Molecularly Imprinted Titanium Dioxide: Synthesis Strategies and Applications in Photocatalytic Degradation of Antibiotics from Marine Wastewater: A Review
Abstract
:1. Introduction
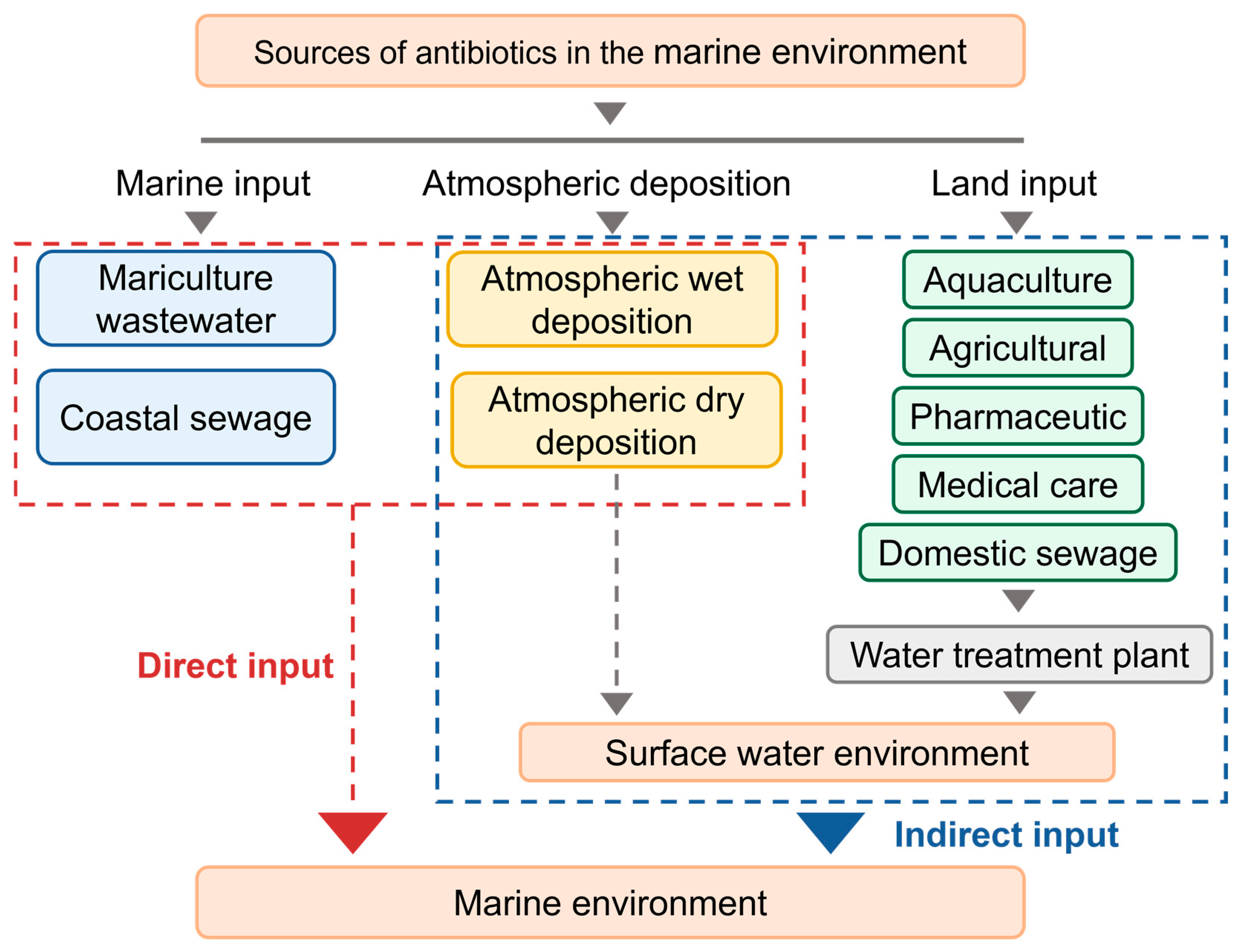
2. Sources and Characterization of Antibiotics in the Marine Environment
2.1. Sources of Marine Antibiotics
2.2. Characteristics of Marine Antibiotics
2.3. Traditional Methods of Antibiotic Removal
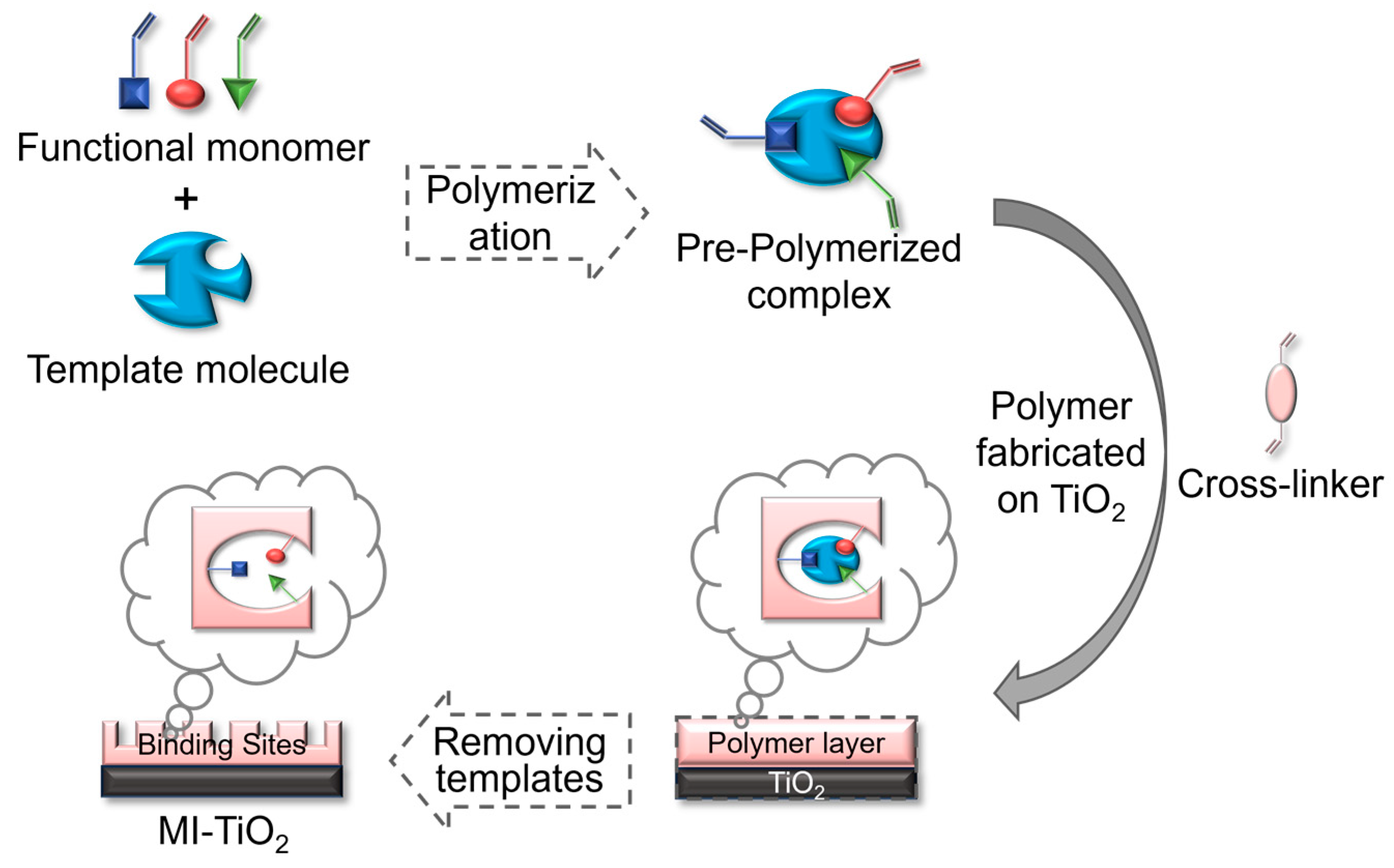
3. Preparation and Modification of Imprinted Titanium Dioxide Catalysts
3.1. Preparation Method
3.1.1. Surface Molecular Imprinting
3.1.2. Molecularly Imprinted Sol-Gel Technology
3.1.3. Other Methods
3.2. Modification Method
3.2.1. Elemental Admixture
3.2.2. Composite Structure Construction
3.2.3. Conformal Modification
3.2.4. Surface Functionalization
3.2.5. Photothermal Synergy
3.2.6. Magnetic/Electric Field-Assisted Catalysis
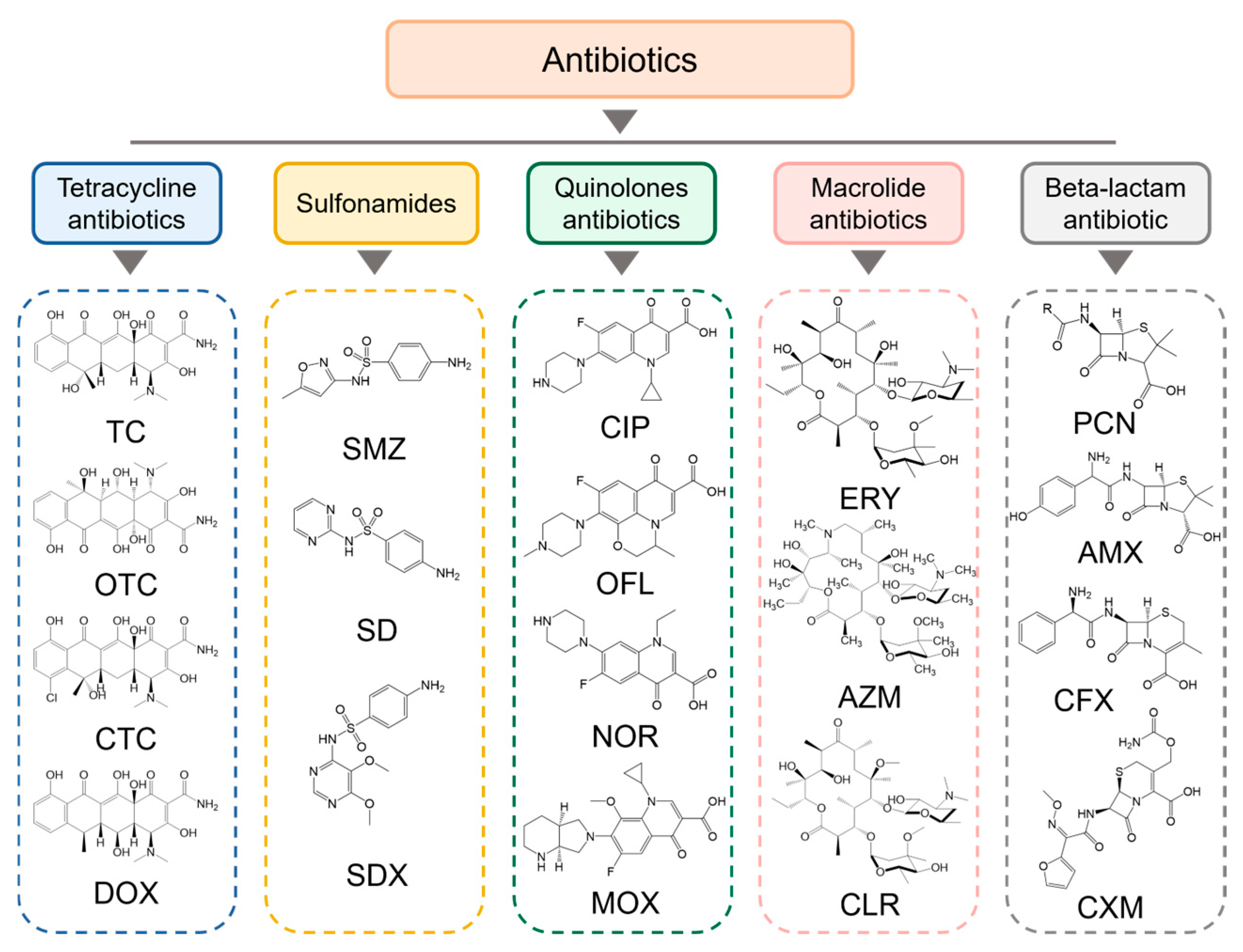
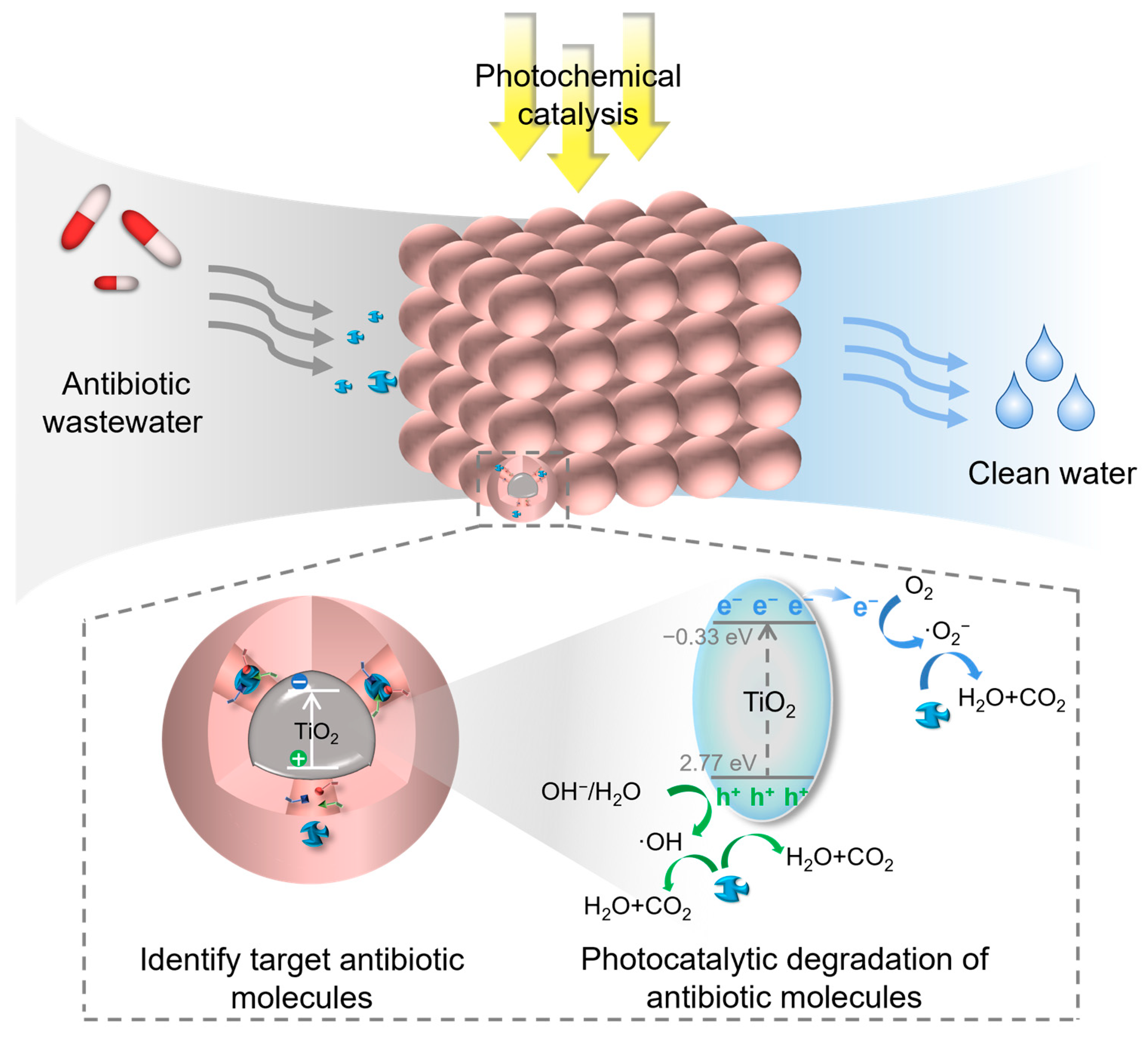
4. Application of Imprinted Titanium Dioxide in Antibiotic Degradation
4.1. Tetracycline Antibiotics
4.2. Sulfonamide Antibiotics
4.3. Quinolone Antibiotics
4.4. Macrolide Antibiotics
4.5. β-Lactam Antibiotics
5. Conclusions and Outlook
- A photo-electro-magnetic synergistic catalytic system was constructed to improve the degradation efficiency and monitor the formation of by-products in real time to ensure environmental safety.
- Artificial intelligence is used to optimize the geometric configuration and doping strategy of imprinted cavities, so as to realize the efficient recognition and degradation of specific antibiotics.
- The long-term performance of MI-TiO2 in complex environments was evaluated by establishing a test platform that simulated real marine conditions (such as dynamic salinity and biofouling).
- The full-cycle environmental footprint of MI-TiO2 from synthesis to abandonment was systematically analyzed to promote the development of sustainable technology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Wang, S.; Luo, X.; Yu, Z.; Zhou, Y. Fenton-like process in antibiotic-containing wastewater treatment: Applications and toxicity evaluation. Chin. Chem. Lett. 2025; in press. [Google Scholar] [CrossRef]
- WHO. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 3 March 2025).
- Anwar, M.; Iqbal, Q.; Saleem, F. Improper disposal of unused antibiotics: An often overlooked driver of antimicrobial resistance. Expert. Rev. Anti-Infect. Ther. 2020, 18, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, H.; Qi, S.; Wang, W.; Lu, H. Synergistic effects of quaternary ammonium compounds and antibiotics on the evolution of antibiotic resistance. Water Res. 2025, 275, 123206. [Google Scholar] [CrossRef]
- Li, K.; Cheng, Y.; Chen, C.; Fan, Y.; Fang, M.; Li, X. Molecularly imprinted composite membranes with the dual imprinted network for highly selective separation of acteoside. Sep. Purif. Technol. 2025, 358, 130203. [Google Scholar] [CrossRef]
- Lu, J.; Qin, Y.Y.; Wu, Y.L.; Zhu, Z.; Chen, M.N.; Yan, Y.S.; Li, C.X. Bio-synthesis of molecularly imprinted membrane with photo-regeneration availability for selective separation applications. Mater. Today Chem. 2022, 24, 100836. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Wang, X. Incorporation of Hydroquinone in the Synthesis of Bi2Ti2O7-TiO2 Contributes to Higher Efficiency of Hydroquinone Degradation: Preparation, Characterization, and Photocatalytic Mechanism. Langmuir 2024, 40, 19260–19269. [Google Scholar] [CrossRef] [PubMed]
- Semra, A.; Seçkin, K.; Cem, E.; Adil, D. Molecularly Imprinted Polymer-Based Sensors for Protein Detection. Polymers 2023, 15, 629. [Google Scholar] [CrossRef]
- Ye, X.; Ge, L.; Jiang, T.; Guo, H.; Chen, B.; Liu, C.; Hayashi, K. Fully Inkjet-Printed Chemiresistive Sensor Array Based on Molecularly Imprinted Sol-Gel Active Materials. ACS Sens. 2022, 7, 1819–1828. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Electrochemical sensors based on magnetic molecularly imprinted polymers: A review. Anal. Chim. Acta 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Ananya, K.; Apichai, P.; Opas, B. A magnetic molecularly imprinted polymer hierarchical composite adsorbent embedded with a zinc oxide carbon foam nanocomposite for the extraction of sulfonamides. Microchem. J. 2022, 179, 107443. [Google Scholar] [CrossRef]
- Kamel, A.H.; Rabboh, H.S.M.A.; Hefnawy, A. Molecularly imprinted polymer-based electrochemical sensors for monitoring the persistent organic pollutants chlorophenols. RSC Adv. 2024, 14, 20163–20181. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-H.; Qian, H.-L.; Yang, C.; Wang, C.; Wang, Z.; Yan, X.-P. Surface imprinted-covalent organic frameworks for efficient solid-phase extraction of fluoroquinolones in food samples. J. Hazard. Mater. 2023, 459, 132031. [Google Scholar] [CrossRef]
- Solmaz, N.; Kheibar, D.; Fereshteh, A.; Rouholah, Z. Red-emissive carbon nanostructure-anchored molecularly imprinted Er-BTC MOF: A biosensor for visual anthrax monitoring. Analyst 2023, 148, 3379–3391. [Google Scholar] [CrossRef]
- Elisabetta, M.; Tiziano, D.G.; Stefano, M.; Martina, C.; Cosimino, M.; Giuseppe, B. Vapor-Phase Synthesis of Molecularly Imprinted Polymers on Nanostructured Materials at Room-Temperature. Small 2023, 19, 2302274. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, L.; Han, C.; Lv, X.; Sun, X.; Wang, T. Hybrid amino-functionalized TiO2/sodium lignosulfonate surface molecularly imprinted polymer for effective scavenging of methylene blue from wastewater. J. Clean. Prod. 2022, 337, 130457. [Google Scholar] [CrossRef]
- Louros, V.L.; Silva, V.; Silva, C.P.; Calisto, V.; Otero, M.; Esteves, V.I.; Freitas, R.; Lima, D.L.D. Sulfadiazine’s photodegradation using a novel magnetic and reusable carbon based photocatalyst: Photocatalytic efficiency and toxic impacts to marine bivalves. J. Environ. Manag. 2022, 313, 115030. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Wang, J.; Ge, C.; Lian, Z. Fast extraction of chloramphenicol from marine sediments by using magnetic molecularly imprinted nanoparticles. Microchim. Acta 2019, 186, 428. [Google Scholar] [CrossRef]
- Zhao, C.; Suyamud, B.; Yuan, Y.; Ghosh, S.; Xu, X.; Hu, J. Effect of non-antibiotic factors on conjugative transfer of antibiotic resistance genes in aquaculture water. J. Hazard. Mater. 2025, 483, 136701. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Liu, L.; Su, Z.; Zhao, J.; Zhang, J.; Cai, Z.; Peñuelas, J.; Huang, X. Plant Diversity Reduces the Risk of Antibiotic Resistance Genes in Agroecosystems. Adv. Sci. 2025, 12, 2410990. [Google Scholar] [CrossRef]
- Peiravi, M.; Zafarnak, S.; Taghvaei, H. Design and fabrication of a microchannel plasma reactor for pharmaceutical wastewater treatment. J. Water Process Eng. 2025, 70, 107007. [Google Scholar] [CrossRef]
- Nugraha, M.W.; Kim, S.; Roddick, F.; Xie, Z.; Fan, L. A review of the recent advancements in adsorption technology for removing antibiotics from hospital wastewater. J. Water Process Eng. 2025, 70, 106960. [Google Scholar] [CrossRef]
- Burgos, J.; Gómez, G.; Leiva, A.M.; Vidal, G. Effects of combined antibiotics on the biomass stability and the occurrence of antibiotic-resistant bacteria in activated sludge used for domestic wastewater treatment. J. Water Process Eng. 2025, 70, 106966. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Shu, X.; Cheng, X.; Yu, H.; Cao, Y.; Hu, Z.; Yu, J.C. A photocatalytic system for the Alleviation of ocean acidification and antibiotic pollution. Sep. Purif. Technol. 2025, 362, 131771. [Google Scholar] [CrossRef]
- Kong, M.; Zhang, Y.; Ma, Y.; Fang, H.; Wang, W.; Shi, G.; Yan, Y.; Zhang, S. Antibiotics and antibiotic resistance change bacterial community compositions in marine sediments. Environ. Res. 2024, 244, 118005. [Google Scholar] [CrossRef]
- Adenike, A.; Martine, B.; Thorsten, B.; Mariana, R.; Oliver, W. Usage of antibiotics in aquaculture and the impact on coastal waters. Mar. Pollut. Bull. 2023, 188, 114645. [Google Scholar] [CrossRef]
- Chen, S.; Liu, D.; Zhang, Q.; Guo, P.; Ding, S.; Shen, J.; Zhu, K.; Lin, W. A Marine Antibiotic Kills Multidrug-Resistant Bacteria without Detectable High-Level Resistance. ACS Infect. Dis. 2021, 7, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Qiu, D.; Zhang, Z.; Wang, Y.; Chen, B.; Zhang, Q.; Wang, T.; Hong, W.; Zhou, N.-Y.; Penuelas, J.; et al. A global atlas of marine antibiotic resistance genes and their expression. Water Res. 2023, 244, 120488. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, Y.; Zhang, R.; Han, M.; Zeng, W.; Wang, Y.; Yu, K.; Yang, Y. Occurrence, source, and the fate of antibiotics in mariculture ponds near the Maowei Sea, South China: Storm caused the increase of antibiotics usage. Sci. Total Environ. 2021, 752, 141882. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, R.; Wang, Y.; Pan, X.; Tang, J.; Zhang, G. Occurrence and distribution of antibiotics in the Beibu Gulf, China: Impacts of river discharge and aquaculture activities. Mar. Environ. Res. 2012, 78, 26–33. [Google Scholar] [CrossRef]
- Chau, H.T.C.; Kadokami, K.; Duong, H.T.; Kong, L.; Nguyen, T.T.; Nguyen, T.Q.; Ito, Y. Occurrence of 1153 organic micropollutants in the aquatic environment of Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 7147–7156. [Google Scholar] [CrossRef]
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Siddique, M.B.A.; Kamal, A.; Coyne, M.S. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2018, 191, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Xu, W.H.; Zhang, G.; Wai, O.W.H.; Zou, S.C.; Li, X.D. Transport and adsorption of antibiotics by marine sediments in a dynamic environment. J. Soils Sediment. 2009, 9, 364–373. [Google Scholar] [CrossRef]
- Jiang, L.; Zhai, W.; Wang, J.; Li, G.; Zhou, Z.; Li, B.; Zhou, H. Antibiotics and antibiotic resistance genes in the water sources of the Wuhan stretch of the Yangtze River: Occurrence, distribution, and ecological risks. Environ. Res. 2023, 239, 117295. [Google Scholar] [CrossRef] [PubMed]
- Biel-Maeso, M.; Baena-Nogueras, R.M.; Corada-Fernández, C.; Lara-Martín, P.A. Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci. Total Environ. 2018, 612, 649–659. [Google Scholar] [CrossRef]
- Kim, S.-C.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2007, 41, 50–57. [Google Scholar] [CrossRef]
- Ali, A.M.; Rønning, H.T.; Alarif, W.; Kallenborn, R.; Al-Lihaibi, S.S. Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Sci. Total Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef]
- He, K.; Hain, E.; Timm, A.; Tarnowski, M.; Blaney, L. Occurrence of antibiotics, estrogenic hormones, and UV-filters in water, sediment, and oyster tissue from the Chesapeake Bay. Sci. Total Environ. 2018, 650, 3101–3109. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Zeng, L.; Zhu, M. Recent progress on the removal of antibiotic pollutants using photocatalytic oxidation process. Crit. Rev. Env. Sci. Technol. 2022, 52, 1401–1448. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.Q.; Nguyen, A.T.Q.; Nguyen, N.T.M.; Nguyen, A.D.; Bui, H.V.; Nguyen-Thanh, L.; Nguyen, M.N. Sorption of oxytetracycline to microsized colloids under concentrated salt solution: A perspective on terrestrial-to-ocean transfer of antibiotics. Sci. Total Environ. 2023, 905, 167005. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Qu, S.; Li, R.; Huo, Z.; Gao, Y.; Luo, Y. Degradation of antibiotics by electrochemical advanced oxidation processes (EAOPs): Performance, mechanisms, and perspectives. Sci. Total Environ. 2023, 856, 159092. [Google Scholar] [CrossRef]
- Ma, L.; Yang, H.; Guan, L.; Liu, X.; Zhang, T. Risks of antibiotic resistance genes and antimicrobial resistance under chlorination disinfection with public health concerns. Environ. Int. 2022, 158, 106978. [Google Scholar] [CrossRef] [PubMed]
- Tavasol, F.; Tabatabaie, T.; Ramavandi, B.; Amiri, F. Design a new photocatalyst of sea sediment/titanate to remove cephalexin antibiotic from aqueous media in the presence of sonication/ultraviolet/hydrogen peroxide: Pathway and mechanism for degradation. Ultrason. Sonochem. 2020, 65, 105062. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, A.; Dhiman, A.; Mola, G.T.; Sharma, G.; Lai, C.W. Recent advances in photocatalytic removal of sulfonamide pollutants from waste water by semiconductor heterojunctions: A review. Mater. Today Chem. 2023, 30, 101603. [Google Scholar] [CrossRef]
- Sajini, T.; Gigimol, M.G.; Mathew, B. A brief overview of molecularly imprinted polymers supported on titanium dioxide matrices. Mater. Today Chem. 2019, 11, 283–295. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Wang, F.; Zhang, X.; Zhu, H.; Zou, H. Preparation of inorganic-framework molecularly imprinted TiO2/SiO2 nanofibers by one-step electrospinning and their highly selective photodegradation. Inorg. Chem. Front. 2023, 10, 4456–4470. [Google Scholar] [CrossRef]
- He, C.; Ma, J.; Xu, H.; Ge, C.; Lian, Z. Selective capture and determination of doxycycline in marine sediments by using magnetic imprinting dispersive solid-phase extraction coupled with high performance liquid chromatography. Mar. Pollut. Bull. 2022, 184, 114215. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.; Situ, W.; Gao, Y.; Huang, X.; Zhou, W.; Li, J.; Song, X. Selective adsorption-photocatalytic synergistic breakdown of sulfamethazine in milk using loaded molecularly imprinted Ag3PO4/TiO2 films. Food Chem. 2025, 467, 142194. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; He, W.; Xue, C.; Peng, W.; Liu, H.; Wang, W.; Shi, Z.; Dai, W.; Yuan, Z.; et al. The optical and electrical properties of V2O5-TiO2/PI Nanocomposite film prepared by the Sol-Gel method. Infrared Phys. Technol. 2025, 145, 105719. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, B.; Liu, B.; Liu, X.; Zhu, L.; Wang, X. Study on the Degradation Performance of Novel Molecularly Imprinted Nd-TiO2 for Oxytetracycline Hydrochloride. Curr. Anal. Chem. 2024, 20, e15734110319990. [Google Scholar] [CrossRef]
- Ferreira, V.R.A.; Azenha, M.A.; Pereira, C.M.; Silva, A.F. Molecularly Imprinted Methyl-Modified Hollow TiO2 Microspheres. Molecules 2022, 27, 8510. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Guo, H.; Ma, W.; Li, J.; Zhou, S.; Lin, S.; Yan, L.; Li, K. Preparation of Molecularly Imprinted Carbon Microspheres by One-Pot Hydrothermal Method and Their Adsorption Properties to Perfluorooctane Sulfonate. Chin. J. Anal. Chem. 2019, 47, 1776–1784. [Google Scholar] [CrossRef]
- Xiong, X.; Li, C.; Yang, X.; Shu, Y.; Jin, D.; Zang, Y.; Shu, Y.; Xu, Q.; Hu, X. In situ grown TiO2 nanorod arrays functionalized by molecularly imprinted polymers for salicylic acid recognition and detection. J. Electroanal. Chem. 2020, 873, 114394. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Li, M.; Jin, Y.; Gu, Z.; Liu, C.; Ogino, K. Fe-doped TiO2/SiO2 nanofibrous membranes with surface molecular imprinted modification for selective photodegradation of 4-nitrophenol. Chin. Chem. Lett. 2018, 29, 527–530. [Google Scholar] [CrossRef]
- Fu, X.; Wen, D.; Shi, Y.; Zhao, S. Fabrication of the mesoporous TiO2 modified Eu-CN system for the adsorption of tetracycline antibiotics. J. Mol. Struct. 2025, 1321, 140077. [Google Scholar] [CrossRef]
- Uthiravel, V.; Narayanamurthi, K.; Raja, V.; Anandhabasker, S.; Kuppusamy, K. Green synthesis and characterization of TiO2 and Ag-doped TiO2 nanoparticles for photocatalytic and antimicrobial applications. Inorg. Chem. Commun. 2024, 170, 113327. [Google Scholar] [CrossRef]
- Kuang, K.; Chen, Y.; Li, Y.; Ji, Y.; Jia, N. N-doped TiO2/Ti3C2-driven self-photocatalytic molecularly imprinted ECL sensor for sensitive and steady detection of dexamethasone. Biosens. Bioelectron. 2024, 247, 115914. [Google Scholar] [CrossRef]
- Qi, H.; Wang, H. Facile synthesis of Pr-doped molecularly imprinted TiO2 mesocrystals with high preferential photocatalytic degradation performance. Appl. Surf. Sci. 2020, 511, 145607. [Google Scholar] [CrossRef]
- Asadbeigi, N.; Givianrad, M.H.; Azar, P.A.; Saber-Tehrani, M. Synthesis, Characterization and Optimization of Highly Selective Molecularly Imprinted Ni and F Co-Doped TiO2 Photocatalyst for Effective Removal and Photocatalytic Decomposition of Paracetamol. J. Water Chem. Technol. 2023, 45, 303–321. [Google Scholar] [CrossRef]
- Rescigno, R.; Sacco, O.; Venditto, V.; Fusco, A.; Donnarumma, G.; Lettieri, M.; Fittipaldi, R.; Vaiano, V. Photocatalytic activity of P-doped TiO2 photocatalyst. Photochem. Photobiol. Sci. 2023, 22, 1223–1231. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Wang, X.; Meng, X. Evaluation of photocatalytic selectivity of Ag/Zn modified molecularly imprinted TiO2 by multiwavelength measurement. Sci. Total Environ. 2020, 703, 134732. [Google Scholar] [CrossRef]
- Vo, T.L.N.; Dao, T.T.; Duong, A.T.; Bui, V.H.; Nguyen, V.H.; Nguyen, D.L.; Nguyen, D.C.; Nguyen, T.H.; Nguyen, H.T. Enhanced photocatalytic degradation of organic dyes using Ce-doped TiO2 thin films. J. Sol-Gel Sci. Technol. 2023, 108, 423–434. [Google Scholar] [CrossRef]
- Rahman, E.H.; Nair, P.S.; Shinoj, V.K.; Shaji, S.; Bunnell, A.; Jobson, N.; Philip, R.R. Enhanced photocatalytic degradation efficiency of TiO2 nanotubes by potassium doping. J. Mater. Sci. Mater. Electron. 2025, 36, 236. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Leon, R. Effect of Doping TiO2 NPs with Lanthanides (La, Ce and Eu) on the Adsorption and Photodegradation of Cyanide-A Comparative Study. Nanomaterials 2023, 13, 1068. [Google Scholar] [CrossRef]
- Yadav, V.; Saini, V.K.; Sharma, H. How different dopants leads to difference in photocatalytic activity in doped TiO2? Ceram. Int. 2020, 46, 27308–27317. [Google Scholar] [CrossRef]
- Akhani, S.B.; Thatikonda, S.K.; Solanki, M.B.; Akhani, T.; Gone, S.; Rathore, M.S. Photoluminescence and photocatalytic activity of sol gel synthesized Mg doped TiO2 nanoparticles. Inorg. Chem. Commun. 2024, 170, 113294. [Google Scholar] [CrossRef]
- Kavi, G.K.; Ravindran, B.; Ravichandran, K.; Thirumurugan, K.; Varshini, M.; Suvathi, S. Combined influence of vanadium doping and rGO composite partnering on the photocatalytic ability of TiO2. Mater. Lett. 2025, 381, 137767. [Google Scholar] [CrossRef]
- Verma, V.; Singh, S.V. Augmentation of photocatalytic degradation of methylene blue dye using lanthanum and iodine Co-doped TiO2 nanoparticles, their regeneration and reuse; and preliminary phytotoxicity studies for potential use of treated water. J. Environ. Chem. Eng. 2023, 11, 111339. [Google Scholar] [CrossRef]
- Deng, Z.; Osuga, R.; Matsubara, M.; Kanie, K.; Muramatsu, A. Morphological effect of TiO2 nanoparticles in TiO2/g-C3N4 heterojunctions on photocatalytic dye degradation. Chem. Lett. 2024, 53, upae171. [Google Scholar] [CrossRef]
- Lin, L.; Shi, L.; Liu, S.; He, J. Preparation of TiO2 Grafted on Graphene and Study on their Photocatalytic Properties. Int. J. Photoenergy 2023, 2023, 8676430. [Google Scholar] [CrossRef]
- Ye, M.; Pan, J.; Guo, Z.; Liu, X.; Chen, Y. Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite. Nanotechnol. Rev. 2020, 9, 558–567. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, L.; Ma, R.; Tomovska, R.; Luo, X.; Xie, X.; Su, T.; Ji, H. TiO2/BiYO3 composites for enhanced photocatalytic hydrogen production. J. Alloys Compd. 2020, 836, 155428. [Google Scholar] [CrossRef]
- Alimard, P.; Gong, C.; Itskou, I.; Kafizas, A. Achieving high photocatalytic NOx removal activity using a Bi/BiOBr/TiO2 composite photocatalyst. Chemosphere 2024, 368, 143728. [Google Scholar] [CrossRef]
- Prabhakaran, P.K.; Balu, S.; Sridharan, G.; Ganapathy, D.; Sundramoorthy, A.K. Development of Eco-friendly CQDs/TiO2 nanocomposite for enhanced photocatalytic degradation of methyl orange dye. Eng. Res. Express 2025, 7, 015002. [Google Scholar] [CrossRef]
- Balagoutham, P.; Vallarasu, K.; Sampathkumar, V.; Anitha, R.; Vijayalakshmi, V. Synergistic photocatalytic activity of LaFeO3/TiO2 nanocomposites for methylene blue degradation under UV Light. J. Mater. Sci. Mater. Electron. 2025, 36, 472. [Google Scholar] [CrossRef]
- Arora, M.; Kaur, H. Effect of doping in TiO2/chitosan composite on adsorptive-photocatalytic removal of gallic acid from water. Chemosphere 2025, 373, 144122. [Google Scholar] [CrossRef]
- He, J.; Hu, Z.; Zhu, X.; Liu, Z. Enhanced photocatalytic treatment of oilfield wastewater using TiO2/MoS2 nanocomposites prepared via sol-gel and hydrothermal methods. React. Kinet. Mech. Catal. 2025, 138, 1153–1174. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Wan, X.; Jiang, N.; Duan, W.; Lei, W.; Nan, Y.; Ding, D.; Xiao, G. Powering efficient dye degradation based on nanostructured FeOOH/TiO2 composite with hydrophilic surfaces and unparalleled photocatalytic performance. Ceram. Int. 2025, 51, 14966–14973. [Google Scholar] [CrossRef]
- Dong, R.; Lu, H.; Mei, W.; Tang, S.; Xu, J. Bi2O3-Modified Rice-Like Brookite TiO2 for Enhancing the Photocatalytic Activity under Visible-Light Irradiation. ChemistrySelect 2025, 10, e202405403. [Google Scholar] [CrossRef]
- Mohammed-Amine, E.; Kaltoum, B.; El Mountassir, E.M.; Abdelaziz, A.T.; Stephanie, R.; Stephanie, L.; Anne, P.; Pascal, W.W.C.; Alrashed, M.M.; Salah, R. Novel sol-gel synthesis of TiO2/BiPO4 composite for enhanced photocatalytic degradation of carbamazepine under UV and visible light: Kinetic, identification of photoproducts and mechanistic insights. J. Water Process Eng. 2025, 70, 107098. [Google Scholar] [CrossRef]
- Meirelles, R.M.; Giroto, A.S.; Furukawa, K.; Gonçalves, M. Valorization of Lignocellulosic Biomass for Photocatalytic Applications: Development of Activated Carbon-TiO2 Composites. ACS Sustain. Resour. Manag. 2025, 2, 524–535. [Google Scholar] [CrossRef]
- Yang, D.; Xia, Y.; Xiao, T.; Xu, Z.; Lei, Y.; Jiao, Y.; Zhu, X.; Feng, W. Constructing Ag-TiO2-g-C3N4 S-scheme heterojunctions for photocatalytic degradation of malachite green. Opt. Mater. 2025, 159, 116652. [Google Scholar] [CrossRef]
- Almutairi, S.T. Enhanced photocatalytic degradation of methylene blue using a ZnO-TiO2/rGO nanocomposite under UV irradiation. Ionics 2025, 31, 2789–2805. [Google Scholar] [CrossRef]
- Liu, S.; Lou, H.; Luo, J.; Albashir, D.; Shi, Y.; Chen, Q. A Novel In Situ Biosynthesized Bacterial Cellulose/MoS2/TiO2 Composite Film for Efficient Removal of Dyes and Pathogenic Bacteria from Industrial Wastewater under Sunlight Illumination. ACS Appl. Mater. Interfaces 2025, 17, 19453–19561. [Google Scholar] [CrossRef]
- Appu, S.; Udayabhanu; Anusha, B.R.; Priyadarshini, H.N.; Alharethy, F.; Srinivas, R.G.; Abhijna; Nagaraju, G.; Prashantha, K. Type-1 heterojunction TiO2 Nanotubes/Ag2CrO4 nanoparticles: Advanced photocatalytic and electrochemical applications. Mater. Chem. Phys. 2025, 337, 130573. [Google Scholar] [CrossRef]
- Wu, J.; Chen, C.; Lin, C.; Kumar, K.; Lu, Y.; Liou, S.Y.H.; Chen, S.; Wei, D.; Dong, C.; Chen, C. Improved photocatalytic efficacy of TiO2 open nanotube arrays: A view by XAS. Appl. Surf. Sci. 2020, 527, 146844. [Google Scholar] [CrossRef]
- Sharafudheen, S.B.; Vijayakumar, C.; Anjana, P.M.; Bindhu, M.R.; Alharbi, N.S.; Khaled, J.M.; Kadaikunnan, S.; Kakarla, R.R.; Aminabhavi, T.M. Biogenically synthesized porous TiO2 nanostructures for advanced anti-bacterial, electrochemical, and photocatalytic applications. J. Environ. Manag. 2024, 366, 121728. [Google Scholar] [CrossRef]
- Liu, L.; Xue, Z.; Gao, T.; Zhao, Q.; Sun, Y.; Wu, Y. Photocatalytic degradation performance of Ag-modified flexible TiO2 nanofiber film. Opt. Mater. 2025, 160, 116720. [Google Scholar] [CrossRef]
- Mendonca, T.A.P.; Nascimento, J.P.C.; Casagrande, G.A.; Vieira, N.C.S.; Goncalves, M. Ethylenediamine-modified activated carbon photocatalyst with the highest TiO2 attachment/dispersion for improved photodegradation of sulfamethazine. Mater. Chem. Phys. 2024, 318, 129203. [Google Scholar] [CrossRef]
- Wu, Y.; Zang, Y.; Xu, L.; Wang, J.; Jia, H.; Miao, F. Synthesis of functional conjugated microporous polymer/TiO2 nanocomposites and the mechanism of the photocatalytic degradation of organic pollutants. J. Mater. Sci. 2021, 56, 7936–7950. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Ma, Y.; Zhu, D.; Li, T.; Wang, J. Nitrate-group-grafting-induced assembly of rutile TiO2 nanobundles for enhanced photocatalytic hydrogen evolution. Chin. J. Catal. 2020, 41, 95–102. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wang, S.; Yang, F.; Zhou, W. Mesoporous black TiO2/MoS2/Cu2S hierarchical tandem heterojunctions toward optimized photothermal-photocatalytic fuel production. Chem. Eng. J. 2022, 427, 131830. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Wang, G.; Wang, K.; Li, J.; Zhao, L. In situ irradiated XPS investigation on S-scheme TiO2/Bi2S3 photocatalyst with high interfacial charge separation for highly efficient photothermal catalytic CO2 reduction. J. Mater. Sci. Technol. 2024, 189, 86–95. [Google Scholar] [CrossRef]
- Su, L.; Liu, X.; Xia, W.; Wu, B.; Li, C.; Xu, B.; Yang, B.; Xia, R.; Zhou, J.; Qian, J.; et al. Simultaneous photothermal and photocatalytic MOF- derived C/TiO2 composites for high-efficiency solar driven purification of sewage. J. Colloid Interface Sci. 2023, 650, 613–621. [Google Scholar] [CrossRef]
- Tang, Z.; Deng, X.; Chen, X.; Jiang, C.; Cai, L.; Guo, T. Ag2S/TiO2 Z-scheme heterojunction under magnetic field: Enhanced photocatalytic degradation of tetracycline. J. Alloys Compd. 2025, 1010, 177752. [Google Scholar] [CrossRef]
- Grzegorska, A.; Ofoegbu, J.C.; Cervera-Gabalda, L.; Gomez-Polo, C.; Sannino, D.; Zielinska-Jurek, A. Magnetically recyclable TiO2/MXene/MnFe2O4 photocatalyst for enhanced peroxymonosulphate-assisted photocatalytic degradation of carbamazepine and ibuprofen under simulated solar light. J. Environ. Chem. Eng. 2023, 11, 110660. [Google Scholar] [CrossRef]
- Gu, X.; Li, C.; Jiang, H.; Li, C.; Hu, Y. Synthesis of Z-scheme amorphous WO3-loaded TiO2 photocatalyst for enhanced photocatalytic degradation of dichloromethane: Internal electric field and mechanism exploration. J. Environ. Chem. Eng. 2024, 12, 113827. [Google Scholar] [CrossRef]
- Riedel, R.; Schowarte, J.; Semisch, L.; Gonzalez-Castano, M.; Ivanova, S.; Arellano-Garcia, H.; Martienssen, M. Improving the photocatalytic degradation of EDTMP: Effect of doped NPs (Na, Y, and K) into the lattice of modified Au/TiO2 nano-catalysts. Chem. Eng. J. 2025, 506, 160109. [Google Scholar] [CrossRef]
- Fu, W.; Wang, S.; Zhang, Y.; Cheng, B.; Wu, Y. 2D/2D F-doped TiO2/CdS S-scheme heterojunction photocatalyst for enhanced photocatalytic H2 generation. J. Mater. Sci. Technol. 2025, 232, 181–190. [Google Scholar] [CrossRef]
- Lin, Z.; Pei, L.; Liu, S.; Jiang, X.; Xu, W.; Li, F.; Wu, X.; Wang, H.; Lu, X. Developments and challenges on crystal forms and morphologies of nano-TiO2 photocatalysts in air and wastewater treatment. J. Water Process Eng. 2025, 70, 106909. [Google Scholar] [CrossRef]
- Chhabria, D.; Sundaram, G.A.; Ganapathy, D.; Balasubramaniyan, P. Unveiling the biomedical and photocatalytic properties of copper(II) imidazole complex-functionalized TiO2 nanoparticles. J. Mol. Liq. 2025, 426, 127368. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Dorraji, M.S.S.; Mohajer, S.; Saeedi, S.N.; Kianfar, M.; Koshelev, A.V.; Arkharova, N.A.; Karimov, D.N. Synergistic photothermal conversion and visible-light photodegradation of antibiotic in S-type TiO2 derived Ti3C2-MXene loaded on NaYF4: Tm3+, Er3+, Yb3+ @BiOI. J. Sci. Adv. Mater. Devices 2025, 10, 100851. [Google Scholar] [CrossRef]
- Yee, L.Y.; Ng, Q.H.; Ab Rahim, S.K.; Hoo, P.Y.; Chang, P.T.; Ahmad, A.L.; Low, S.C.; Shuit, S.H. A Novel Tri-Functionality pH-Magnetic-Photocatalytic Hybrid Organic-Inorganic Polyoxometalates Augmented Microspheres for Polluted Water Treatment. Membranes 2023, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, C.; Wan, J.; Zhang, X.; Cheng, Y.; Jin, J.; Zhang, H.; Duan, C.; Fang, Y. Study on the matching of adsorption rate and photocatalytic rate under electric field synergy to enhance the degradation performance of cyclohexane. Chem. Eng. Res. Des. 2025, 215, 386–397. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Chen, Z.; Wang, C.; Li, S.; Gui, D.; Zhu, X. Dye-encapsulated metal-organic frameworks as highly sensitive fluorescent sensors for tetracycline antibiotics in water. Mater. Lett. 2024, 363, 136243. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Du, Z.; Jin, M.; Yao, J.; Zhang, Z. MnFe2O4/zeolite composite catalyst for activating peroxymonosulfate to efficiently degrade antibiotic. Mater. Lett. 2023, 344, 134460. [Google Scholar] [CrossRef]
- Lou, Z.; Wen, X.; Song, L.; Yan, C.; Chen, H.; Lu, T.; Yu, J.; Xu, X.; Li, J. Oxygen vacancy engineered molecular imprinted TiO2 for preferential florfenicol remediation by electro-reductive approach: Enhanced dehalogenation performance and elimination of antibiotic resistance genes. Appl. Catal. B Environ. 2023, 336, 122923. [Google Scholar] [CrossRef]
- Chen, X.; Li, Q.; Yuan, T.; Ma, M.; Ye, Z.; Wei, X.; Fang, X.; Mao, S. Highly Specific Antibiotic Detection on Water-Stable Black Phosphorus Field-Effect Transistors. Acs Sens. 2023, 8, 858–866. [Google Scholar] [CrossRef]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, K.T.; Le, P.H. TiO2 and Au-TiO2 Nanomaterials for Rapid Photocatalytic Degradation of Antibiotic Residues in Aquaculture Wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Xu, S.; Tian, H.; Wang, C. Efficient identification and degradation of tetracycline hydrochloride from water by molecularly imprinted core-shell structured SiO2@TiO2. New J. Chem. 2023, 47, 13106–13116. [Google Scholar] [CrossRef]
- Fu, J.; Li, S.; Li, Q.; Bell, E.; Yang, D.; Li, T.; Li, Y.; He, J.; Zhou, L.; Zhang, Q.; et al. Preparation of surface molecular-imprinted MOFs for selective degradation of tetracycline antibiotics in wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133575. [Google Scholar] [CrossRef]
- Fu, J.; Yu, X.; Li, Z.; Zhang, Y.; Zhu, W.; Liu, J. Study on the Degradation of Chlortetracycline Hydrochloride in Mariculture Wastewater by Zn0.75Mn0.75Fe1.5O4/ZnFe2O4/ZnO Photocatalyst. Water Air Soil Pollut. 2021, 232, 12. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Duan, L.; Guo, P.; Li, X.; Cui, Q.; Wang, H.; Jiang, Q. Preparation of Si doped molecularly imprinted TiO2 photocatalyst and its degradation to antibiotic wastewater. Integr. Ferroelectr. 2016, 168, 170–182. [Google Scholar] [CrossRef]
- Li, D.; Yuan, R.; Zhou, B.; Chen, H. Selective photocatalytic removal of sulfonamide antibiotics: The performance differences in molecularly imprinted TiO2 synthesized using four template molecules. J. Clean. Prod. 2023, 383, 135470. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, Z.; Liu, H.; Li, Y.; Wu, J.; Sun, P.; Shen, G. Single-atom iron cocatalyst for highly enhancing TiO2 photocatalytic degradation of antibiotics and antibiotic-resistant genes. Chem. Eng. J. 2024, 482, 148906. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, J.; Liu, L.; Wu, R.; Ding, L.; Jiang, J.; Pang, J.; Li, Y.; Ren, N.; Yang, S. Selective removal of sulfamethoxazole by a novel double Z-scheme photocatalyst: Preferential recognition and degradation mechanism. Environ. Sci. Ecotechnol. 2024, 17, 100308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, X.; Chi, Y.; Wang, Y.; Sun, X.; Yue, Q.; Gao, B.; Xu, S. Molecularly imprinted carbon nanosheets supported TiO2: Strong selectivity and synergic adsorption-photocatalysis for antibiotics removal. J. Hazard. Mater. 2020, 383, 121211. [Google Scholar] [CrossRef]
- Qin, D.; Zhao, M.; Wang, J.; Lian, Z. Selective extraction and detection of norfloxacin from marine sediment and seawater samples using molecularly imprinted silica sorbents coupled with HPLC. Mar. Pollut. Bull. 2020, 150, 110677. [Google Scholar] [CrossRef]
- Zheng, L.; Liao, W.; Wu, J.; Long, Q.; Luo, Y.; Li, X.; Huang, L.; Jia, L.; Li, H.; Liu, K. Selective adsorption and photodegradation of residual norfloxacin in water using a mTiO2 based inorganic molecularly imprinted magnetic photocatalyst. New J. Chem. 2024, 48, 15567–15576. [Google Scholar] [CrossRef]
- Li, S.; Fang, L.; Ye, M.; Zhang, Y. Enhanced adsorption of norfloxacin on modified TiO2 particles prepared via surface molecular imprinting technique. Desalination Water Treat. 2016, 57, 408–418. [Google Scholar] [CrossRef]
- Xie, W.; Wu, Y.; Yan, W.; Ma, Y.; Meng, H.; Wang, G.; Zhang, L.; Jia, G.; Li, W.; Xiao, Y.; et al. The erythromycin sorption removal at environmentally relevant concentration based on molecular imprinted polymer: Performance and mechanism. Environ. Pollut. 2023, 336, 122425. [Google Scholar] [CrossRef]
- Cizmic, M.; Ljubas, D.; Rozman, M.; Asperger, D.; Curkovic, L.; Babic, S. Photocatalytic Degradation of Azithromycin by Nanostructured TiO2 Film: Kinetics, Degradation Products, and Toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Satulu, V.; Pandele, A.M.; Ionica, G.I.; Bobirica, L.; Bonciu, A.F.; Scarlatescu, A.; Bobirica, C.; Orbeci, C.; Voicu, S.I.; Mitu, B.; et al. Robust CA-GO-TiO2/PTFE Photocatalytic Membranes for the Degradation of the Azithromycin Formulation from Wastewaters. Polymers 2024, 16, 1368. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Chen, R.; Jiao, Y.; Zhao, K.; Liu, Y.; Zhu, G. Carbonized polymer dots-based molecular imprinting: An adsorbent with enhanced selectivity for highly efficient recognition and removal of ceftiofur sodium from complex samples. J. Hazard. Mater. 2024, 473, 134637. [Google Scholar] [CrossRef]
- Mehralipour, J.; Bagheri, S.; Gholami, M. Synthesis and characterization of rGO/Fe0/Fe3O4/TiO2 nanocomposite and application of photocatalytic process in the decomposition of penicillin G from aqueous. Heliyon 2023, 9, e18172. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, G.; Kong, J.; Guo, Y.; Xian, Z.; Dai, Y.; Wang, J.; Gong, T.; Sun, C.; Xian, Q. Sheet-on-sheet TiO2/Bi2MoO6 heterostructure for enhanced photocatalytic amoxicillin degradation. J. Hazard. Mater. 2022, 421, 126634. [Google Scholar] [CrossRef]
| Sea (Country) | Antibiotic | Concentration (ng∙L−1) | Reference |
|---|---|---|---|
| Beibu Gulf (China) | Sulfamethoxazole | 0–15.9 | [29] |
| Trimethoprim | 0–4.11 | ||
| Erythromycin | 2.59–47.6 | ||
| Bohai Bay (China) | Tetracyclines | 41.5–222.4 | [30] |
| Maowei Sea (China) | Demeclocycline | 276 ± 71.6 | [31] |
| Norfloxacin | 1.56 ± 1.46 | ||
| Enrofloxacin | 0.85 ± 0.65 | ||
| Hailing Island (south coast of England) | Oxytetracycline | 0–16,000 | [32] |
| Trimethoprim | 0–20 | ||
| North coast of the Persian Gulf (Iran) | Norfloxacin | 1.21–51.5 | [33] |
| Baltic Sea (Northern Europe) | Sulfamethoxazole | 0–311 | [34] |
| Trimethoprim | 0–279 | ||
| Po Valley (Italy) | Clarithromycin | 0–128.1 | [35] |
| Ciprofloxacin | 0–124 | ||
| Cadiz Bay (Spain) | Azithromycin | 0–1.2 | [36] |
| Erythromycin | 0–0.3 | ||
| South Sea (Korea) | Norfloxacin | 0–0.5 | [37] |
| Lincomycin | 0–438 | ||
| Red Sea (Saudi Arabia) | Sulfamethoxazole | 31.5–62.4 | [38] |
| Metronidazole | 51.0–178.6 | ||
| Eastern Mediterranean (Greece) | Clarithromycin | 0–1.5 | [39] |
| Amoxicillin | 0–127.8 | ||
| Chesapeake Bay (United States) | Azithromycin | 0–2.7 | [40] |
| Norfloxacin | 0–94.1 |
| Element | Photocatalyst | Pollutant | Degradation (%) | Reference |
|---|---|---|---|---|
| Pr | Pr-MIP-TMCs | Dinitrophenol | 92 | [61] |
| Ni and F | Ni-F-TiO2 | acetaminophen | 84 | [62] |
| P | 0.071PT | Escherichia coli | 90 | [63] |
| Ag and Zn | Ag/Zn-MIP-TiO2 | Ethyl hydroxybenzoate | 99 | [64] |
| Ce | Ce-TiO2 | Tetracycline | 86 | [65] |
| K | TNT-K5 | Methylene blue | 97 | [66] |
| La | La/TiO2 | Cyanide | 98 | [67] |
| B | B-TiO2 | Diclofenac sodium | 98 | [68] |
| Mg | Mg-doped TiO2 | Methyl orange | 95 | [69] |
| V | (TiO2:V)/rGO | Rhodamine B | 95 | [70] |
| La and I | LICT | Methylene blue | 98 | [71] |
| Material | Photocatalyst | Pollutant | Degradation (%) | Reference |
|---|---|---|---|---|
| CQDs | TiO2/CQDs | Methyl orange | 85 | [77] |
| LaFeO3 | LaFeO3/TiO2 | Methylene blue | 96 | [78] |
| Chitosan | TiO2/Chitosan | Gallic acid | 81 | [79] |
| MoS2 | TiO2/MoS2 | Oilfield suspended solids | 93 | [80] |
| FeOOH | FeOOH/TiO2 | Rodamine B | 84 | [81] |
| Bi2O3 | Bi2O3/brookite TiO2 | Ofloxacin | 91 | [82] |
| BiPO4 | TiO2/BiPO4 | Kamasipin | 88 | [83] |
| Activated Charcoal | AC-TiO2 | N-Acetyl-p-Aminophenol (APAP) | 82 | [84] |
| g-C3N4 | g-C3N4-TiO2-Ag | Malachite green | 66 | [85] |
| ZnO and rGO | ZnO-TiO2/rGO | Methylene blue | 100 | [86] |
| MoS2 | BC/MoS2/TiO2 | Escherichia coli | 100 | [87] |
| Ag2CrO4 | Ag2CrO4/TiO2 | NO2− | 100 | [88] |
| Method | Photocatalyst | Target Substance | Photocatalytic Performance | Reference |
|---|---|---|---|---|
| Conformal modification | CR- TiO2 NPs | Phenol red | Degradation rate 94% | [90] |
| Ag/TiO2 nanofiber film | Rhodamine B | Degradation rate 73% | [91] | |
| Surface functionalization | AC-ET/90TiO2 | Sulfadimethoxine | Degradation rate 90% | [92] |
| CMP/TiO2 | Ciprofloxacin | Degradation rate 97% | [93] | |
| Rutile TiO2 | Hydrogen production | Hydrogen precipitation rate 402 μmol·h−1 | [94] | |
| Photothermal Synergy | TiO2/MoS2/Cu2S | Hydrogen production | Hydrogen precipitation rate 3377 μmol·h−1 | [95] |
| TiO2/BiS | CO2 reduction | Reduction rate 8 μmol·h−1 | [96] | |
| UiO-66-NH2(Ti) | Methyl orange | Degradation rate 93% | [97] | |
| Magnetic/Electric field assisted catalysis | Ag2S/TiO2 | Tetracycline | Degradation rate 96% | [98] |
| TiO2/Ti3C2/MnFe2O4 | Ibuprofen | Degradation rate 100% | [99] | |
| WO3/TiO2 | Dichloromethane | Degradation rate 98% | [100] |
| Modification Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Element doping | High carrier separation efficiency; high selectivity | Doping amount is difficult to control; high cost | [101] |
| Composite structure construction | Versatility; high stability | Complicated preparation process; difficult to recover | [102] |
| Conformal modification | High surface area; strong adsorption properties | Difficult preparation; poor structural stability | [103] |
| Surface functionalization | High selectivity; high dispersibility | Poor modification stability; side reactions | [104] |
| Photothermal synergistic effect | High reaction rate; strong light absorption | High energy consumption; high material cost | [105] |
| Magnetic/electric field assisted catalysis | High separation and recovery; high reaction efficiency | High energy consumption; limited scope of application | [106,107] |
| Antibiotic Type | Antibiotic | Photocatalyst | Selectivity Factor | Degradation (%) | Reference |
|---|---|---|---|---|---|
| Tetracycline antibiotics | Tetracycline | MIP-TiO2@SiO2 | - | 82 | [113] |
| Oxytetracycline | MIP-Nd-TiO2 | 1.7 | 92 | [53] | |
| Tetracycline | TMIP | 3.4 | 100 | [114] | |
| Oxytetracycline | TiO2/SiO2/OTC | - | 81 | [116] | |
| Sulfonamide antibiotics | Sulfamethoxazole | MIP-TiO2/SMZ | 4.0 | 99 | [117] |
| Sulfadiazine | MIP-TiO2/SD | 1.3 | 95 | [117] | |
| Sulfamethoxazole | MFTC | 2.8 | 97 | [119] | |
| Quinolone antibiotics | Ciprofloxacin | CT-MI | 3.2 | 86 | [120] |
| Norfloxacin | MIFTA | 3.1 | 88 | [122] | |
| Norfloxacin | MIPs | 3.4 | 77 | [123] | |
| Macrolide antibiotics | Erythromycin | EMIP | 2.6 | 80 | [124] |
| Azithromycin | CA-GO-TiO2/PTFE | - | 80 | [126] | |
| β-lactam antibiotics | Ceftiofur sodium | CPDs-NH@MIP | 5.6 | 82 | [127] |
| Penicillin | rGO/Fe0/Fe3O4/TiO2 | - | 96 | [128] | |
| Amoxicillin | TNBM-80 | - | 95 | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Jin, Y.; Zhao, L.; Zhang, Y.; Ren, B.; Song, X.; Liu, R. Molecularly Imprinted Titanium Dioxide: Synthesis Strategies and Applications in Photocatalytic Degradation of Antibiotics from Marine Wastewater: A Review. Materials 2025, 18, 2161. https://doi.org/10.3390/ma18092161
Han X, Jin Y, Zhao L, Zhang Y, Ren B, Song X, Liu R. Molecularly Imprinted Titanium Dioxide: Synthesis Strategies and Applications in Photocatalytic Degradation of Antibiotics from Marine Wastewater: A Review. Materials. 2025; 18(9):2161. https://doi.org/10.3390/ma18092161
Chicago/Turabian StyleHan, Xue, Yu Jin, Luyang Zhao, Yuying Zhang, Binqiao Ren, Xiaoxiao Song, and Rui Liu. 2025. "Molecularly Imprinted Titanium Dioxide: Synthesis Strategies and Applications in Photocatalytic Degradation of Antibiotics from Marine Wastewater: A Review" Materials 18, no. 9: 2161. https://doi.org/10.3390/ma18092161
APA StyleHan, X., Jin, Y., Zhao, L., Zhang, Y., Ren, B., Song, X., & Liu, R. (2025). Molecularly Imprinted Titanium Dioxide: Synthesis Strategies and Applications in Photocatalytic Degradation of Antibiotics from Marine Wastewater: A Review. Materials, 18(9), 2161. https://doi.org/10.3390/ma18092161








