Biosafety and Blood Compatibility of Graphene Oxide Particles in In Vitro Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Dynamic Light Scattering (DLS)
2.3. Atomic Force Microscopy (AFM)
2.4. Scanning Electron Microscopy (SEM)
2.5. Ames Mutagenicity Assay
2.6. In Vitro Toxicity Assay
2.6.1. Cell Culture
2.6.2. Cytotoxicity Assay
2.7. Blood Compatibility Assessment
2.7.1. Sample Preparation
2.7.2. Hemolytic Assay
2.7.3. Platelet Aggregation Assay
2.7.4. Coagulation Assay
2.8. Statistical Analysis
3. Results
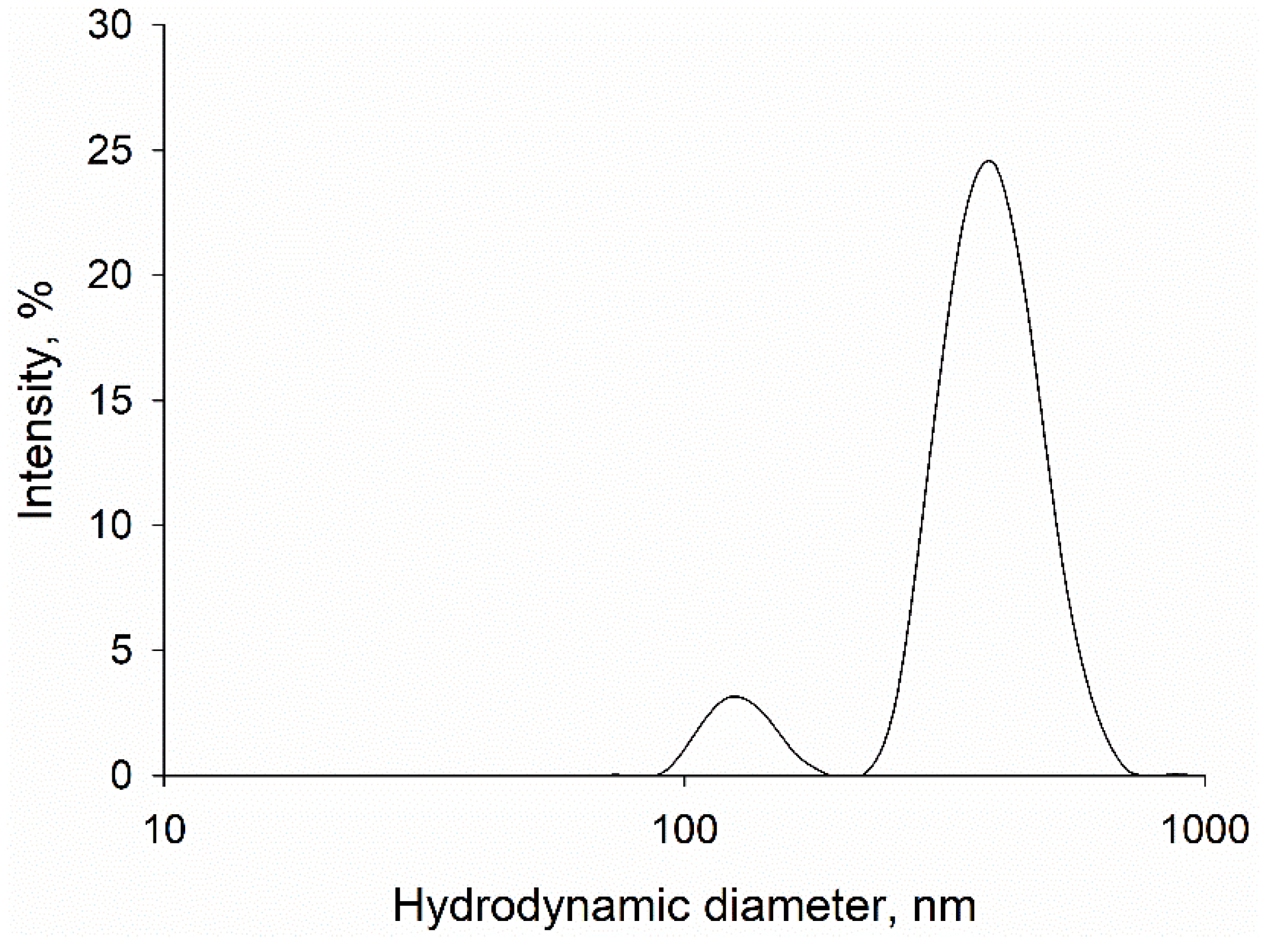
3.1. Particle Characterization
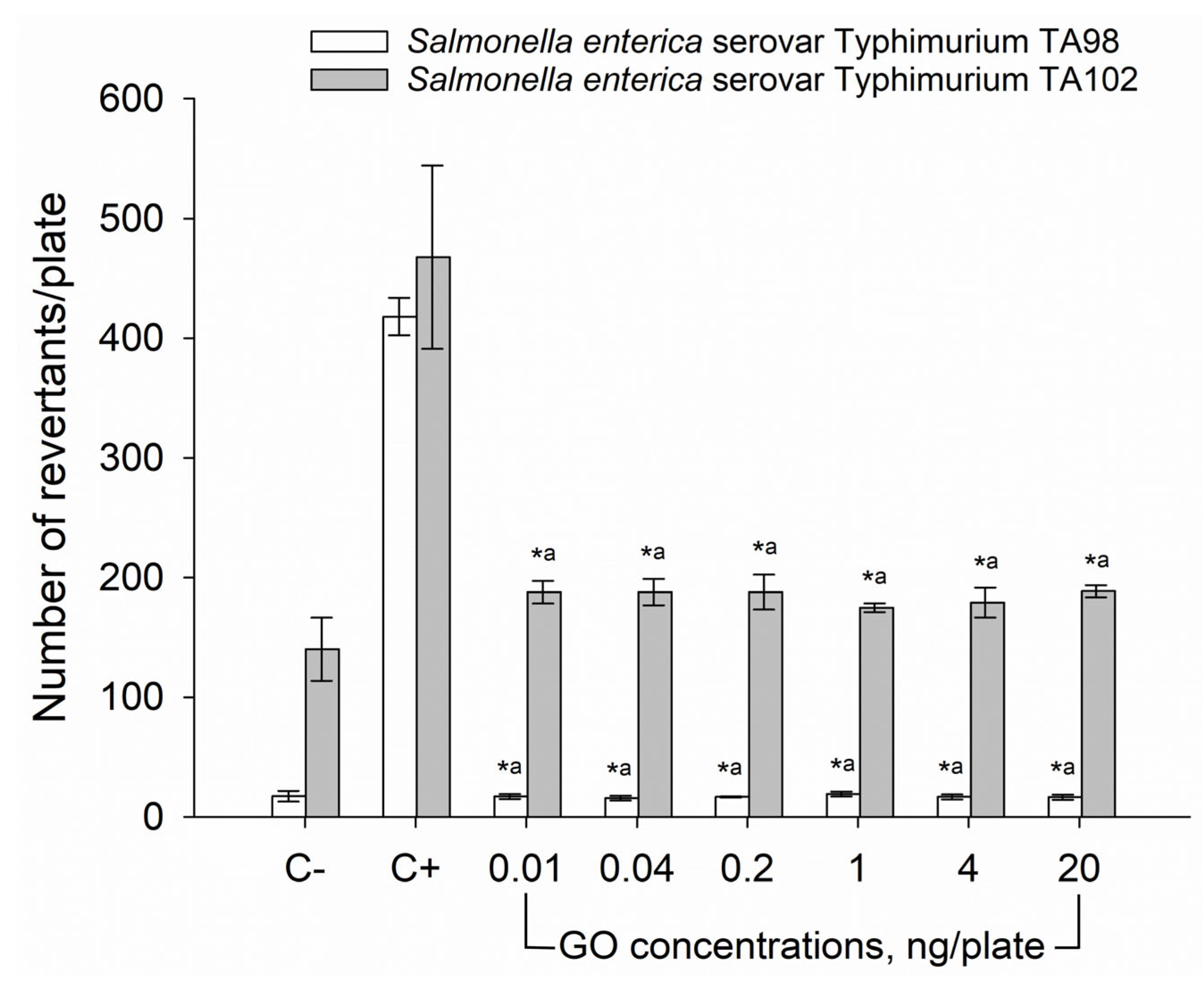
3.2. Ames Mutagenicity Test
3.3. AlamarBlue In Vitro Cytotoxicity Test
3.4. Blood Compatibility
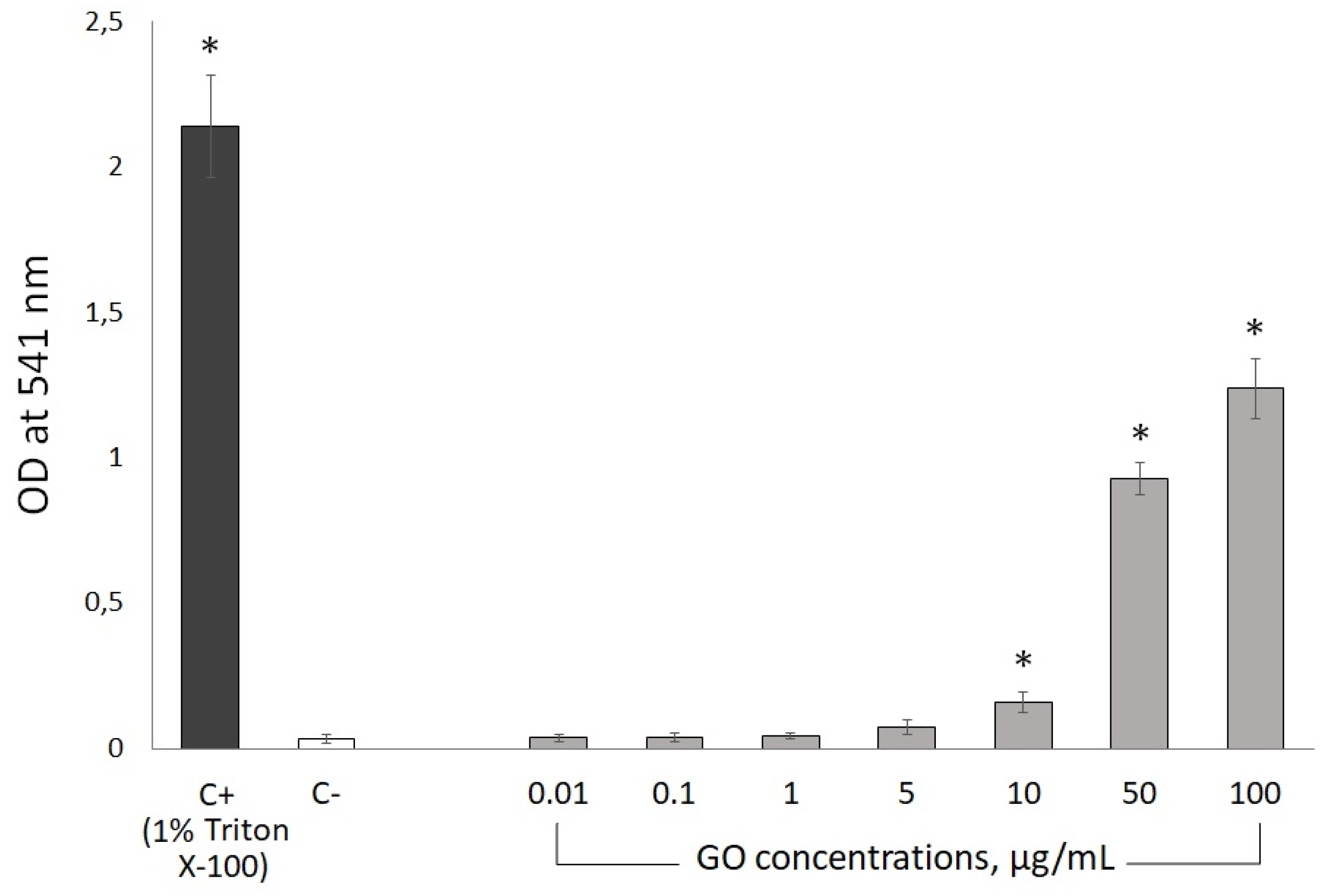
3.4.1. Hemolytic Activity
3.4.2. Platelet-Rich Plasma (PRP) Aggregation Kinetics
3.4.3. Procoagulant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikram, R.; Jan, B.M.; Ahmad, W. An Overview of Industrial Scalable Production of Graphene Oxide and Analytical Approaches for Synthesis and Characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Shin, D.S.; Kim, H.G.; Ahn, H.S.; Jeong, H.Y.; Kim, Y.J.; Odkhuu, D.; Tsogbadrakh, N.; Lee, H.B.R.; Kim, B.H. Distribution of Oxygen Functional Groups of Graphene Oxide Obtained from Low-Temperature Atomic Layer Deposition of Titanium Oxide. RSC Adv. 2017, 7, 13979–13984. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- AbouAitah, K.; Sabbagh, F.; Kim, B.S. Graphene Oxide Nanostructures as Nanoplatforms for Delivering Natural Therapeutic Agents: Applications in Cancer Treatment, Bacterial Infections, and Bone Regeneration Medicine. Nanomaterials 2023, 13, 2666. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Kim, S.; Min, D.H. Biosensors Based on Graphene Oxide and Its Biomedical Application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Bidram, E.; Zarrabi, A.; Amini, A.; Cheng, C. Graphene Oxide and Its Derivatives as Promising In-Vitro Bio-Imaging Platforms. Sci. Rep. 2020, 10, 18052. [Google Scholar] [CrossRef]
- Seifi, T.; Reza Kamali, A. Antiviral Performance of Graphene-Based Materials with Emphasis on COVID-19: A Review. Med. Drug Discov. 2021, 11, 100099. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Iravani, S.; Varma, R.S. Graphene and Graphene Oxide with Anticancer Applications: Challenges and Future Perspectives. MedComm 2022, 3, e118. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, B.; Liu, S.; Tan, C.; Tan, Y.; Jiang, Y. A New Strategy Involving the Use of Peptides and Graphene Oxide for Fluorescence Turn-on Detection of Proteins. Sensors 2018, 18, 385. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.M.; Jovanović, S.P.; Mašković, P.Z.; Mojsin, M.M.; Stevanović, M.J.; Danko, M.; Mičušík, M.; Jovanović, D.J.; Kleinová, A.; Špitalský, Z.; et al. Graphene Oxide Size and Structure Pro-Oxidant and Antioxidant Activity and Photoinduced Cytotoxicity Relation on Three Cancer Cell Lines. J. Photochem. Photobiol. B 2019, 200, 111647. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Zarezadeh, R.; Nouri, M.; Sadigh, A.R.; Pouremamali, F.; Asemi, Z.; Kafil, H.S.; Alemi, F.; Yousefi, B. Graphene Oxide: A Promising Material for Regenerative Medicine and Tissue Engineering. Biomol. Concepts 2021, 11, 182–200. [Google Scholar] [CrossRef] [PubMed]
- Sukhodub, L.; Fediv, V.; Kumeda, M.; Sukhodub, L.; Kulchynskyi, V.; Tkachuk, I.; Cherepanov, V.; Prylutskyy, Y. Electrical Properties of Biodegradable Chitosan-Calcium Phosphate Nerve Conduits Doped with Inorganic Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132425. [Google Scholar] [CrossRef]
- Jiang, T.; Amadei, C.A.; Lin, Y.; Gou, N.; Rahman, S.M.; Lan, J.; Vecitis, C.D.; Gu, A.Z. Dependence of Graphene Oxide (Go) Toxicity on Oxidation Level, Elemental Composition, and Size. Int. J. Mol. Sci. 2021, 22, 10578. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vinodhini, J.; Palanisamy, S.; Manivannan, N. Toxicity Aspects of Nanomaterials. In Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications: Volume 1–4; Springer: Berlin/Heidelberg, Germany, 2023; Volume 4, pp. 2535–2551. [Google Scholar] [CrossRef]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing Biocompatibility of Graphene Oxide-Based Nanocarriers: A Review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene Oxide Touches Blood: In Vivo Interactions of Bio-Coronated 2D Materials. Nanoscale Horiz. 2019, 4, 464–471. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and Biocompatibility Studies of Graphene Oxide in Mice after Intravenous Administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/Microsome Mutagenicity Assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Woziwodzka, A.; Gwizdek-Wiśniewska, A.; Piosik, J. Caffeine, Pentoxifylline and Theophylline Form Stacking Complexes with IQ-Type Heterocyclic Aromatic Amines. Bioorg. Chem. 2011, 39, 10–17. [Google Scholar] [CrossRef]
- Halenova, T.; Raksha, N.; Savchuk, O.; Ostapchenko, L.; Prylutskyy, Y.; Ritter, U.; Scharff, P. Evaluation of the Biocompatibility of Water-Soluble Pristine C60 Fullerenes in Rabbit. Bionanoscience 2020, 10, 721–730. [Google Scholar] [CrossRef]
- Ochedowski, O.; Bussmann, B.K.; Schleberger, M. Graphene on Mica-Intercalated Water Trapped for Life. Sci. Rep. 2014, 4, 6003. [Google Scholar] [CrossRef] [PubMed]
- Nemes-Incze, P.; Osváth, Z.; Kamarás, K.; Biró, L.P. Anomalies in Thickness Measurements of Graphene and Few Layer Graphite Crystals by Tapping Mode Atomic Force Microscopy. Carbon 2008, 46, 1435–1442. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene Oxide: An Efficient Material and Recent Approach for Biotechnological and Biomedical Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Volkov, Y.; McIntyre, J.; Prina-Mello, A. Graphene Toxicity as a Double-Edged Sword of Risks and Exploitable Opportunities: A Critical Analysis of the Most Recent Trends and Developments. 2d Mater. 2017, 4, 022001. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional Graphene Oxide Nanoparticles for Drug Delivery in Cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef]
- Matvienko, T.; Sokolova, V.; Prylutska, S.; Harahuts, Y.; Kutsevol, N.; Kostjukov, V.; Evstigneev, M.; Prylutskyy, Y.; Epple, M.; Ritter, U. In Vitro Study of the Anticancer Activity of Various Doxorubicin-Containing Dispersions. Bioimpacts 2018, 9, 57. [Google Scholar] [CrossRef]
- Borowik, A.; Prylutskyy, Y.; Kawelski, Ł.; Kyzyma, O.; Bulavin, L.; Ivankov, O.; Cherepanov, V.; Wyrzykowski, D.; Kaźmierkiewicz, R.; Gołuński, G.; et al. Does C 60 Fullerene Act as a Transporter of Small Aromatic Molecules? Colloids Surf. B Biointerfaces 2018, 164, 134–143. [Google Scholar] [CrossRef]
- Skivka, L.M.; Prylutska, S.V.; Rudyk, M.P.; Khranovska, N.M.; Opeida, I.V.; Hurmach, V.V.; Prylutskyy, Y.I.; Sukhodub, L.F.; Ritter, U. C60 Fullerene and Its Nanocomplexes with Anticancer Drugs Modulate Circulating Phagocyte Functions and Dramatically Increase ROS Generation in Transformed Monocytes. Cancer Nanotechnol. 2018, 9, 8. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; MacOsko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Zhai, G. The Effect of Incubation Conditions on the Hemolytic Properties of Unmodified Graphene Oxide with Various Concentrations. RSC Adv. 2016, 6, 68322–68334. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S.; Kim, S. An Insight into Recent Advances on Platelet Function in Health and Disease. Int. J. Mol. Sci. 2022, 23, 6022. [Google Scholar] [CrossRef] [PubMed]
- Podolska, M.J.; Barras, A.; Alexiou, C.; Frey, B.; Gaipl, U.; Boukherroub, R.; Szunerits, S.; Janko, C.; Muñoz, L.E. Graphene Oxide Nanosheets for Localized Hyperthermia-Physicochemical Characterization, Biocompatibility, and Induction of Tumor Cell Death. Cells 2020, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, M.K.; Kulkarni, P.P.; Sonkar, V.K.; Grácio, J.J.A.; Dash, D. Amine-Modified Graphene: Thrombo-Protective Safer Alternative to Graphene Oxide for Biomedical Applications. ACS Nano 2012, 6, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, M.K.; Nayak, M.K.; Kumari, S.; Shrivastava, S.; Grácio, J.J.A.; Dash, D. Thrombus Inducing Property of Atomically Thin Graphene Oxide Sheets. ACS Nano 2011, 5, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Kenry; Loh, K.P.; Lim, C.T. Molecular Interactions of Graphene Oxide with Human Blood Plasma Proteins. Nanoscale 2016, 8, 9425–9441. [Google Scholar] [CrossRef]
- Feng, R.; Yu, Y.; Shen, C.; Jiao, Y.; Zhou, C. Impact of Graphene Oxide on the Structure and Function of Important Multiple Blood Components by a Dose-Dependent Pattern. J. Biomed. Mater. Res. A 2015, 103, 2006–2014. [Google Scholar] [CrossRef]



| ADP-Induced Platelet Aggregation Parameters | |||||
|---|---|---|---|---|---|
| Amax (%) | V0 (%·s−1) | Tmax (s) | A6min (%) | ||
| 5 min incubation of PRP with GO particles | |||||
| control | 53 ± 2 | 1.2 ± 0.2 | 100 ± 7 | 30 ± 2 | |
| GO concentration (μg/mL) | 1 | 55 ± 2 | 1.2 ± 0.1 | 95 ± 5 | 30 ± 1 |
| 5 | 54 ± 1 | 1.1 ± 0.2 | 110 ± 6 | 35 ± 2 | |
| 10 | 55 ± 2 | 1.1 ± 0.1 | 110 ± 4 | 45 ± 3 * | |
| 50 | 56 ± 3 | 1.1 ± 0.1 | 118 ± 5 | 49 ± 2 * | |
| 100 | 57 ± 3 | 0.9 ± 0.1 | 120 ± 8 | 55 ± 3 * | |
| 60 min incubation of PRP with GO particles | |||||
| control | 55 ± 3 | 0.9 ± 0.2 | 95 ± 5 | 35 ± 2 | |
| GO concentration (μg/mL) | 1 | 60 ± 3 | 1.0 ± 0.1 | 100 ± 5 | 55 ± 3 * |
| 5 | 60 ± 2 | 1.1 ± 0.2 | 95 ± 4 | 55 ± 2 * | |
| 10 | 55 ± 2 | 0.9 ± 0.1 | 110 ± 6 | 49 ± 3 * | |
| 50 | 45± 2 * | 0.8 ± 0.1 | 115 ± 7 * | 39 ± 2 | |
| 100 | 43 ± 2 * | 0.6 ± 0.1 * | 140 ± 5 * | 40 ± 3 | |
| Coagulation Tests | ||||
|---|---|---|---|---|
| aPPT | PT | TT | ||
| 5 min incubation of rabbit plasma with GO particles | ||||
| control | 16.9 ± 1.3 | 7.9 ± 0.9 | 11.3 ± 1.2 | |
| GO concentration (μg/mL) | 0.01 | 18.7 ± 1.3 | 8.8 ± 0.5 | 9.3 ± 1.1 |
| 0.05 | 20.6 ± 0.9 | 7.4 ± 0.7 | 8.8 ± 1.3 | |
| 0.1 | 17.4 ± 0.5 | 7.3 ± 0.9 | 9.0 ± 0.9 | |
| 0.5 | 16.5 ± 1.1 | 7.4 ± 0.6 | 8.9 ± 1.1 | |
| 1 | 16.1 ± 0.9 | 8.5 ± 0.8 | 8.5 ± 0.9 | |
| 5 | 15.9 ± 1.3 | 7.3 ± 0.6 | 8.3 ± 0.6 | |
| 10 | 17.8 ± 1.2 | 7.2 ± 0.7 | 11.0 ± 0.7 | |
| 30 min incubation of rabbit plasma with GO particles | ||||
| control | 17.6 ± 1.2 | 8.8 ± 0.7 | 7.0 ± 0.7 | |
| GO concentration (μg/mL) | 0.01 | 15.5 ± 1.2 | 8.1 ± 0.9 | 8.2 ± 0.8 |
| 0.05 | 15.6 ± 1.3 | 6.9 ± 1.0 | 6.8 ± 0.9 | |
| 0.1 | 18.4 ± 1.1 | 7.7 ± 0.8 | 7.7 ± 0.5 | |
| 0.5 | 17.8 ± 1.2 | 7.3 ± 0.7 | 7.8 ± 0.4 | |
| 1 | 20.4 ± 0.9 | 7.0 ± 0.9 | 8.3 ± 0.5 | |
| 5 | 16.0 ± 0.8 | 7.4 ± 1.1 | 7.5 ± 0.3 | |
| 10 | 19.1 ± 1.1 | 6.9 ± 0.8 | 7.8 ± 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prylutskyy, Y.; Bełdzińska, P.; Derewońko, N.; Halenova, T.; Raksha, N.; Zakrzewski, M.; Gołuński, G.; Prylutska, S.; Ritter, U.; Savchuk, O.; et al. Biosafety and Blood Compatibility of Graphene Oxide Particles in In Vitro Experiments. Materials 2025, 18, 2128. https://doi.org/10.3390/ma18092128
Prylutskyy Y, Bełdzińska P, Derewońko N, Halenova T, Raksha N, Zakrzewski M, Gołuński G, Prylutska S, Ritter U, Savchuk O, et al. Biosafety and Blood Compatibility of Graphene Oxide Particles in In Vitro Experiments. Materials. 2025; 18(9):2128. https://doi.org/10.3390/ma18092128
Chicago/Turabian StylePrylutskyy, Yuriy, Patrycja Bełdzińska, Natalia Derewońko, Tetiana Halenova, Nataliia Raksha, Marcin Zakrzewski, Grzegorz Gołuński, Svitlana Prylutska, Uwe Ritter, Olexii Savchuk, and et al. 2025. "Biosafety and Blood Compatibility of Graphene Oxide Particles in In Vitro Experiments" Materials 18, no. 9: 2128. https://doi.org/10.3390/ma18092128
APA StylePrylutskyy, Y., Bełdzińska, P., Derewońko, N., Halenova, T., Raksha, N., Zakrzewski, M., Gołuński, G., Prylutska, S., Ritter, U., Savchuk, O., & Piosik, J. (2025). Biosafety and Blood Compatibility of Graphene Oxide Particles in In Vitro Experiments. Materials, 18(9), 2128. https://doi.org/10.3390/ma18092128








