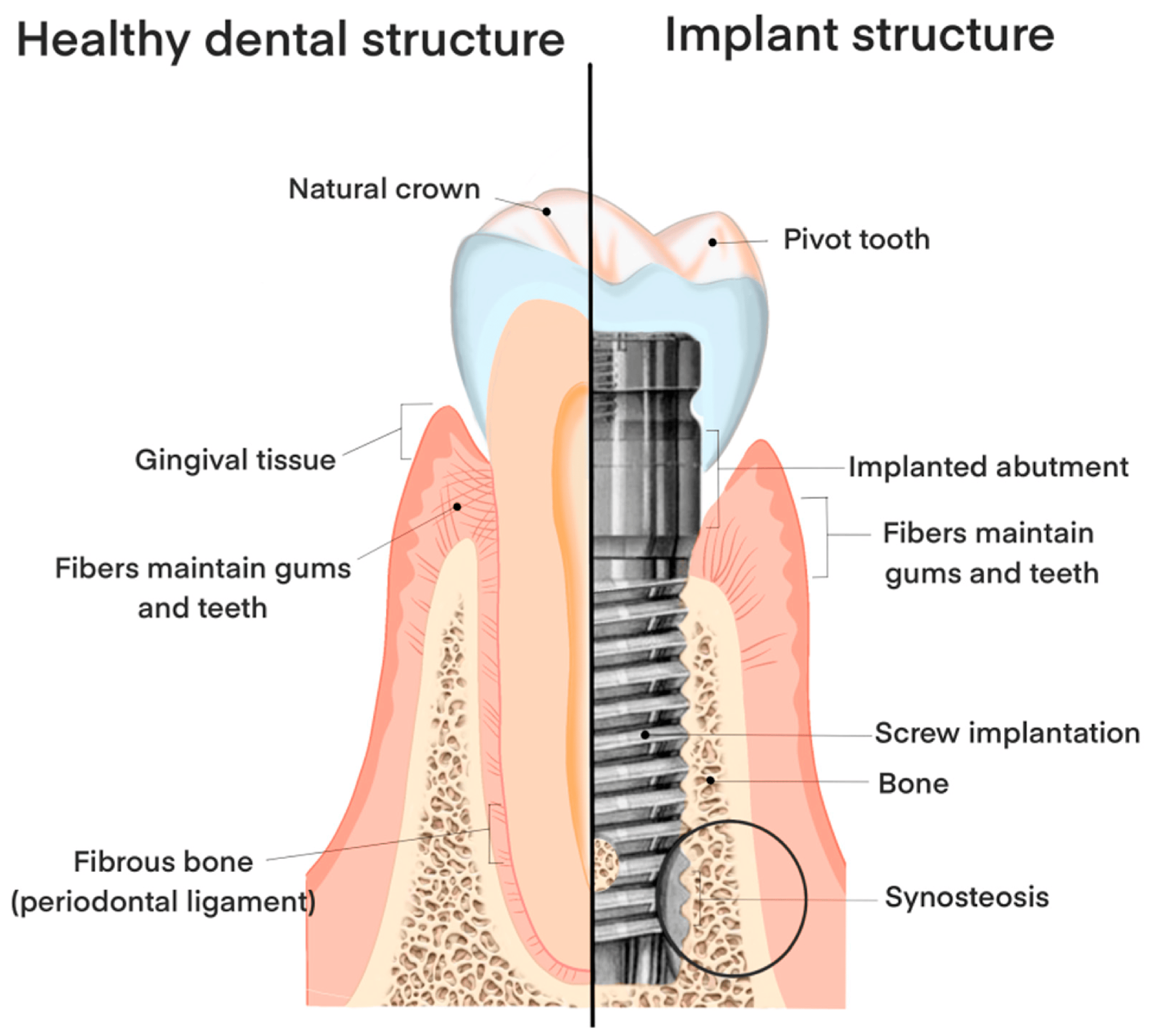
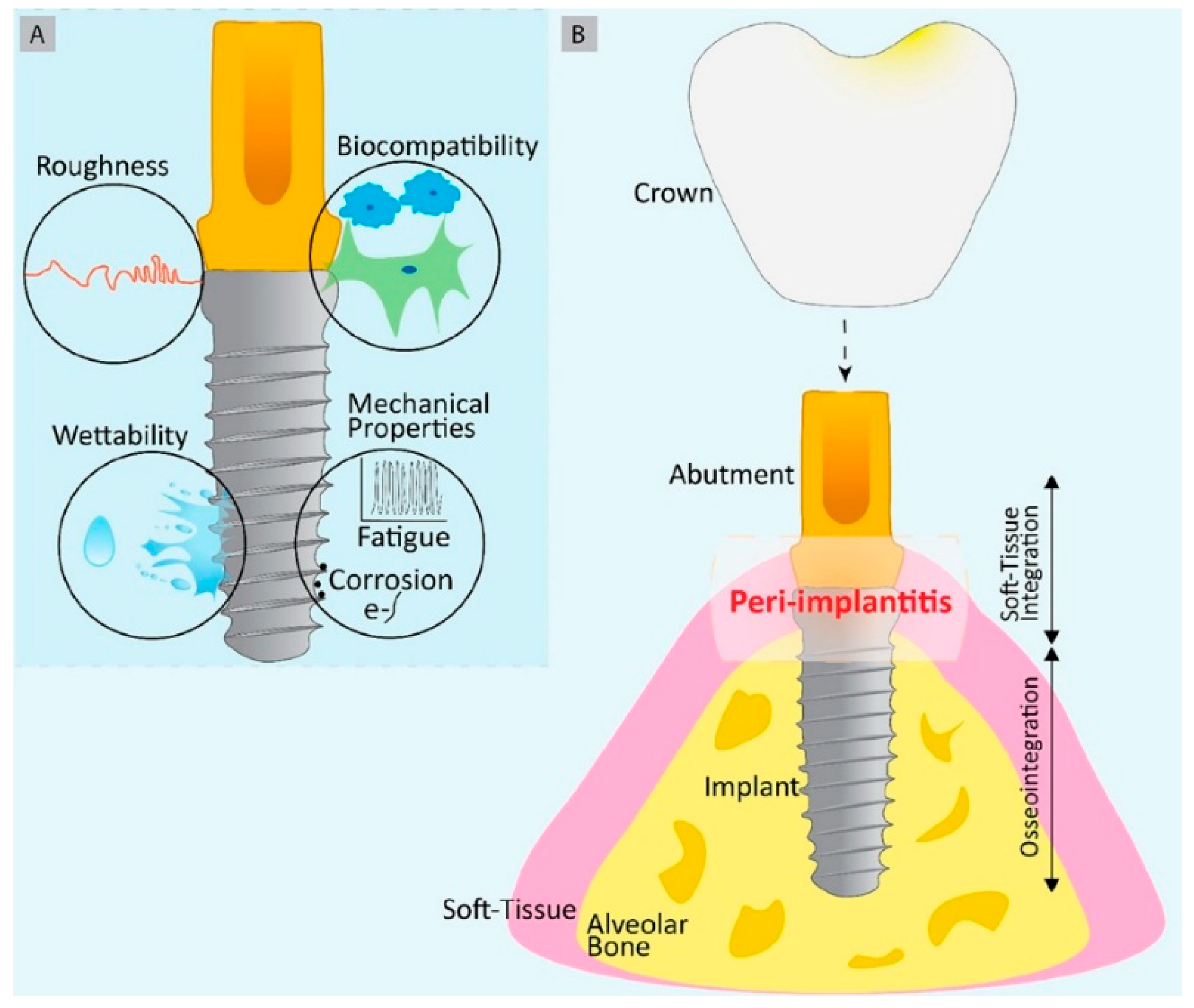
2. Challenges Associated with Dental Implants
2.1. Post Operative Infection
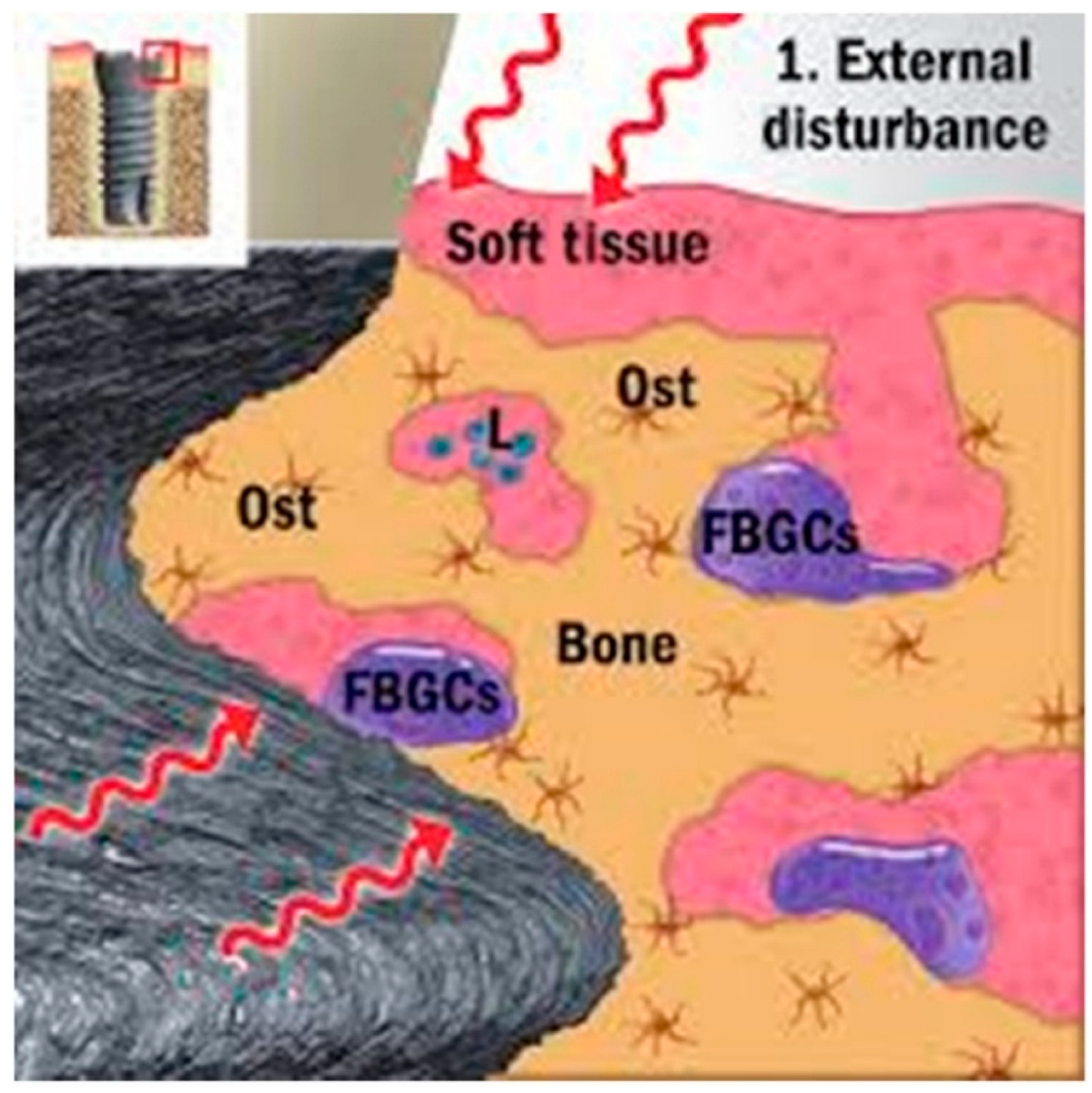
2.2. Implant Rejection
2.3. Allergic Reactions
2.4. Peri-Implantitis
2.5. Implant Failure
2.6. Bone Loss or Resorption
2.7. Esthetic Issue
3. Implant-Related Dental Infections
3.1. Microbiota of Oral Cavity and Dental Implants
3.2. Biofilms on Implants
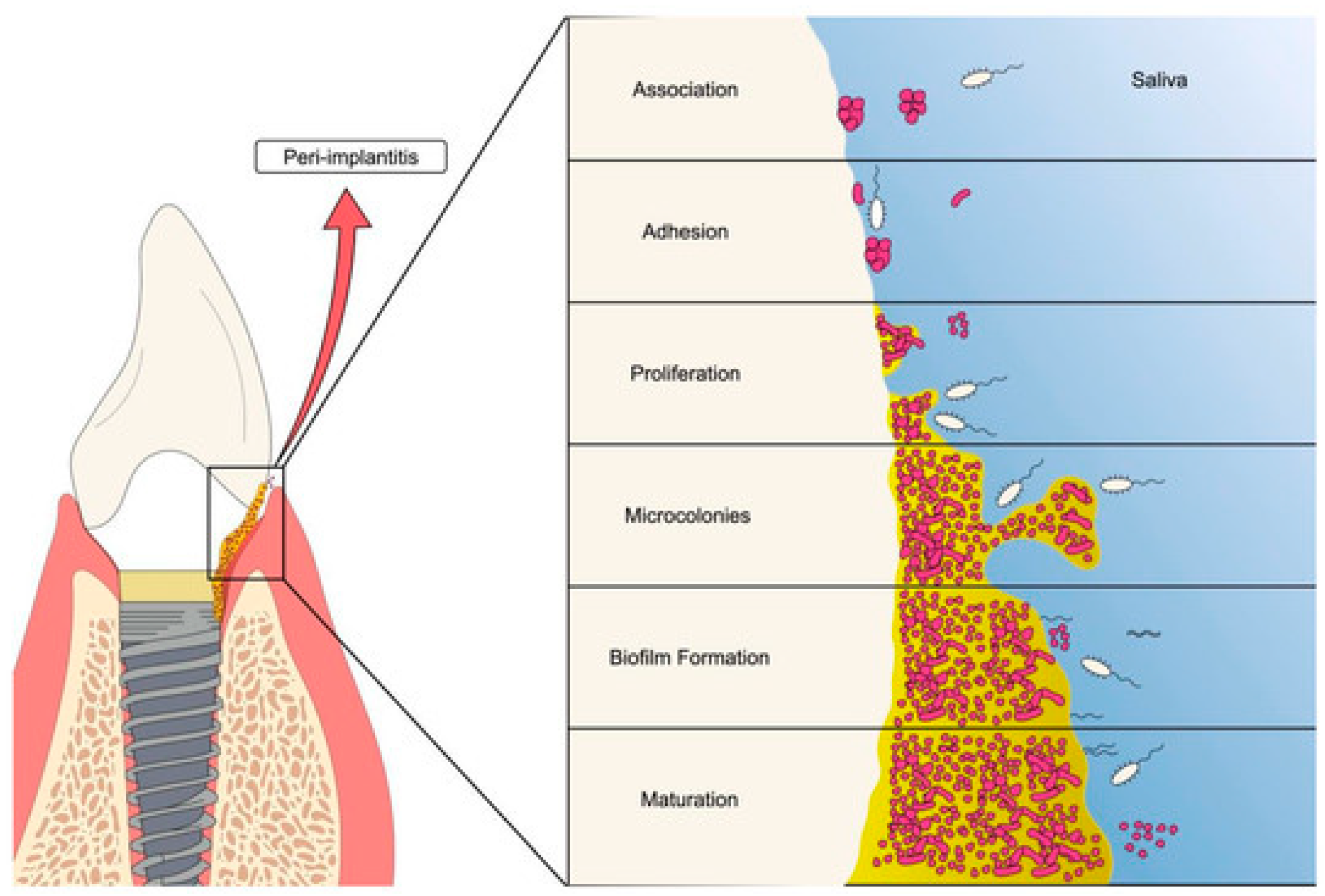
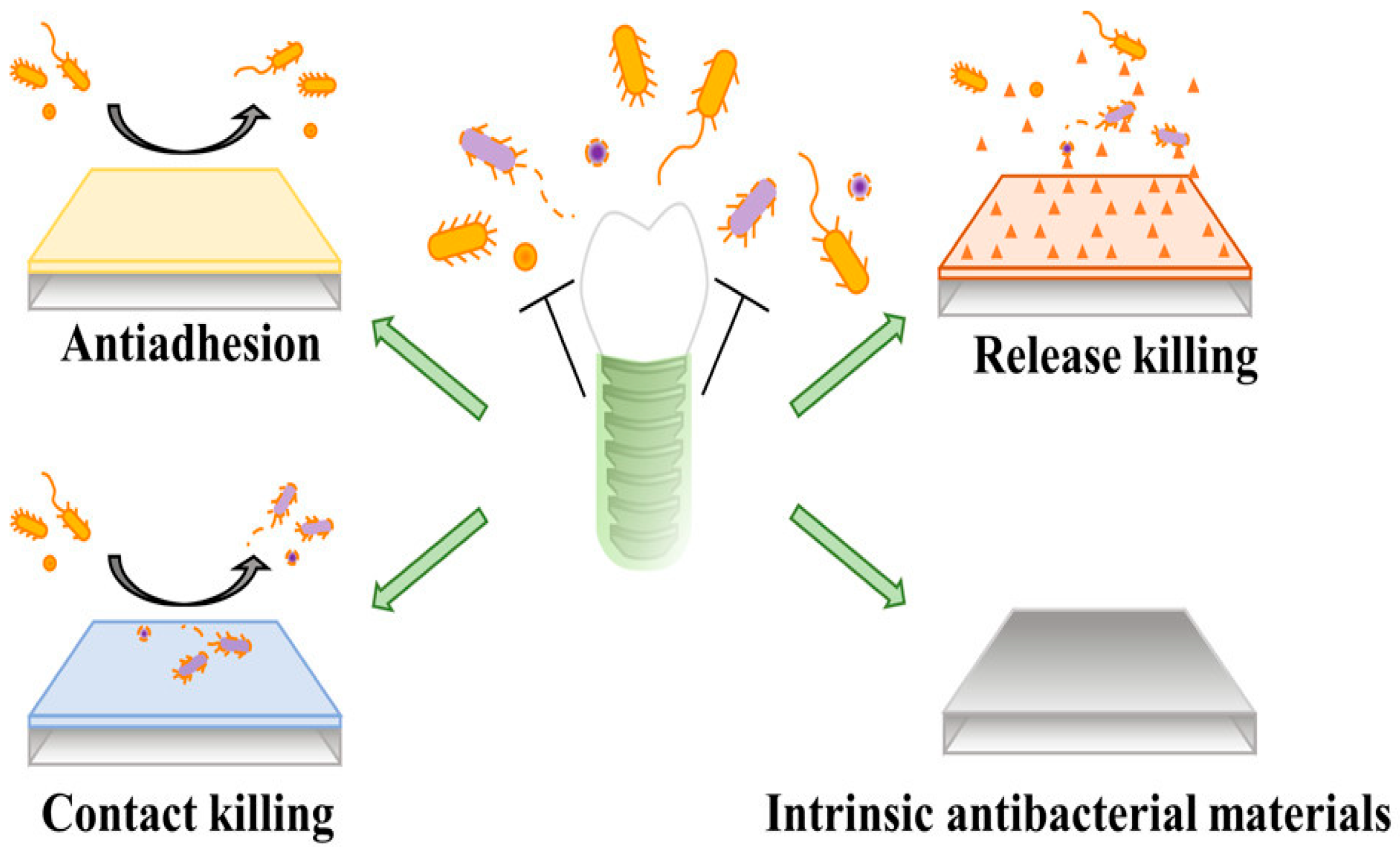
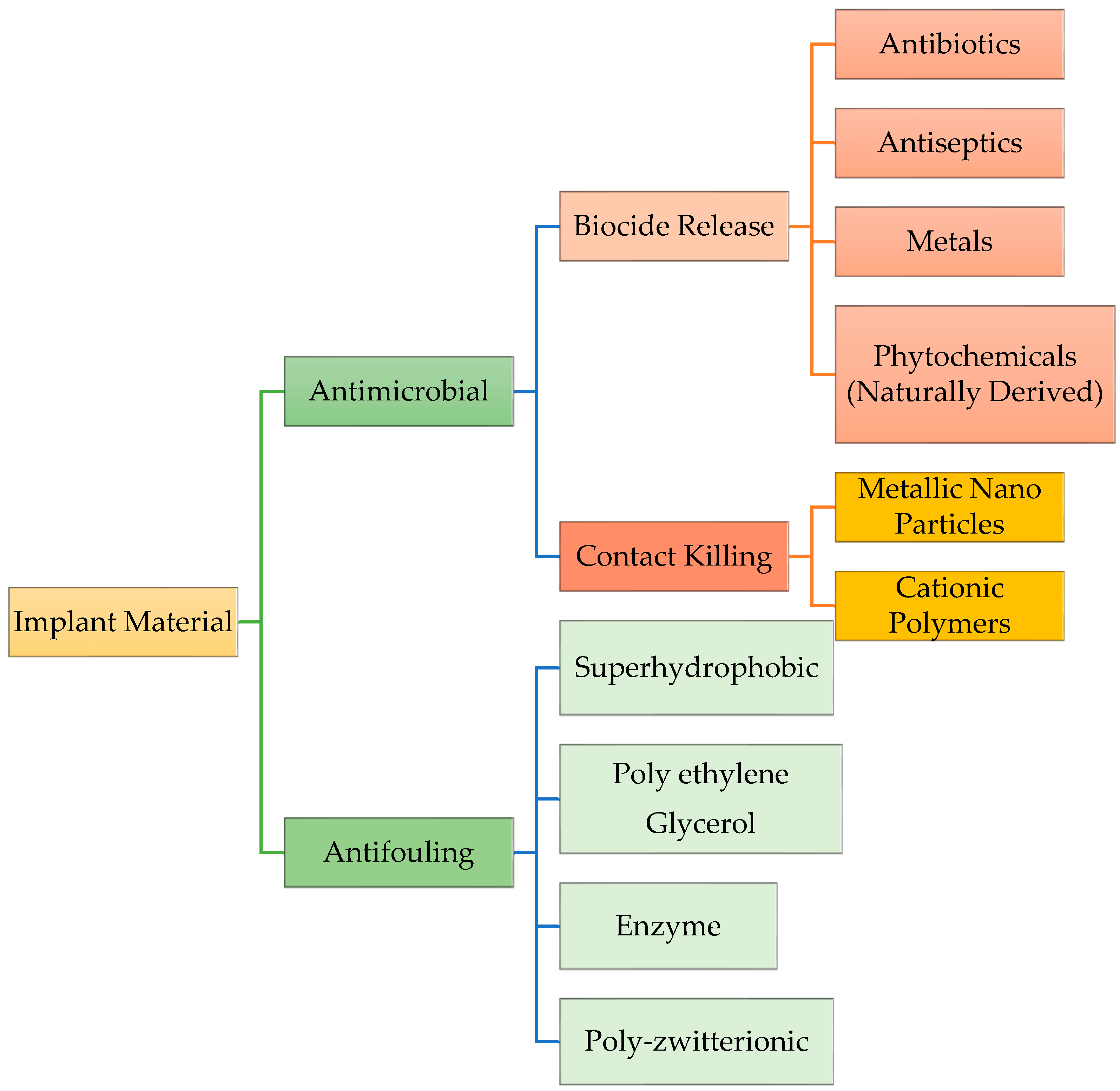
4. Dental Antimicrobial Approaches
4.1. AMPs
4.2. Metal-Releasing Coatings
4.3. Phytochemicals Used in Dental Materials (Phytodentistry)
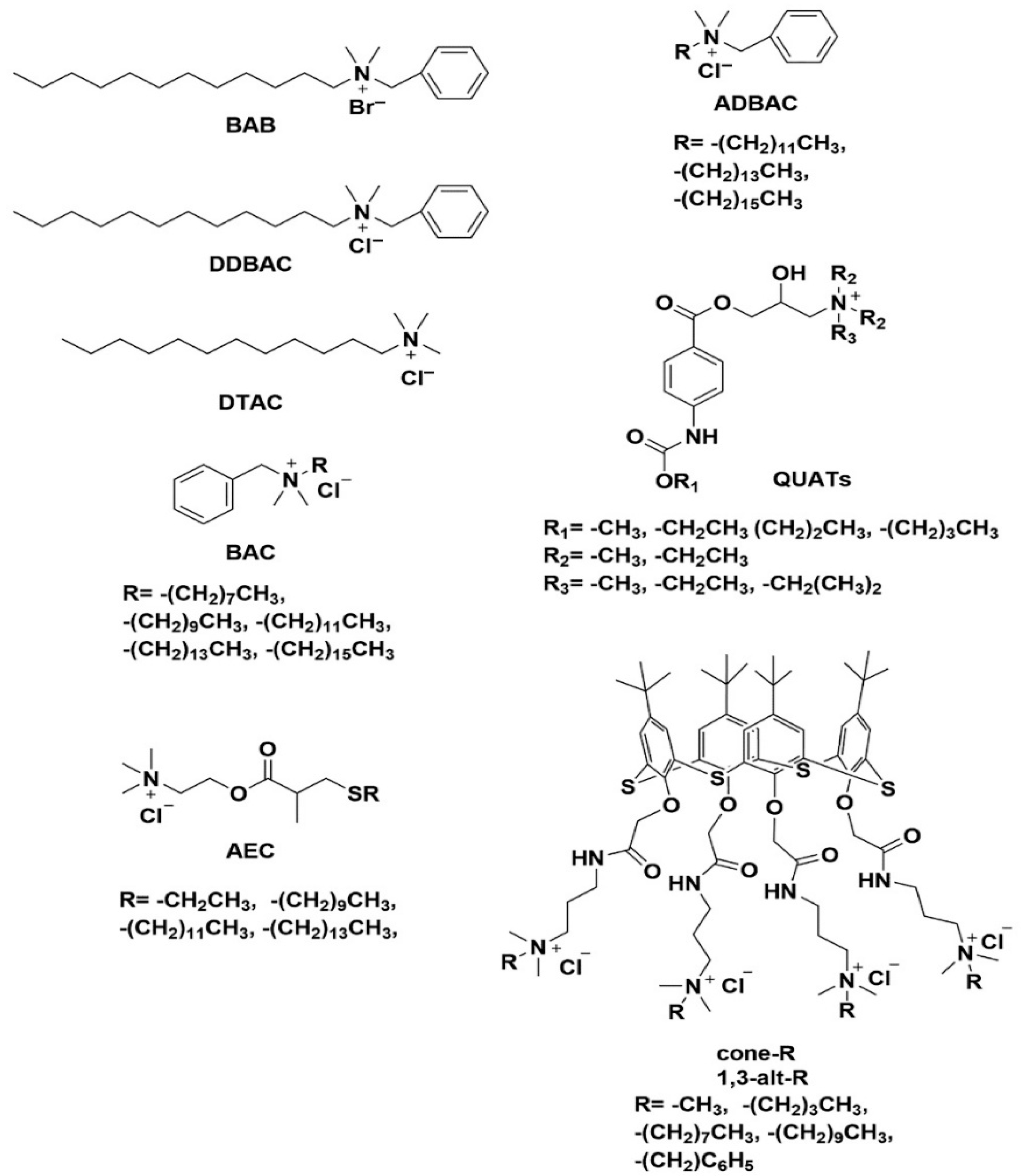
4.4. Quaternary Ammonium Compounds
- The additive contains the following compounds: n-dodecyl dimethyl benzyl ammonium chloride (CAS Reg. No. 139-07-1); n-dodecyl dimethyl ethylbenzyl ammonium chloride (CAS Reg. No. 27479-28-3); n-hexadecyl dimethyl benzyl ammonium chloride (CAS Reg. No. 122-18-9); n-octadecyl dimethyl benzyl ammonium chloride (CAS Reg. No. 122-19-0); n-tetradecyl dimethyl benzyl ammonium chloride (CAS Reg. No. 139-08-2); n-tetradecyl dimethyl ethylbenzyl ammonium chloride (CAS Reg. No. 27479-29-4).
- The composition meets the following specifications: pH (5 percent active solution) 7.0–8.0; total amines, maximum 1 percent as combined free amines and amine hydrochlorides.
- The compound is used as an antimicrobial agent, as defined [91] orally in food.
| Name of QAS | Target Bacterial Strain | Human Cell Toxicity | Reference | |
|---|---|---|---|---|
| Alkyl Dimethyl benzyl Ammonium Chloride (ADBAC) | S. aureus; MIC: 0.6 μg mL−1 | In chronic trials with beagles, mice, and rats, repeated dosage oral toxicity studies found no harmful effects at 10–93.1 mg/kg-day for DDAC and 3.7–188 mg/kg-day for ADBAC (C > 12). At modest adverse impact levels, DDAC and ADBAC (C > 12) consistently cause decreased food intake, average body weight, body weight growth, and localized discomfort. | [93,94] | |
| Dodecyl dimethyl benzyl ammonium chloride (DDBAC) | Listeria monocytogenes; E. coli; S. aureus | Cell viability (NIH-3T3 assays) was 39.7% within 24 hrs incubation at dose of 500 μg/mL, respectively. | [95] | |
| P-tert-butylthiacalix [4]arene (1,3-alt-R) | S. aureus, B. subtills, E.coli, P. aeruginosa | Toxicity tests on human skin cells showed less toxicity as compared to ref. drugs. | [96] | |
| Ammonium-esterified acrylate (AEC) | S. aureus; MIC: 3 ppm, E.coli; MIC: 31 ppm, P. aeruginosa; MIC: 250 ppm, Candida albicans, Aspergillus niger; Klebsiella pneumoniae; Acinetobacter baummanii | _ | [97] | |
| Didecyl dimethylammonium chloride (DDAC) | S. aureus; MIC: 1.63 µM, E.coli; MIC: 15. 63 µM, P. aeruginosa; MIC: 500 µM; K. pneumoniae; MIC: 11 µM, Enterococcus sp.;MIC: 3 µM. | Cell viability assays confirm a trend of a higher cytotoxicity in correlation with an increasing carbon chain length of the compounds. DDACs prove more effective as surface disinfectants than antiseptics, due to their hazardous potential and wide range of selectivity for bacteria. | [98] | |
| N,N-dialkyl-N-(2-hydroxyethyl)-N-methylammonium salts (NDMAC) | S. aureus; MIC: 0.9 µM, E.coli; MIC: 7.8 µM, P. aeruginosa; MIC: 500 µM | |||
| N-[N′(3-gluconamide)propyl-N′-alkyl]propyl-N,N-dimethyl-N-alkyl ammonium bromide (CDDGPB) | S. aureus; MIC: 150 ppm, E.coli; MIC: 150 ppm, | The mortality of mice test group was the highest, with an LD50 of mice larger than 100 mg/kg, indicating that the surfactant has medium toxicity. The mortality of mice in the C10DDGPB test group was significantly lower than that in the C12DDGPB test group. No obvious blackening or body stiffness was observed in any of the tested animals during the 14 day observation period. | [99] | |
5. Bioactive Dental Materials
5.1. Properties of Biomaterials
5.2. Metallic Substrates
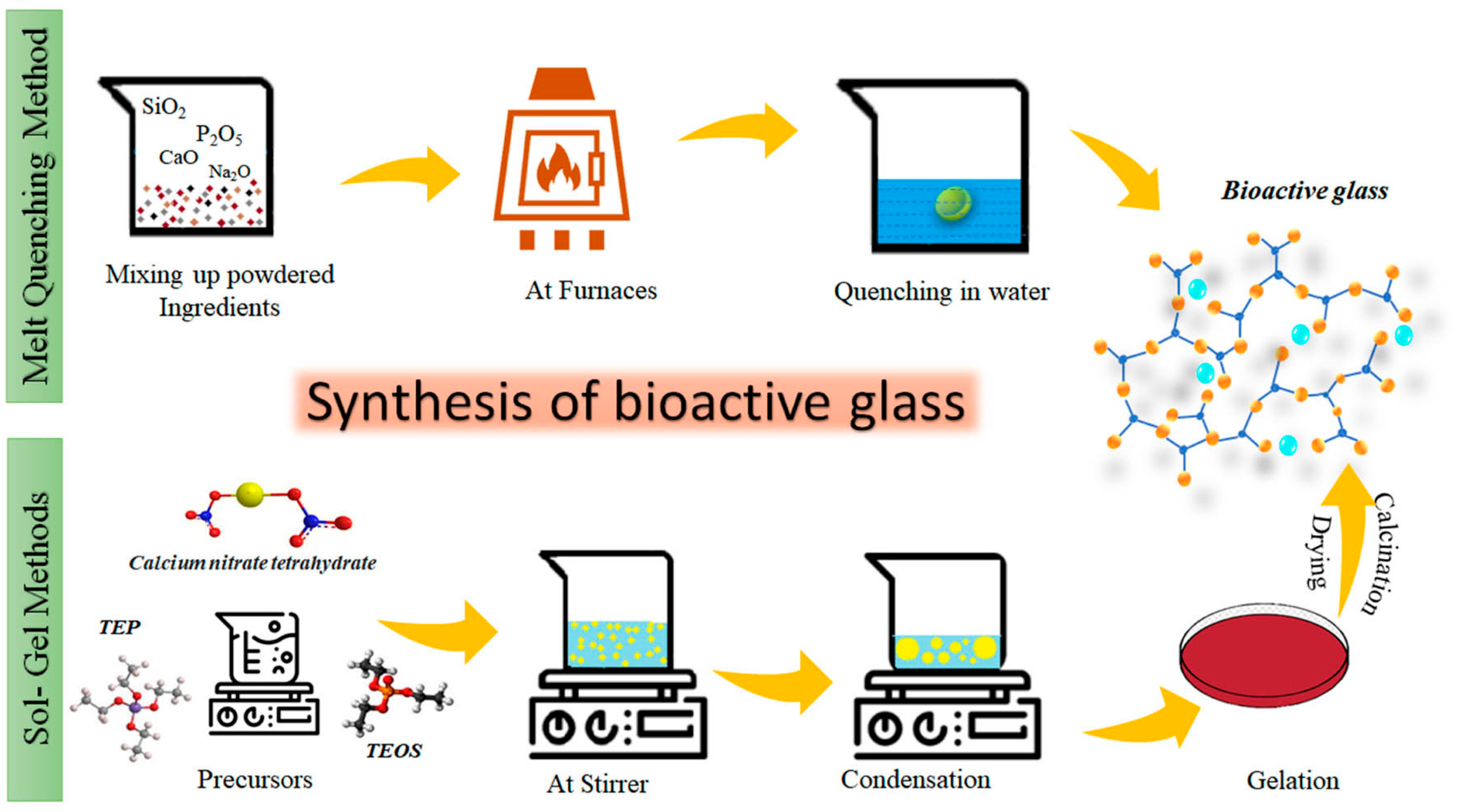
5.3. Bioactive Glass
5.4. Implant Coatings Made from Bioactive Glass
5.4.1. Coating Synthesis
- Sol–gel process;
- In situ polymerization;
- Chemical vapor deposition (CVD);
- Hydrolysis.
5.4.2. Sol–Gel Coating Process
- Preparation of the sol
- Gelation
- Aging and drying
- Thermal treatment (if required)
5.4.3. Advantages of the Sol–Gel Process [153,154,155]
- Precise control: Allows fine-tuning of material composition and properties.
- Simple/efficient: Suitable for applications where high temperatures may degrade components. Very high production efficiency. Low initial investment while having high quality products.
- Versatility: Can produce various material forms (thin films, coatings, fibers, and powders).
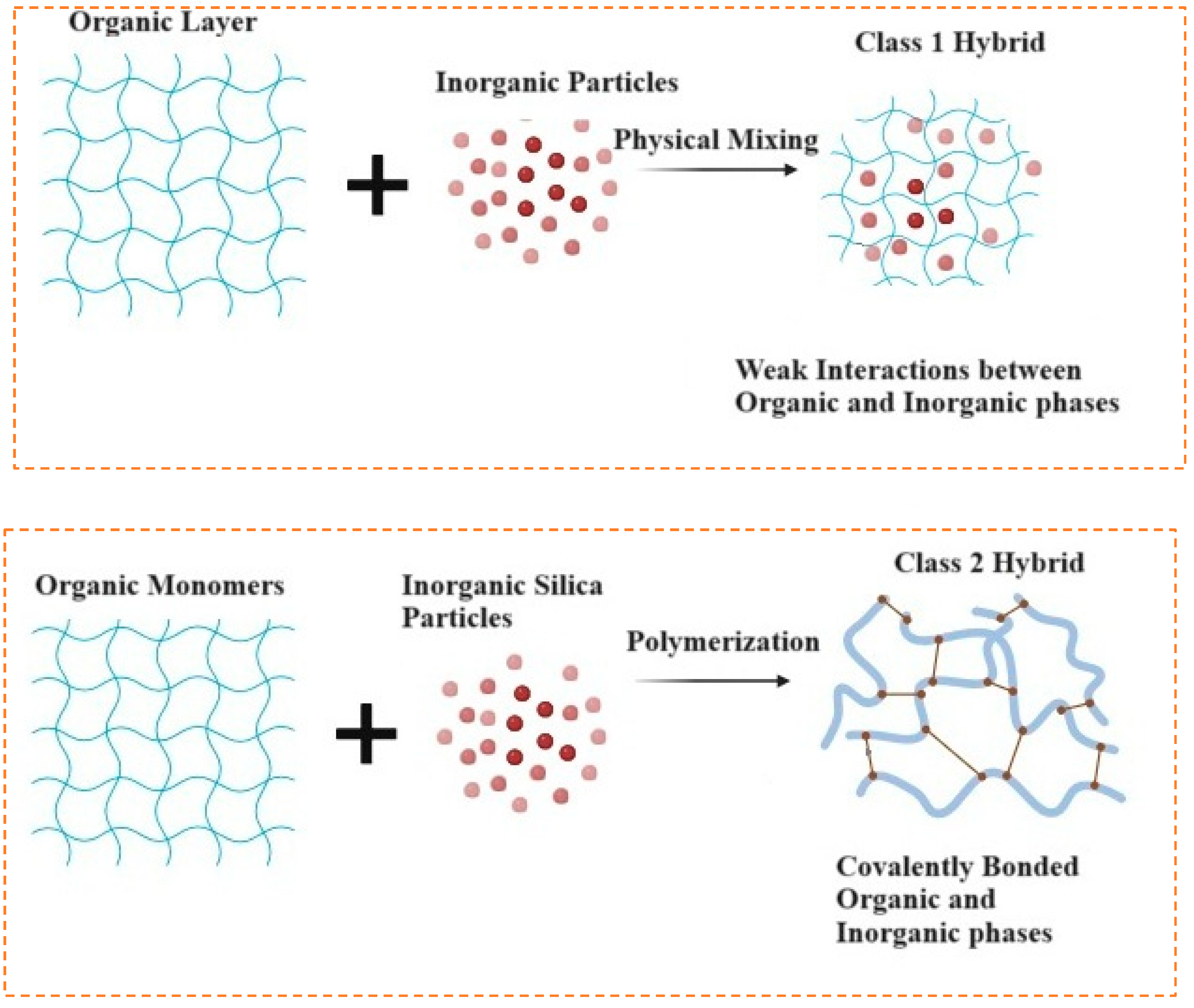
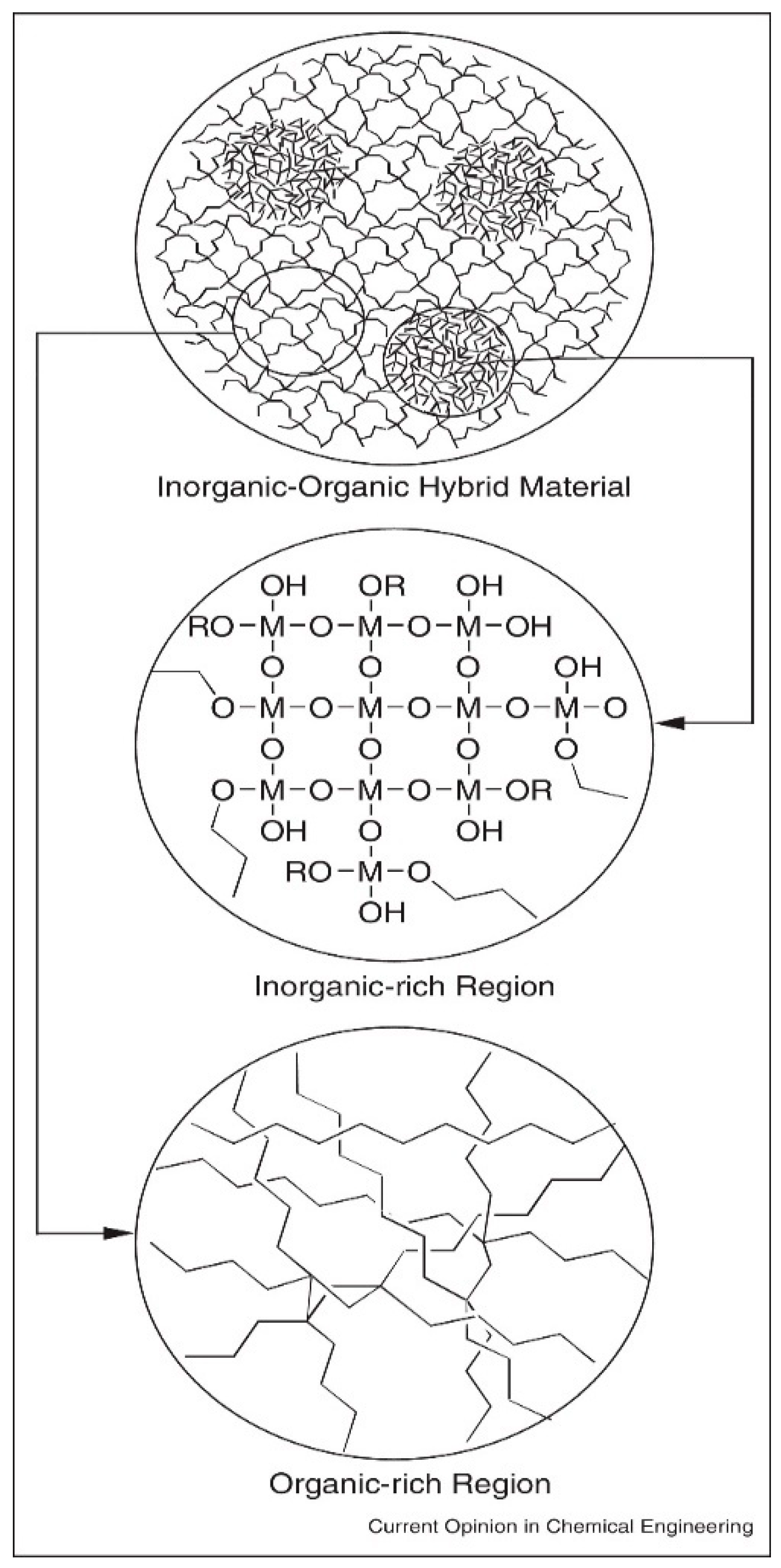
- Purity and homogeneity: Ensures uniform chemical distribution of organic and inorganic phases as shown in Figure 11.
5.4.4. Combination of the Sol–Gel Method with Coating Techniques
5.5. Sol–Gel-Based Antimicrobial Materials
6. Gaps and Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Association of Oral and Maxillofacial Surgeons. Oral and Maxillofacial Surgeons: The Experts in Face, Mouth and Jaw Surgery. Available online: https://myoms.org/what-we-do/ (accessed on 1 November 2025).
- Gupta, A.; Dhanraj, M.; Sivagami, G. Status of surface treatment in endosseous implant: A literary overview. Indian J. Dent. Res. 2010, 21, 433. [Google Scholar] [CrossRef]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50. [Google Scholar] [CrossRef]
- Lambris, J.D.; Paoletti, R. Advances in Experimental Medicine and Biology. Available online: http://www.springer.com/series/5584 (accessed on 9 November 2025).
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Montanaro, L.; Speziale, P.; Campoccia, D.; Ravaioli, S.; Cangini, I.; Pietrocola, G.; Giannini, S.; Arciola, C.R. Scenery of Staphylococcus Implant Infections in Orthopedics. Future Microbiol. 2011, 6, 1329–1349. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pan, Y.; Gao, Y.; Pang, H.; Sun, H.; Cheng, L.; Liu, J. Research Progress on the Preparation Process and Material Structure of 3D-Printed Dental Implants and Their Clinical Applications. Coatings 2024, 14, 781. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Pan-Canadian Action Plan on Antimicrobial Resistance; Public Health Agency of Canada: Ottawa, ON, Canada, 2023. [Google Scholar]
- Zoutman, D.; McDonald, S.; Vethanayagan, D. Total and Attributable Costs of Surgical-Wound Infections at a Canadian Tertiary-Care Center. Infect. Control Hosp. Epidemiol. 1998, 19, 254–259. [Google Scholar] [CrossRef]
- Camps-Font, O.; Martín-Fatás, P.; Clé-Ovejero, A.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Postoperative infections after dental implant placement: Variables associated with increased risk of failure. J. Periodontol. 2018, 89, 1165–1173. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-Centered Infection: Microbial Adhesion Versus Tissue Integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Schierholz, J.M.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Bartold, P.M.; Huang, Y. The role of foreign body response in peri-implantitis: What is the evidence? Periodontology 2000 2022, 90, 176–185. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Thomas, P.; Summer, B. Implant allergy. Allergol. Sel. 2017, 1, 59–64. [Google Scholar] [CrossRef]
- Wiltshire, W.A.; Ferreira, M.R.; Ligthelm, A.J. Allergies to dental materials. Vital 2007, 4, 39. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89, S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Prathapachandran, J.; Suresh, N. Management of peri-implantitis. Dent. Res. J. 2012, 9, 516–521. [Google Scholar] [CrossRef]
- Hasan, J.; Bright, R.; Hayles, A.; Palms, D.; Zilm, P.; Barker, D.; Vasilev, K. Preventing Peri-implantitis: The Quest for a Next Generation of Titanium Dental Implants. Am. Chem. Soc. 2022, 8, 4697–4737. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Lee, B.-A.; Choi, S.-H.; Kim, Y.-T. Evaluation of failed implants and reimplantation at sites of previous dental implant failure: Survival rates and risk factors. J. Periodontal Implant Sci. 2022, 52, 230. [Google Scholar] [CrossRef]
- Zhang, Y.; Pajares, A.; Lawn, B.R. Fatigue and damage tolerance of Y-TZP ceramics in layered biomechanical systems. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 166–171. [Google Scholar] [CrossRef]
- Kashi, A.; Saha, S. Failure mechanisms of medical implants and their effects on outcomes. In Biointegration of Medical Implant Materials, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 407–432. [Google Scholar]
- Lambert, F.E.; Weber, H.; Susarla, S.M.; Belser, U.C.; Gallucci, G.O. Descriptive Analysis of Implant and Prosthodontic Survival Rates With Fixed Implant–Supported Rehabilitations in the Edentulous Maxilla. J. Periodontol. 2009, 80, 1220–1230. [Google Scholar] [CrossRef]
- Shetty, P.; Yadav, P.; Tahir, M.; Saini, V. Implant Design and Stress Distribution. Int. J. Oral Implantol. Clin. Res. 2016, 7, 34–39. [Google Scholar] [CrossRef]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental Implant Complications. Semin. Ultrasound CT MRI 2015, 36, 427–433. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S278–S285. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.M.; Faggion, C.M.; Duncan, W.J. The Frequency of Peri-Implant Diseases: A Systematic Review and Meta-Analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Bolukbasi, N.; Ozdemir, T.; Oksuz, L.; Gurler, N. Bacteremia following dental implant surgery: Preliminary results. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Pye, A.D.; Lockhart, D.E.A.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef]
- Pang, C.M.; Hong, P.; Guo, H.; Liu, W.-T. Biofilm Formation Characteristics of Bacterial Isolates Retrieved from a Reverse Osmosis Membrane. Environ. Sci. Technol. 2005, 39, 7541–7550. [Google Scholar] [CrossRef]
- Goldberg, J. Biofilms and antibiotic resistance: A genetic linkage. Trends Microbiol. 2002, 10, 264. [Google Scholar] [CrossRef]
- Peng, J.-S.; Tsai, W.-C.; Chou, C.-C. Inactivation and removal of Bacillus cereus by sanitizer and detergent. Int. J. Food Microbiol. 2002, 77, 11–18. [Google Scholar] [CrossRef]
- Chen, M.J.; Zhang, Z.; Bott, T.R. Direct measurement of the adhesive strength of biofilms in pipes by micromanipulation. Biotechnol. Tech. 1998, 12, 875–880. [Google Scholar] [CrossRef]
- Dickinson, G.M.; Bisno, A.L. Infections associated with indwelling devices: Infections related to extravascular devices. Antimicrob. Agents Chemother. 1989, 33, 602–607. [Google Scholar] [CrossRef]
- Muller, E.; Hübner, J.; Gutierrez, N.; Takeda, S.; Goldmann, D.A.; Pier, G.B. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect. Immun. 1993, 61, 551–558. [Google Scholar] [CrossRef]
- Mack, D.; Fischer, W.; Krokotsch, A.; Leopold, K.; Hartmann, R.; Egge, H.; Laufs, R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996, 178, 175–183. [Google Scholar] [CrossRef]
- Sutherland, I. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Dunne, W.M. Bacterial Adhesion: Seen Any Good Biofilms Lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef]
- Aboelnaga, N.; Elsayed, S.W.; Abdelsalam, N.A.; Salem, S.; Saif, N.A.; Elsayed, M.; Ayman, S.; Nasr, M.; Elhadidy, M. Deciphering the dynamics of methicillin-resistant Staphylococcus aureus biofilm formation: From molecular signaling to nanotherapeutic advances. Cell Commun. Signal. 2024, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Korber, D.R.; Lawrence, J.R.; Lappin-Scott, H.M.; Costerton, J.W. Growth of Microorganisms on Surfaces. In Microbial Biofilms; Cambridge University Press: Cambridge, UK, 1995; pp. 15–45. [Google Scholar] [CrossRef]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Qiu, W.; Fang, F. Overview of Antibacterial Strategies of Dental Implant Materials for the Prevention of Peri-Implantitis. Bioconjug. Chem. 2021, 32, 627–638. [Google Scholar] [CrossRef]
- Li, G.; Jiang, H.; Yang, F. A Novel Diffuse Plasma Jet Without Airflow and Its Application in the Real-Time Surface Modification of Titanium. IEEE Trans. Plasma Sci. 2022, 50, 4603–4611. [Google Scholar] [CrossRef]
- Zhu, J.; Chu, W.; Luo, J.; Yang, J.; He, L.; Li, J. Dental Materials for Oral Microbiota Dysbiosis: An Update. Front. Cell. Infect. Microbiol. 2022, 12, 900918. [Google Scholar] [CrossRef]
- An, S.-J.; Namkung, J.-U.; Ha, K.-W.; Jun, H.-K.; Kim, H.Y.; Choi, B.-K. Inhibitory effect of d-arabinose on oral bacteria biofilm formation on titanium discs. Anaerobe 2022, 75, 102533. [Google Scholar] [CrossRef] [PubMed]
- Masurier, N.; Tissot, J.-B.; Boukhriss, D.; Jebors, S.; Pinese, C.; Verdié, P.; Amblard, M.; Mehdi, A.; Martinez, J.; Humblot, V.; et al. Site-specific grafting on titanium surfaces with hybrid temporin antibacterial peptides. J. Mater. Chem. B 2018, 6, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Dixit, J.; Prakash, D. Modulatory effects of Cissus quadrangularis on periodontal regeneration by bovine-derived hydroxyapatite in intrabony defects: Exploratory clinical trial. J. Int. Acad. Periodontol. 2008, 10, 59–65. [Google Scholar]
- Wan, R.; Chu, S.; Wang, X.; Lei, L.; Tang, H.; Hu, G.; Dong, L.; Li, D.; Gu, H. Study on the osteogenesis of rat mesenchymal stem cells and the long-term antibacterial activity of Staphylococcus epidermidis on the surface of silver-rich TiN/Ag modified titanium alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3008–3021. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jin, W.; Qasim, A.M.; Gao, A.; Peng, X.; Li, W.; Feng, H.; Chu, P.K. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Hardes, J.; Streitburger, A.; Ahrens, H.; Nusselt, T.; Gebert, C.; Winkelmann, W.; Battmann, A.; Gosheger, G. The Influence of Elementary Silver Versus Titanium on Osteoblasts Behaviour In Vitro Using Human Osteosarcoma Cell Lines. Sarcoma 2007, 2007, 026539. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Du, W.-L.; Niu, S.-S.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Xia, C.; Cai, D.; Tan, J.; Li, K.; Qiao, Y.; Liu, X. Synergistic Effects of N/Cu Dual Ions Implantation on Stimulating Antibacterial Ability and Angiogenic Activity of Titanium. ACS Biomater. Sci. Eng. 2018, 4, 3185–3193. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jin, G.; Ouyang, L.; Wang, D.; Qiao, Y.; Liu, X. Antibacterial activity, osteogenic and angiogenic behaviors of copper-bearing titanium synthesized by PIII&D. J. Mater. Chem. B 2016, 4, 1296–1309. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Yang, K.; Hao, X. Effects of Cu2+ and Cu+ on the proliferation, differentiation and calcification of primary mouse osteoblasts in vitro. Chin. J. Inorg. Chem. 2010, 26, 2251–2258. [Google Scholar]
- Zhang, D.; Ren, L.; Zhang, Y.; Xue, N.; Yang, K.; Zhong, M. Antibacterial activity against Porphyromonas gingivalis and biological characteristics of antibacterial stainless steel. Colloids Surf. B Biointerfaces 2013, 105, 51–57. [Google Scholar] [CrossRef]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7, S515–S527. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Use of Copper Cast Alloys To Control Escherichia coli O157 Cross-Contamination during Food Processing. Appl. Environ. Microbiol. 2006, 72, 4239–4244. [Google Scholar] [CrossRef]
- Zevenhuizen, L.P.T.M.; Dolfing, J.; Eshuis, E.J.; Scholten-Koerselman, I.J. Inhibitory effects of copper on bacteria related to the free ion concentration. Microb. Ecol. 1979, 5, 139–146. [Google Scholar] [CrossRef]
- Almoudi, M.M.; Hussein, A.S.; Hassan, M.I.A.; Zain, N.M. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J. 2018, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Q.; Zhao, M.; Dong, L.; Wu, J.; Li, D. Effects of Zn and Ag Ratio on Cell Adhesion and Antibacterial Properties of Zn/Ag Coimplanted TiN. ACS Biomater. Sci. Eng. 2019, 5, 3303–3310. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Oishi, H.; Suketa, Y. Stimulatory effect of zinc on bone formation in tissue culture. Biochem. Pharmacol. 1987, 36, 4007–4012. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Kokkonen, H.; Cassinelli, C.; Verhoef, R.; Morra, M.; Schols, H.A.; Tuukkanen, J. Differentiation of Osteoblasts on Pectin-Coated Titanium. Biomacromolecules 2008, 9, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Jawad, R.; Mushtaq, Q.; Spalletta, A.; Martin, P.; Ishtiaq, U. Determination of antibacterial and antioxidant potential of organic crude extracts from Malus domestica, Cinnamomum verum and Trachyspermum ammi. Sci. Rep. 2025, 15, 976. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Vanitha, A.; Swamy, M.M.; Ravishankar, G.A. Antioxidant and Antimicrobial Activity of Cissus quadrangularis L. J. Med. Food 2003, 6, 99–105. [Google Scholar] [CrossRef]
- Kim, H.; Kim, C.; Jhon, G.; Moon, I.; Choi, S.; Cho, K.; Chai, J.; Kim, C. The Effect of Safflower Seed Extract on Periodontal Healing of 1-Wall Intrabony Defects in Beagle Dogs. J. Periodontol. 2002, 73, 1457–1466. [Google Scholar] [CrossRef]
- Dobrin, A.; Popa, G. Evaluation of the Antimicrobial Activity of Carthamus Tinctorius Extracts Against Nosocomial Microorganisms. Sci. Papers Ser. B Hortic. 2022, 1, 799–811. [Google Scholar]
- Merolli, A.; Nicolais, L.; Ambrosio, L.; Santin, M. A degradable soybean-based biomaterial used effectively as a bone filler in vivo in a rabbit. Biomed. Mater. 2010, 5, 015008. [Google Scholar] [CrossRef]
- Chaleshtori, S.A.H.; Kachoie, M.A.; Jazi, S.M.H. Antibacterial effects of the methanolic extract of Glycine max (Soybean). Microbiol. Res. 2017, 8, 7319. [Google Scholar] [CrossRef]
- Agrawal, A.; Reche, A.; Agrawal, S.; Paul, P. Applications of Chitosan Nanoparticles in Dentistry: A Review. Cureus 2023, 15, e49934. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C.-H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida albicans, Candida tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef]
- Tahsin, K.; Xu, W.; Watson, D.; Rizkalla, A.; Charpentier, P. Antimicrobial Denture Material Synthesized from Poly(methyl methacrylate) Enriched with Cannabidiol Isolates. Molecules 2025, 30, 943. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Liao, S.; Wen, Z.T.; Fan, Y. Synthesis and characterization of antibacterial dental monomers and composites. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100B, 1151–1162. [Google Scholar] [CrossRef]
- Xu, X.; Costin, S. Chapter 10. Antimicrobial Polymeric Dental Materials. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; Royal Society of Chemistry: London, UK, 2013; pp. 279–309. [Google Scholar] [CrossRef]
- Imazato, S.; Imai, T.; Russell, R.R.B.; Torii, M.; Ebisu, S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J. Biomed. Mater. Res. 1998, 39, 511–515. [Google Scholar] [CrossRef]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R.B. Incorporation of Bacterial Inhibitor into Resin Composite. J. Dent. Res. 1994, 73, 1437–1443. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, S.; Zhou, X.; Wang, H.; Xu, H.; Cheng, L. The Use of Quaternary Ammonium to Combat Dental Caries. Materials 2015, 8, 3532–3549. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Suh, B.I.; Yang, J. Antibacterial dental restorative materials: A review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar]
- US Food and Drug Admistration. Sec. 172.165 Quaternary Ammonium Chloride Combination. January 1985. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.165 (accessed on 30 January 2025).
- Zhou, Z.; Zhou, S.; Zhang, X.; Zeng, S.; Xu, Y.; Nie, W.; Zhou, Y.; Xu, T.; Chen, P. Quaternary Ammonium Salts: Insights into Synthesis and New Directions in Antibacterial Applications. Bioconjug. Chem. 2023, 34, 302–325. [Google Scholar] [CrossRef]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of Disinfectant Quaternary Ammonium Compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.; DeLeo, P.; Pechacek, N.; Freemantle, M. Human health hazard assessment of quaternary ammonium compounds: Didecyl dimethyl ammonium chloride and alkyl (C12–C16) dimethyl benzyl ammonium chloride. Regul. Toxicol. Pharmacol. 2020, 116, 104717. [Google Scholar] [CrossRef]
- Ye, X.; Qin, X.; Yan, X.; Guo, J.; Huang, L.; Chen, D.; Wu, T.; Shi, Q.; Tan, S.; Cai, X. π–π conjugations improve the long-term antibacterial properties of graphene oxide/quaternary ammonium salt nanocomposites. Chem. Eng. J. 2016, 304, 873–881. [Google Scholar] [CrossRef]
- Padnya, P.L.; Terenteva, O.; Akhmedov, A.; Iksanova, A.; Shtyrlin, N.; Nikitina, E.; Krylova, E.; Shtyrlin, Y.G.; Stoikov, I. Thiacalixarene based quaternary ammonium salts as promising antibacterial agents. Bioorg. Med. Chem. 2021, 29, 115905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kong, H.; Zhang, X.; Jia, H.; Ma, X.; Miao, H.; Mu, Y.; Zhang, G. Design and production of environmentally degradable quaternary ammonium salts. Green Chem. 2021, 23, 6548–6554. [Google Scholar] [CrossRef]
- Benkova, M.; Soukup, O.; Prchal, L.; Sleha, R.; Eleršek, T.; Novak, M.; Sepčić, K.; Gunde-Cimerman, N.; Dolezal, R.; Bostik, V.; et al. Synthesis, Antimicrobial Effect and Lipophilicity-Activity Dependence of Three Series of Dichained N-Alkylammonium Salts. ChemistrySelect 2019, 4, 12076–12084. [Google Scholar] [CrossRef]
- Zhi, L.; Shi, X.; Zhang, E.; Gao, C.; Gai, H.; Wang, H.; Liu, Z.; Zhang, T. Synthesis and Performance of Double-Chain Quaternary Ammonium Salt Glucosamide Surfactants. Molecules 2022, 27, 2149. [Google Scholar] [CrossRef]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef]
- Xie, D.; Weng, Y.; Guo, X.; Zhao, J.; Gregory, R.L.; Zheng, C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent. Mater. 2011, 27, 487–496. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H.K. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef]
- Zhou, C.; Weir, M.D.; Zhang, K.; Deng, D.; Cheng, L.; Xu, H.H.K. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dent. Mater. 2013, 29, 859–870. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Wu, J.; Weir, M.D.; Xu, H.H.K. Novel antibacterial orthodontic cement containing quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2014, 42, 1193–1201. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y.; et al. Antibacterial Effect of Dental Adhesive Containing Dimethylaminododecyl Methacrylate on the Development of Streptococcus mutans Biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.; Weir, M.D.; Ge, Y.; Li, M.; Li, Y.; Xu, X.; Zheng, L.; et al. Effect of Antibacterial Dental Adhesive on Multispecies Biofilms Formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Bradshaw, D.J. Dental plaque as a biofilm. J. Ind. Microbiol. 1995, 15, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.; Ito, S. Collagen Degradation by Host-derived Enzymes during Aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Chen, C.; Weir, M.D.; Cheng, L.; Lin, N.J.; Lin-Gibson, S.; Chow, L.C.; Zhou, X.; Xu, H.H. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent. Mater. 2014, 30, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, L.; Wu, E.J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 504–513. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of Quaternary Ammonium Chain Length on Antibacterial Bonding Agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J. Dent. 2013, 41, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, P.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 2014, 10, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, F.; Weir, M.D.; Xu, H.H.K. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. J. Dent. 2013, 41, 1122–1131. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, J.; Melo, M.A.S.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234. [Google Scholar] [CrossRef]
- Karakullukcu, A.B.; Taban, E.; Ojo, O.O. Biocompatibility of biomaterials and test methods: A review. Mater. Test. 2023, 65, 545–559. [Google Scholar] [CrossRef]
- Al-Shalawi, F.D.; Ariff, A.H.M.; Jung, D.-W.; Ariffin, M.K.A.M.; Kim, C.L.S.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef]
- Binlateh, T.; Thammanichanon, P.; Rittipakorn, P.; Thinsathid, N.; Jitprasertwong, P. Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen. Biomimetics 2022, 7, 34. [Google Scholar] [CrossRef]
- Talha, M.; Behera, C.K.; Sinha, O.P. A review on nickel-free nitrogen containing austenitic stainless steels for biomedical applications. Mater. Sci. Eng. C 2013, 33, 3563–3575. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, S.; Xu, G.; Shen, S.; Li, Y.; Zhang, M.; Wu, X. Corrosion behavior of mesoporous bioglass-ceramic coated magnesium alloy under applied forces. J. Mech. Behav. Biomed. Mater. 2016, 56, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhu, R.-F.; Lu, Y.-P.; Xiao, G.-Y.; He, K.; Yuan, Y.; Ma, X.-N.; Li, Y. Effect of sandblasting intensity on microstructures and properties of pure titanium micro-arc oxidation coatings in an optimized composite technique. Appl. Surf. Sci. 2014, 292, 204–212. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F. Biocompatibility improvement of titanium implants by coating with hybrid materials synthesized by sol-gel technique. J. Biomed. Mater. Res. A 2014, 102, 4473–4479. [Google Scholar] [CrossRef]
- Midha, S.; Kim, T.B.; Van Den Bergh, W.; Lee, P.D.; Jones, J.R.; Mitchell, C.A. Preconditioned 70S30C bioactive glass foams promote osteogenesis in vivo. Acta Biomater. 2013, 9, 9169–9182. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Kickelbick, G. Introduction to Hybrid Materials. In Hybrid Materials; Wiley: Hoboken, NJ, USA, 2006; pp. 1–48. [Google Scholar] [CrossRef]
- Novak, B.M. Hybrid Nanocomposite Materials—Between inorganic glasses and organic polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Grosso, D.; Ribot, F.; Boissiere, C.; Sanchez, C. Molecular and supramolecular dynamics of hybrid organic–inorganic interfaces for the rational construction of advanced hybrid nanomaterials. Chem. Soc. Rev. 2011, 40, 829–848. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559. [Google Scholar] [CrossRef]
- Khurshid, Z.; Husain, S.; Alotaibi, H.; Rehman, R.; Zafar, M.S.; Farooq, I.; Khan, A.S. Novel Techniques of Scaffold Fabrication for Bioactive Glasses. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Woodhead Publishing: Cambridge, UK, 2019; pp. 497–519. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Habibah, T.U.; Amlani, D.V.; Brizuela, M. Hydroxyapatite Dental Material; StatPearls: Tampa, FL, USA, 2025. [Google Scholar]
- Islam, M.A.; Hossain, N.; Hossain, S.; Khan, F.; Hossain, S.; Arup, M.R.; Chowdhury, M.A.; Rahman, M. Advances of Hydroxyapatite Nanoparticles in Dental Implant Applications. Int. Dent. J. 2025, 75, 2272–2313. [Google Scholar] [CrossRef]
- Al-Noaman, A.; Rawlinson, S.C.F.; Hill, R.G. MgF2-containing glasses as a coating for titanium dental implant. I- Glass powder. J. Mech. Behav. Biomed. Mater. 2022, 125, 104948. [Google Scholar] [CrossRef]
- Oliver, J.A.N.; Su, Y.; Lu, X.; Kuo, P.H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Rohr, N.; Nebe, J.B.; Schmidli, F.; Müller, P.; Weber, M.; Fischer, H.; Fischer, J. Influence of bioactive glass-coating of zirconia implant surfaces on human osteoblast behavior in vitro. Dent. Mater. 2019, 35, 862–870. [Google Scholar] [CrossRef]
- Zhang, M.; Pu, X.; Chen, X.; Yin, G. In-vivo performance of plasma-sprayed CaO–MgO–SiO2-based bioactive glass-ceramic coating on Ti–6Al–4V alloy for bone regeneration. Heliyon 2019, 5, e02824. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, K.; Xie, Y.; Pan, H.; Zhao, J.; Huang, L.; Zheng, X. Different response of osteoblastic cells to Mg2+, Zn2+ and Sr2+ doped calcium silicate coatings. J. Mater. Sci. Mater. Med. 2016, 27, 56. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, I.; Aitasalo, K.; Pöllönen, M.; Varpula, M. Reconstruction of orbital floor fractures using bioactive glass. J. Cranio-Maxillofac. Surg. 2000, 28, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Farooq, I.; Awais, M.; Najeeb, S.; Khurshid, Z.; Zohaib, S. Bioactive Surface Coatings for Enhancing Osseointegration of Dental Implants. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Woodhead Publishing: Cambridge, UK, 2019; pp. 313–329. [Google Scholar] [CrossRef]
- Yoshimura, M.; Suda, H.; Okamoto, K.; Ioku, K. Hydrothermal synthesis of biocompatible whiskers. J. Mater. Sci. 1994, 29, 3399–3402. [Google Scholar] [CrossRef]
- Silva, C.C.; Pinheiro, A.G.; Miranda, M.A.R.; Góes, J.C.; Sombra, A.S.B. Structural properties of hydroxyapatite obtained by mechanosynthesis. Solid State Sci. 2003, 5, 553–558. [Google Scholar] [CrossRef]
- Saeri, M.R.; Afshar, A.; Ghorbani, M.; Ehsani, N.; Sorrell, C.C. The wet precipitation process of hydroxyapatite. Mater. Lett. 2003, 57, 4064–4069. [Google Scholar] [CrossRef]
- Shih, W.-J.; Chen, Y.-F.; Wang, M.-C.; Hon, M.-H. Crystal growth and morphology of the nano-sized hydroxyapatite powders synthesized from CaHPO4·2H2O and CaCO3 by hydrolysis method. J. Cryst. Growth 2004, 270, 211–218. [Google Scholar] [CrossRef]
- Kim, I.-S.; Kumta, P.N. Sol–gel synthesis and characterization of nanostructured hydroxyapatite powder. Mater. Sci. Eng. B 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Sol–gel derived organic–inorganic hybrid materials: Synthesis, characterizations and applications. J. Solgel Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kamil, M.P.; Fatimah, S.; Nisa, N.; Ko, Y.G. Recent advances in hybrid organic-inorganic materials with spatial architecture for state-of-the-art applications. Prog. Mater. Sci. 2020, 112, 100663. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactivity, Degradation, and Mechanical Properties of Poly(vinylpyrrolidone-co-triethoxyvinylsilane)/Tertiary Bioactive Glass Hybrids. ACS Appl. Bio Mater. 2018, 1, 1369–1381. [Google Scholar] [CrossRef]
- Cinici, B.; Yaba, S.; Kurt, M.; Yalcin, H.C.; Duta, L.; Gunduz, O. Fabrication Strategies for Bioceramic Scaffolds in Bone Tissue Engineering with Generative Design Applications. Biomimetics 2024, 9, 409. [Google Scholar] [CrossRef]
- Gao, Y.; Seles, M.A.; Rajan, M. Role of bioglass derivatives in tissue regeneration and repair: A review. Rev. Adv. Mater. Sci. 2023, 62, 20220318. [Google Scholar] [CrossRef]
- Tshikovhi, A.; Koao, L.F.; Malevu, T.D.; Linganiso, E.C.; Motaung, T.E. Dopants concentration on the properties of various host materials by sol-gel method: Critical review. Results Mater. 2023, 19, 100447. [Google Scholar] [CrossRef]
- Tinoco Navarro, L.K.; Jaroslav, C. Enhancing Photocatalytic Properties of TiO2 Photocatalyst and Heterojunctions: A Comprehensive Review of the Impact of Biphasic Systems in Aerogels and Xerogels Synthesis, Methods, and Mechanisms for Environmental Applications. Gels 2023, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Barrino, F. Hybrid Organic–Inorganic Materials Prepared by Sol–Gel and Sol–Gel-Coating Method for Biomedical Use: Study and Synthetic Review of Synthesis and Properties. Coatings 2024, 14, 425. [Google Scholar] [CrossRef]
- Zvonkina, I.; Soucek, M. Inorganic–organic hybrid coatings: Common and new approaches. Curr. Opin. Chem. Eng. 2016, 11, 123–127. [Google Scholar] [CrossRef]
- Tranquillo, E.; Bollino, F. Surface Modifications for Implants Lifetime extension: An Overview of Sol-Gel Coatings. Coatings 2020, 10, 589. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Bononi, M.; Colombini, E.; Giovanardi, R.; Veronesi, P.; Tranquillo, E. Coating of Titanium Substrates with ZrO2 and ZrO2-SiO2 Composites by Sol-Gel Synthesis for Biomedical Applications: Structural Characterization, Mechanical and Corrosive Behavior. Coatings 2019, 9, 200. [Google Scholar] [CrossRef]
- Mendhe, A.C. Spin Coating: Easy Technique for Thin Films. In Simple Chemical Methods for Thin Film Deposition: Synthesis and Applications; Springer: Singapore, 2023; pp. 387–424. [Google Scholar]
- Gvishi, R.; Sokolov, I. 3D sol–gel printing and sol–gel bonding for fabrication of macro- and micro/nano-structured photonic devices. J. Solgel Sci. Technol. 2020, 95, 635–648. [Google Scholar] [CrossRef]
- Kawashita, M.; Tsuneyama, S.; Miyaji, F.; Kokubo, T.; Kozuka, H.; Yamamoto, K. Antibacterial silver-containing silica glass prepared by sol–gel method. Biomaterials 2000, 21, 393–398. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Kostova, Y.; Yordanova, L.; Nenova, E.; Shestakova, P.; Ivanova, I.; Pavlova, E. Sol–Gel Synthesis of Silica–Poly (Vinylpyrrolidone) Hybrids with Prooxidant Activity and Antibacterial Properties. Molecules 2024, 29, 2675. [Google Scholar] [CrossRef]
- Bryaskova, R.; Pencheva, D.; Kale, G.M.; Lad, U.; Kantardjiev, T. Synthesis, characterisation and antibacterial activity of PVA/TEOS/Ag-Np hybrid thin films. J. Colloid Interface Sci. 2010, 349, 77–85. [Google Scholar] [CrossRef]
- Copello, G.J.; Teves, S.; Degrossi, J.; D’Aquino, M.; Desimone, M.F.; Diaz, L.E. Antimicrobial activity on glass materials subject to disinfectant xerogel coating. J. Ind. Microbiol. Biotechnol. 2006, 33, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Rothrock, A.R.; Schoenfisch, M.H. Nitric oxide-releasing sol–gels as antibacterial coatings for orthopedic implants. Biomaterials 2005, 26, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ubhale, Y.S.; More, A.P. Antimicrobial sol–gel coating: A review. J. Coat. Technol. Res. 2024, 2, 527–548. [Google Scholar] [CrossRef]
- Gunawidjaja, P.N.; Lo, A.Y.H.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Stevensson, B.; Grins, J.; Vallet-Regí, M.; Edén, M. Biomimetic Apatite Mineralization Mechanisms of Mesoporous Bioactive Glasses as Probed by Multinuclear 31P, 29Si, 23Na and 13C Solid-State NMR. J. Phys. Chem. C 2010, 114, 19345–19356. [Google Scholar] [CrossRef]
- Hench, L.L. Chronology of Bioactive Glass Development and Clinical Applications. New J. Glass Ceram. 2013, 3, 67–73. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Moreno, M.G.; Butini, M.E.; Maiolo, E.M.; Sessa, L.; Trampuz, A. Antimicrobial activity of bioactive glass S53P4 against representative microorganisms causing osteomyelitis—Real-time assessment by isothermal microcalorimetry. Colloids Surf. B Biointerfaces 2020, 189, 110853. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-10:2021; Biological Evaluation of Medical Devices Part 10: Tests for Skin Sensitization. ISO: Geneva, Switzerland, 2021.
- ISO 10993-3:2014; Biological Evaluation of Medical Devices Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity. ISO: Geneva, Switzerland, 2014.
- ISO 10993-6:2016; Biological Evaluation of Medical Devices Part 6: Tests for Local Effects after Implantation. ISO: Geneva, Switzerland, 2016.
- ISO 10993-11:2017; Biological Evaluation of Medical Devices Part 11: Tests for Systemic Toxicity. ISO: Geneva, Switzerland, 2017.
- ISO 2409:2020; Paints and Varnishes—Cross-Cut Test. ISO: Geneva, Switzerland, 2020.
- ASTM D4541-22; Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers. ASTM International: West Conshohocken, PA, USA, 2022.
- ISO 14801:2016; Dentistry—Implants—Dynamic Loading Test for Endosseous Dental Implants. ISO: Geneva, Switzerland, 2016.
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011.
- JIS Z 2801; Antibacterial Products—Test for Antibacterial Activity and Efficacy. Japanese Standards Association (JSA): Tokyo, Japan, 2010.
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Grabińska-Sota, E. Genotoxicity and biodegradation of quaternary ammonium salts in aquatic environments. J. Hazard. Mater. 2011, 195, 182–187. [Google Scholar] [CrossRef]


| Type of Implant (No. of Patients/Implants) | Most Prevalent Microbes Detected (% Sites Infected with Bacteria) |
|---|---|
| Brånemark: System is a well-established and widely used dental implant system based on the principle of osseointegration. The original Brånemark implant was a cylindrical, pure titanium implant with smooth, polished screw-like threads. | Prevotella intermedia/P. nigrescens 60% Actinobacillus actinomycetemcomitans 60% Staphylococci, coliforms, Candida spp. 55% |
| Not stated. | Bacteroides forsythus 59% Spirochetes 54% Fusobacterium spp. 41% Peptostreptococcus micros 39% Porphyromonas gingivalis 27% |
| Titanium hollow cylinder implants (7/not stated). | Bacteroides spp., Fusobacterium spp., spirochetes, fusiform bacilli, motile and curved rods (% not stated) |
| Not stated (13/20). | Staphylococcus spp. 55% |
| Not stated (21/28). | P. nicrescens, P. micros, Fusobacterium nucleatum (% not stated) |
| IMZ: The IMZ (IntraMobil Zylinder) implant system was notable for its two-part design, which included an inner elastic intramobile element that aimed to mimic the natural flexibility of teeth. This design was meant to reduce stress on the bone and improve load distribution. However, IMZ implants are now considered outdated and are rarely used in modern implants. | Bacteroides spp. 89% Actinobacillus actinomycetemcomitans 89% Fusobacterium nucleatum 22% Capnocytophaga spp. 27.8% Eikenella corrodens 17% |
| Astra: Widely used in implant dentistry by OsseoSpeed™ surfaces, in micro thread technology with conical design, reducing complications like peri-implantitis. Astra implants come in various lengths and diameters, making them versatile for different clinical cases, including single tooth replacement, multiple teeth, and full-arch reconstructions. ITI Staumann: Made of a titanium–zirconium alloy that is stronger than pure titanium, allowing for smaller implants with high strength—ideal for patients with limited bone. SLActive® Surface, modified hydrophilic implant surface speeds up osseointegration, reducing healing time. Esthetic finishing in visible areas. MorsTaper Connection for antimicrobial effects. | Actinomyces spp. 83% F. nucleatum 70% P. intermedia/nigrescens group 60% Steptococcus anginosus (milleri) group 70% P. micros 63% Enterococcus spp. 30% Yeast spp. 30% |
| PEPTIDE NAME | APPLICATION | DESCRIPTION |
|---|---|---|
| LACTOPEROXIDASE | Toothpaste, mouthwash, and gel | Used as a saliva substitute and showed improvement of xerostomic symptoms and a reduction in streptococci. |
| GERM CLEAN | Oral spray | Oral spray containing GERM CLEAN showed an inhibitory effect on the initial adhesion, acid production, extracellular polysaccharides production, and biofilm formation of Streptococcus mutans. |
| C16G2 | Oral rinse | C16G2 oral rinse showed a decrease in plaque, salivary S. mutans, lactic acid production, and enamel demineralization. |
| TET213 | Dental implant coating | CaP-Tet213 and CaP-HHC36 coating showed antimicrobial activity against Staphylococcus aureus and Pseudomonas aeruginosa. |
| HHC36 | ||
| β-DEFENSIN-2 | Coated recombinant human β-defensin-2 on titanium surfaces yielded antimicrobial activities and prevented bacterial colonization. | |
| HUMAN Β-DEFENSIN-3 CONTAINING CHIMERIC PEPTIDES | Chimeric peptide containing human β-defensing-3 coating prevented biofilm formation by inhibition of initial colonizing Streptococci. | |
| LL-37 | Nanopore coating loaded with LL-37 showed diverse antibacterial and osteogenic induction abilities. |
| Metal | Features | Toxicity Profile | Antimicrobial Ability |
|---|---|---|---|
| Silver | TiN/Ag-modified titanium alloy produced via multiarc ion-plating and ion implantation exhibited stable antimicrobial activity against Staphylococcus epidermidis for over 12 weeks in vitro [54]. A study using PIII to embed Ag into Ti, Si, and SiO2 surfaces found that electron transfer between Ag nanoparticles and Ti is the initial step in the antibacterial mechanism [55]. | Silver at low concentrations was not cytotoxic for osteoblast in vitro [55]. Studies showed that Ag+, Zn2+, and Hg2+ ions are very cytotoxic, even at low concentrations [56]. | Effective against S. choleraesuis, E. coli [57], S. aureus, and S. epidermis [58]. |
| Copper | N/Cu-incorporated Ti produced by PIII showed strong antibacterial activity against Staphylococcus aureus and Escherichia coli, along with enhanced angiogenic properties from Cu and excellent corrosion resistance from TiN [59]. Another study found that the form of Cu (metallic Cu or CuNPs) in coatings depends on synthesis parameters, with metallic Cu showing superior antibacterial activity and biocompatibility compared to CuNPs [60]. The study emphasized that preparation technology parameters critically influence a surface’s antibacterial performance and biocompatibility. | Essential metal ion functioning of organs and metabolic processes [61]. Cu deficiency results in anemia, heart disease, arthritis, and osteoporosis, etc. [62]. Cu ion promotes osteoblast proliferation, differentiation, and migration [63]. High concentrations of Cu ions inhibit growth and cause cell death and toxicity on humans [64]. | Effective against MRSA [65] and E. coli [66] within a few hours. Copper inhibited K. aerogenes [67] and S. aureus [65]. |
| Zinc | Zinc, ZnO, nano ZnO, and Zn2+ ion release are antibacterial agents. Used as dental and formulated into dental hygiene products to control plaque, such as mouth rinses and toothpaste [68]. Ti surface with Zn–Ag increased ratio of Zn and made up for the inhibition of Ag on cell adhesion and growth of fibroblast-like cells [56]. | Zn ion is not harmful to cells, and it has been known for a long time that zinc can help bones grow. Zinc is an important part of making DNA, enzymes working, nucleic acid processing, biomineralization, and hormone action [69]. | Effective against S. aureus; E. coli; S. choleraesuis [55], P. phosphoreum [70], and S. epidermis [71]. |
| Phytochemical | Material | Application | Antimicrobial Efficacy |
|---|---|---|---|
| Malus domestica L. | Titanium implant coating [72]. | Dental implantology | Streptococcus mutans, Salmonella typhi bacteria responsible for dental caries and periodontal diseases [73]. Escherichia coli, Salmonella, and Listeria monocytogenes |
| Cissus quadrangularis L. | Periodontal filler in association with hydroxyapatite [74]. | Periodontal regeneration | Gram-positive bacteria [75]: Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Streptococcus species |
| Carthamus tinctorius L. | Periodontal materials combined with a collagen sponge; periodontal filler integrated with a polylactide-glycolic acid bioresorbable barrier [76]. | Periodontal regeneration. | Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumonia), Acinetobacter baumannii (A. baumannii), Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus) and Salmonella spp. [77] |
| Glycine max L. | Bone filler [78]. | Alveolar bone regeneration | K. pneumoniae, L. monocytogenes S. aureus [79] |
| Chitosan | The cell walls of fungal mycelia are composed of chitin, glucan, and glycoproteins. In species such as Aspergillus niger, Mucor rouxii, and Penicillium notatum, chitin can constitute up to 45% of the cell wall. Chitosan is produced by deacetylating chitin. | Guided tissue regeneration (GTR), hydrogel made of chitosan was developed with the purpose of delivering amelogenin, dentin bonding, and adhesion, coating of dental implants [80]. | Prevents biofilm formation of S. aureus, P. Aeruginosa, Proteus mirabilis, and E. coli [81]. Antifungal against Candida albicans, Candida tropicalis, and other Candida species [82]. |
| Cannabidiol (CBD), derived from the Cannabis plant | PMMA restorations. | To minimize denture-associated infections, antimicrobial enhancements to PMMA, the primary material for dentures, were coated with CBD nanoparticles [83]. | Antimicrobial activity against the following: Staphylococcus aureus, Escherichia coli, Streptococcus agalactiae [83]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tahsin, K.N.; Rizkalla, A.; Charpentier, P. Antimicrobial Materials Used in Coating Dental Implant Surfaces: State of the Art and Future Prospectives. Materials 2026, 19, 403. https://doi.org/10.3390/ma19020403
Tahsin KN, Rizkalla A, Charpentier P. Antimicrobial Materials Used in Coating Dental Implant Surfaces: State of the Art and Future Prospectives. Materials. 2026; 19(2):403. https://doi.org/10.3390/ma19020403
Chicago/Turabian StyleTahsin, Kazi Naziba, Amin Rizkalla, and Paul Charpentier. 2026. "Antimicrobial Materials Used in Coating Dental Implant Surfaces: State of the Art and Future Prospectives" Materials 19, no. 2: 403. https://doi.org/10.3390/ma19020403
APA StyleTahsin, K. N., Rizkalla, A., & Charpentier, P. (2026). Antimicrobial Materials Used in Coating Dental Implant Surfaces: State of the Art and Future Prospectives. Materials, 19(2), 403. https://doi.org/10.3390/ma19020403







