Iron Oxide Magnetic Nanoparticles Synthesized by Laser Target Evaporation Method for the Needs of Cancer Immunotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetic Nanoparticles and Their Colloidal Suspensions
2.2. Magnetic Measurements
2.3. Cells Preparation and Measurements
2.3.1. Generation of Human Monocyte-Derived Dendritic Cells
2.3.2. Flow Cytometry
2.3.3. Prussian Blue Staining
2.3.4. MNPs Cytotoxicity Test
3. Results and Discussion
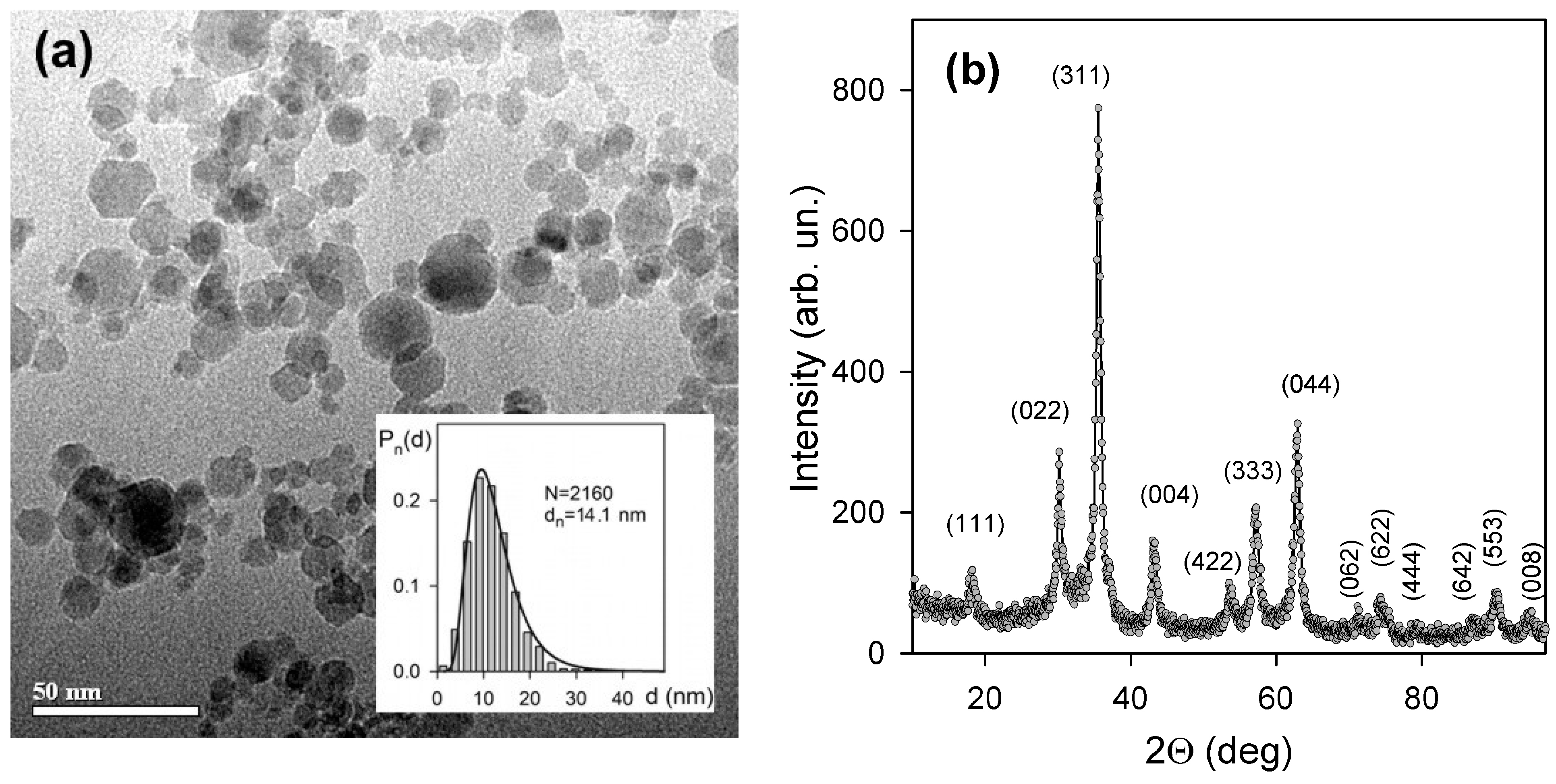
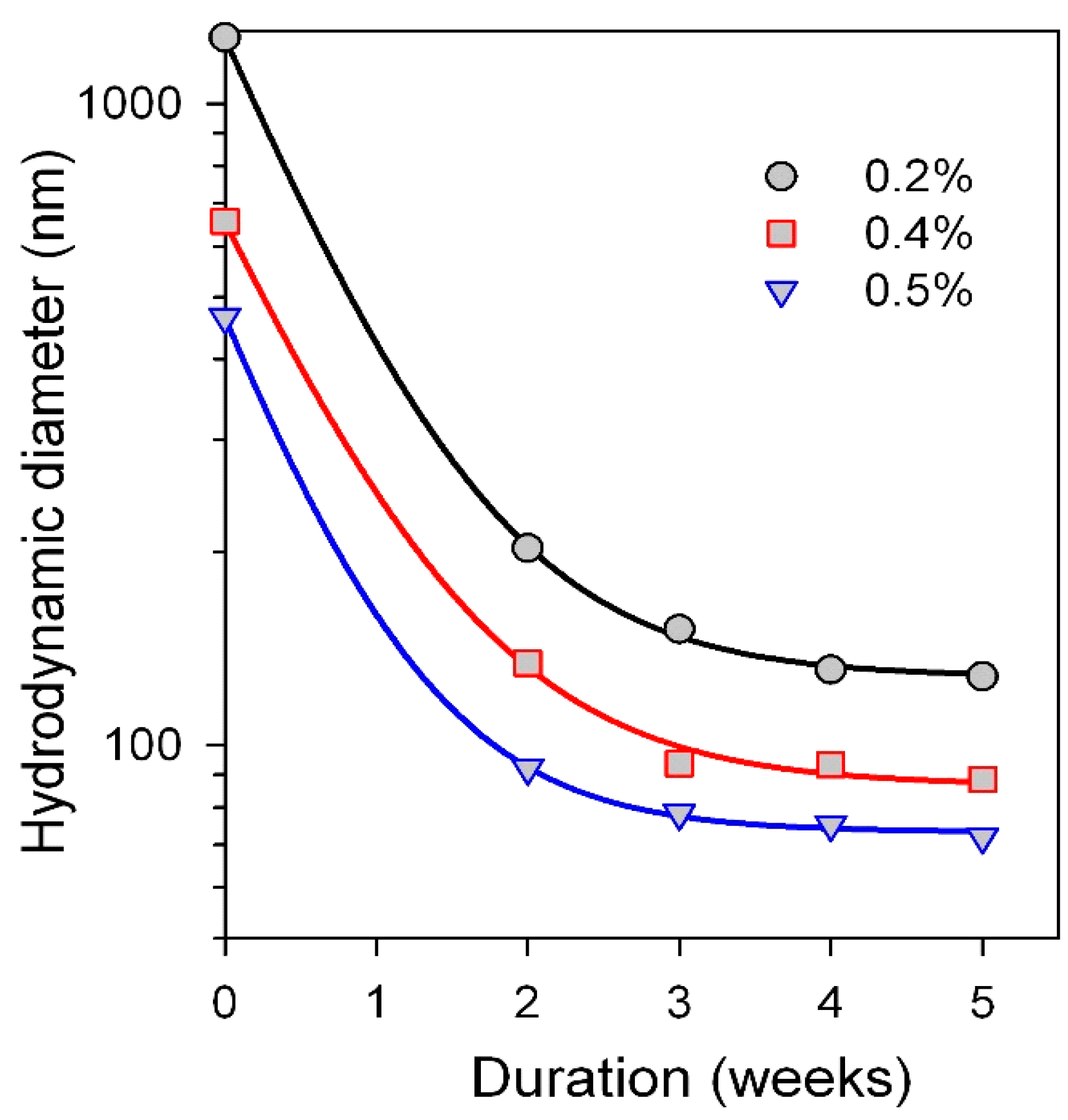
3.1. Characterization of Physicochemical Properties of MNPs and Suspensions
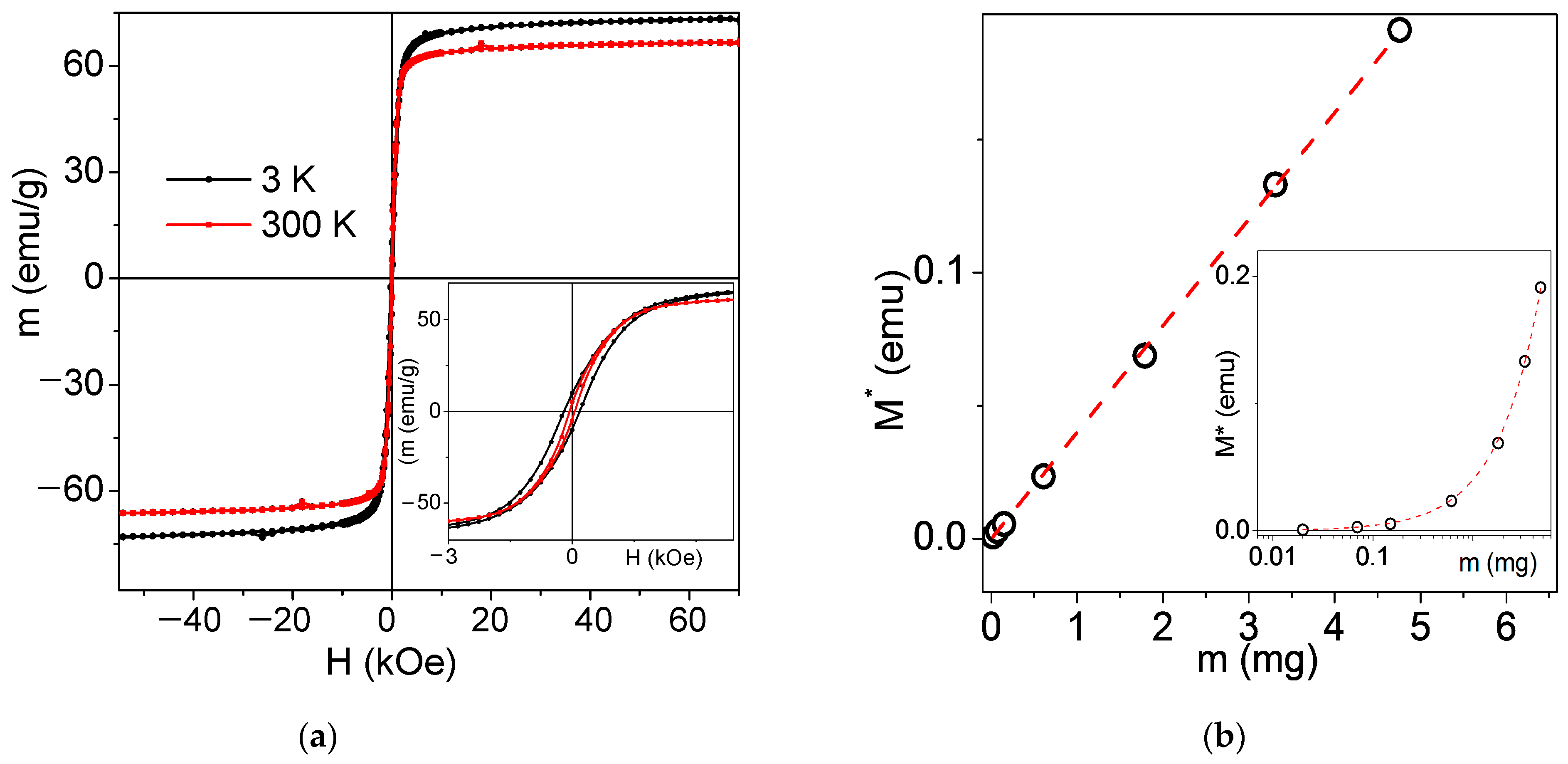
3.2. Magnetic Properties of MNPs
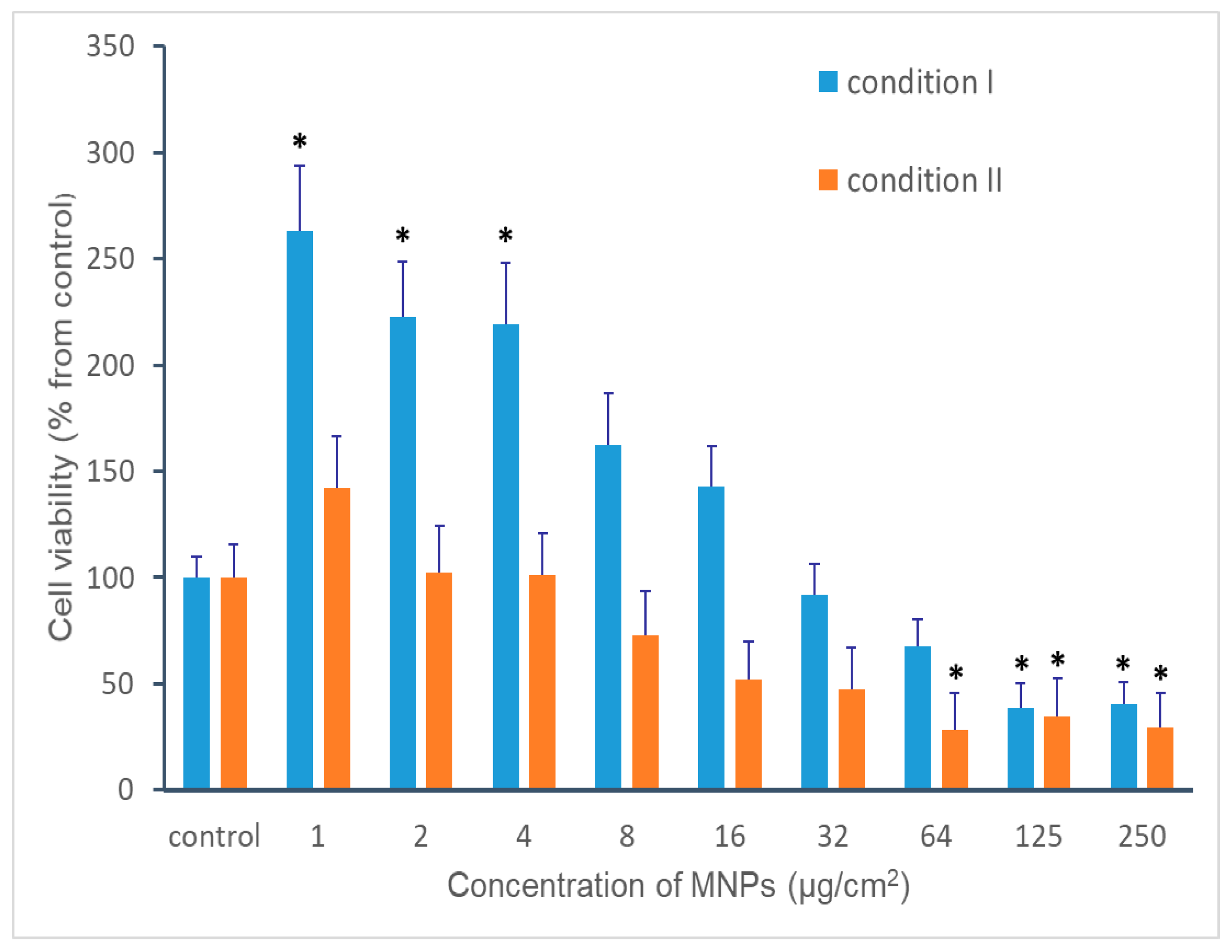
3.3. Cytotoxic Effects of MNPs Concentration on moDCs Viability and Estimations of the Magnetic Measurements Limits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| moDCs | monocyte-derived dendritic cells |
| MNPs | magnetic nanoparticles |
| LTE | laser target evaporation |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscopy |
| SQUID | Superconducting Quantum Interference Device |
| PSD | particle size distribution |
| DMEM | Dulbecco’s Modified Eagle Medium |
| PBMC | Peripheral blood mononuclear cells |
| DBPS | Dulbecco’s phosphate buffer saline |
| EBSS | Earle’s balanced salt solution |
References
- Pruna, A.; Poliac, I.; Busquets-Mataix, D.; Ruotolo, A. Effect of electrodeposition conditions on adsorption and photocatalytic properties of ZnO. Materials 2025, 18, 497. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.P.; Ferreira, R.; Cardoso, S. Spintronic sensors. Proc. IEEE 2016, 104, 1894–1918. [Google Scholar] [CrossRef]
- Zamani Kouhpanji, M.R.; Stadler, B.J.H. Magnetic nanowires for quantitative detection of biopolymers. AIP Adv. 2020, 10, 125231. [Google Scholar] [CrossRef]
- Burks, E.C.; Gilbert, D.A.; Murray, P.D.; Flores, C.; Felter, T.E.; Charnvanichborikarn, S.; Kucheyev, S.O.; Colvin, J.D.; Yin, G.; Liu, K. 3D nanomagnetism in low density interconnected nanowire networks. Nano Lett. 2020, 21, 716–722. [Google Scholar] [CrossRef]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the application of magnetic nanoparticles for sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar] [CrossRef]
- Beato, J.; Pérez-Landazábal, J.; Gómez-Polo, C. Enhanced magnetic nanoparticle detection sensitivity in non-linear magnetoimpedance-based sensor. IEEE Sens. J. 2018, 18, 8701–8708. [Google Scholar] [CrossRef]
- Barrera, G.; Celegato, F.; Vassallo, M.; Martella, D.; Coïsson, M.; Olivetti, E.S.; Martino, L.; Sözeri, H.; Manzin, A.; Tiberto, P. Microfluidic detection of spions and co-ferrite ferrofluid using amorphous wire magneto-impedance sensor. Sensors 2024, 24, 4902–4916. [Google Scholar] [CrossRef]
- Safronov, A.P.; Beketov, I.V.; Komogortsev, S.V.; Kurlyandskaya, G.V.; Medvedev, A.I.; Leiman, D.V.; Larranaga, A.; Bhagat, S.M. Spherical magnetic nanoparticles fabricated by laser target evaporation. AIP Adv. 2013, 3, 052135. [Google Scholar] [CrossRef]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in cancer medicine. Phys. Today 2012, 65, 38–42. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials 2019, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.M.; De Geest, B.G.; Louage, B.; Lybaert, L.; De Koker, S.; Koudelka, Z.; Sapelkin, A.; Sukhorukov, G.B. Magnetically engineered microcapsules as intracellular anchors for remote control over cellular mobility. Adv. Mater. 2013, 25, 6945–6950. [Google Scholar] [CrossRef] [PubMed]
- Zverev, V.I.; Pyatakov, A.P.; Shtil, A.A.; Tishin, A.M. Novel applications of magnetic materials and technologies for medicine. J. Magn. Magn. Mater. 2018, 459, 182–186. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.; Pu, H. Magnetic impedance biosensor: A review. Biosens. Bioelectron. 2017, 90, 418–435. [Google Scholar] [CrossRef]
- Llandro, J.; Palfreyman, J.J.; Ionescu, A.; Barnes, C.H.W. Magnetic biosensor technologies for medical applications: A review. Med. Biol. Eng. Comput. 2010, 48, 977–998. [Google Scholar] [CrossRef]
- Alsharif, N.A.; Martiinez-Banderas, A.; Merzaban, J.; Ravasi, T.; Kosel, J. Biofunctionalizing magnetic nanowires toward targeting and killing leukemia cancer cells. IEEE Trans. Magn. 2019, 55, 1–5. [Google Scholar] [CrossRef]
- Devkota, J.; Howell, P.; Mukherjee, P.; Srikanth, H.; Mohapatra, S.; Phan, M.H. Magneto-reactance based detection of MnO nanoparticle-embedded Lewis lung carcinoma cells. J. Appl. Phys. 2015, 117, 17D123. [Google Scholar] [CrossRef]
- Osipov, V.V.; Kotov, Y.A.; Ivanov, M.G.; Samatov, O.M.; Lisenkov, V.V.; Platonov, V.V.; Murzakaev, A.M.; Medvedev, A.I.; Azarkevich, E.I. Laser synthesis of nanopowder. Laser Synth. Nanopowders 2006, 16, 116–125. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Makarova, E.B.; Shabadrov, P.A.; Fadeyev, F.A.; Shklyar, T.F.; Safronov, A.P.; Komogortsev, S.V.; Kurlyandskaya, G.V. Magnetic nanoparticles as a strong contributor to the biocompatibility of ferrogels. Phys. Metals Metallogr. 2020, 121, 299–304. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Melnikov, G.Y.; Makarova, E.B.; Fadeyev, F.A.; Sedneva-Lugovets, D.V.; Shabadrov, P.A.; Volchkov, S.O.; Mekhdieva, K.R.; Safronov, A.P.; Fernández Armas, S.; et al. Effects of constant magnetic field to the proliferation rate of human fibroblasts grown onto different substrates: Tissue culture polystyrene, polyacrylamide hydrogel and ferrogels γ-Fe2O3 magnetic nanoparticles. Nanomaterials 2020, 10, 1697. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Novoselova, I.P.; Schupletsova, V.V.; Andrade, R.; Dunec, N.A.; Litvinova, L.S.; Safronov, A.P.; Yurova, K.A.; Kulesh, N.A.; Dzyuman, A.N.; et al. Nanoparticles for magnetic biosensing systems. J. Magn. Magn. Mater. 2017, 431, 249–254. [Google Scholar] [CrossRef]
- Fadeyev, F.A.; Blyakhman, F.A.; Safronov, A.P.; Melnikov, G.Y.; Nikanorova, A.D.; Novoselova, I.P.; Kurlyandskaya, G.V. Biological impact of -Fe2O3 magnetic nanoparticles obtained by laser target evaporation: Focus on magnetic biosensor applications. Biosensors 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Kulesh, N.A.; Novoselova, I.P.; Safronov, A.P.; Beketov, I.V.; Samatov, O.M.; Kurlyandskaya, G.V.; Morozova, M.V.; Denisova, T.P. Total reflection X-ray fluorescence spectroscopy as a tool for evaluation of iron concentration in ferrofluids and yeast samples. J. Magn. Magn. Mater. 2016, 415, 39–44. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Safronov, A.P.; Shcherbinin, S.V.; Beketov, I.V.; Blyakhman, F.A.; Makarova, E.B.; Korch, M.A.; Svalov, A.V. Magnetic nanoparticles obtained by electrophysical technique: Focus on biomedical applications. Phys. Solid State 2021, 63, 1444–1458. [Google Scholar] [CrossRef]
- Bol, K.F.; Schreibelt, G.; Rabold, K.; Wculek, S.K.; Schwarze, J.K.; Dzionek, A.; Teijeira, A.; Kandalaft, L.E.; Romero, P.; Coukos, G.; et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J. Immunother. Cancer 2019, 7, 109. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Z.; Wu, C.; Lin, J.; Liu, N. Magnetic nanoparticles enhance the cellular immune response of dendritic cell tumor vaccines by realizing the cytoplasmic delivery of tumor antigens. Bioeng. Transl. Med. 2022, 8, e10400. [Google Scholar] [CrossRef]
- Grippin, A.J.; Wummer, B.; Wildes, T.; Dyson, K.; Trivedi, V.; Yang, C.; Sebastian, M.; Mendez-Gomez, H.R.; Padala, S.; Grubb, M.; et al. Dendritic cell-activating magnetic nanoparticles enable early prediction of antitumor response with magnetic resonance imaging. ACS Nano 2019, 13, 13884–13898. [Google Scholar] [CrossRef]
- de Vries, I.J.; Lesterhuis, W.J.; Barentsz, J.O.; Verdijk, P.; van Krieken, J.H.; Boerman, O.C.; Oyen, W.J.; Bonenkamp, J.J.; Boezeman, J.B.; Adema, G.J.; et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 2005, 23, 1407–1413. [Google Scholar] [CrossRef]
- Jin, H.; Qian, Y.; Dai, Y.; Qiao, S.; Huang, C.; Lu, L.; Luo, Q.; Chen, J.; Zhang, Z. Magnetic enrichment of dendritic cell vaccine in lymph node with fluorescent-magnetic nanoparticles enhanced cancer immunotherapy. Theranostics 2016, 6, 2000–2014. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic cells and their role in immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Sabado, R.L.; Bhardwaj, N. Directing dendritic cell immunotherapy towards successful cancer treatment. Immunotherapy 2010, 2, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, T.; Morisaki, T.; Kubo, M.; Morisaki, S.; Nakamura, Y.; Onishi, H. Lymph nodes as anti-tumor immunotherapeutic tools: Intranodal-tumor-specific antigen-pulsed dendritic cell vaccine immunotherapy. Cancers 2022, 14, 2438. [Google Scholar] [CrossRef] [PubMed]
- Khranovska, N.; Skachkova, O.; Gorbach, O.; Inomistova, M.; Orel, V. Magnetically sensitive nanocomplex enhances antitumor efficacy of dendritic cell-based immunotherapy. Exp. Oncol. 2021, 43, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Cinar, M.U.; Fan, H.; Pröll, M.; Tesfaye, D.; Tholen, E.; Looft, C.; Hölker, M.; Schellander, K.; Uddin, M.J. Comparison of the innate immune responses of porcine monocyte-derived dendritic cells and splenic dendritic cells stimulated with LPS. Innate Immun. 2015, 21, 242–254. [Google Scholar] [CrossRef]
- Donini, M.; Pettinella, F.; Zanella, G.; Gaglio, S.; Laudanna, C.; Jimenez-Carretero, M.; Jimenez-Lopez, C.; Perduca, M.; Dusi, S. Effects of magnetic nanoparticles on the functional activity of human monocytes and dendritic cells. Int. J. Mol. Sci. 2023, 24, 1358. [Google Scholar] [CrossRef]
- Philip, J. Magnetic nanofluids (Ferrofluids): Recent advances, applications, challenges, and future directions. Adv. Colloid Interface Sci. 2023, 311, 102810. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, H.; Zheng, J. Ferrofluids transport in bioinspired nanochannels: Application to electrochemical biosensing with magnetic-controlled detection. Biosens. Bioelectron. 2022, 201, 113963. [Google Scholar] [CrossRef]
- Figueroa, G.; Parira, T.; Laverde, A.; Casteleiro, G.; El-Mabhouh, A.; Nair, M.; Agudelo, M. Characterization of human monocyte-derived dendritic cells by imaging flow cytometry: A comparison between two monocyte isolation protocols. J. Vis. Exp. 2016, 18, 54296. [Google Scholar]
- Mikloska, Z.; Bosnjak, L.; Cunningham, A.L. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 2001, 75, 5958–5964. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J. Properties of immature and mature dendritic cells: Phenotype, morphology, phagocytosis, and migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef]
- Burban, E.A.; Fadeyev, F.A.; Safronov, A.P.; Blyakhman, F.A.; Terzian, T.V.; Neznakhin, D.S.; Yushkov, A.A.; Kurlyandskaya, G.V. Determination of limits for evaluating the degree of internalization of γ-Fe2O3 nanoparticles by cultures of human mesenchymal stomal cells. Colloid J. 2024, 86, 836–847. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Portnov, D.S.; Beketov, I.V.; Larrañaga, A.; Safronov, A.P.; Orue, I.; Medvedev, A.I.; Chlenova, A.A.; Sanchez-Ilarduya, M.B.; Martinez-Amesti, A.; et al. Nanostructured materials for magnetic biosensing. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Rubio, I.; Insausti, M.; Garaio, E.; Gil De Muro, I.; Plazaola, F.; Rojo, T.; Lezama, L. Fe3O4 nanoparticles prepared by the seeded-growth route for hyperthermia: Electron magnetic resonance as a key tool to evaluate size distribution in magnetic nanoparticles. Nanoscale 2014, 6, 7542–7552. [Google Scholar] [CrossRef] [PubMed]
- Goiriena-Goikoetxea, M.; García-Arribas, A.; Rouco, M.; Svalov, A.V.; Barandiaran, J.M. High-yield fabrication of 60 nm Permalloy nanodiscs in well-defined magnetic vortex state for biomedical applications. Nanotechnology 2016, 27, 175302. [Google Scholar] [CrossRef]
- Darton, N.J.; Ionescu, A.; Llandro, J. Magnetic Nanoparticles in Biosensing and Medicine; Cambridge University Press: Cambridge, UK, 2019; p. 279. [Google Scholar]
- De Cos, D.; Lete, N.; Fdez-Gubieda, M.L.; Garcia-Arribas, A. Study of the influence of sensor permeability in the detection of a single magnetotactic bacterium. J. Magn. Magn. Mater. 2020, 500, 166346. [Google Scholar] [CrossRef]
- Mikushin, P.; Fadeyev, F.; Starodumov, I.; Bugayova, A.; Shklyar, T.; Blyakhman, F. Application of computer vision algorithms for assessing the content of magnetic nanoparticles uptaken by dendritic cells in-vitro. IEEE Xplore, 2025; accepted. [Google Scholar]
- Kurlyandskaya, G.V.; Litvinova, L.S.; Safronov, A.P.; Schupletsova, V.V.; Tyukova, I.S.; Khaziakhmatova, O.G.; Slepchenko, G.B.; Yurova, K.A.; Cherempey, E.G.; Kulesh, N.A.; et al. Water-based suspensions of iron oxide nanoparticles with electrostatic or steric stabilization by chitosan: Fabrication, characterization and biocompatibility. Sensors 2017, 17, 2605. [Google Scholar] [CrossRef]
- Uchiyama, T.; Mohri, K.; Honkura, Y.; Panina, L.V. Recent advances of pico-Tesla resolution magneto-impedance sensor based on amorphous wire CMOS IC MI sensor. IEEE Trans. Magn. 2012, 48, 3833–3839. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Buznikov, N.A.; Svalov, A.V. Giant Magnetoimpedance: 30 years since rediscovery and next steps. Phys. Met. Metallogr. 2024, 125 (Suppl. S1), S33–S61. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Xu, W.; Zhang, M.; Zhou, Q.; Chen, W. The function and magnetic resonance imaging of immature dendritic cells under ultrasmall superparamagnetic iron oxide (USPIO)-labeling. Biotechnol. Lett. 2017, 39, 1079–1089. [Google Scholar] [CrossRef]
- Goya, G.F.; Marcos-Campos, I.; Fernández-Pacheco, R.; Sáez, B.; Godino, J.; Asín, L.; Lambea, J.; Tabuenca, P.; Mayordomo, J.I.; Larrad, L.; et al. Dendritic cell uptake of iron-based magnetic nanoparticles. Cell Biol. Int. 2008, 32, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhu, Y.; Zhang, M.; Xiao, Y.; Du, X.; Liu, H.; Wang, S. The induction of maturation on dendritic cells by TiO2 and Fe(3)O(4)@TiO(2) nanoparticles via NF-κB signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 39, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Liu, Y.P.; Ho, J.H.; Hsu, S.C.; Lee, O.K. Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 1499–1506. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blyakhman, F.; Fadeyev, F.; Safronov, A.; Terziyan, T.; Burban, E.; Shklyar, T.; Kurlyandskaya, G. Iron Oxide Magnetic Nanoparticles Synthesized by Laser Target Evaporation Method for the Needs of Cancer Immunotherapy. Materials 2025, 18, 2142. https://doi.org/10.3390/ma18092142
Blyakhman F, Fadeyev F, Safronov A, Terziyan T, Burban E, Shklyar T, Kurlyandskaya G. Iron Oxide Magnetic Nanoparticles Synthesized by Laser Target Evaporation Method for the Needs of Cancer Immunotherapy. Materials. 2025; 18(9):2142. https://doi.org/10.3390/ma18092142
Chicago/Turabian StyleBlyakhman, Felix, Fedor Fadeyev, Alexander Safronov, Tatiana Terziyan, Ekaterina Burban, Tatyana Shklyar, and Galina Kurlyandskaya. 2025. "Iron Oxide Magnetic Nanoparticles Synthesized by Laser Target Evaporation Method for the Needs of Cancer Immunotherapy" Materials 18, no. 9: 2142. https://doi.org/10.3390/ma18092142
APA StyleBlyakhman, F., Fadeyev, F., Safronov, A., Terziyan, T., Burban, E., Shklyar, T., & Kurlyandskaya, G. (2025). Iron Oxide Magnetic Nanoparticles Synthesized by Laser Target Evaporation Method for the Needs of Cancer Immunotherapy. Materials, 18(9), 2142. https://doi.org/10.3390/ma18092142







