Assessing the Performance of Different Treatment Methods in Removing Tetracycline from Wastewater: Efficiency and Cost Evaluation
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Batch Adsorption Experiment
2.3. Photocatalytic Degradation Experiment
2.4. Ozonation Experiment
2.5. Municipal and Hospital Real Wastewater Treatment
3. Results and Discussion
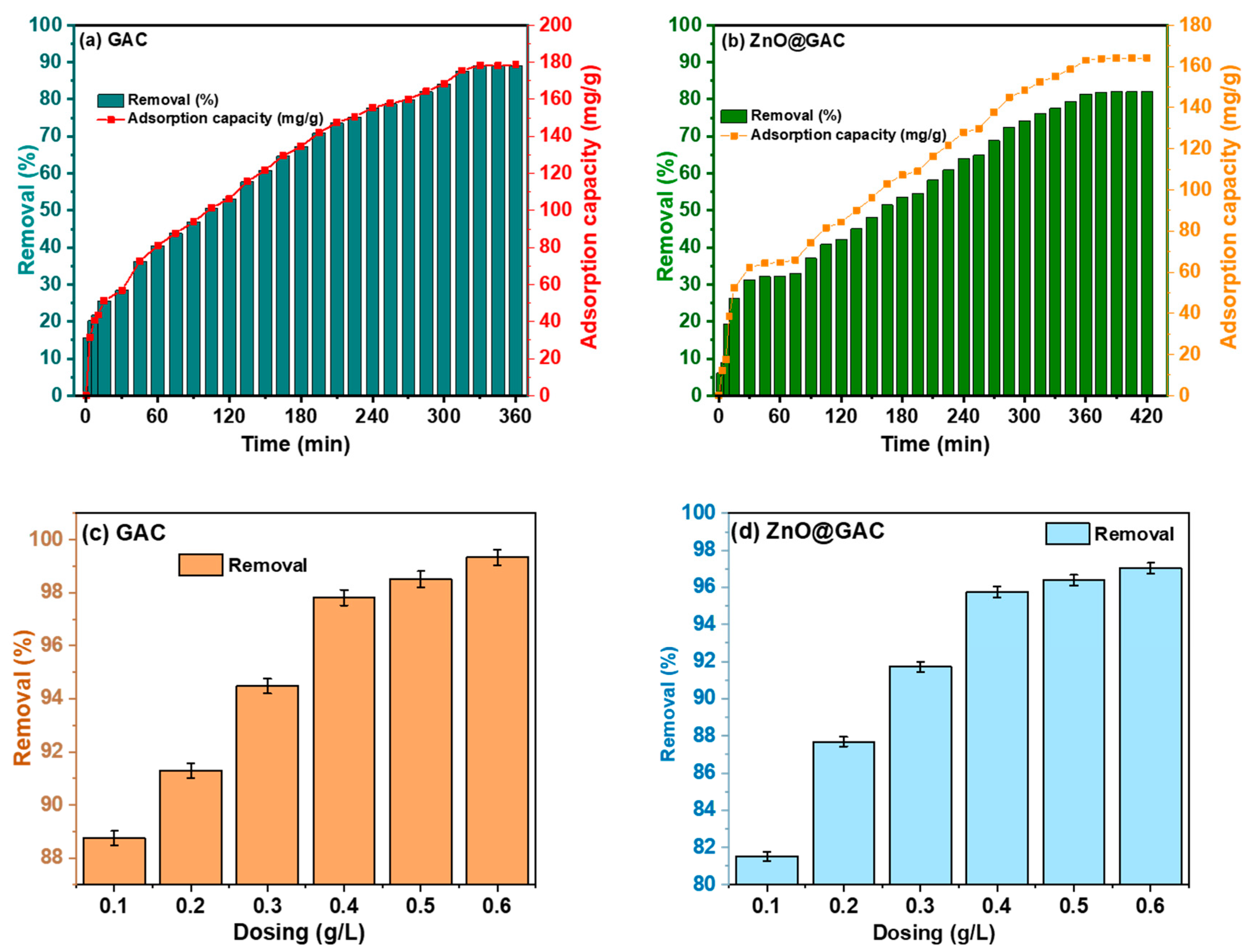
3.1. Adsorptive Performance
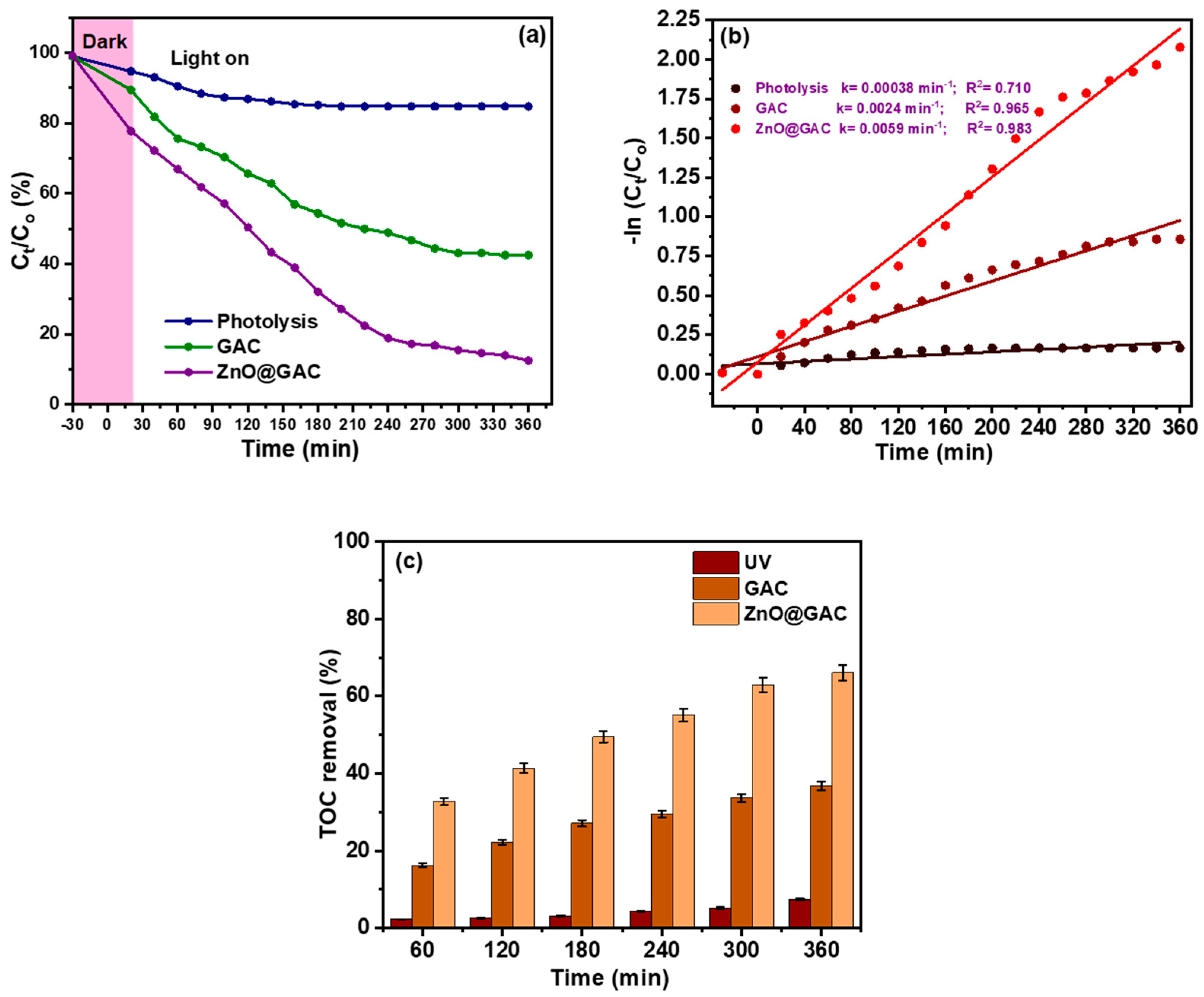
3.2. Photocatalytic Performance
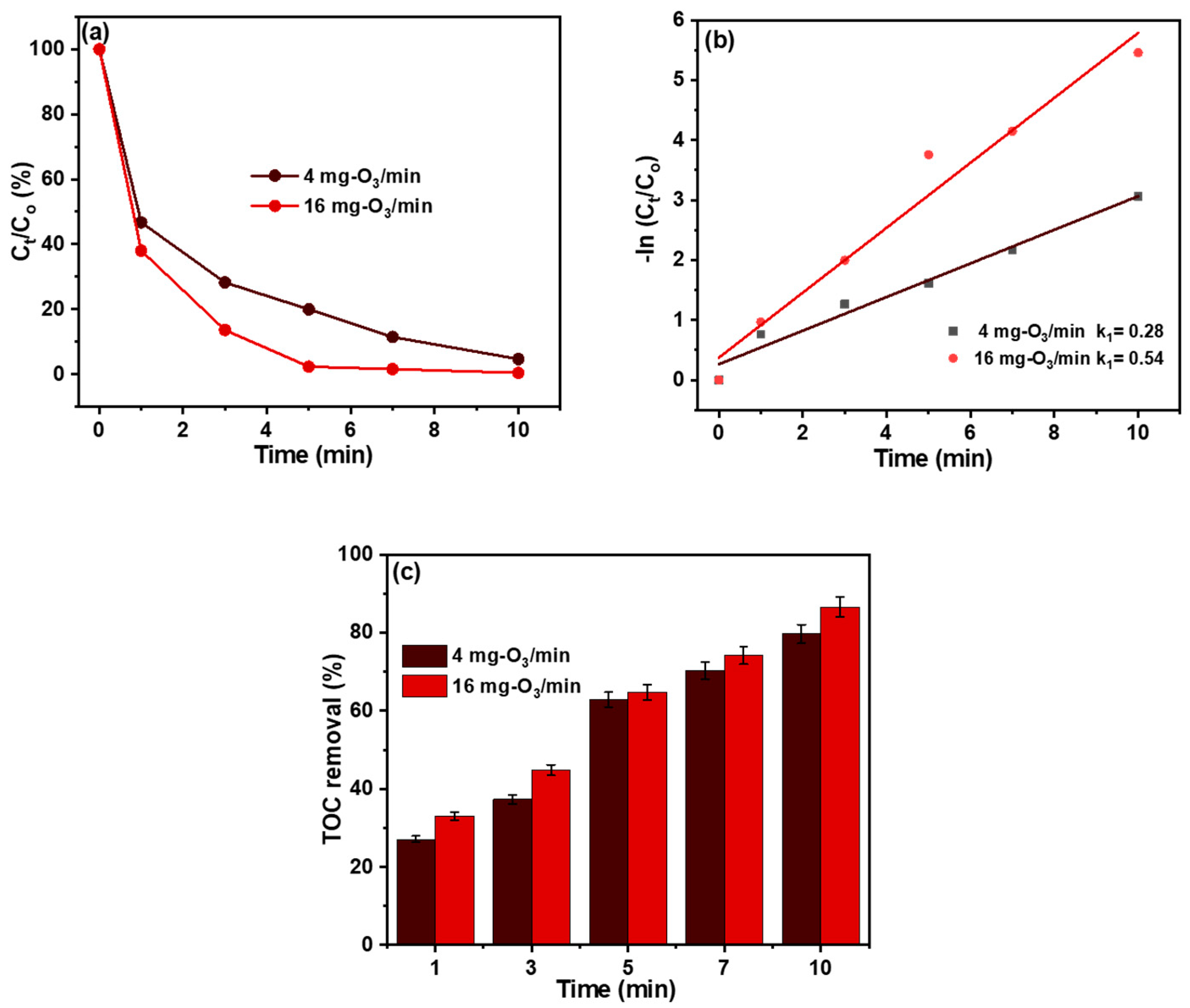
3.3. Ozonation Performance
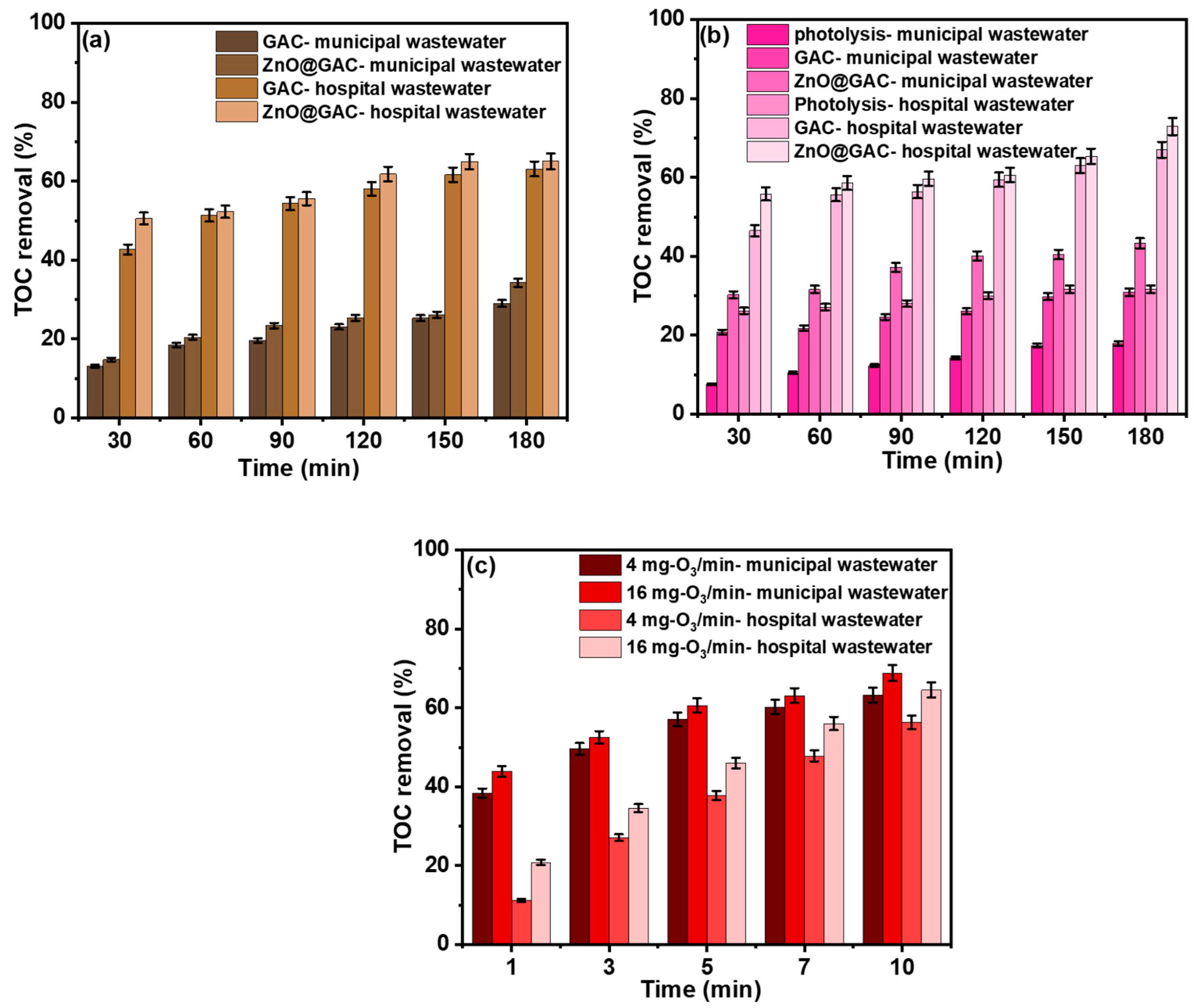
3.4. Comparative Study of Different Techniques in Real-Wastewater Treatment
3.5. Cost Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Valizadeh, K.; Bateni, A.; Sojoodi, N.; Rafiei, R.; Behroozi, A.H.; Maleki, A. Preparation and characterization of chitosan-curdlan composite magnetized by zinc ferrite for efficient adsorption of tetracycline antibiotics in water. Int. J. Biol. Macromol. 2023, 235, 123826. [Google Scholar] [CrossRef] [PubMed]
- Siahbandi, M.S.; Moradi, O.; Akbari–adergani, B.; Azar, P.A.; Tehrani, M.S. Fabrication and implementation of bimetallic Fe/Zn nanoparticles (mole ratio 1:1) loading on hydroxyethylcellulose—Graphene oxide for removal of tetracycline antibiotic from aqueous solution. Chemosphere 2023, 312, 137184. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.A.R.; Kalaee, M.R.; Moradi, O.; Nosratinia, F.; Abdouss, M. Synthesis of novel zeolitic imidazolate framework (ZIF-67)-zinc oxide (ZnO) nanocomposite (ZnO@ZIF-67) and potential adsorption of pharmaceuticals (tetracycline (TCC)) from water. J. Mol. Struct. 2022, 1251, 132013. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Tian, J.; Liu, C.; Zhang, S.; Cao, L.; Zhou, Y.; Zhang, S. Effective removal of tetracycline antibiotics from water by magnetic functionalized biochar derived from rice waste. Environ. Pollut. 2023, 330, 121681. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Fang, Y.; Huang, Q.; Wang, Y. Efficient adsorption of tetracycline from aqueous solution using copper and zinc oxides modified porous boron nitride adsorbent. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131372. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Moghaddam, N.S.M.; Rahimi, S.M.; Hajjizadeh, M.; Nasseh, N. Hexadecyltrimethylammonium-activated and zinc oxide-coated nano-bentonite: A promising photocatalyst for tetracycline degradation. Sustain. Energy Technol. Assess. 2022, 53, 102451. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, J.; Li, P.; Du, Z.; Qi, X.; Chen, X.; Mu, R.; Zeng, C.; Ma, Y.; Zhang, Z. Effective adsorptive removal of tetracycline from aqueous solution by Zn-BTC@SBC derived from sludge: Experimental study and density functional theory (DFT) calculations. J. Mol. Liq. 2023, 384, 122283. [Google Scholar] [CrossRef]
- Sun, M.; Ma, Y.; Yang, Y.; Zhu, X. Effect of iron impregnation ratio on the properties and adsorption of KOH activated biochar for removal of tetracycline and heavy metals. Bioresour. Technol. 2023, 380, 129081. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, Z.; Dong, Y.; Zhao, J.; Zhang, H.; Li, X.; Zhang, C.; Tang, X.; Guo, X.; Zhu, L. Efficient removal of tetracycline by a novel bimetallic nickel/copper-loaded biochar: The crucial roles of π-π interaction and complexation. Appl. Surf. Sci. 2023, 640, 158372. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Lau, S.Y.; Danquah, M.K.; Zhang, J.; Chiong, T.; Meunier, L.; Rahman, M.M. Selective adsorption of organic dyes from aqueous environment using fermented maize extract-enhanced graphene oxide-durian shell derived activated carbon composite. Chemosphere 2023, 339, 139742. [Google Scholar] [CrossRef]
- Jingrui, X.; Alam, M.A.; Jing, W.; Wenchao, W.; Yusof, Z.N.B.; Daroch, M.; Zhang, D.; Lifen, L.; Russel, M. Enhanced removal of tetracycline from synthetic wastewater using an optimal ratio of co-culture of Desmodesmus sp. and Klebsiella pneumoniae. Bioresour. Technol. 2022, 351, 127056. [Google Scholar] [CrossRef] [PubMed]
- Phuong, D.M.; Duong, T.A.; Huong, N.T.; Khoa, N.V.; Hanh, N.T.; Phuong, N.M.; Pham, T.-D.; Trang, H.T.; Noi, N.V. Enhancement of visible light photocatalytic removal of residual tetracycline by Ni doped WO3 nano structures. Inorg. Chem. Commun. 2023, 157, 111329. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Yin, Z.; Wang, T.; Liu, S.; Cao, S. One-pot synthesis of porous TiO2/BiOI adsorbent with high removal efficiency and excellent recyclability towards tetracyclines. Ceram. Int. 2023, 49, 22139–22148. [Google Scholar] [CrossRef]
- Xie, H.; Sun, J.; Zhou, S.; Liu, C.; Jiang, Q.; Hu, H.; Li, C.; Zhang, Z.; Qin, J.; Kong, Y. Construction of plum-branch-like BiOI/NH2-MIL-68(In) Z-scheme heterojunctions for removal of tetracycline hydrochloride from wastewater via synergistic adsorption-photocatalysis process. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131887. [Google Scholar] [CrossRef]
- Zhang, P.; Xiang, M.; Jiang, W.; Liu, H.; Chen, Y.; Fang, Y.; Wu, L.; Guo, H.; Wang, Y. Configuration modulation of vermiculite by exfoliation coupled Cu(II) anchoring for boosting removal of tetracycline via synergy of adsorption and photocatalysis. Chem. Eng. J. 2023, 473, 145143. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Tian, Y.; Lin, Y.; Hu, Y.H. Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light. Chem. Eng. J. 2021, 406, 126747. [Google Scholar] [CrossRef]
- Yuan, Z.S.; Zou, J.X.; Zhao, X.L.; Shi, J.Y.; Guo, C.S.; Yan, M. Cadmium sulfide cage photocatalysis coupled electroactive biofilm for synergistic promotion of tetracycline degradation and electricity production. J. Mater. Sci. Technol. 2023, 166, 86–97. [Google Scholar]
- Ma, J.; Zhao, B.; Shao, N.; Jiang, P.; Yang, H.; Li, B. Rational design of a novel magnetically recoverable and environment-friendly Z-scheme SnFe2O4/Bi2WO6 heterojunction with enhanced photocatalytic performance for rhodamine B degradation and toxicity elimination. Mater. Today Chem. 2023, 30, 101538. [Google Scholar] [CrossRef]
- Wang, H.; Quan, X.; Xiong, Q.; Yin, L.; Tian, Y.; Zhang, J. Enhanced performance of β-cyclodextrin modified Cu2O nanocomposite for efficient removal of tetracycline and dyes: Synergistic role of adsorption and photocatalysis, 2023. Appl. Surf. Sci. 2023, 621, 156735. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Reddy, C.V.; Syed, K.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; Shim, J. Ultra-small zinc oxide nanosheets anchored onto sodium bismuth sulfide nanoribbons as solar-driven photocatalysts for removal of toxic pollutants and phtotoelectrocatalytic water oxidation. Chemosphere 2021, 267, 128559. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Dong, X.; Zhao, Y.; Li, F.; Cen, Q. Efficient adsorption of tetracycline hydrochloride on biochar-ceramsite composite: Optimization of response surface methodology and investigation of adsorption mechanism. Mater. Today Sustain. 2023, 24, 100525. [Google Scholar] [CrossRef]
- Ismail, M.S.; Yahya, M.D.; Auta, M.; Obayomi, K.S. Facile preparation of amine -functionalized corn husk derived activated carbon for effective removal of selected heavy metals from battery recycling wastewater. Heliyon 2022, 8, e09516. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Qian, Y.; Jiang, P.; Zhu, Z.; Mu, L.; Lu, X.; Zhu, J. Biomass-derived mesoporous and super-hydrophilic carbon manufactured by cycling-pressure-switching air activation process towards ultrahigh adsorption efficiency of tetracycline. Sustain. Mater. Technol. 2022, 32, e00430. [Google Scholar] [CrossRef]
- Zelekew, O.A.; Haitosa, H.H.; Chen, X.; Wu, Y.-N. Recent progress on plant extract-mediated biosynthesis of ZnO-based nanocatalysts for environmental remediation: Challenges and future outlooks. Adv. Colloid Interface Sci. 2023, 317, 102931. [Google Scholar] [CrossRef]
- Li, J.-q.; Zhou, Z.-w.; Li, X.; Yang, Y.-l.; Gao, J.-f.; Yu, R.; Wang, H.-p.; Wang, N. Synergistically boosting sulfamerazine degradation via activation of peroxydisulfate by photocatalysis of Bi2O3-TiO2/PAC under visible light irradiation. Chem. Eng. J. 2022, 428, 132613. [Google Scholar] [CrossRef]
- Hayati, F.; Moradi, S.; Saei, S.F.; Madani, Z.; Giannakis, S.; Isari, A.A.; Kakavandi, B. A novel, Z-scheme ZnO@AC@FeO photocatalyst suitable for the intensification of photo-mediated peroxymonosulfate activation: Performance, reactivity, and bisphenol A degradation pathways. J. Environ. Manag. 2022, 321, 115851. [Google Scholar] [CrossRef]
- Samy, M.; Kumi, A.G.; Salama, E.; ElKady, M.; Mensah, K.; Shokry, H. Heterogeneous activation of persulfate by a novel nano-magnetite/ZnO/activated carbon nanohybrid for carbofuran degradation: Toxicity assessment, water matrices, degradation mechanism and radical and non-radical pathways. Process Saf. Environ. Prot. 2023, 169, 337–351. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Lau, S.Y.; Zahir, A.; Meunier, L.; Jianhua, Z.; Dada, A.O.; Rahman, M.M. Removing methylene blue from water: A study of sorption effectiveness onto nanoparticles-doped activated carbon. Chemosphere 2023, 313, 137533. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Lau, S.Y.; Xie, Z.; Gray, S.R.; Zhang, J. In-situ hydrothermal fabrication of ZnO-loaded GAC nanocomposite for efficient Rhodamine B dye removal via synergistic photocatalytic and adsorptive performance. Nanomaterials 2024, 14, 1234. [Google Scholar] [CrossRef]
- Ghani, A.A.; Kim, B.; Nawaz, M.; Devarayapalli, K.C.; Lim, Y.; Kim, G.; Lee, D.S. Adsorption and electrochemical regeneration of 2D magnetic MXene nanosheets loaded with tetracycline. Chem. Eng. J. 2023, 467, 143473. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; Xiong, T.; Zuo, M.; Luo, L.; Qin, P.; Lei, M.; Liang, Y.; Gong, X.; Zou, D.; et al. Photocatalytic degradation of tetracycline by N-CQDs modified S-g-C3N4 nanotubes and its product toxicity evaluation. Sep. Purif. Technol. 2023, 314, 123533. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, Z.; Hu, C.; Zheng, J.; Peng, H.; Liu, B. Fabrication of unconventional S-scheme NiAl LDH/Ag6Si2O7 heterojunction photocatalysts: Outstanding photocatalytic performance and photocatalytic mechanism for tetracycline degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131806. [Google Scholar] [CrossRef]
- Nie, Y.; Zhao, C.; Zhou, Z.; Kong, Y.; Ma, J. Hydrochloric acid-modified fungi-microalgae biochar for adsorption of tetracycline hydrochloride: Performance and mechanism. Bioresour. Technol. 2023, 383, 129224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; Wang, C.; Li, Y.; Zhu, Q.; Zhang, S.; Tian, C.; Sun, X.; Huang, W. Constructing bifunctional TiO2 from NH2-MIL-125(Ti) for excellent photocatalytic tetracycline degradation. J. Alloys Compd. 2023, 965, 171396. [Google Scholar] [CrossRef]
- Hu, H.; Jin, J.; Xu, M.; Xu, C.; Cheng, Y.; Ji, W.; Ding, Z.; Shao, M.; Wan, Y. Novel Z-scheme Bi3O4Br/NH2-MIL-125(Ti) composite for efficient photocatalytic degradation of tetracycline. Opt. Mater. 2023, 135, 113262. [Google Scholar] [CrossRef]
- Zhao, N.; Ma, Q.; Zhang, B.; Liu, D.; Wei, Y.; Li, M.; Yu, T.; Li, H.; Shen, Y.; Yuan, P. New insight into removal of tetracycline by a two-dimensional graphene-like carbon adsorbent prepared using one-step pyrolysis of spent bleaching earth: Adsorption behaviors, mechanisms and cost analysis. Sep. Purif. Technol. 2023, 327, 124950. [Google Scholar] [CrossRef]
- Xie, J.; Tang, Y.; Chen, F.; Hao, C.C. A visible light responsive Bi2S3/MIL-53(Fe) heterojunction with enhanced photocatalytic activity for degradation of tetracycline. J. Phys. Chem. Solids 2023, 181, 111551. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.; Zhang, X.; Wang, S.; Duan, K.; Li, X.; Hu, Y.; Cheng, H. Direct Z-scheme In2O3/AgI heterojunction with oxygen vacancies for efficient molecular oxygen activation and enhanced photocatalytic degradation of tetracycline. Chem. Eng. J. 2023, 466, 143319. [Google Scholar] [CrossRef]
- Lu, T.; Gao, Y.; Yang, Y.; Ming, H.; Huang, Z.; Liu, G.; Zheng, D.; Zhang, J.; Hou, Y. Efficient degradation of tetracycline hydrochloride by photocatalytic ozonation over Bi2WO6. Chemosphere 2021, 283, 131256. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhang, J.; Lu, C.; Huang, Q.; Wu, J.; Liu, F. Degradation of tetracycline in aqueous media by ozonation in an internal loop-lift reactor. J. Hazard. Mater. 2011, 192, 35–43. [Google Scholar] [CrossRef]
- Ling, Y.; Li, B.; Liu, H.; Hu, H.; Wu, Y.; Zhang, B.; Zhao, T.; Huang, S.; Niu, L. Uncommonly efficient degradation performance of photocatalytic ozonation towards tetracycline over synthesizing 3-D g-C3N4 nanosheet based on Si-O-Co framework. Process Saf. Environ. Prot. 2023, 172, 513–522. [Google Scholar] [CrossRef]
- Su, Y.; Wang, X.; Dong, S.; Fu, S.; Zhou, D.; Rittmann, B.E. Towards a simultaneous combination of ozonation and biodegradation for enhancing tetracycline decomposition and toxicity elimination. Bioresour. Technol. 2020, 304, 123009. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, C.-Y.; Liao, G.Y. Degradation of antibiotic tetracycline by ultrafine-bubble ozonation process. J. Water Process Eng. 2020, 37, 101463. [Google Scholar] [CrossRef]
- Golrizkhatami, F.; Taghavi, L.; Nasseh, N.; Panahi, H.A. Synthesis of novel MnFe2O4/BiOI green nanocomposite and its application to photocatalytic degradation of tetracycline hydrochloride: (LC-MS analyses, mechanism, reusability, kinetic, radical agents, mineralization, process capability, and purification of actual pharmaceutical wastewater). J. Photochem. Photobiol. A Chem. 2023, 444, 114989. [Google Scholar]
- Xing, J.; Huang, J.; Wang, X.; Yang, F.; Bai, Y.; Li, S.; Zhang, X. Removal of low-concentration tetracycline from water by a two-step process of adsorption enrichment and photocatalytic regeneration. J. Environ. Manag. 2023, 343, 118210. [Google Scholar] [CrossRef]
- Song, X.; He, J.; Wang, Y.; Wang, J.; Zhang, S. A novel MIL-125(Ti)-based nanocomposite for enhanced adsorption and catalytic degradation of tetracycline hydrochloride: Synergetic mechanism of calcination and the nitrogen-containing reticulated surface layer. J. Colloid Interface Sci. 2023, 645, 918–932. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, J.; Zhou, Y.; Luo, Z.; Sun, Y.; Wan, Z.; Luo, L.; Tsang, D.C.W.; Dionysiou, D.D. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction. Chem. Eng. J. 2020, 383, 123149. [Google Scholar] [CrossRef]
| Materials | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| ZnO | 74 | 0.00234 | 2.87 |
| GAC | 474 | 0.268 | 2.26 |
| ZnO@GAC | 453 | 0.273 | 2.42 |
| Treatment Methods | Pollutants/Removal (%) | |||||
|---|---|---|---|---|---|---|
| Trimethoprim | Metronidazole | Sulfamethoxazole | Tebuconazole | Propylparaben | Carbamazepine | |
| Wastewater + UV light only | <LOD | <LOD | 83 | <LOD | 14 | <LOD |
| GAC + UV light + wastewater | <LOD | <LOD | <LOD | <LOD | 90 | <LOD |
| GAC@ZnO + UV light + wasterwater | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| GAC + wastewater (adsorption) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| GAC@ZnO + wastewater (adsorption) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Wasterwater + ozone (4 mg.O3/min) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Wasterwater + ozone (16 mg.O3/min) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Techniques | Cost Description | Unit Cost (USD) | Quantity Used | Total Cost (USD) | |
|---|---|---|---|---|---|
| 1. | Adsorption | ZnO@GAC | 5.59/20.34 g | 0.5 g | 0.14 |
| Cost of electricity (magnetic stirrer) | 0.19/1 kWh | 550 W × 6 | 0.63 | ||
| Total cost for adsorbing 0.5 L of TC (50 mg/L) | - | - | - | 0.77 | |
| Total amount needed to adsorb 100 L of TC (50 mg/L) | 154.0 | ||||
| 2. | Photocatalytic degradation | ZnO@GAC | 5.59/20.34 g | 0.5 g | 0.14 |
| Cost of electricity (magnetic stirrer) | 0.19/1 kWh | 550 W × 7 | 0.73 | ||
| Cost of electricity (light bulbs) | 0.19/1 kWh | (8 W × 6) × 7 | 0.064 | ||
| Total cost for degrading 0.5 L of TC (50 mg/L) | - | - | - | 0.93 | |
| Total amount needed to degrade 100 L of TC (50 mg/L) | - | - | - | 186.80 | |
| 3 | Ozonation | Energy consumption per unit of TC (EE/O) at 4 mg-O3/min | 0.19/1 kWh | 178.57 kWh | 33.93 |
| Energy consumption per unit of TC (EE/O) at 16 mg-O3/min | 0.19/1 kWh | 178.57 kWh | 17.59 | ||
| Total cost for ozonating 0.5 L of TC (50 mg/L) at 4 mg-O3/min | - | - | - | 33.93 | |
| Total amount needed to ozonate 100 L of TC (50mg/L) at 4 mg-O3/min | 6789 | ||||
| Total cost for treating 0.5 L of TC (50 mg/L) at 16 mg-O3/min | - | - | - | 17.59 | |
| Total amount needed to ozonate 100 L of TC (50 mg/L) at 16 mg-O3/min | 3518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obayomi, K.S.; Xie, Z.; Gray, S.R.; Zhang, J. Assessing the Performance of Different Treatment Methods in Removing Tetracycline from Wastewater: Efficiency and Cost Evaluation. Materials 2025, 18, 2134. https://doi.org/10.3390/ma18092134
Obayomi KS, Xie Z, Gray SR, Zhang J. Assessing the Performance of Different Treatment Methods in Removing Tetracycline from Wastewater: Efficiency and Cost Evaluation. Materials. 2025; 18(9):2134. https://doi.org/10.3390/ma18092134
Chicago/Turabian StyleObayomi, Kehinde Shola, Zongli Xie, Stephen R. Gray, and Jianhua Zhang. 2025. "Assessing the Performance of Different Treatment Methods in Removing Tetracycline from Wastewater: Efficiency and Cost Evaluation" Materials 18, no. 9: 2134. https://doi.org/10.3390/ma18092134
APA StyleObayomi, K. S., Xie, Z., Gray, S. R., & Zhang, J. (2025). Assessing the Performance of Different Treatment Methods in Removing Tetracycline from Wastewater: Efficiency and Cost Evaluation. Materials, 18(9), 2134. https://doi.org/10.3390/ma18092134










