Preparation of NaA Zeolite Composite Polyacrylonitrile Membranes (TiO2-NaA@PANMs) Doped with TiO2 and Adsorption Study of Sr2+
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
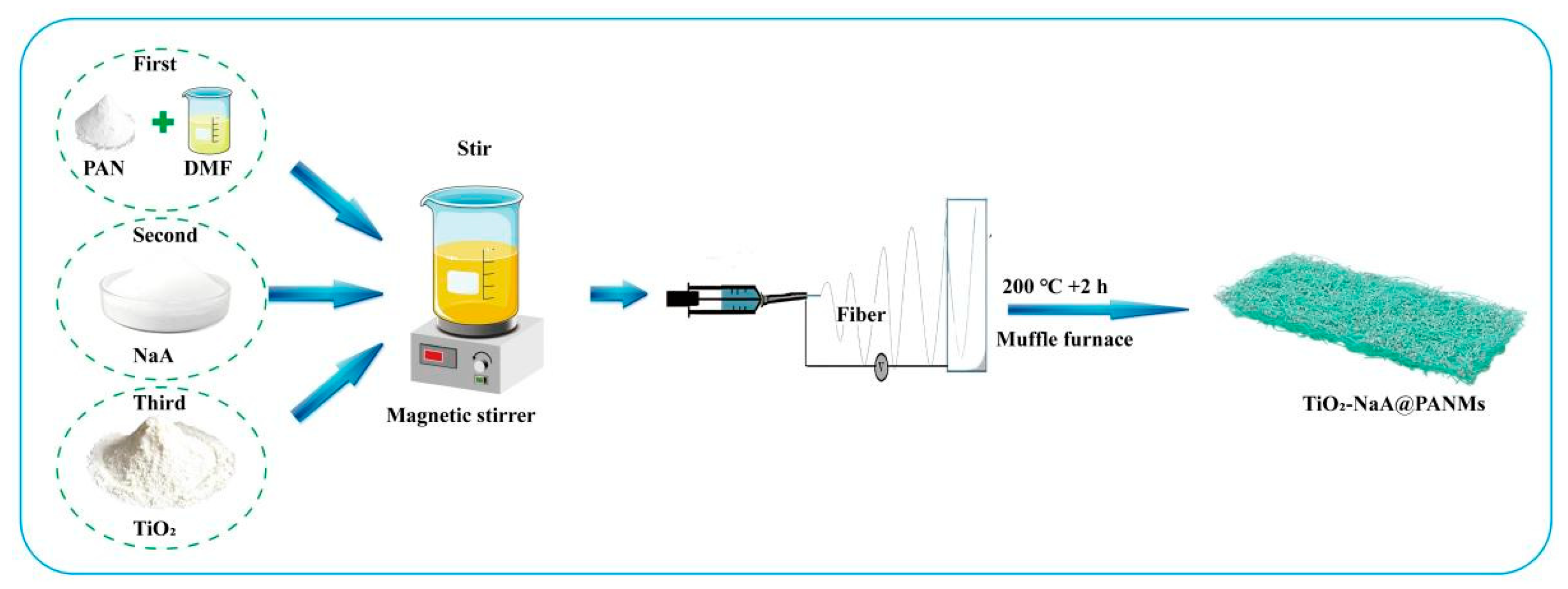
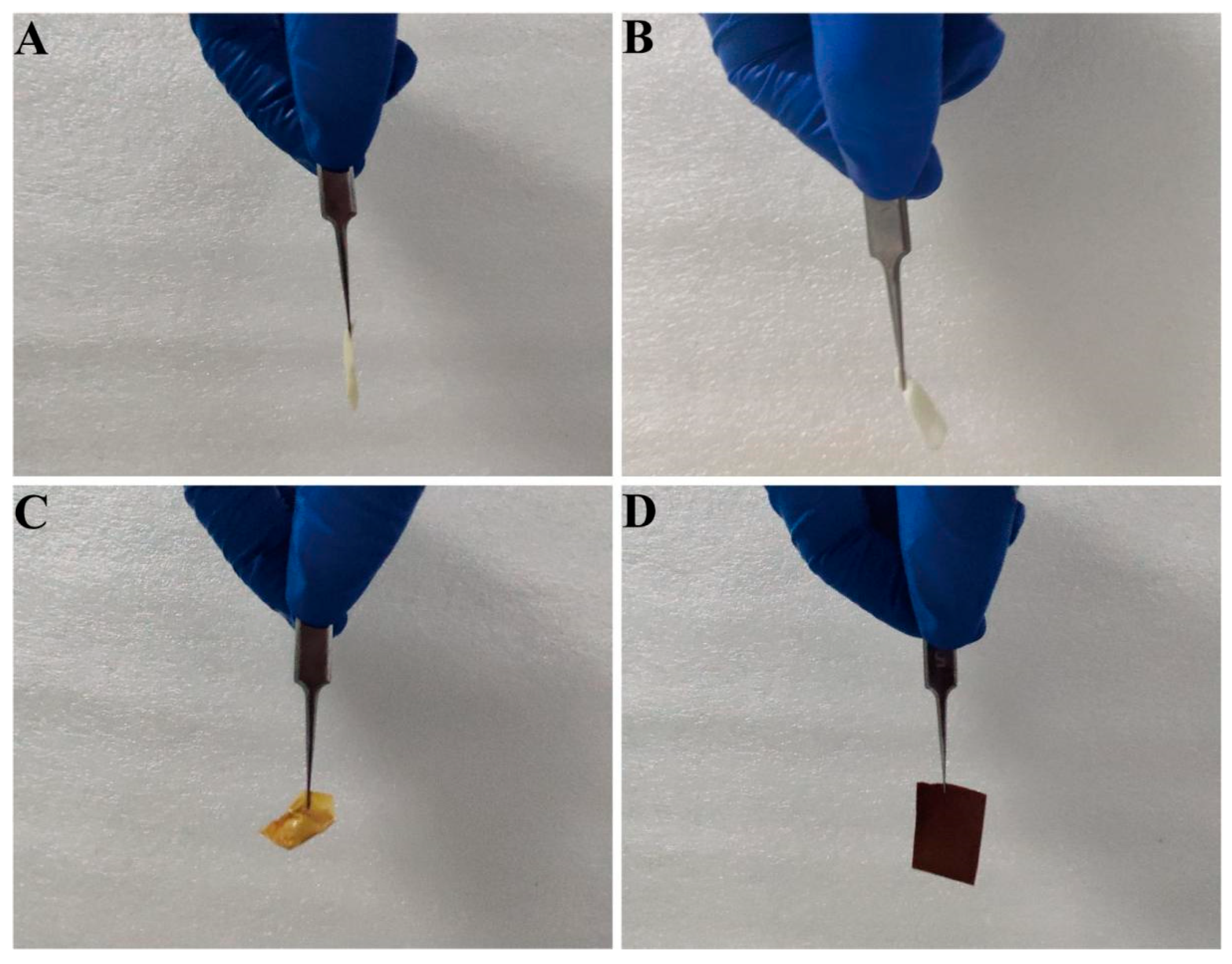
2.2. Preparation of NaA@PANMs
2.3. Characterization
2.4. Adsorption Experiment
2.5. Adsorption Parameters
3. Results and Discussions
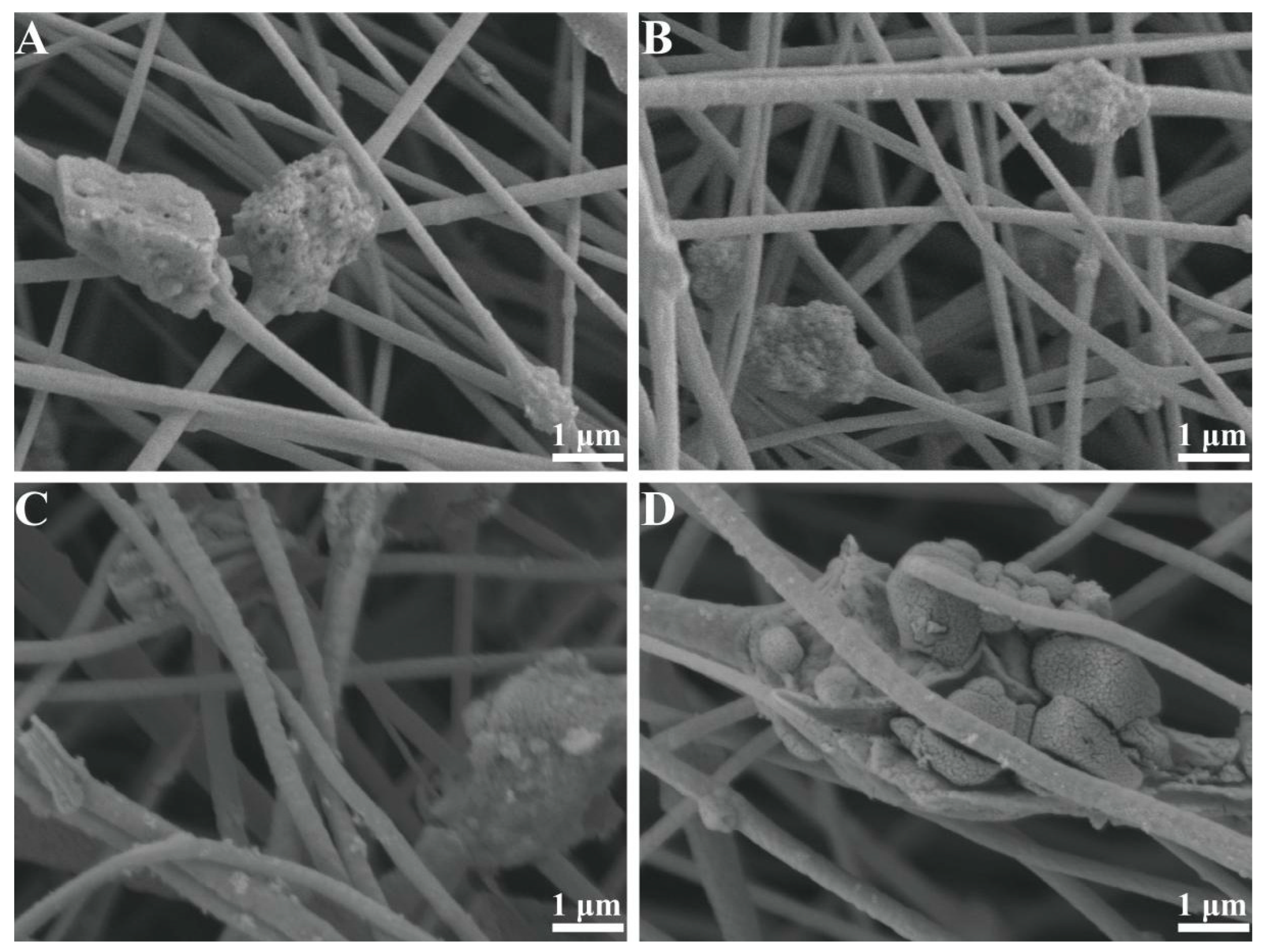
3.1. Effect of TiO2 to PAN Mass Ratio on the Morphology of TiO2-NaA@PANMs
3.2. Effect of Oxidation Temperature on the Morphology of TiO2-NaA@PANMs
3.3. XRD and FT-IR Analysis of TiO2-NaA@PANMs
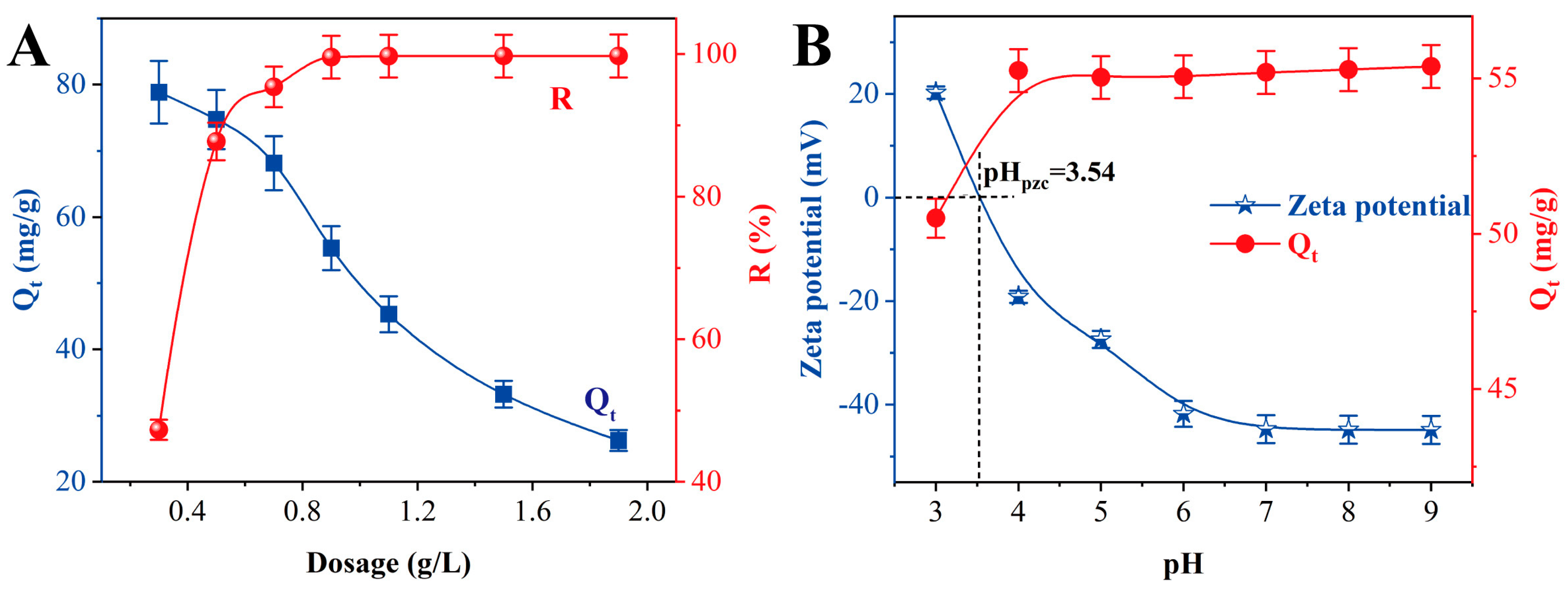
3.4. Effect of TiO2-NaA@PANMs Dosage and pH on Sr2+ Adsorption
3.5. Adsorption Kinetics
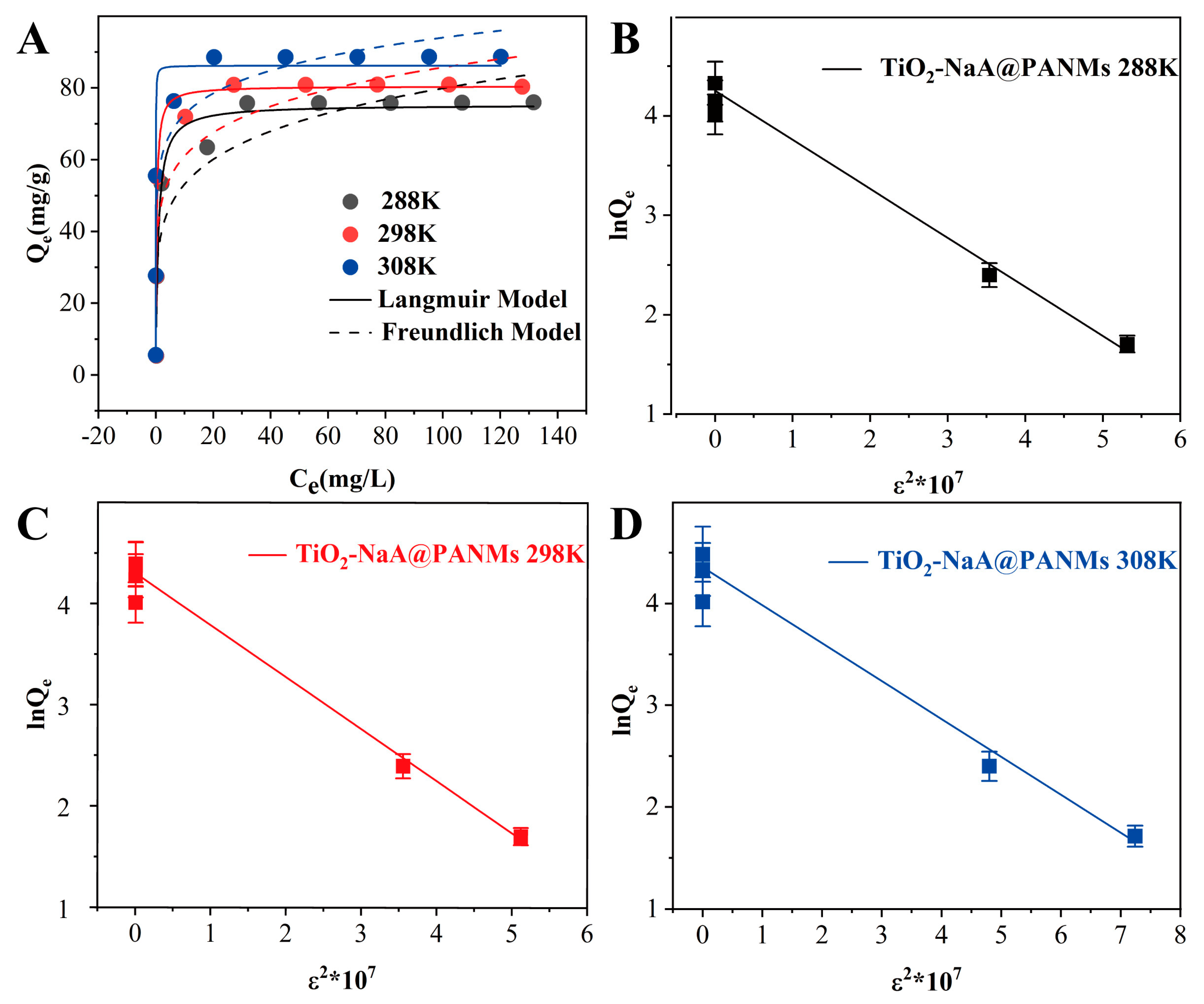
3.6. Adsorption Isotherm
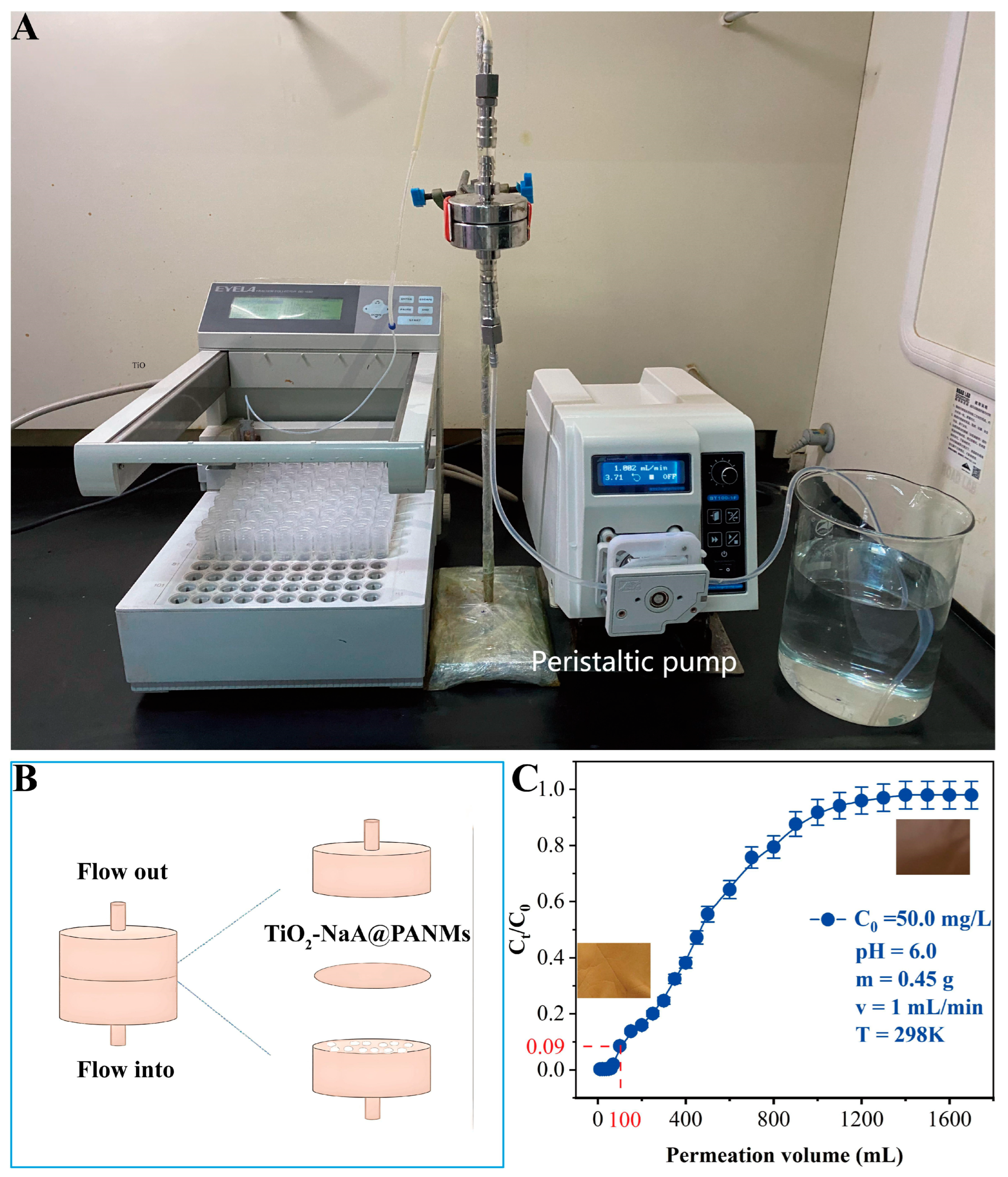
3.7. Dynamic Adsorption
3.8. Desorption and Circulation
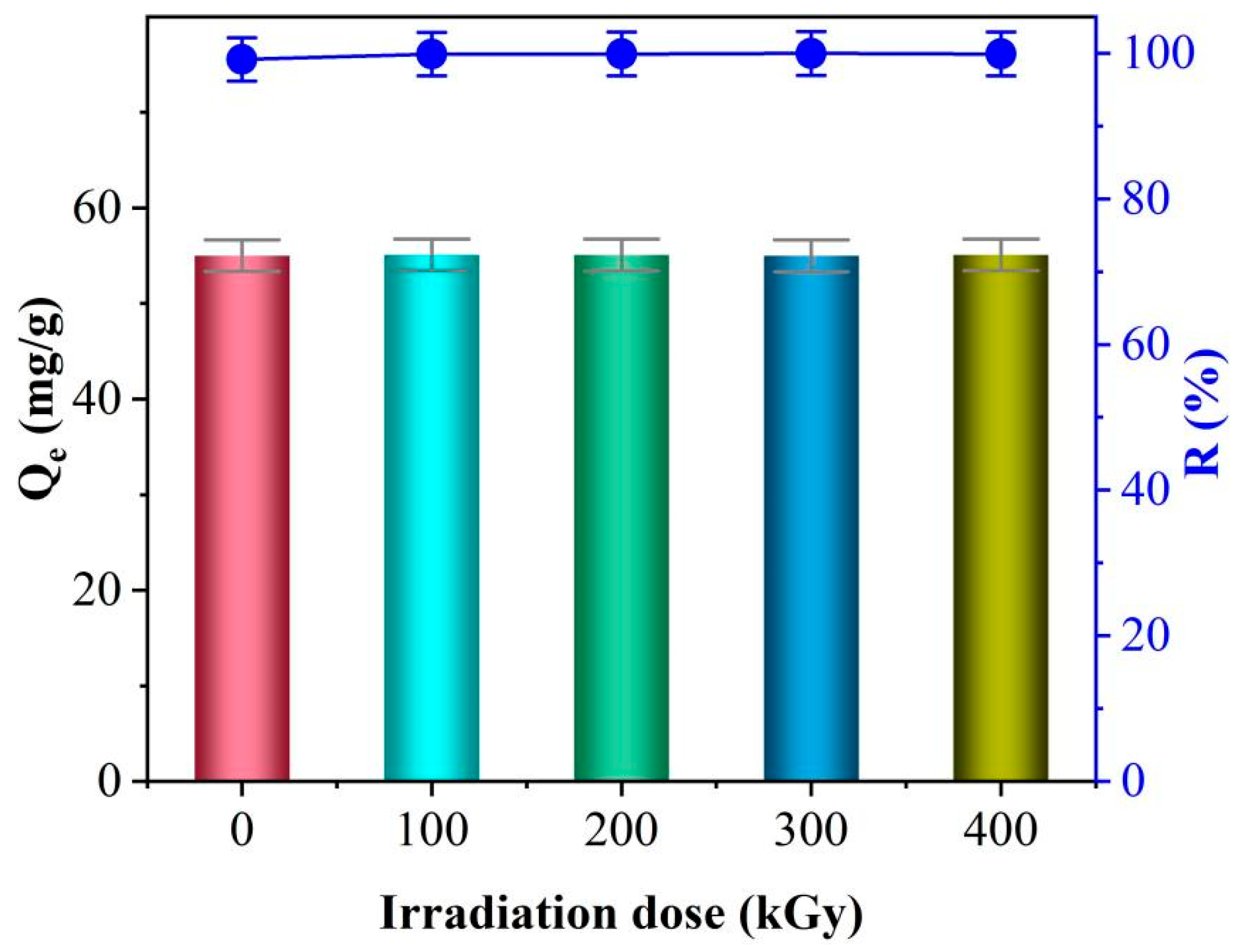
3.9. Effect of Irradiation on the Adsorption Properties of TiO2-NaA@PANMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ammar, A.; Nouira, A.; Mouridi, Z.E.; Boughribil, S. Recent Trends in the Phytoremediation of Radionuclide Contamination of Soil by Cesium and Strontium: Sources, Mechanisms and Methods: A Comprehensive Review. Chemosphere 2024, 359, 142273. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, K.; Kozai, N.; Takahashi, Y. A New Technique for Removing Strontium from Seawater by Coprecipitation with Barite. J. Hazard. Mater. 2018, 359, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, L.; Wang, D.; Chen, J.; Guo, Q.; Zhao, Y.; Zhao, F.; He, L.; Li, J.; Zhang, W.; et al. Sulfhydryl-modified Zeolite A with Exceptional Strontium Adsorption Selectivity. Sci. China Chem. 2025, 68, 1–12. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Cangemi, J.R. 68-Year-old Man with Peptic Ulcer and Diarrhea. Mayo Clin. Proc. 1997, 72, 879–882. [Google Scholar] [CrossRef]
- Wu, Q.; Luan, H.; Xiao, F. Targeted Synthesis of Zeolites from Calculated Interaction Between Zeolite Structure and Organic Template. Natl. Sci. Rev. 2022, 9, nwac023. [Google Scholar] [CrossRef]
- Wang, K.; Wang, F.; Chen, F.; Cui, X.; Wei, Y.; Shao, L.; Yu, M. One-Pot Preparation of NaA Zeolite Microspheres for Highly Selective and Continuous Removal of Sr(II) from Aqueous Solution. ACS Sustain. Chem. Eng. 2019, 7, 2459–2470. [Google Scholar] [CrossRef]
- Vatanpour, V.; Pasaoglu, M.E.; Kose-Mutlu, B.; Koyuncu, I. Polyacrylonitrile in the Preparation of Separation Membranes: A Review. Ind. Amp Eng. Chem. Res. 2023, 62, 6537–6558. [Google Scholar] [CrossRef]
- Mogre, C.; Thakurdesai, A.U.; van Ommen, J.R.; Salameh, S. Long-term Fluidization of Titania Nanoparticle Agglomerates. Powder Technol. 2017, 316, 441–445. [Google Scholar] [CrossRef]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Novel Hydrophobic PVDF Membranes Prepared by Nonsolvent Induced Phase Separation for Membrane Distillation. J. Membr. Sci. 2019, 596, 117575. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, Z.; Zhang, Y.; Qin, S.; Yan, F.; Li, J. Fabrication of Polysulfone Membrane Via Thermally Induced Phase Separation Process. Mater. Lett. 2017, 195, 190–193. [Google Scholar] [CrossRef]
- Liu, B.; Sun, H.; Peng, T.; Zhi, X. 3D Core-Shell Poly(aniline-Co-pyrrole)/reduced Graphene Oxide Composite for Supercapacitor Performance. Diam. Relat. Mater. 2021, 118, 108498. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, G.; Zhu, P.; Ma, J.; Chen, W.; Liu, Z.; Kong, T. Omniphobic ZIF-8@Hydrogel Membrane by Microfluidic-Emulsion-Templating Method for Wound Healing. Adv. Funct. Mater. 2020, 30, 1909389. [Google Scholar] [CrossRef]
- Huang, T.; Niu, Y.; Zhang, F.; Zhang, L.; Chen, S.; Yu, Q.J. Preparation and Characterization of Cyclodextrin Functionalized Polydimethylsiloxane Films Via Interfacial Self-Assembly. Appl. Mater. Today 2017, 9, 176–183. [Google Scholar] [CrossRef]
- Liu, W.; Yang, G.; Huang, M.; Liang, J.; Zeng, B.; Fu, C.; Wu, H. Ultrarobust and Biomimetic Hierarchically Macroporous Ceramic Membrane For Oil-Water Separation Templated by Emulsion-Assisted Self-Assembly Method. ACS Appl. Mater. Interfaces 2020, 12, 35555–35562. [Google Scholar] [CrossRef]
- Attia, H.; Johnson, D.J.; Wright, C.J.; Hilal, N. Robust Superhydrophobic Electrospun Membrane Fabricated by Combination of Electrospinning and Electrospraying Techniques for Air Gap Membrane Distillation. Desalination 2018, 446, 70–82. [Google Scholar] [CrossRef]
- Han, H.; Hong, H.; Park, S.M.; Kim, D.S. Metal–Electrolyte Solution Dual-Mode Electrospinning Process for in Situ Fabrication of Electrospun Bilayer Membrane. Adv. Mater. Interfaces 2020, 7, 2000571. [Google Scholar] [CrossRef]
- Ding, L.; Li, R.; Gao, Y.; Yan, B.; Zhang, C.; Zhang, G.; Yu, P.; Long, Y.; Zhang, J. Electrospun Nanofibers for Fragile Artifact Conservation. Compos. Commun. 2024, 46, 101824. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Ni, J.; Li, L. Electrospinning for Flexible Sodium-Ion Batteries. Energy Storage Mater. 2021, 45, 704–719. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Gao, Z.; Li, X.; Cui, W.; Li, R.; Ramakrishna, S.; Zhang, J.; Long, Y. Review on Electrospinning Anode and Separators for Lithium Ion Batteries. Renew. Sustain. Energy Rev. 2023, 189, 113939. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Huang, M.; Liu, Z. Rational Fabrication of Metal Phosphide Nanoparticles Immobilized in Electrospun Carbon Nanofibers for Efficient Ph-Universal Hydrogen Evolution and Overall Water Splitting. Int. J. Hydrogen Energy 2024, 63, 556–565. [Google Scholar] [CrossRef]
- Kumar, L.; Nandan, B.; Sarkar, S.; Koenig, T.A.F.; Pohl, D.; Tsuda, T.; Zainuddin, M.S.B.; Humenik, M.; Scheibel, T.; Horechyy, A. Enhanced Photocatalytic Performance of Coaxially Electrospun Titania Nanofibers Comprising Yolk-Shell Particles. J. Colloid. Interface Sci. 2024, 674, 560–575. [Google Scholar] [CrossRef]
- Babitha, S.; Rachita, L.; Karthikeyan, K.; Shoba, E.; Janani, I.; Poornima, B.; Sai, K.P. Electrospun Protein Nanofibers in Healthcare: A Review. Int. J. Pharm. 2017, 523, 52–90. [Google Scholar] [CrossRef]
- Dziemidowicz, K.; Sang, Q.; Wu, J.; Zhang, Z.; Zhou, F.; Lagaron, J.M.; Mo, X.; Parker, G.J.M.; Yu, D.; Zhu, L.; et al. Electrospinning for healthcare: Recent advancements. J. Mater. Chem. B 2021, 9, 939–951. [Google Scholar] [CrossRef]
- Niu, B.; Zhan, L.; Shao, P.; Xiang, N.; Sun, P.; Chen, H.; Gao, H. Electrospinning of Zein-Ethyl Cellulose Hybrid Nanofibers with Improved Water Resistance for Food Preservation. Int. J. Biol. Macromol. 2019, 142, 592–599. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, G.; Xu, B.; Liu, H.; Tian, H.; Li, J.; Ouyang, Y.; Xiang, A.; Kumar, R. Electrospun Natural Polypeptides Based Nanofabrics Enriched with Antioxidant Polyphenols for Active Food Preservation. Food Chem. 2023, 405, 134991. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, K.; Luo, J.; Tang, Y. Electrospinning of Boron Nitride Nanofibers with High Temperature Stability. Scr. Mater. 2019, 170, 116–119. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, X.; Huo, H.; Zhang, B.; Peng, G.; Ge, J.; Guo, H.; Liu, Y. Interlaminar Reinforced Carbon Fiber/epoxy Composites by Electrospun Ultrafine Hybrid Fibers. Compos. Part. B Eng. 2024, 281, 111578. [Google Scholar] [CrossRef]
- Zhou, A.; Du, J.; Shi, Y.; Wang, Y.; Zhang, T.; Fu, Q.; Shan, H.; Ji, T.; Xu, S.; Liu, Q.; et al. Hierarchical Porous Carbon Nanofibrous Membranes with Elaborated Chemical Surfaces for Efficient Adsorptive Removal of Volatile Organic Compounds from Air. J. Colloid. Interface Sci. 2024, 673, 860–873. [Google Scholar] [CrossRef]
- Wu, S.; Shi, W.; Li, K.; Cai, J.; Xu, C.; Gao, L.; Lu, J.; Ding, F. Chitosan-based Hollow Nanofiber Membranes with Polyvinylpyrrolidone and Polyvinyl Alcohol for Efficient Removal and Filtration of Organic Dyes and Heavy Metals. Int. J. Biol. Macromol. 2023, 239, 124264. [Google Scholar] [CrossRef]
- EL-Rafei, A.M. Synthesis and Characterization of High Surface Area Mesoporous Manganese Oxides Nanofibers Prepared by Electrospinning Technique. J. Aust. Ceram. Soc. 2023, 59, 633–643. [Google Scholar] [CrossRef]
- Wei, E.; Wang, K.; Muhammad, Y.; Chen, S.; Dong, D.; Wei, Y.; Fujita, T. Preparation and Conversion Mechanism of Different Geopolymer-Based Zeolite Microspheres and Their Adsorption Properties for Pb2+. Sep. Purif. Technol. 2021, 282, 119971. [Google Scholar] [CrossRef]
- Wang, K.; Lemougna, P.N.; Tang, Q.; Li, W.; Cui, X. Lunar Regolith Can Allow the Synthesis of Cement Materials with Near-Zero Water Consumption. Gondwana Res. 2016, 44, 1–6. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.; Van Deventer, J.; Lukey, G. The effect of Composition and Temperature on the Properties of Fly Ash- and Kaolinite -Based Geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Zhu, S.; Wang, D. Synthesis and Characterization of Low-Cost Zeolite NaA from Coal Gangue by Hydrothermal Method. Adv. Powder Technol. 2021, 32, 791–801. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Zhang, H.; Kim, Y.K.; Harbottle, D.; Lee, J.W. A high-strength polyvinyl alcohol hydrogel membrane crosslinked by sulfosuccinic acid for strontium removal via filtration. J. Environ. Chem. Eng. 2019, 7, 102824. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Shi, L.; Zheng, X.; Wang, J. Adsorption of Sr(II) from Water by Mercerized Bacterial Cellulose Membrane Modified with EDTA. J. Hazard. Mater. 2018, 364, 645–653. [Google Scholar] [CrossRef]
- Zheng, B.; Yin, J.; Zhu, L.; Zhou, B.; Shen, H.; Harbottle, D.; Hunter, T.N.; Sheng, Y.; Zhu, D.; Zhang, H. Thiol-rich and Ion-Imprinted Alginate Hydrogel As a Highly Adsorptive and Recyclable Filtration Membrane for Rapid and Selective Sr(II) Removal. Chem. Eng. J. 2023, 465, 142752. [Google Scholar] [CrossRef]
- Eom, H.H.; Kim, Y.; Harbottle, D.; Lee, J.W. Immobilization of KTS-3 on an Electrospun Fiber Membrane for Efficient Removal of Cs+ and Sr2+. J. Environ. Chem. Eng. 2021, 9, 105991. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, K.; Li, X.; Huang, L.; Liang, J.; Zheng, G.; Shan, G. Nickel-metal-organic Framework Nanobelt Based Composite Membranes for Efficient Sr2+ Removal from Aqueous Solution. Environ. Sci. Ecotechnol. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Huo, J.; Yu, G.; Wang, J. Efficient Removal of Co(II) and Sr(II) from Aqueous Solution Using Polyvinyl Alcohol/graphene Oxide/mno2 Composite As a Novel Adsorbent. J. Hazard. Mater. 2021, 411, 125117. [Google Scholar] [CrossRef]
- Yi, M.; Wang, K.; Wei, H.; Wei, D.; Wei, X.; Wei, B.; Shao, L.; Fujita, T.; Cui, X. Efficient preparation of red mud-based geopolymer microspheres (RM@GMs) and adsorption of fluoride ions in wastewater. J. Hazard. Mater. 2023, 442, 130027. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Muhammad, Y.; Wang, K.; Yi, M.; He, C.; Wei, Y.; Fujita, T. Facile fabrication of metakaolin/slag-based zeolite microspheres (M/SZMs) geopolymer for the efficient remediation of Cs+ and Sr2+ from aqueous media. J. Hazard. Mater. 2021, 406, 124292. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, S.; Qiu, R.; Muhammad, Y.; Shao, L.; Li, X.; Wei, Y.; Fujita, T. Convenient preparation of activated carbon modified phosphoric acid-activated geopolymer microspheres (C@PAAGMs) for the efficient adsorption of ReO4−: Implications for TcO4− elimination. Compos. Part B-Eng. 2022, 247, 110296. [Google Scholar] [CrossRef]

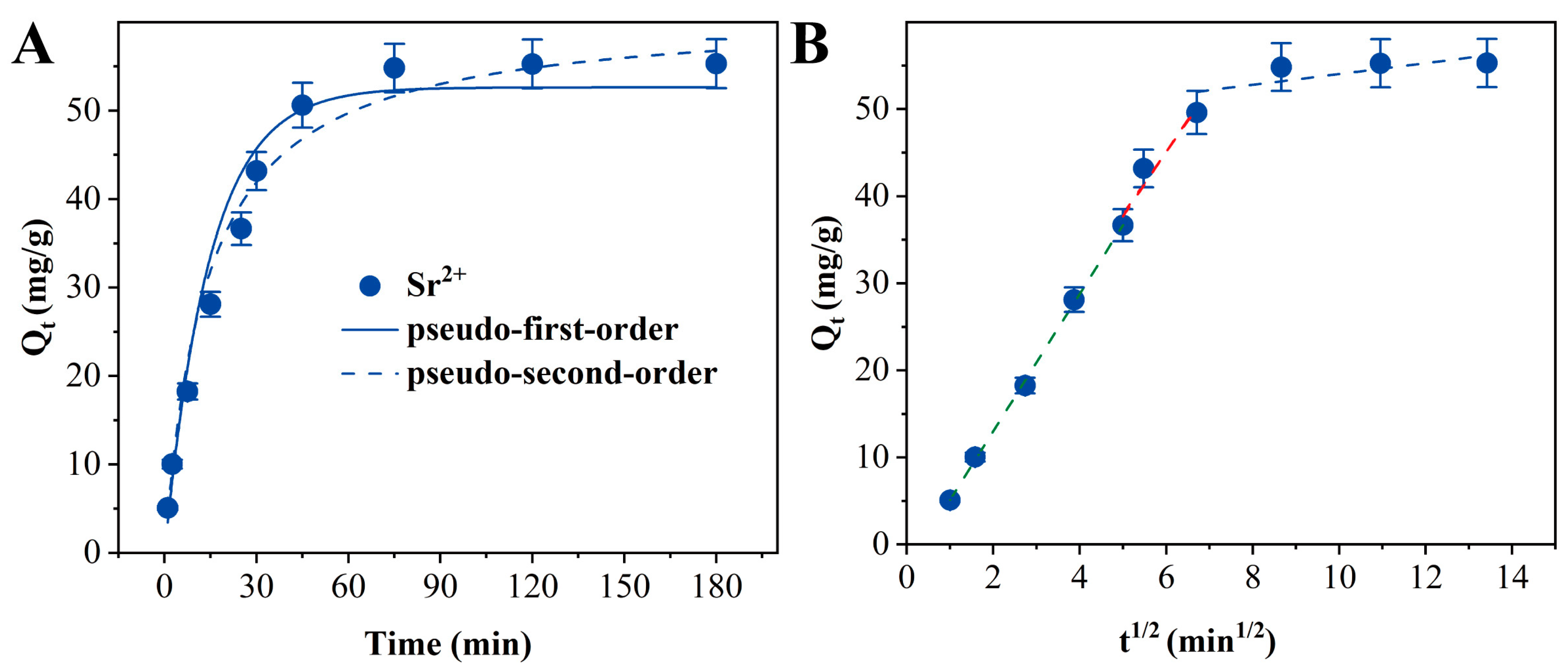

| Pseudo-First-Order | Pseudo-Second-Order | IPD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ion Type | Qe,exp | Qe,cal | K1 (×10−4) | R2 | Qe,cal | K2 (×10−4) | R2 | Kid | C | R2 |
| Sr2+ | 55.00 | 52.62 | 676.7 | 0.963 | 57.08 | 12.8 | 0.979 | 7.41 | 0.65 | 0.968 |
| Isotherm Model | Parameter | Temperature (K) | ||
|---|---|---|---|---|
| 288 | 298 | 308 | ||
| Langmuir | Qe,exp | 75.76 | 80.89 | 88.56 |
| Qmax | 75.30 | 80.49 | 86.22 | |
| KL | 1.20 | 3.41 | 49.17 | |
| R2 | 0.958 | 0.934 | 0.910 | |
| Freundlich | n | 5.66 | 6.77 | 8.92 |
| KF | 35.40 | 43.43 | 56.12 | |
| R2 | 0.820 | 0.757 | 0.824 | |
| D-R | Qm | 73.78 | 78.07 | 82.76 |
| β (×10−6) | 4.93 | 5.13 | 3.73 | |
| E | 0.35 | 0.36 | 0.27 | |
| R2 | 0.986 | 0.991 | 0.959 | |
| Temperature (K) | Thermodynamic Parameters | ||
|---|---|---|---|
| ΔGθ (kJ·mol−1) | ΔHθ (kJ·mol−1) | ΔSθ (J·mol−1·K−1) | |
| 288 | −2.15 | 23.1 | 87.0 |
| 298 | −2.70 | ||
| 308 | −3.76 | ||
| Adsorbing Material | Dosage | pH | Maximum Adsorbing Capacity | Reference |
|---|---|---|---|---|
| 1.00 g/L | 6 | 45.80 mg/g | [35] |
| 1.00 g/L | 6 | 44.86 mg/g | [36] |
| 1.00 g/L | 6 | 151.7 mg/g | [37] |
| 1.00 g/L | 6 | 32.4 mg/g | [38] |
| 0.40 g/L | 7 | 72.00 mg/g | [39] |
| 0.40 g/L | 7 | 28.81 mg/g | [40] |
| NaA@PANMs | 0.90 g/L | 6 | 80.89 mg/g | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wei, E.; Ji, R.; Wang, K. Preparation of NaA Zeolite Composite Polyacrylonitrile Membranes (TiO2-NaA@PANMs) Doped with TiO2 and Adsorption Study of Sr2+. Materials 2025, 18, 2151. https://doi.org/10.3390/ma18092151
Liu Y, Wei E, Ji R, Wang K. Preparation of NaA Zeolite Composite Polyacrylonitrile Membranes (TiO2-NaA@PANMs) Doped with TiO2 and Adsorption Study of Sr2+. Materials. 2025; 18(9):2151. https://doi.org/10.3390/ma18092151
Chicago/Turabian StyleLiu, Yu, Erna Wei, Riwen Ji, and Kaituo Wang. 2025. "Preparation of NaA Zeolite Composite Polyacrylonitrile Membranes (TiO2-NaA@PANMs) Doped with TiO2 and Adsorption Study of Sr2+" Materials 18, no. 9: 2151. https://doi.org/10.3390/ma18092151
APA StyleLiu, Y., Wei, E., Ji, R., & Wang, K. (2025). Preparation of NaA Zeolite Composite Polyacrylonitrile Membranes (TiO2-NaA@PANMs) Doped with TiO2 and Adsorption Study of Sr2+. Materials, 18(9), 2151. https://doi.org/10.3390/ma18092151






