Plasma Modification Effects of Thermoplastic Starch (TPS) Surface Layer: Film Wettability and Sterilization
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Wettability and Surface Free Energy
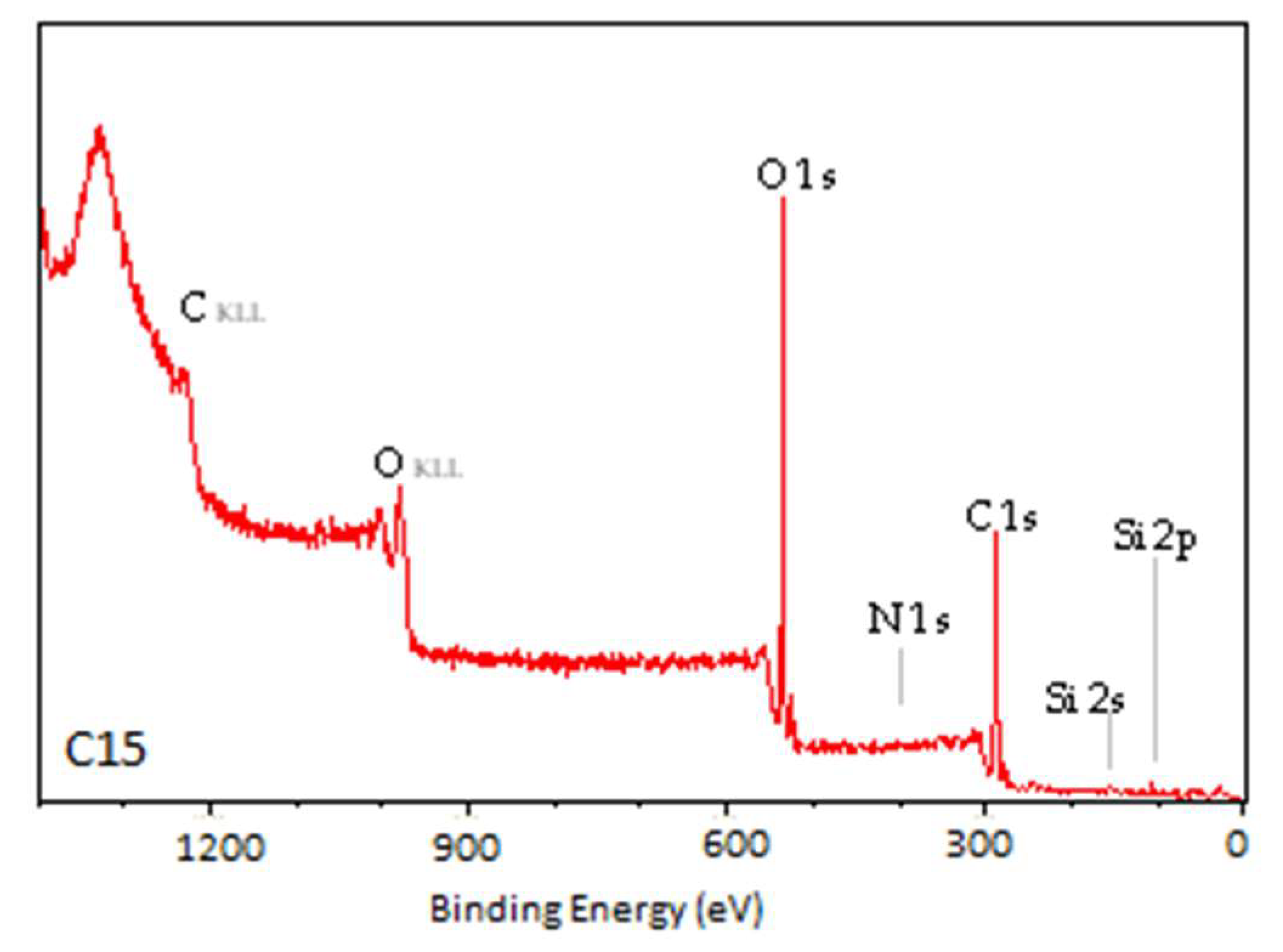
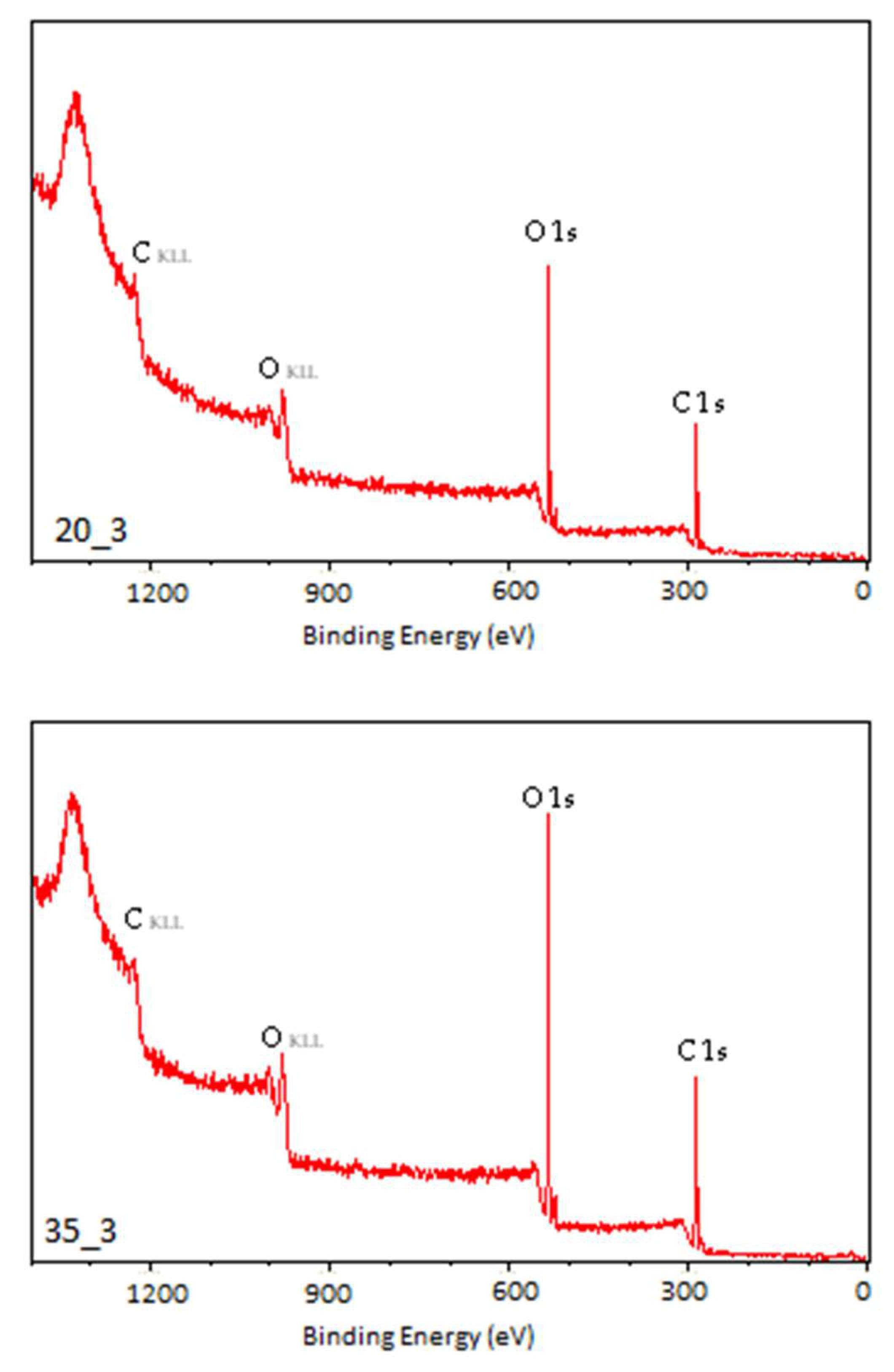
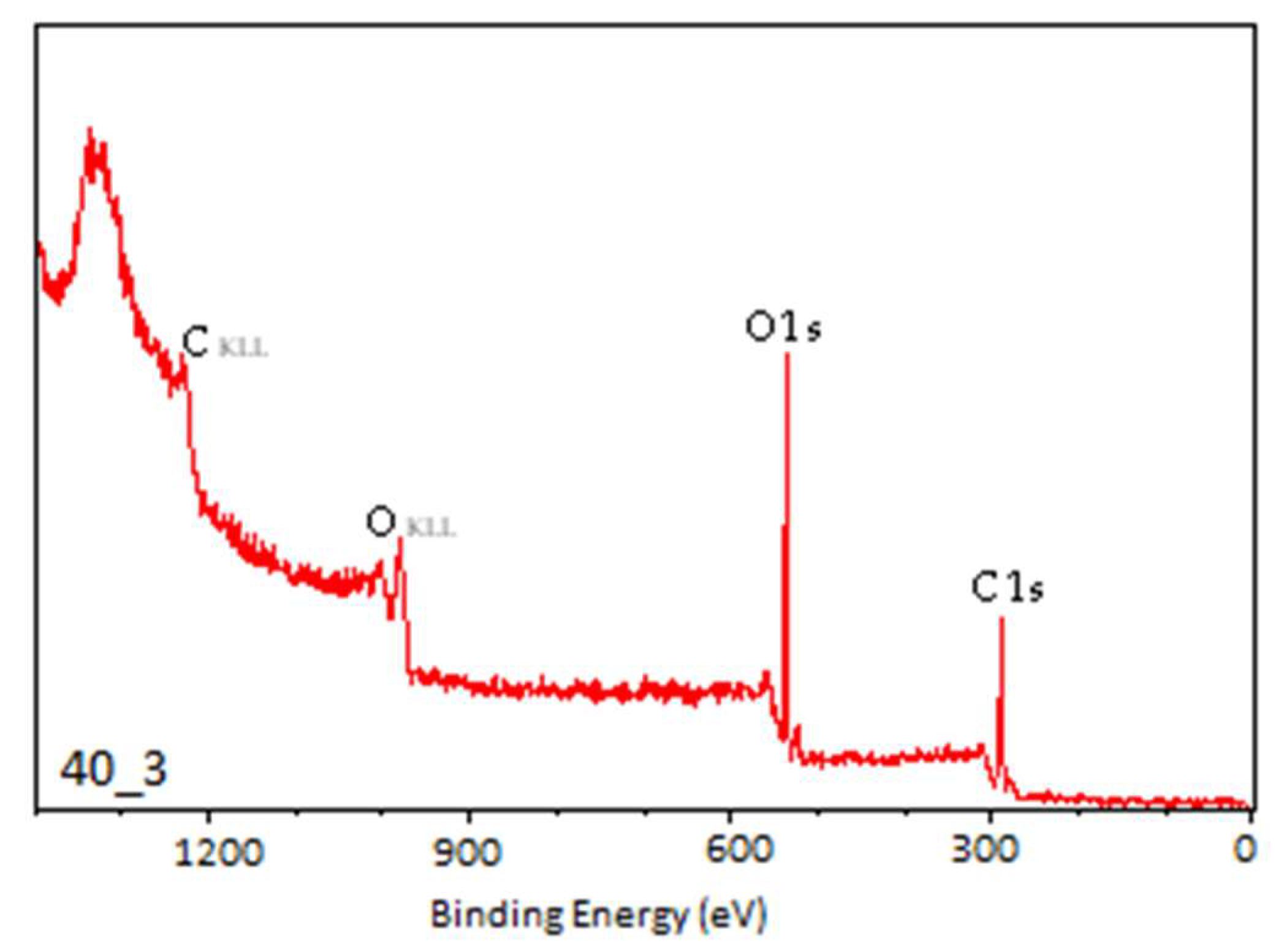
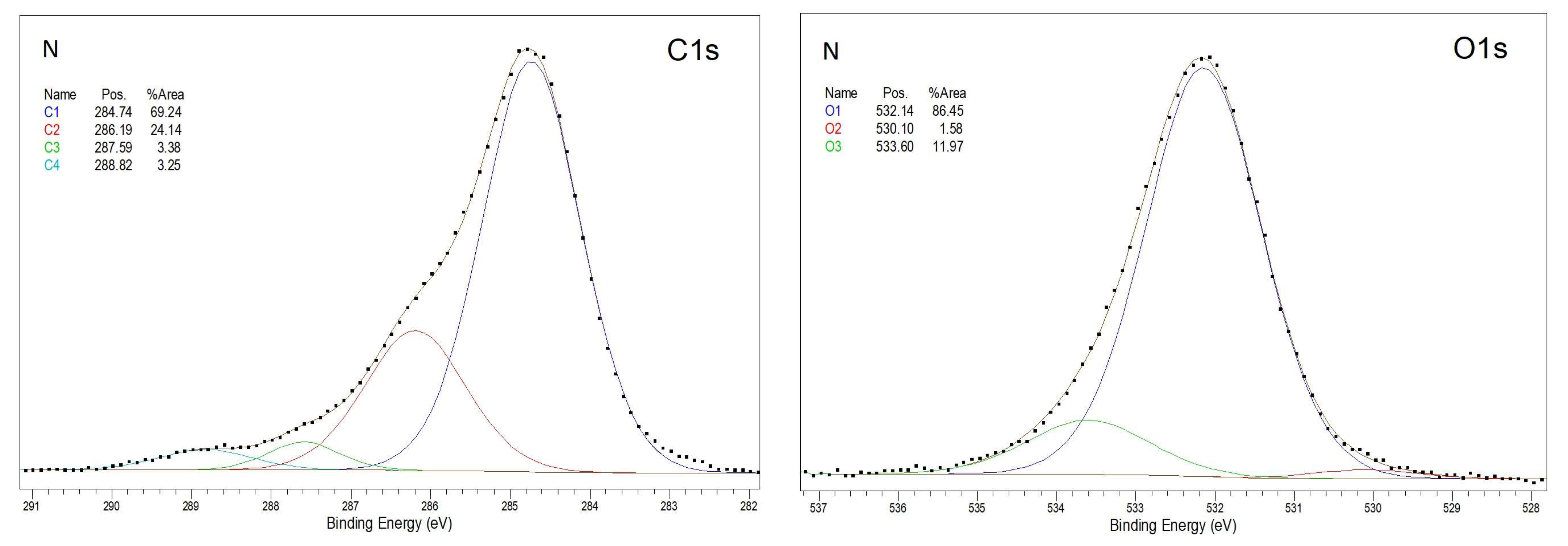
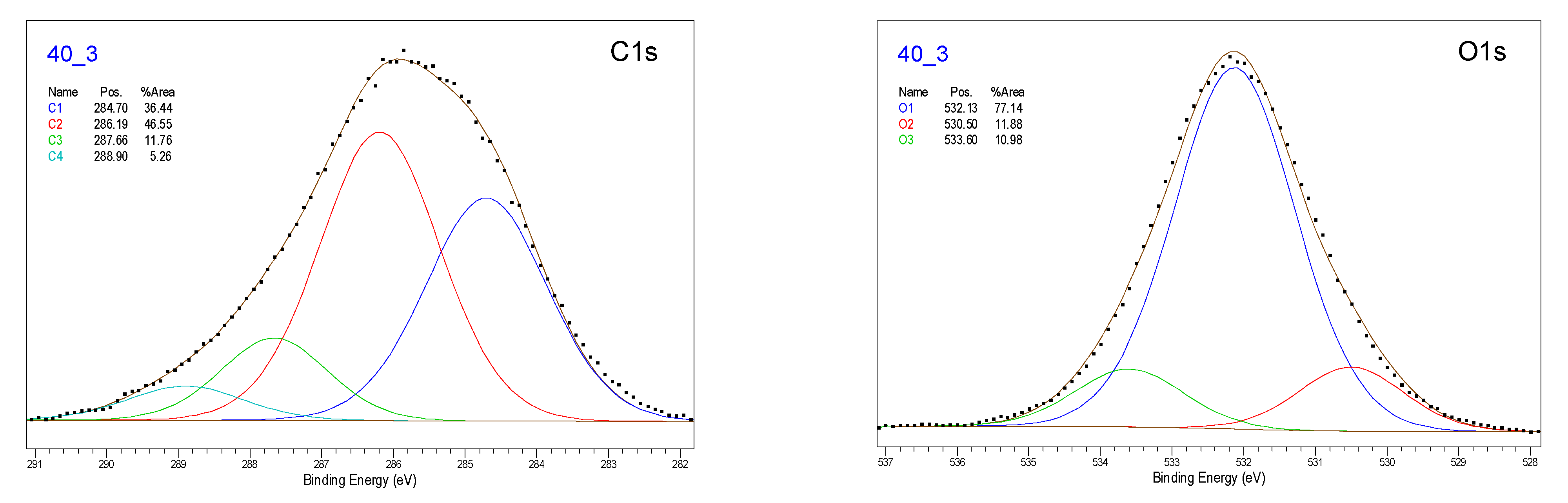
3.2. X-Ray Photoelectron Spectroscopy
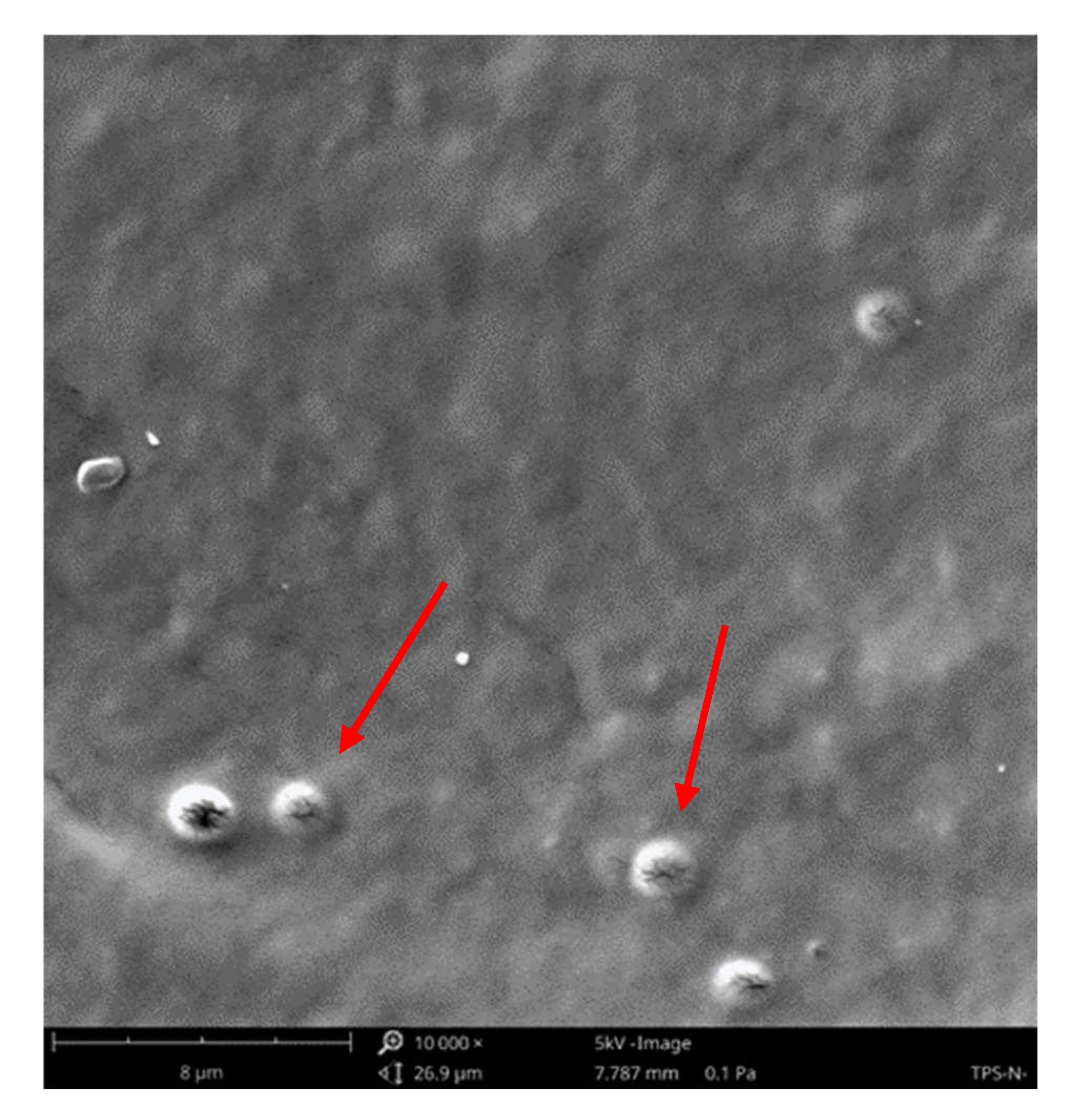
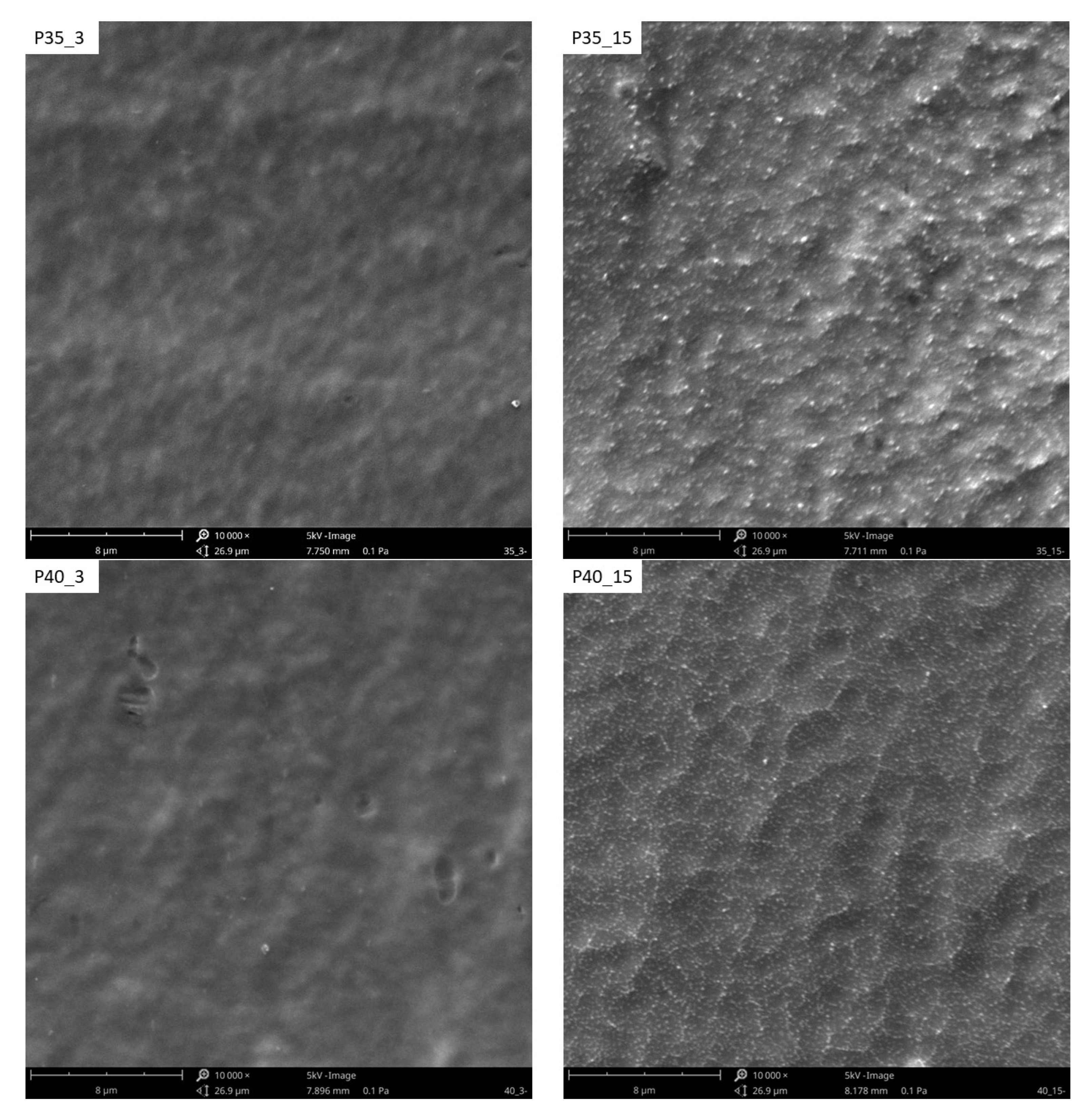
3.3. The Surface Morphology
3.4. Plasma Sterilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-based biodegradable packaging materials: A review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Gamarano, D.d.S.; Pereira, I.M.; da Silva, M.C.; Mottin, A.C.; Ayres, E. Crystal structure transformations in extruded starch plasticized with glycerol and urea. Polym. Bull. 2020, 77, 4971–4992. [Google Scholar] [CrossRef]
- Altayan, M.M.; Al Darouich, T.; Karabet, F. On the plasticization process of potato starch: Preparation and characterization. Food Biophys. 2017, 12, 397–403. [Google Scholar] [CrossRef]
- Denardin, C.C.; da Silva, L.P. Starch granules structure and its regards with physicochemical properties. Ciência Rural 2009, 39, 945–954. [Google Scholar] [CrossRef]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers Derived from Biomass Resources: A Short Review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Stepczyńska, M.; Rytlewski, P.; Moraczewski, K.; Pawłowska, A.; Karasiewicz, T. Novel Biocomposite of Starch and Flax Fiber Modified with Tannic Acid with Biocidal Properties. Polymers 2024, 16, 1108. [Google Scholar] [CrossRef]
- Baek, S.K.; Kim, S.; Song, K. Bin Cowpea starch films containing maqui berry extract and their application in salmon packaging. Food Packag. Shelf Life 2019, 22, 100394. [Google Scholar] [CrossRef]
- Martins, I.M.G.; Magina, S.P.; Oliveira, L.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. New biocomposites based on thermoplastic starch and bacterial cellulose. Compos. Sci. Technol. 2009, 69, 2163–2168. [Google Scholar] [CrossRef]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and food packaging: A review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Stepczyńska, M. Surface Modification by Low Temperature Plasma: Sterilization of Biodegradable Materials. Plasma Process. Polym. 2016, 13, 1078–1086. [Google Scholar] [CrossRef]
- Stepczyńska, M. Research of biocidal effect of corona discharges on poly(lactic acid) packaging films. J. Food Eng. 2014, 126, 56–61. [Google Scholar] [CrossRef]
- Vassallo, E.; Pedroni, M.; Aloisio, M.; Pietralunga, S.M.; Donnini, R.; Saitta, F.; Fessas, D. Plasma Treatment of Different Biodegradable Polymers: A Method to Enhance Wettability and Adhesion Properties for Use in Industrial Packaging. Plasma 2024, 7, 91–105. [Google Scholar] [CrossRef]
- Heidemann, H.M.; Dotto, M.E.R.; Laurindo, J.B.; Carciofi, B.A.M.; Costa, C. Cold plasma treatment to improve the adhesion of cassava starch films onto PCL and PLA surface. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 580, 123739. [Google Scholar] [CrossRef]
- Stepczyńska, M.; Zenkiewicz, M. Effects of corona treatment on the properties of the surface layer of polylactide. Polimery/Polymers 2014, 59, 220–226. [Google Scholar] [CrossRef]
- Ajmal, M.; Asad, M.; Huo, W.; Shao, Y.; Lu, W. Enhancing degradation of PLA-made rigid biodegradable plastics with non-thermal plasma treatment. J. Clean. Prod. 2024, 479, 143985. [Google Scholar] [CrossRef]
- Laput, O.; Vasenina, I.; Salvadori, M.C.; Savkin, K.; Zuza, D.; Kurzina, I. Low-temperature plasma treatment of polylactic acid and PLA/HA composite material. J. Mater. Sci. 2019, 54, 11726–11738. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Fazeli, M.; Florez, J.P.; Simão, R.A. Improvement in adhesion of cellulose fibers to the thermoplastic starch matrix by plasma treatment modification. Compos. Part B Eng. 2019, 163, 207–216. [Google Scholar] [CrossRef]
- Zhu, F. Plasma modification of starch. Food Chem. 2017, 232, 476–486. [Google Scholar] [CrossRef]
- Bastos, D.C.; Santos, A.E.F.; da Silva, M.L.V.J.; Simão, R.A. Hydrophobic corn starch thermoplastic films produced by plasma treatment. Ultramicroscopy 2009, 109, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Effect of high voltage dielectric barrier discharge (DBD) atmospheric cold plasma treatment on physicochemical and functional properties of taro (Colocasia esculenta) starch. Int. J. Biol. Macromol. 2023, 253, 126772. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Moisan, M.; Barbeau, J.; Crevier, M.C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization. Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Smith, H.M.M. History of Plasmas. Nature 1971, 233, 1971. [Google Scholar]
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Shul, R.J.; Pearton, S.J. (Eds.) Handbook of Advanced Plasma Processing Techniques; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Koga-Ito, C.Y.; Kostov, K.G.; Miranda, F.S.; Milhan, N.V.M.; Azevedo Neto, N.F.; Nascimento, F.; Pessoa, R.S. Cold Atmospheric Plasma as a Therapeutic Tool in Medicine and Dentistry. Plasma Chem. Plasma Process. 2024, 44, 1393–1429. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, M.; Chuang, R.; Zhang, H.; Li, H.; Xu, L.; Xia, N.; Xiao, C.; Rayan, A.M.; Ghamry, M. Mechanistic applications of low-temperature plasma in starch-based biopolymer film: A review. Food Chem. 2025, 479, 143739. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, H. The effect of cold plasma on starch: Structure and performance. Carbohydr. Polym. 2024, 340, 122254. [Google Scholar] [CrossRef]
- Zille, A.; Oliveira, F.R.; Souto, P.A.P. Plasma treatment in textile industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Fridman, A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008; ISBN 9780521847353. [Google Scholar]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Dharini, M.; Jaspin, S.; Mahendran, R. Cold plasma reactive species: Generation, properties, and interaction with food biomolecules. Food Chem. 2023, 405, 134746. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, E.; Başaran, P.; Kartal, S.; Akan, T. Cold plasma application to fresh green leafy vegetables: Impact on microbiology and product quality. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4484–4515. [Google Scholar] [CrossRef]
- Sani, I.K.; Aminoleslami, L.; Mirtalebi, S.S.; Sani, M.A.; Mansouri, E.; Eghbaljoo, H.; Jalil, A.T.; Thanoon, R.D.; Khodaei, S.M.; Mohammadi, F.; et al. Cold plasma technology: Applications in improving edible films and food packaging. Food Packag. Shelf Life 2023, 37, 101087. [Google Scholar] [CrossRef]
- Anuntagool, J.; Srangsomjit, N.; Thaweewong, P.; Alvarez, G. A review on dielectric barrier discharge nonthermal plasma generation, factors affecting reactive species, and microbial inactivation. Food Control 2023, 153, 109913. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Mahmoodi-Eshkaftaki, M. Potential applications of atmospheric-pressure dielectric barrier discharge cold plasma for fruit preservation: Advantages, effects on quality characteristics, and limitations. Innov. Food Sci. Emerg. Technol. 2024, 94, 103675. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q.; Ma, C.; Zhang, J.; Zhao, X.; Song, Z. Review on microwave–metal discharges and their applications in energy and industrial processes. Appl. Energy 2016, 175, 141–157. [Google Scholar] [CrossRef]
- Blackman, B.R.K.; Kinloch, A.J.; Watts, J.F. The plasma treatment of thermoplastic fibre composites for adhesive bonding. Composites 1994, 25, 332–341. [Google Scholar] [CrossRef]
- George, A.; Shen, B.; Craven, M.; Wang, Y.; Kang, D.; Wu, C.; Tu, X. A Review of Non-Thermal Plasma Technology: A novel solution for CO2 conversion and utilization. Renew. Sustain. Energy Rev. 2021, 135, 109702. [Google Scholar] [CrossRef]
- Kim, H.-H. Nonthermal plasma processing for air-pollution control: A historical review, current issues, and future prospects. Plasma Process. Polym. 2004, 1, 91–110. [Google Scholar] [CrossRef]
- Napartovich, A.P. Overview of atmospheric pressure discharges producing nonthermal plasma. Plasmas Polym. 2001, 6, 1–14. [Google Scholar] [CrossRef]
- Rytlewski, P.; Stepczyńska, M.; Moraczewski, K.; Malinowski, R.; Karasiewicz, T.; Sikorska, W.; Żenkiewicz, M. Flax fibers reinforced polycaprolactone modified by triallyl isocyanurate and electron radiation. Polym. Compos. 2019, 40, 481–488. [Google Scholar] [CrossRef]
- ISO 8296:2003; Plastics—Film and Sheeting—Determination of Wetting Tension. 2003. Available online: https://www.iso.org/standard/38451.html (accessed on 1 April 2025).
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Dann, J.R. Forces involved in the adhesive process: I. Critical surface tensions of polymeric solids as determined with polar liquids. J. Colloid Interface Sci. 1970, 32, 302–320. [Google Scholar] [CrossRef]
- Altay, B.N.; Ma, R.; Fleming, P.D.; Joyce, M.J.; Anand, A.; Chen, T.; Keskin, B.; Maddipatla, D.; Turkani, V.S.; Kotkar, P.R.; et al. Surface Free Energy Estimation: A New Methodology for Solid Surfaces. Adv. Mater. Interfaces 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Zenkiewicz, M. Comparative study on the surface free energy of a solid calculated by different methods. Polym. Test. 2007, 26, 14–19. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Zenkiewicz, M. New method of analysis of the surface free energy of polymeric materials calculated with Owens-Wendt and Neumann methods. Polimery/Polymers 2006, 51, 584–587. [Google Scholar] [CrossRef]
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. 2011. Available online: https://www.iso.org/standard/54431.html (accessed on 1 April 2025).
- McFarland, J. The nephelometer:an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. 1907, 49, 1176–1178. [Google Scholar]
- Wang, Y.; Liu, Y.; Zhao, Y.; Sun, Y.; Duan, M.; Wang, H.; Dai, R.; Liu, Y.; Li, X.; Jia, F. Bactericidal efficacy difference between air and nitrogen cold atmospheric plasma on Bacillus cereus: Inactivation mechanism of Gram-positive bacteria at the cellular and molecular level. Food Res. Int. 2023, 173, 113204. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.F. The Plasma Chemistry of Polymer Surfaces: Advanced Techniques for Surface Design; John Willey and Sons: Weinheim, Germany, 2012; ISBN 978-3-527-64802-3. [Google Scholar]
- Phan, L.T.; Yoon, S.M.; Moon, M.W. Plasma-based nanostructuring of polymers: A review. Polymers 2017, 9, 1–24. [Google Scholar] [CrossRef]
- Hasan, J.; Chatterjee, K. Recent advances in engineering topography mediated antibacterial surfaces. Nanoscale 2015, 7, 15568–15575. [Google Scholar] [CrossRef]
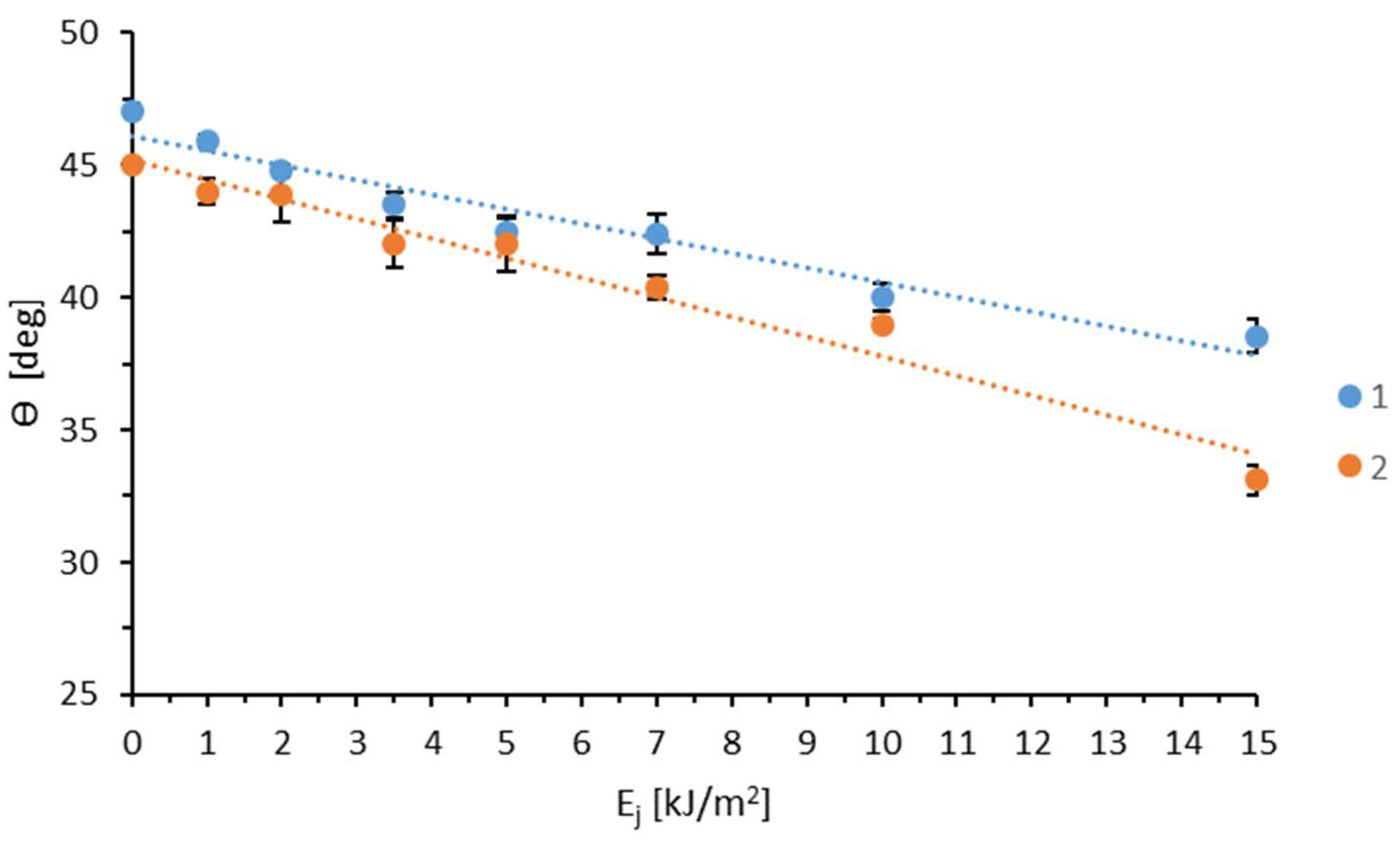
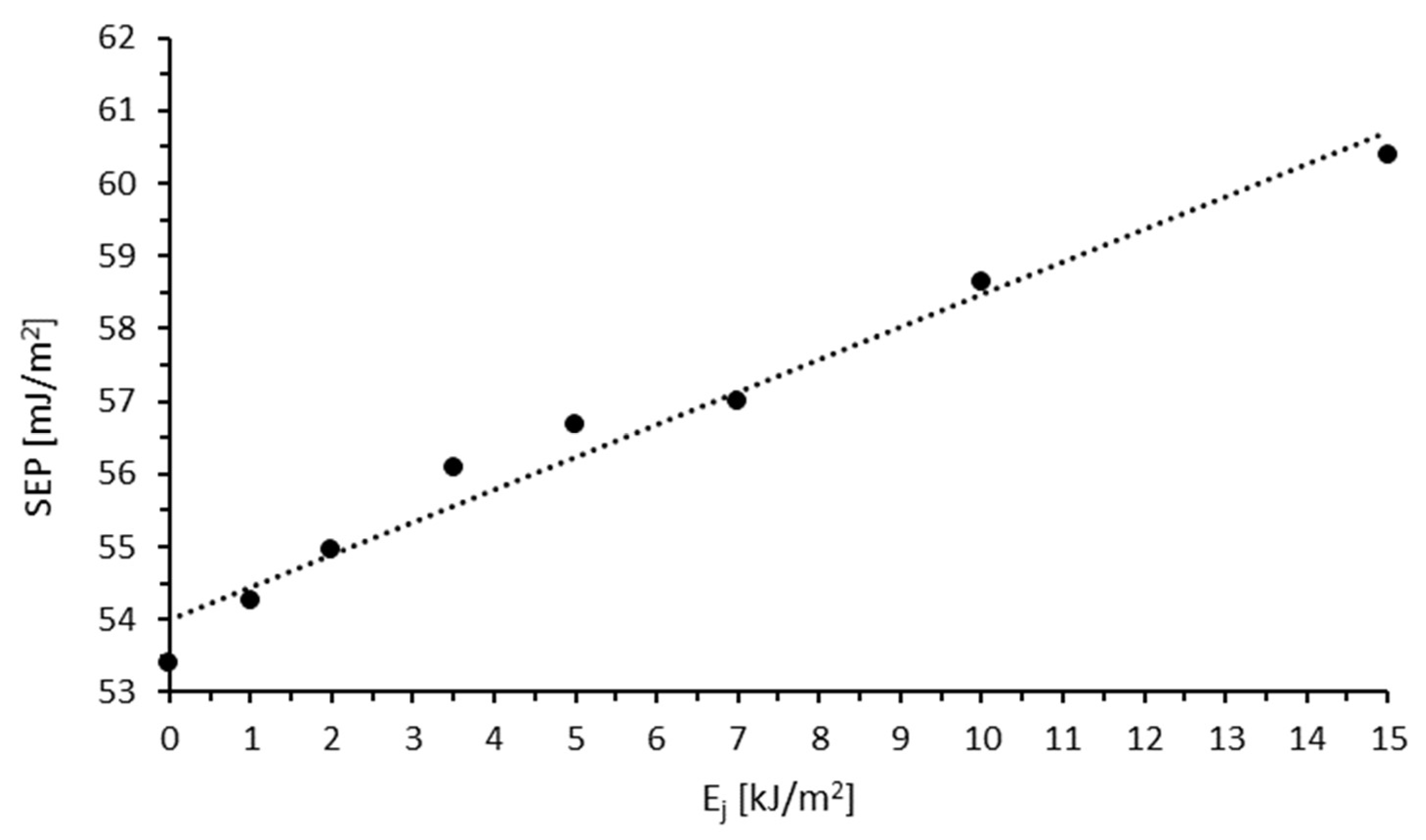
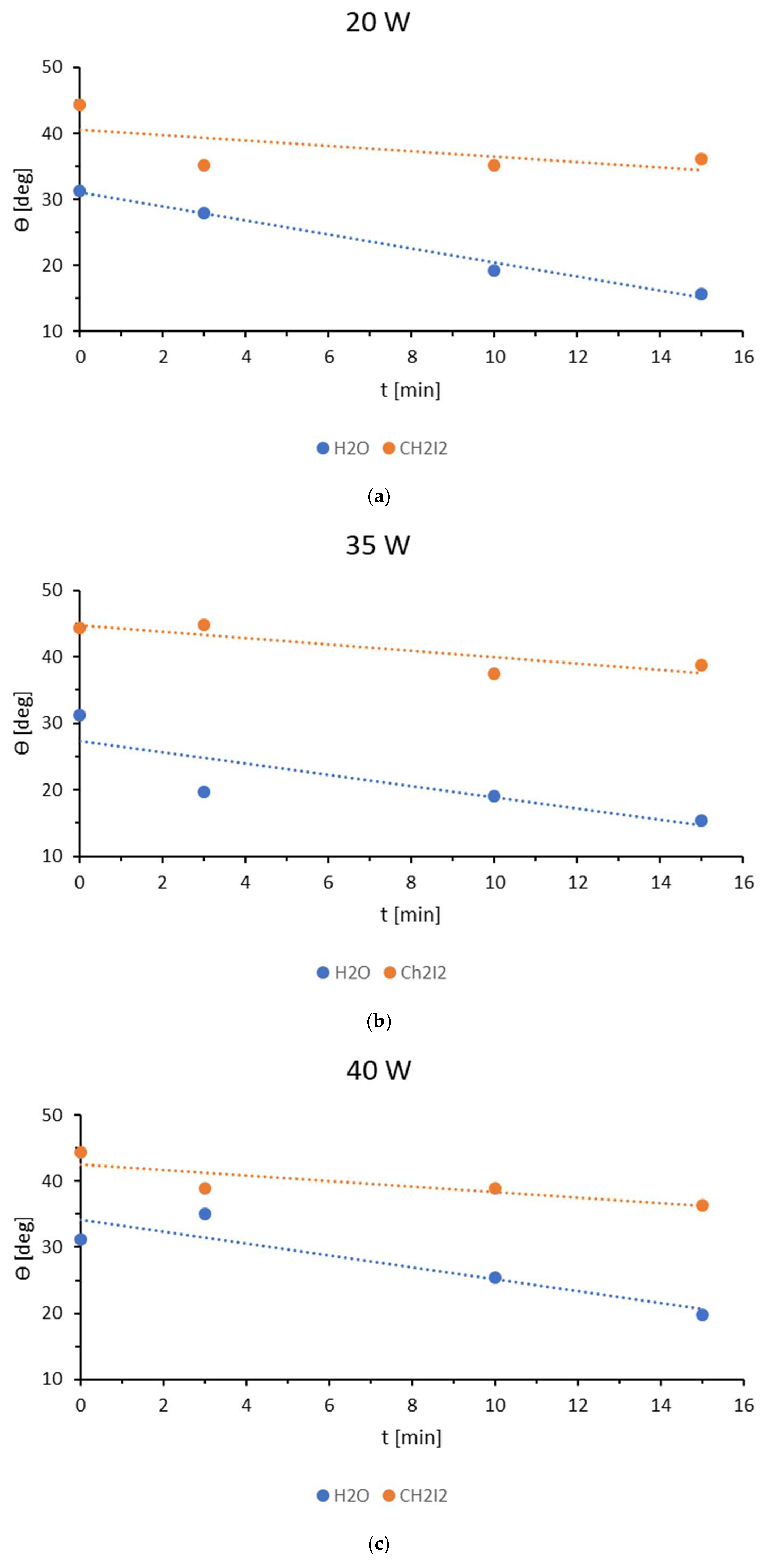
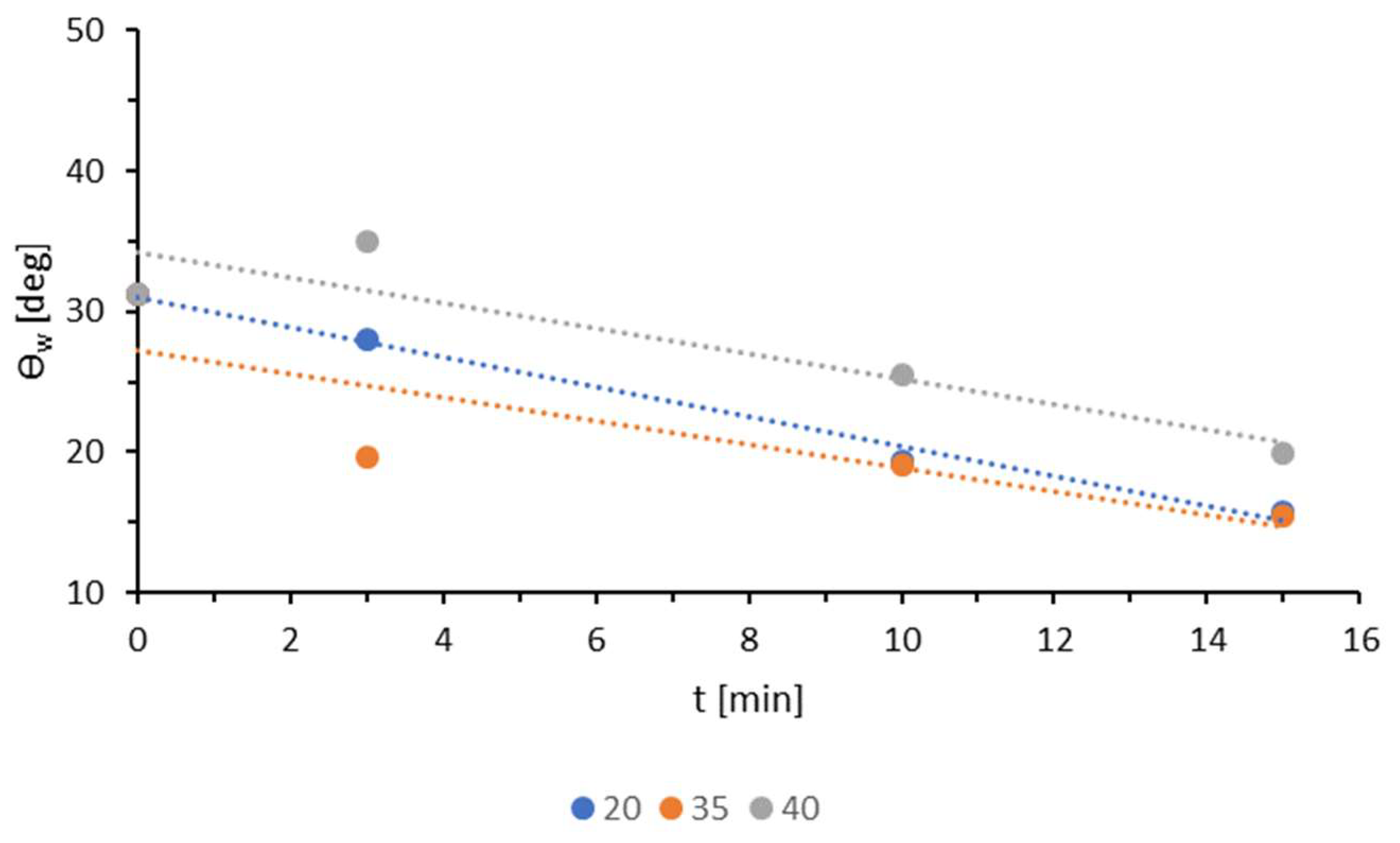
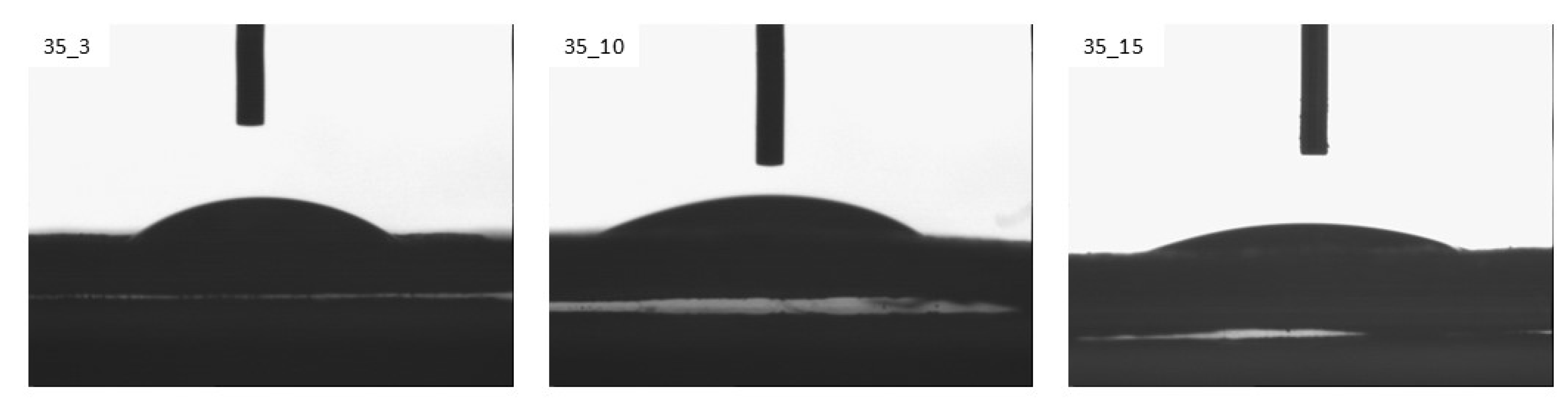
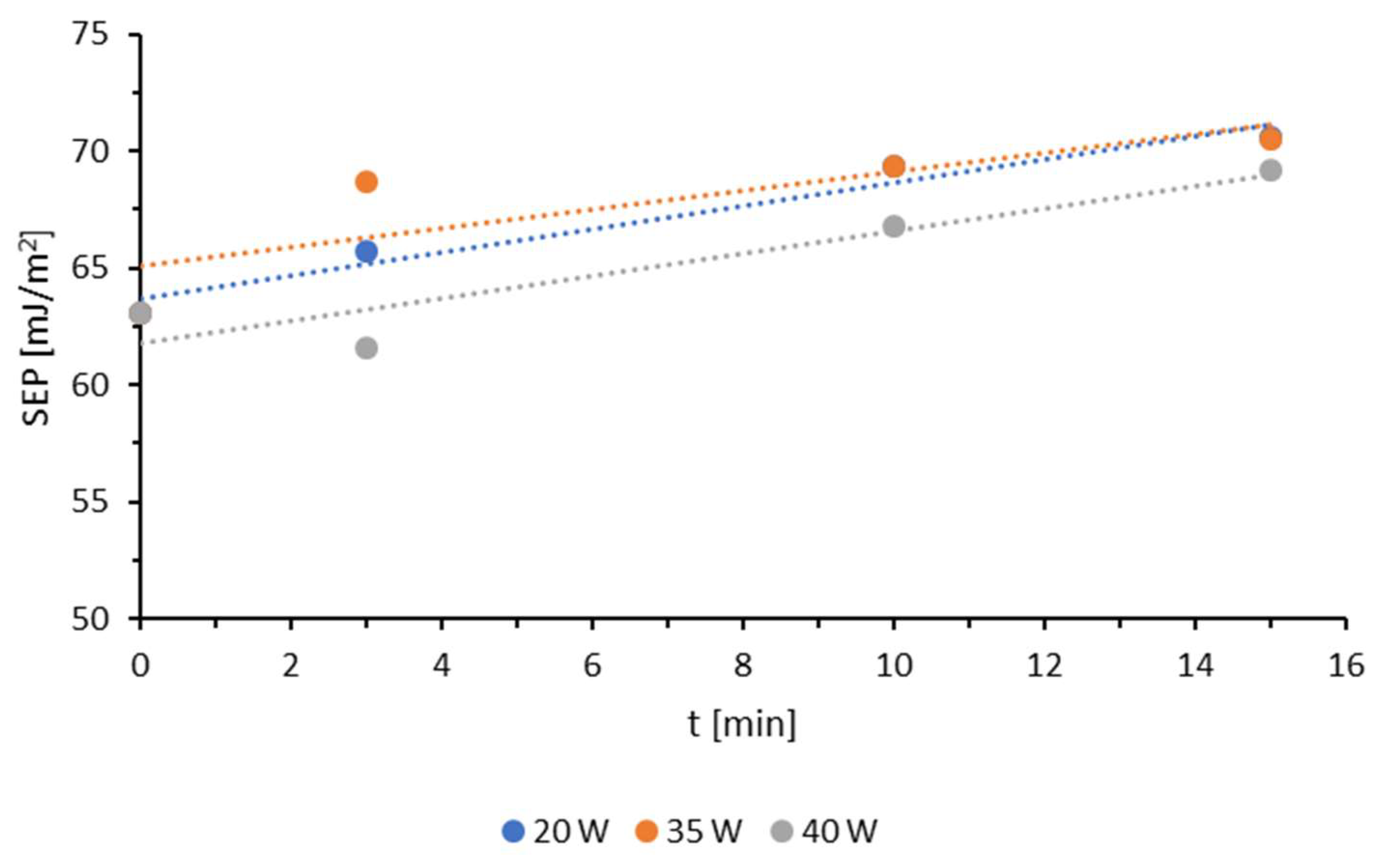
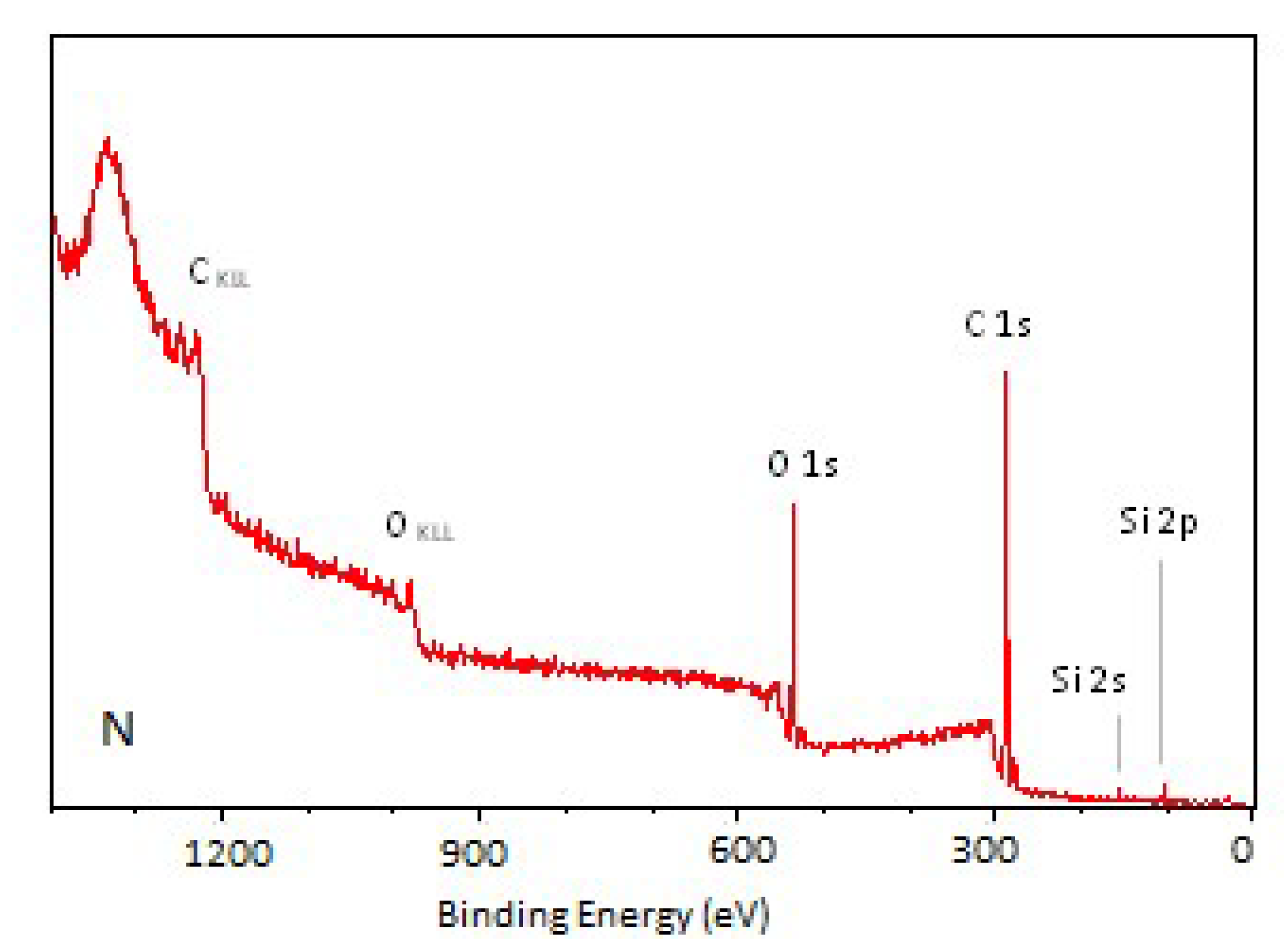
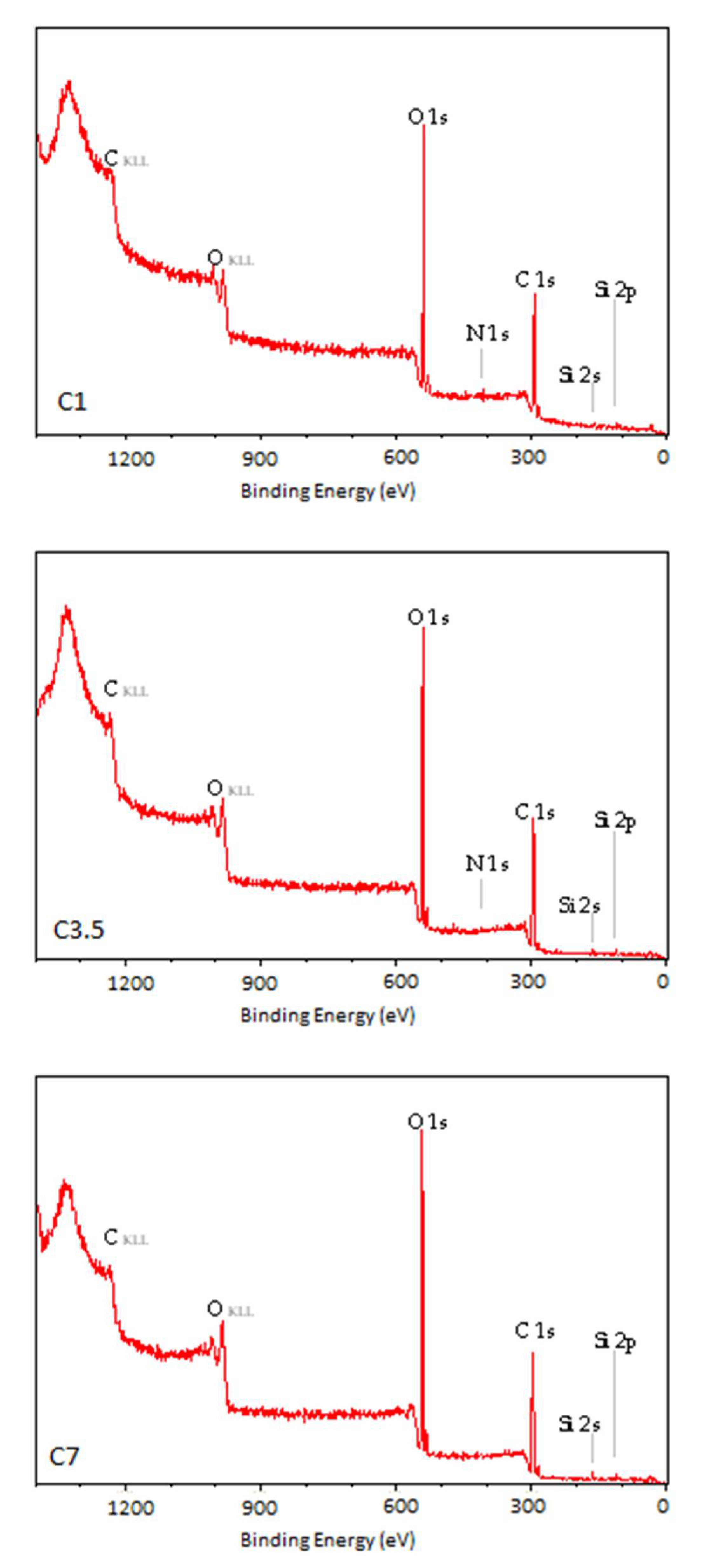




| Sample | Ej [kJ/m2] | v [m/min] |
|---|---|---|
| C1 | 1 | 96 |
| C2 | 2 | 48 |
| C3.5 | 3.5 | 27.4 |
| C5 | 5 | 19.2 |
| C7 | 7 | 13.7 |
| C10 | 10 | 9.6 |
| C15 | 15 | 6.4 |
| Sample | P [W] | t [min] |
|---|---|---|
| P20_3 | 20 | 3 |
| P20_10 | 20 | 10 |
| P20_15 | 20 | 15 |
| P35_3 | 35 | 3 |
| P35_10 | 35 | 10 |
| P35_15 | 35 | 15 |
| P40_3 | 40 | 3 |
| P40_10 | 40 | 10 |
| P40_15 | 40 | 15 |
| Test Liquid | V [μL] | ΔV [μL/min] | τ [s] |
|---|---|---|---|
| Water | 3–7 | 5 | 0.5 |
| Diiodomethane | 1–3 | 5 | 0.5 |
| Sample | C1 | C2 | C3 | C4 | O1 | O2 | O3 |
|---|---|---|---|---|---|---|---|
| N | 55.73 | 19.43 | 2.72 | 2.61 | 14.57 | 0.27 | 2.02 |
| C1 | 35.20 | 24.02 | 8.72 | 1.65 | 22.57 | 1.37 | 2.10 |
| C3.5 | 23.88 | 26.67 | 10.34 | 1.44 | 31.68 | 1.53 | 1.89 |
| C7 | 19.77 | 27.53 | 10.33 | 2.70 | 34.20 | 0.99 | 2.12 |
| C15 | 15.70 | 30.10 | 12.61 | 1.30 | 33.32 | 2.44 | 2.01 |
| P20_3 | 13.53 | 34.32 | 11.78 | 0.94 | 35.42 | 1.78 | 2.23 |
| P35_3 | 13.98 | 33.39 | 12.59 | 2.47 | 33.46 | 1.16 | 2.95 |
| P40_3 | 22.80 | 29.11 | 7.36 | 3.29 | 28.88 | 4.45 | 4.11 |
| Sample | %O | %C | %Si | %N | O/C [%] |
|---|---|---|---|---|---|
| N | 16.86 | 80.49 | 2.65 | 0.00 | 20.94 |
| C1 | 26.04 | 69.59 | 3.39 | 0.98 | 37.41 |
| C3.5 | 35.10 | 62.33 | 2.16 | 0.41 | 56.31 |
| C7 | 37.31 | 60.33 | 2.36 | 0.00 | 61.84 |
| C15 | 37.77 | 59,71 | 1.96 | 0.56 | 63.25 |
| P20_3 | 39.43 | 60.57 | 0.00 | 0.00 | 60.09 |
| P35_3 | 37.57 | 62.43 | 0.00 | 0.00 | 60.17 |
| P40_3 | 37.44 | 62.56 | 0.00 | 0.00 | 59.84 |
| Sample | Escherichia coli | Staphylococcus aureus | Salmonella enteritidis |
|---|---|---|---|
| N | 0 | 0 | 0 |
| C1 | 2 | 1 | 2 |
| C3.5 | 2 | 1 | 2 |
| C7 | 3 | 1 | 3 |
| C15 | 3 | 2 | 3 |
| P20_3 | 1 | 1 | 2 |
| P35_3 | 1 | 1 | 2 |
| P40_3 | 2 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepczyńska, M.; Śpionek, A. Plasma Modification Effects of Thermoplastic Starch (TPS) Surface Layer: Film Wettability and Sterilization. Materials 2025, 18, 2156. https://doi.org/10.3390/ma18092156
Stepczyńska M, Śpionek A. Plasma Modification Effects of Thermoplastic Starch (TPS) Surface Layer: Film Wettability and Sterilization. Materials. 2025; 18(9):2156. https://doi.org/10.3390/ma18092156
Chicago/Turabian StyleStepczyńska, Magdalena, and Aleksandra Śpionek. 2025. "Plasma Modification Effects of Thermoplastic Starch (TPS) Surface Layer: Film Wettability and Sterilization" Materials 18, no. 9: 2156. https://doi.org/10.3390/ma18092156
APA StyleStepczyńska, M., & Śpionek, A. (2025). Plasma Modification Effects of Thermoplastic Starch (TPS) Surface Layer: Film Wettability and Sterilization. Materials, 18(9), 2156. https://doi.org/10.3390/ma18092156






