Novel Applications of Natural Biomaterials in Dentistry—Properties, Uses, and Development Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Characteristics of Selected Natural Biomaterials Used in Dentistry
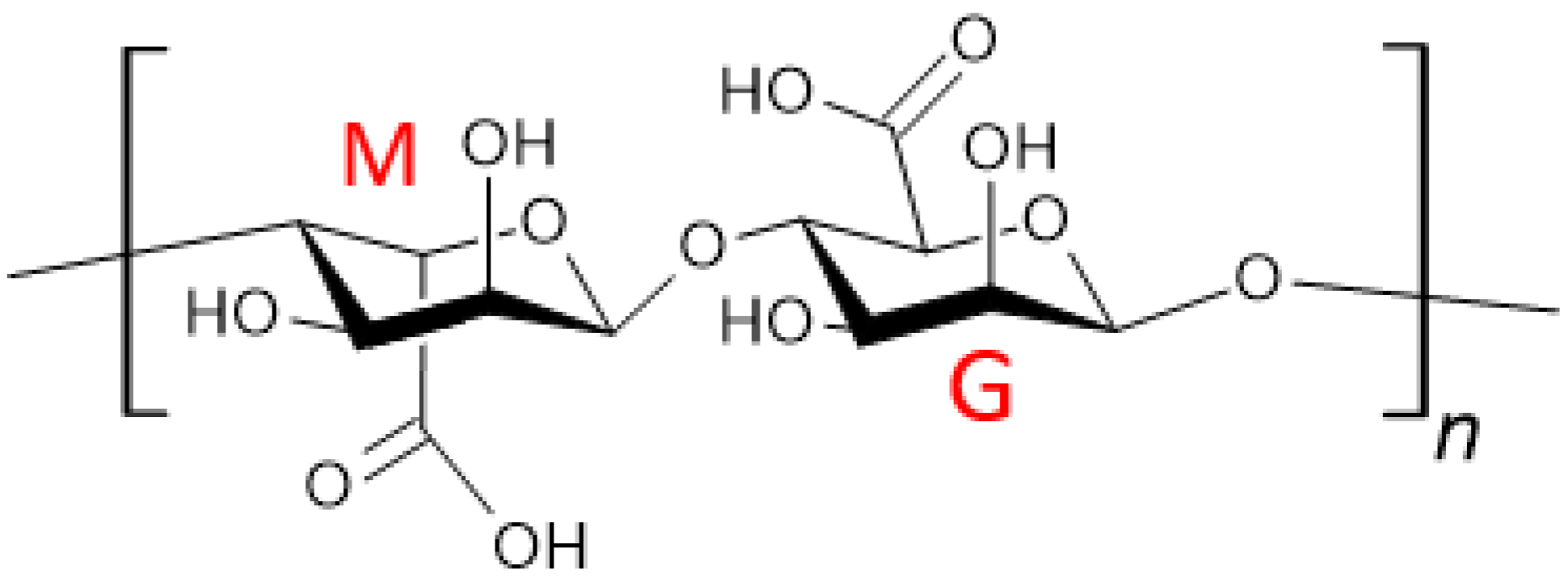
3.1. Alginate
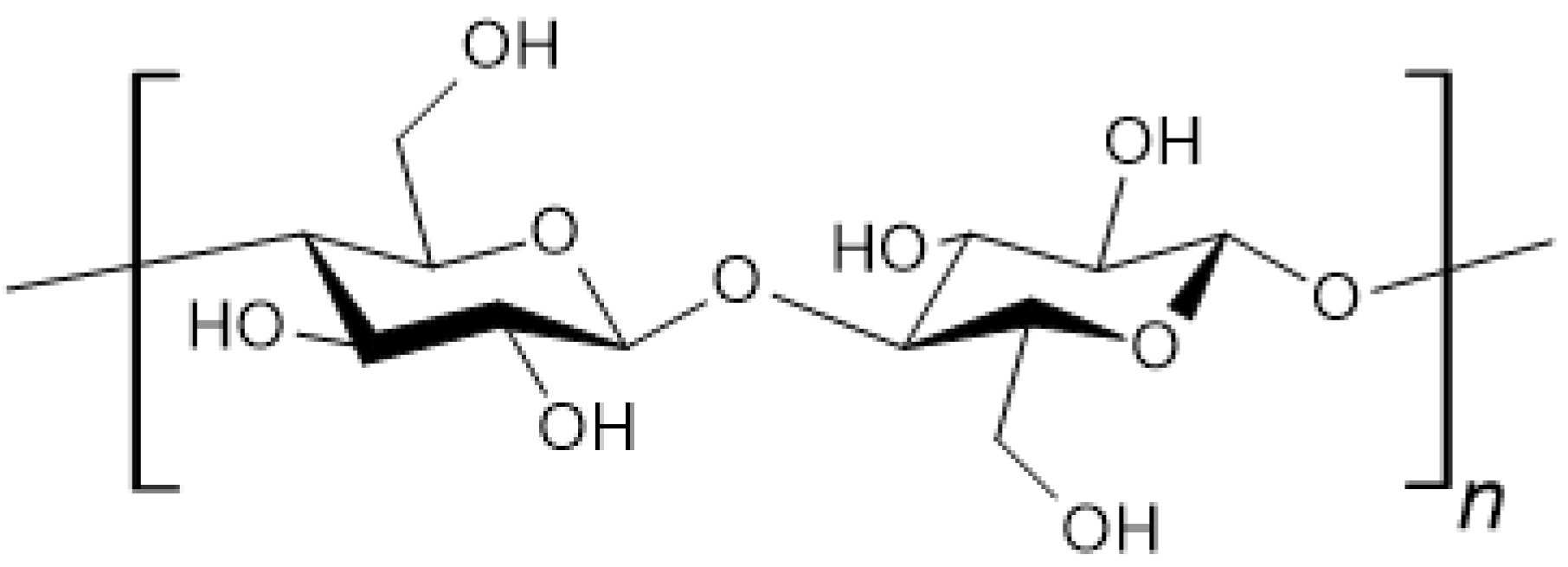
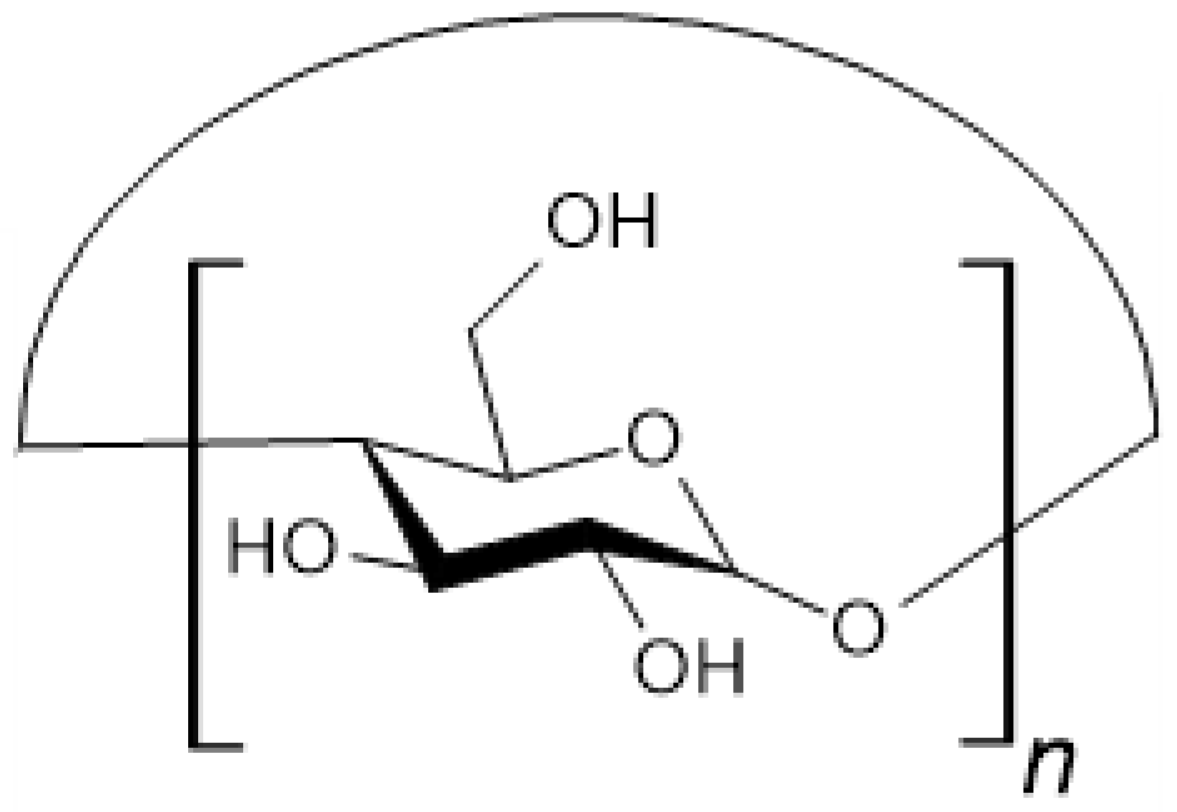
3.2. Cellulose
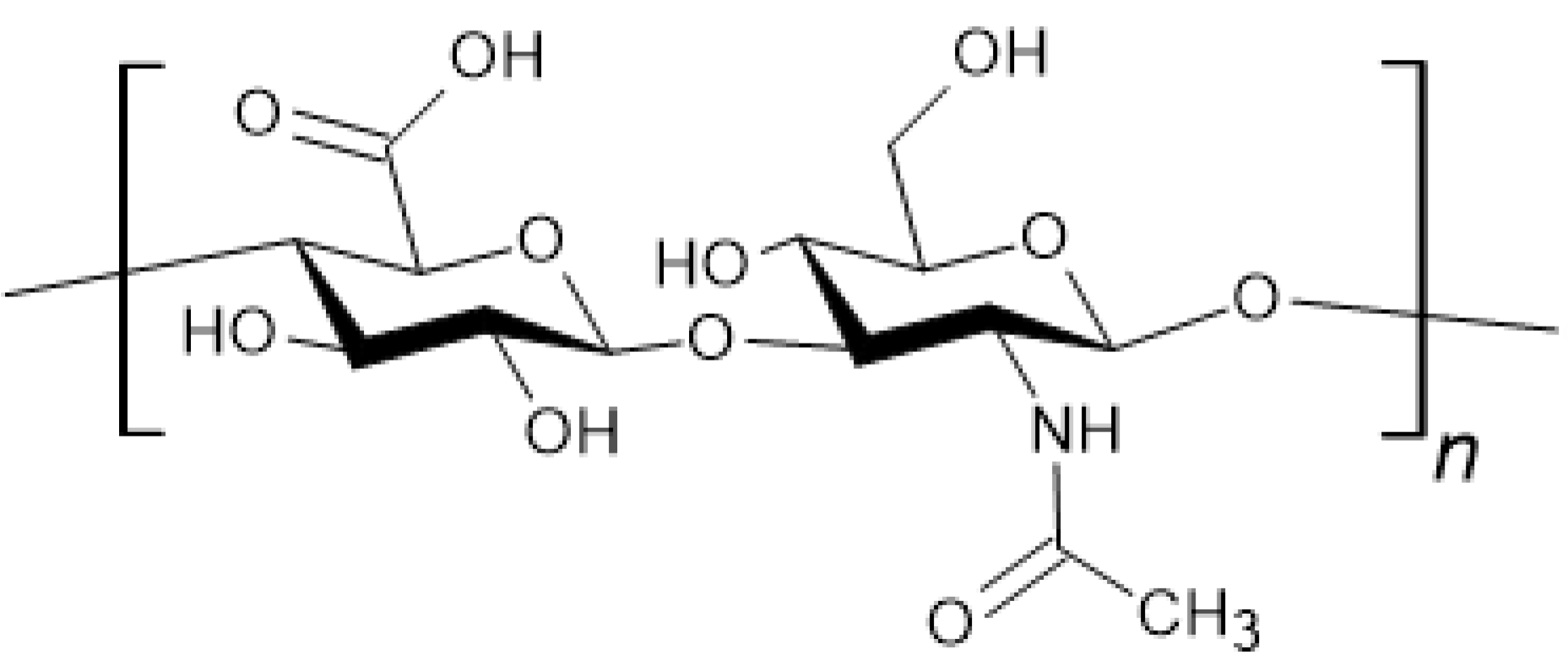
3.3. Chitosan
3.4. Collagen
3.5. Cyclodextrins
3.6. Gelatin
3.7. Hyaluronic Acid
3.8. Hydroxyapatite
3.9. Silk
3.10. Comparison of Biomaterials
4. Natural Biomaterials in Selected Areas of Stomatology
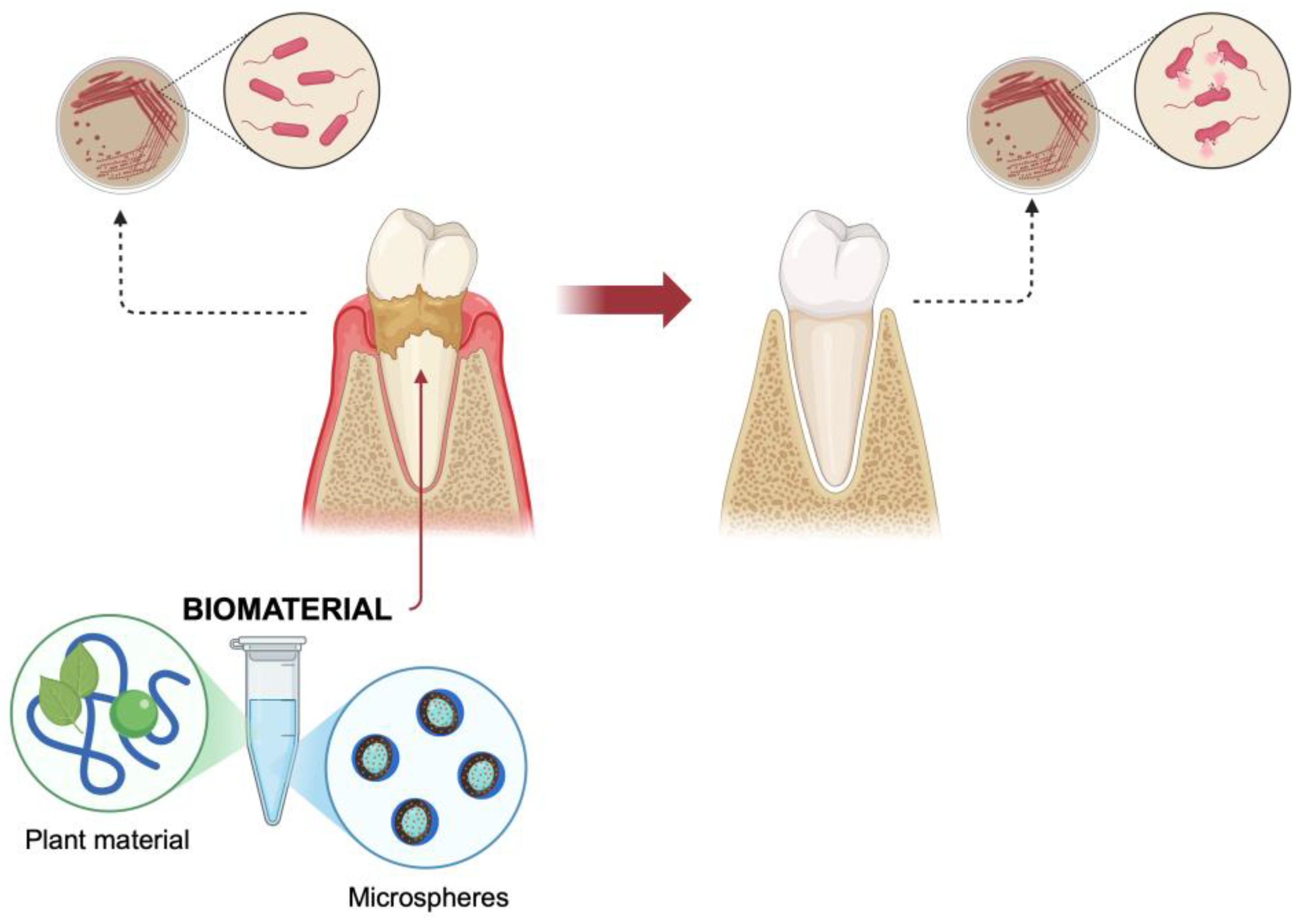
4.1. Periodontal Disease Prevention and Regeneration
4.2. Dental Caries Prevention
4.3. Bone Substitutes in Implantology
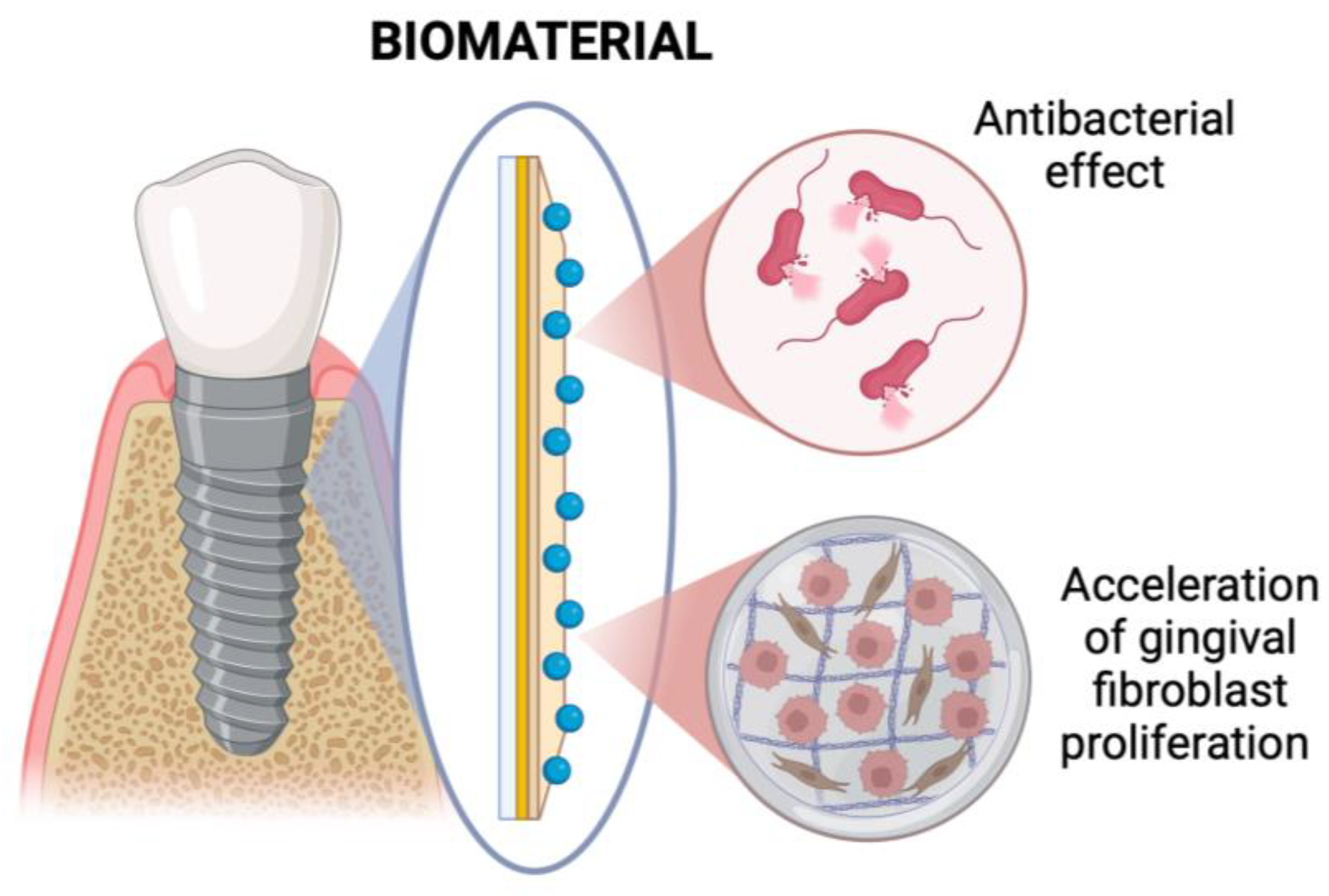
4.4. Dental Implants Coating
5. Development Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Jiang, L.; Zeng, Y.; Han, Y.; Sha, C.; Xie, X.; Li, H.; Zhou, J.; Lin, W. 3D bioprinting of collagen-based materials for oral medicine. Collagen Leather 2023, 5, 23. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Galler, K.M.; Brandl, F.P.; Kirchhof, S.; Widbiller, M.; Eidt, A.; Buchalla, W.; Göpferich, A.; Schmalz, G. Suitability of Different Natural and Synthetic Biomaterials for Dental Pulp Tissue Engineering. Tissue Eng. Part A 2018, 24, 234–244. [Google Scholar] [CrossRef]
- Varoni, E.M.; Iriti, M.; Rimondini, L. Plant Products for Innovative Biomaterials in Dentistry. Coatings 2012, 2, 179–194. [Google Scholar] [CrossRef]
- Aguilar-Ayala, F.J.; Aguilar-Pérez, F.J.; Nic-Can, G.I.; Rojas-Herrera, R.; Chuc-Gamboa, G.; Aguilar-Pérez, D.; Rodas-Junco, B.A. A Molecular View on Biomaterials and Dental Stem Cells Interactions: Literature Review. Appl. Sci. 2022, 12, 5815. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sionkowska, A. State of Innovation in Alginate-Based Materials. Mar. Drugs 2023, 21, 353. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, W.; Mei, Y.; Yun, Y.; Wang, B.; Zhong, Q.; Chen, H.; Chen, W. Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate. Molecules 2019, 24, 4374. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef]
- Urtuvia, V.; Maturana, N.; Acevedo, F.; Peña, C.; Díaz-Barrera, A. Bacterial alginate production: An overview of its biosynthesis and potential industrial production. World J. Microbiol. Biotechnol. 2017, 33, 198. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Shahid, A.; Hossain, T.; Sheikh, S.; Rahman, S.; Uddin, N.; Rahim, A.; Khan, R.A.; Hossain, I. Sources, extractions, and applications of alginate: A review. Discov. Appl. Sci. 2024, 6, 443. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef]
- Remminghorst, U.; Rehm, B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Ren, K.; Zhu, Y.; Yang, S. Alginate: Microbial production, functionalization, and biomedical applications. Int. J. Biol. Macromol. 2023, 242, 125048. [Google Scholar] [CrossRef]
- Mutch, A.L.; Yang, J.; Ferro, V.; AnithA, A.; Grøndahl, L. Sulfated Alginate for Biomedical Applications. Macromol. Biosci. 2024, 24, e2400237. [Google Scholar] [CrossRef]
- Lukova, P.; Kokova, V.; Baldzhieva, A.; Murdjeva, M.; Katsarov, P.; Delattre, C.; Apostolova, E. Alginate from Ericaria crinita Possesses Antioxidant Activity and Attenuates Systemic Inflammation via Downregulation of Pro-Inflammatory Cytokines. Mar. Drugs 2024, 22, 482. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Laino, L.; Troiano, G.; Amoroso, G.; Crimi, S.; Matarese, M.; D’amico, C.; Siniscalchi, E.N.; et al. Alginate Materials and Dental Impression Technique: A Current State of the Art and Application to Dental Practice. Mar. Drugs 2019, 17, 18. [Google Scholar] [CrossRef]
- Syed, M.; Chopra, R.; Sachdev, V. Allergic Reactions to Dental Materials-A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE04–ZE09. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Hou, L.; Ching, Y.C.; Ching, K.Y.; Hai, N.D.; Chuah, C.H. A review of recent advances of cellulose-based intelligent-responsive hydrogels as vehicles for controllable drug delivery system. Int. J. Biol. Macromol. 2024, 264, 130525. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. Multifunctional cellulose-based biomaterials for dental applications: A sustainable approach to oral health and regeneration. Ind. Crop. Prod. 2023, 203, 117142. [Google Scholar] [CrossRef]
- Kononova, S.V.; Khripunov, A.K.; Romanov, V.N.; Orekhov, A.S.; Mikhutkin, A.A.; Vlasova, E.N.; Lukasov, M.S.; Klechkovskaya, V.V. Animal Cellulose with Hierarchical Structure Isolated from Halocynthia aurantium Tunic as the Basis for High-Performance Pressure-Resistant Nanofiltration Membrane. Membranes 2022, 12, 975. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 428253. [Google Scholar] [CrossRef]
- Jedvert, K.; Heinze, T. Cellulose Modification and Shaping—A Review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Srivastava, D.; Ahopelto, J.; Karttunen, A.J. Thermodynamic Properties of Crystalline Cellulose Allomorphs Studied with Dispersion-Corrected Density Functional Methods. Molecules 2022, 27, 6240. [Google Scholar] [CrossRef]
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.-C.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef]
- Bajpai, S.; Pathak, V.; Soni, B. Minocycline-loaded cellulose nano whiskers/poly(sodium acrylate) composite hydrogel films as wound dressing. Int. J. Biol. Macromol. 2015, 79, 76–85. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact. Mater. 2022, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Nimeskern, L.; Martínez Ávila, H.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N.; Velasco, H.; Mecerreyes, D.; Antonio, R.; et al. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Qu, S.; Ma, X.; Yu, S.; Wang, R. Chitosan as a biomaterial for the prevention and treatment of dental caries: Antibacterial effect, biomimetic mineralization, and drug delivery. Front. Bioeng. Biotechnol. 2023, 11, 1234758. [Google Scholar] [CrossRef]
- Agrawal, A.; Reche, A.; Agrawal, S.; Paul, P. Applications of Chitosan Nanoparticles in Dentistry: A Review. Cureus 2023, 15, e49934. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Hegde, S.; Manaktala, N. Chitosan nanoparticle applications in dentistry: A sustainable biopolymer. Front. Chem. 2024, 12, 1362482. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Laskowska, J.; Karolewicz, B.; Owczarek, A. The Importance of Chitosan Coatings in Dentistry. Mar. Drugs 2023, 21, 613. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.-G.; Park, H.-J.; Liu, C.-G.; Liu, C.-S.; Meng, X.-H.; Yu, L.-J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Sabar, S.; Wilson, L.D.; Jawad, A.H.; Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and Chitosan Nanoparticles: Parameters Enhancing Antifungal Activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef]
- Pardo-Castaño, C.; Bolaños, G. Solubility of chitosan in aqueous acetic acid and pressurized carbon dioxide-water: Experimental equilibrium and solubilization kinetics. J. Supercrit. Fluids 2019, 151, 63–74. [Google Scholar] [CrossRef]
- Ding, K.; Wang, Y.; Wang, H.; Yuan, L.; Tan, M.; Shi, X.; Lyu, Z.; Liu, Y.; Chen, H. 6-O-Sulfated Chitosan Promoting the Neural Differentiation of Mouse Embryonic Stem Cells. ACS Appl. Mater. Interfaces 2014, 6, 20043–20050. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863. [Google Scholar] [CrossRef]
- Chen, Q.; Pei, Y.; Tang, K.; Albu-Kaya, M.G. Structure, extraction, processing, and applications of collagen as an ideal component for biomaterials—A review. Collagen Leather 2023, 5, 20. [Google Scholar] [CrossRef]
- Gauza-Włodarczyk, M.; Kubisz, L.; Włodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef]
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartmann, D.J. Reactions to a Bovine Collagen Implant. Clinical and Immunologic Study in 705 Patients. J. Am. Acad. Dermatol. 1989, 21, 1203–1208. [Google Scholar] [CrossRef]
- Badylak, S.F.; Gilbert, T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef]
- Goo, H.C.; Hwang, Y.-S.; Choi, Y.R.; Cho, H.N.; Suh, H. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials 2003, 24, 5099–5113. [Google Scholar] [CrossRef]
- Honvo, G.; Lengelé, L.; Charles, A.; Reginster, J.-Y.; Bruyère, O. Role of Collagen Derivatives in Osteoarthritis and Cartilage Repair: A Systematic Scoping Review With Evidence Mapping. Rheumatol. Ther. 2020, 7, 703–740. [Google Scholar] [CrossRef] [PubMed]
- Skoracka, J.; Bajewska, K.; Kulawik, M.; Suchorska, W.; Kulcenty, K. Advances in cartilage tissue regeneration: A review of stem cell therapies, tissue engineering, biomaterials, and clinical trials. Excli. J. 2024, 23, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2023, 324, 121500. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Santos Braga, S. Cyclodextrins as Multi-Functional Ingredients in Dentistry. Pharmaceutics 2023, 15, 2251. [Google Scholar] [CrossRef]
- Napiórkowska, E. Overview of cyclodextrins and medicinal products containing cyclodextrins currently registered in Poland. Prospect. Pharm. Sci. 2023, 21, 30–37. [Google Scholar] [CrossRef]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Samuelsen, L.; Holm, R.; Schönbeck, C. Simultaneous determination of cyclodextrin stability constants as a function of pH and temperature—A tool for drug formulation and process design. J. Drug Deliv. Sci. Technol. 2021, 65, 102675. [Google Scholar] [CrossRef]
- Kulawik, A.; Rosiak, N.; Miklaszewski, A.; Cielecka-Piontek, J.; Zalewski, P. Investigation of cyclodextrin as potential carrier for lycopene. Arh. za Farm. 2024, 74, 178–205. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, E.; Maleki, A.; Madkhali, O.A. Drug Delivery of Gelatin Nanoparticles as a Biodegradable Polymer for the Treatment of Infectious Diseases: Perspectives and Challenges. Polymers 2023, 15, 4327. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef]
- Sisso, A.M.; Boit, M.O.; DeForest, C.A. Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. Part A 2020, 108, 1112. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kim, Y.; Lee, S.H.; Yoon, Y.; Kim, W.-H.; Kweon, O.-K. Effect of Gelatin on Osteogenic Cell Sheet Formation Using Canine Adipose-Derived Mesenchymal Stem Cells. Cell Transplant. 2017, 26, 115–123. [Google Scholar] [CrossRef]
- Irfan, N.I.; Mohd Zubir, A.Z.; Suwandi, A.; Haris, M.S.; Jaswir, I.; Lestari, W. Gelatin-based hemostatic agents for medical and dental application at a glance: A narrative literature review. Saudi Dent. J. 2022, 34, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-based biomaterials and gelatin as an additive for chronic wound repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Buckley, C.; Murphy, E.J.; Montgomery, T.R.; Major, I. Hyaluronic Acid: A Review of the Drug Delivery Capabilities of This Naturally Occurring Polysaccharide. Polymers 2022, 14, 3442. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Z.; Zhang, Z.; Xu, Y.; Hillpot, E.; Lin, Y.S.; Zakusilo, F.T.; Lu, J.Y.; Ablaeva, J.; Biashad, S.A.; et al. Evolution of high-molecular-mass hyaluronic acid is associated with subterranean lifestyle. Nat. Commun. 2023, 14, 8054. [Google Scholar] [CrossRef]
- Miglani, A.; Vishnani, R.; Reche, A.; Buldeo, J.; Wadher, B. Hyaluronic Acid: Exploring Its Versatile Applications in Dentistry. Cureus 2023, 15, e46349. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Landi, M.R.; Massa, A.; D’amora, U.; Guarino, V. Hyaluronic Acid in Biomedical Fields: New Trends from Chemistry to Biomaterial Applications. Int. J. Mol. Sci. 2022, 23, 14372. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek LA, M.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 624967. [Google Scholar] [CrossRef]
- La Gatta, A.; Salzillo, R.; Catalano, C.; D’agostino, A.; Pirozzi, A.V.A.; De Rosa, M.; Schiraldi, C. Hyaluronan-based hydrogels as dermal fillers: The biophysical properties that translate into a “volumetric” effect. PLoS ONE 2019, 14, e0218287. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Piccotti, F.; Guarda-Nardini, L. Hyaluronic Acid In the Treatment of TMJ Disorders: A Systematic Review of the Literature. J. Craniomandib. Pract. 2010, 28, 166–176. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Drabczyk, A.; Florkiewicz, W.; Głąb, M.; Kudłacik-Kramarczyk, S.; Słota, D.; Tomala, A.; Tyliszczak, B. Review of the Applications of Biomedical Compositions Containing Hydroxyapatite and Collagen Modified by Bioactive Components. Materials 2021, 14, 2096. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anchan, R.V.; Murugan, S.S.; Anil, S.; Kim, S.-K. Natural hydroxyapatite-based nanobiocomposites and their biomaterials-to-cell interaction for bone tissue engineering. Nanoscale Res. Lett. 2024, 19, 169. [Google Scholar] [CrossRef]
- Firdaus Hussin, M.S.; Abdullah, H.Z.; Idris, M.I.; Abdul Wahap, M.A. Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef]
- Pretorius, N.; Forrest, A.; Walsh, K. A review of near infrared spectroscopic features of teeth, bone and artificial hydroxyapatite. J. Near Infrared Spectrosc. 2023, 31, 227–240. [Google Scholar] [CrossRef]
- Imran, E.; Mei, M.L.; Li, K.C.; Ratnayake, J.; Ekambaram, M.; Cooper, P.R. Dental Applications of Ion-Substituted Hydroxyapatite: A Review of the Literature. Dent. J. 2024, 12, 304. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.-F.; Shiu, S.-T.; Hung, C.-Y.; Chan, Y.-H.; Chee, T.-J.; Huang, P.-C.; Lai, P.-C.; Feng, S.-W. Osseointegration Potential Assessment of Bone Graft Materials Loaded with Mesenchymal Stem Cells in Peri-Implant Bone Defects. Int. J. Mol. Sci. 2024, 25, 862. [Google Scholar] [CrossRef]
- Rau, J.; Cacciotti, I.; De Bonis, A.; Fosca, M.; Komlev, V.; Latini, A.; Santagata, A.; Teghil, R. Fe-doped hydroxyapatite coatings for orthopedic and dental implant applications. Appl. Surf. Sci. 2014, 307, 301–305. [Google Scholar] [CrossRef]
- Krishnan, V.; Bhatia, A.; Varma, H. Development, characterization and comparison of two strontium doped nano hydroxyapatite molecules for enamel repair/regeneration. Dent. Mater. 2016, 32, 646–659. [Google Scholar] [CrossRef]
- Rajabnejadkeleshteri, A.; Kamyar, A.; Khakbiz, M.; Bakalani, Z.L.; Basiri, H. Synthesis and characterization of strontium fluor-hydroxyapatite nanoparticles for dental applications. Microchem. J. 2020, 153, 104485. [Google Scholar] [CrossRef]
- Opris, H.; Bran, S.; Dinu, C.; Baciut, M.; Prodan, D.A.; Mester, A.; Baciut, G. Clinical applications of avian eggshell-derived hydroxyapatite. Bosn. J. Basic Med. Sci. 2020, 20, 430–437. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef] [PubMed]
- Lubojański, A.; Zakrzewski, W.; Samól, K.; Bieszczad-Czaja, M.; Świtała, M.; Wiglusz, R.; Watras, A.; Mielan, B.; Dobrzyński, M. Application of Nanohydroxyapatite in Medicine—A Narrative Review. Molecules 2024, 29, 5628. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Gayathri, V.; Sowmya, S.; Augustine, D.; Alamoudi, A.; Zidane, B.; Hassan Mohammad Albar, N.; Bhandi, S. Nanohydroxyapatite in dentistry: A comprehensive review. Saudi Dent. J. 2023, 35, 741–752. [Google Scholar] [CrossRef]
- Li, M.; You, J.; Qin, Q.; Liu, M.; Yang, Y.; Jia, K.; Zhang, Y.; Zhou, Y. A Comprehensive Review on Silk Fibroin as a Persuasive Biomaterial for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 2660. [Google Scholar] [CrossRef]
- Neubauer, V.J.; Döbl, A.; Scheibel, T. Silk-Based Materials for Hard Tissue Engineering. Materials 2021, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, K.; Kucharczyk, K.; Florczak, A.; Dondajewska, E.; Mackiewicz, A.; Dams-Kozlowska, H. Silk as an innovative biomaterial for cancer therapy. Rep. Pract. Oncol. Radiother. 2014, 20, 87–98. [Google Scholar] [CrossRef]
- Deptuch, T.; Dams-Kozlowska, H. Silk Materials Functionalized via Genetic Engineering for Biomedical Applications. Materials 2017, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef]
- Florczak, A.; Grzechowiak, I.; Deptuch, T.; Kucharczyk, K.; Kaminska, A.; Dams-Kozlowska, H. Silk Particles as Carriers of Therapeutic Molecules for Cancer Treatment. Materials 2020, 13, 4946. [Google Scholar] [CrossRef]
- Zafar, M.S.; Al-Samadani, K.H. Potential use of natural silk for bio-dental applications. J. Taibah Univ. Med. Sci. 2014, 9, 171–177. [Google Scholar] [CrossRef]
- Bottagisio, M.; Palombella, S.; Lopa, S.; Sangalli, F.; Savadori, P.; Biagiotti, M.; Sideratou, Z.; Tsiourvas, D.; Lovati, A.B. Vancomycin-nanofunctionalized peptide-enriched silk fibroin to prevent methicillin-resistant Staphylococcus epidermidis-induced femoral nonunions in rats. Front. Cell. Infect. Microbiol. 2023, 12, 1056912. [Google Scholar] [CrossRef]
- Kochhar, D.; DeBari, M.K.; Abbott, R.D. The Materiobiology of Silk: Exploring the Biophysical Influence of Silk Biomaterials on Directing Cellular Behaviors. Front. Bioeng. Biotechnol. 2021, 9, 697981. [Google Scholar] [CrossRef]
- Gautam, J.; Sood, A.; Chaudhry, S.; Ghumman, S.; Chopra, A. Beyond the Needle: A Comparative Evaluation of Silk Sutures and Cyanoacrylate for Periodontal Flap Closure. Cureus 2024, 16, e56604. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Sah, A.K.; Dewangan, M.; Suresh, P.K. Potential of chitosan-based carrier for periodontal drug delivery. Colloids Surf. B Biointerfaces 2019, 178, 185–198. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Dvořák, J.; Rosiak, N.; Tykarska, E.; Szymańska, E.; Winnicka, K.; Ruchała, M.A.; Cielecka-Piontek, J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics 2021, 13, 417. [Google Scholar] [CrossRef]
- Ausenda, F.; Rasperini, G.; Acunzo, R.; Gorbunkova, A.; Pagni, G. New Perspectives in the Use of Biomaterials for Periodontal Regeneration. Materials 2019, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Scholz, M.; Reske, T.; Böhmer, F.; Hornung, A.; Grabow, N.; Lang, H. In vitro chlorhexidine release from alginate based microbeads for periodontal therapy. PLoS ONE 2017, 12, e0185562. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Cielecka-Piontek, J. Chitosan as a Functional Carrier for the Local Delivery Anti-Inflammatory Systems Containing Scutellariae baicalensis radix Extract. Pharmaceutics 2022, 14, 2148. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Koumentakou, I.; Lazaridou, M.; Bikiaris, D.; Miklaszewski, A.; Plech, T.; Cielecka-Piontek, J. 3D-Printed Chitosan-Based Scaffolds with Scutellariae baicalensis Extract for Dental Applications. Pharmaceutics 2024, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Lee, J.Y.; Yeom, H.R.; Kim, K.H.; Lee, S.C.; Shim, I.K.; Chung, C.P.; Lee, S.J. Injectable Polysaccharide Microcapsules for Prolonged Release of Minocycline for the Treatment of Periodontitis. Biotechnol. Lett. 2005, 27, 1761–1766. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Rodriguez-Nogales, A.; Ortiz-Cullera, V.; Algieri, F.; Garrido-Mesa, J.; Zorrilla, P.; Rodriguez-Cabezas, M.E.; Garrido-Mesa, N.; Utrilla, M.P.; De Matteis, L.; et al. Silk fibroin nanoparticles constitute a vector for controlled release of resveratrol in an experimental model of inflammatory bowel disease in rats. Int. J. Nanomed. 2014, 9, 4507–4520. [Google Scholar] [CrossRef]
- Giménez-Siurana, A.; García, F.G.; Bernabeu, A.P.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; López-Jornet, P. Chemoprevention of Experimental Periodontitis in Diabetic Rats with Silk Fibroin Nanoparticles Loaded with Resveratrol. Antioxidants 2020, 9, 85. [Google Scholar] [CrossRef]
- Imber, J.-C.; Roccuzzo, A.; Stähli, A.; Saulacic, N.; Deschner, J.; Sculean, A.; Bosshardt, D.D. Immunohistochemical Evaluation of Periodontal Regeneration Using a Porous Collagen Scaffold. Int. J. Mol. Sci. 2021, 22, 10915. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, J.C.; Mehr, M.; Buxton, P.G.; Best, S.M.; Cameron, R.E. Optimising collagen scaffold architecture for enhanced periodontal ligament fibroblast migration. J. Mater. Sci. Mater. Med. 2018, 29, 166. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Feng, Y.; Jiang, P.; Su, J.; Huang, C. Dual micelles-loaded gelatin nanofibers and their application in lipopolysaccharide-induced periodontal disease. Int. J. Nanomed. 2019, 14, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ho, H.T.; Huynh, N.C.; Dien, V.H.; Vo, T.L. Hyaluronic acid 0.2% application enhanced periodontitis treatment in non-surgical phase. J. Stomatol. 2021, 74, 76–83. [Google Scholar] [CrossRef]
- Mohammad, C.A.; Mirza, B.A.; Mahmood, Z.S.; Zardawi, F.M. The Effect of Hyaluronic Acid Gel on Periodontal Parameters, Pro-Inflammatory Cytokines and Biochemical Markers in Periodontitis Patients. Gels 2023, 9, 325. [Google Scholar] [CrossRef]
- Tabary, N.; Chai, F.; Blanchemain, N.; Neut, C.; Pauchet, L.; Bertini, S.; Delcourt-Debruyne, E.; Hildebrand, H.F.; Martel, B. A chlorhexidine-loaded biodegradable cellulosic device for periodontal pockets treatment. Acta Biomater. 2014, 10, 318–329. [Google Scholar] [CrossRef]
- Fornazier, M.; Gontijo de Melo, P.; Pasquini, D.; Otaguro, H.; Pompêu, G.C.S.; Ruggiero, R. Additives Incorporated in Cellulose Acetate Membranes to Improve Its Performance as a Barrier in Periodontal Treatment. Front. Dent. Med. 2021, 2, 776887. [Google Scholar] [CrossRef]
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.-T.V.; Thomas, S.; Huang, Y.-C.; Kong, Z.-L. Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci. Rep. 2020, 10, 18037. [Google Scholar] [CrossRef]
- Berta, G.N.; Romano, F.; Vallone, R.; Abbadessa, G.; Di Scipio, F.; Defabianis, P. An Innovative Strategy for Oral Biofilm Control in Early Childhood Based on a Resveratrol-Cyclodextrin Nanotechnology Approach. Materials 2021, 14, 3801. [Google Scholar] [CrossRef]
- Lim, Y.R.I.; Preshaw, P.M.; Lim, L.P.; Ong, M.M.A.; Lin, H.-S.; Tan, K.S. Pterostilbene complexed with cyclodextrin exerts antimicrobial and anti-inflammatory effects. Sci. Rep. 2020, 10, 9072. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Warreth, A. Dental Caries and Its Management. Int. J. Dent. 2023, 2023, 9365845. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Xue, M.; Kang, Y.; Liu, D.; Wen, Y.; Zhao, D.; Guan, B. Engineered Biomaterials Trigger Remineralization and Antimicrobial Effects for Dental Caries Restoration. Molecules 2023, 28, 6373. [Google Scholar] [CrossRef] [PubMed]
- O’hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Peng, J.; Jiang, N.; Zhang, W.; Liu, S.; Li, J.; Duan, D.; Li, Y.; Peng, C.; Yan, Y.; et al. Chitosan Phytate Nanoparticles: A Synergistic Strategy for Effective Dental Caries Prevention. ACS Nano 2024, 18, 13528–13537. [Google Scholar] [CrossRef]
- Drago, L.; Del Fabbro, M.; Bortolin, M.; Vassena, C.; De Vecchi, E.; Taschieri, S. Biofilm Removal and Antimicrobial Activity of Two Different Air-Polishing Powders: An In Vitro Study. J. Periodontol. 2014, 85, e363–e369. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Diagnosis and Prevention Strategies for Dental Caries. J. Lifestyle Med. 2013, 3, 107–109. [Google Scholar]
- Samprasit, W.; Kaomongkolgit, R.; Sukma, M.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Mucoadhesive electrospun chitosan-based nanofibre mats for dental caries prevention. Carbohydr. Polym. 2015, 117, 933–940. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Qarabai, F.A.K.; Shahabuddin, F.S.; Al-Shaikh, T.M.; Makharita, R.R. Antibacterial Activity of Ulva/Nanocellulose and Ulva/Ag/Cellulose Nanocomposites and Both Blended with Fluoride against Bacteria Causing Dental Decay. Polymers 2023, 15, 1047. [Google Scholar] [CrossRef]
- Silvestre, A.L.P.; dos Santos, A.M.; de Oliveira, A.B.; Ferrisse, T.M.; Brighenti, F.L.; Meneguin, A.B.; Chorilli, M. Evaluation of photodynamic therapy on nanoparticles and films loaded-nanoparticles based on chitosan/alginate for curcumin delivery in oral biofilms. Int. J. Biol. Macromol. 2023, 240, 124489. [Google Scholar] [CrossRef]
- Estes Bright, L.M.; Garren, M.R.S.; Ashcraft, M.; Kumar, A.; Husain, H.; Brisbois, E.J.; Handa, H. Dual Action Nitric Oxide and Fluoride Ion-Releasing Hydrogels for Combating Dental Caries. ACS Appl. Mater. Interfaces 2022, 14, 21916–21930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, Y.; Zheng, H.; Zhou, Z.; Wu, Z.; Shen, D.; Wang, Y.; Zhang, Y.; Wang, Z.; Fu, B. A Hydroxypropyl Methylcellulose Film Loaded with AFCP Nanoparticles for Inhibiting Formation of Enamel White Spot Lesions. Int. J. Nanomed. 2021, 16, 7623–7637. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; da Rocha, M.G.; Freire, R.S.; Nunes, P.I.G.; Nunes, J.V.S.; Fernandes, M.R.; Pereira-Neto, W.A.; Sidrim, J.J.C.; Santos, F.A.; Rocha, M.F.G.; et al. Chitosan microparticles loaded with essential oils inhibit duo-biofilms of Candida albicans and Streptococcus mutans. J. Appl. Oral Sci. 2023, 31, e20230146. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Padial-Molina, M.; Lopez-Chaichio, L.; Gutiérrez-Garrido, L.; Martín-Morales, N.; O’Valle, F. Algae-derived hydroxyapatite behavior as bone biomaterial in comparison with anorganic bovine bone: A split-mouth clinical, radiological, and histologic randomized study in humans. Clin. Oral Implant. Res. 2020, 31, 536–548. [Google Scholar] [CrossRef]
- Ratnayake, J.T.; Ross, E.D.; Dias, G.J.; Shanafelt, K.M.; Taylor, S.S.; Gould, M.L.; Guan, G.; Cathro, P.R. Preparation, characterisation and in-vitro biocompatibility study of a bone graft developed from waste bovine teeth for bone regeneration. Mater. Today Commun. 2020, 22, 100732. [Google Scholar] [CrossRef]
- Giordano-Kelhoffer, B.; Rodríguez-Gonzalez, R.; Perpiñan-Blasco, M.; Buitrago, J.O.; Bosch, B.M.; Perez, R.A. A Novel Chitosan Composite Biomaterial with Drug Eluting Capacity for Maxillary Bone Regeneration. Materials 2023, 16, 685. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Kuo, P.-J.; Lin, Y.-H.; Lin, C.-Y.; Lin, J.C.-Y.; Chiu, H.-C.; Hung, T.-F.; Lin, H.-Y.; Huang, H.-M. Fabrication of Low-Molecular-Weight Hyaluronic Acid–Carboxymethyl Cellulose Hybrid to Promote Bone Growth in Guided Bone Regeneration Surgery: An Animal Study. Polymers 2022, 14, 3211. [Google Scholar] [CrossRef]
- Koike, T.; Sha, J.; Bai, Y.; Matsuda, Y.; Hideshima, K.; Yamada, T.; Kanno, T. Efficacy of Bacterial Cellulose as a Carrier of BMP-2 for Bone Regeneration in a Rabbit Frontal Sinus Model. Materials 2019, 12, 2489. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningtyas, E.; Hsu, L.-C.; Lan, W.-C.; Wen, S.-C.; Ou, K.-L.; Chou, H.-H.; Huang, M.-S.; Sugiatno, E. Application of a Promising Bone Graft Substitute in Bone Tissue Regeneration: Characterization, Biocompatibility, and In Vivo Animal Study. BioMed Res. Int. 2019, 2019, 1614024. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Pan, Y.H.; Salamanca, E.; Lin, Y.T.; Chang, W.J. Prevention of Bone Resorption by HA/β-TCP + Collagen Composite after Tooth Extraction: A Case Series. Int. J. Environ. Res. Public Health 2019, 16, 4616. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, L.; Cong, M.; Liu, L.; Yan, G.; Li, Z.; Li, B.; Yu, W.; Sun, H.; Yang, B. Injectable thermosensitive chitosan/gelatin-based hydrogel carried erythropoietin to effectively enhance maxillary sinus floor augmentation in vivo. Dent. Mater. 2020, 36, e229–e240. [Google Scholar] [CrossRef]
- Cosola, S.; Di Dino, B.; Traini, T.; Kim, Y.-S.; Park, Y.-M.; Marconcini, S.; Covani, U.; Vinci, R. Radiographic and Histomorphologic Evaluation of the Maxillary Bone after Crestal Mini Sinus Lift Using Absorbable Collagen—Retrospective Evaluation. Dent. J. 2022, 10, 58. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Inchingolo, A.D.; Mancini, A.; Annicchiarico, C.; Inchingolo, F.; Dipalma, G.; et al. Surface Coatings of Dental Implants: A Review. J. Funct. Biomater. 2023, 14, 287. [Google Scholar] [CrossRef]
- Guillaume, B. Dental implants: A review. Morphologie 2016, 100, 189–198. [Google Scholar] [CrossRef]
- Knopf-Marques, H.; Barthes, J.; Lachaal, S.; Mutschler, A.; Muller, C.; Dufour, F.; Rabineau, M.; Courtial, E.-J.; Bystroňová, J.; Marquette, C.; et al. Multifunctional polymeric implant coatings based on gelatin, hyaluronic acid derivative and chain length-controlled poly(arginine). Mater. Sci. Eng. C 2019, 104, 109898. [Google Scholar] [CrossRef]
- Atluri, K.; Banas, J.A.; Chakka, J.; Avila-Ortiz, G.; Elangovan, S.; Salem, A.K. Metronidazole-loaded chitosan coating for dental implants. Emergent Mater. 2024, 7, 1057–1070. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Choi, A.-J.; Park, J.-E.; Jang, Y.-S.; Lee, M.-H. Antibacterial Activity and Biocompatibility with the Concentration of Ginger Fraction in Biodegradable Gelatin Methacryloyl (GelMA) Hydrogel Coating for Medical Implants. Polymers 2022, 14, 5317. [Google Scholar] [CrossRef]
- Lin, M.-H.; Wang, Y.-H.; Kuo, C.-H.; Ou, S.-F.; Huang, P.-Z.; Song, T.-Y.; Chen, Y.-C.; Chen, S.-T.; Wu, C.-H.; Hsueh, Y.-H.; et al. Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, G.; Yang, Z.; Li, X.; Feng, Z.; Zhao, Y. Chitosan/Hyaluronic Acid/MicroRNA-21 Nanoparticle-Coated Smooth Titanium Surfaces Promote the Functionality of Human Gingival Fibroblasts. Int. J. Nanomed. 2022, 17, 3793–3807. [Google Scholar] [CrossRef] [PubMed]
- Erturk, P.A.; Altuntas, S.; Irmak, G.; Buyukserin, F. Bioinspired Collagen/Gelatin Nanopillared Films as a Potential Implant Coating Material. ACS Appl. Bio Mater. 2022, 5, 4913–4921. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dizaj, S.; Torab, A.; Kouhkani, S.; Sharifi, S.; Negahdari, R.; Bohlouli, S.; Fattahi, S.; Salatin, S. Gelatin–Curcumin Nanocomposites as a Coating for Implant Healing Abutment: In Vitro Stability Investigation. Clin. Pract. 2023, 13, 88–101. [Google Scholar] [CrossRef]



| Biomaterial | Main Application | Clinical Effectiveness | Limitations | Cost-Effectiveness | Level of Available Evidence |
|---|---|---|---|---|---|
| Alginate | Dental impressions, wound dressings, drug delivery, tissue engineering | Good for dental impressions; wound dressings; tissue engineering scaffolds | Poor mechanical properties; needs crosslinking for load-bearing applications | High (low cost, widely available) | High (well-established use in dentistry and wound healing) |
| Cellulose | Wound dressings, tissue engineering scaffolds, bone repair, cartilage regeneration | Good scaffold material; used for wound dressings and cartilage/bone engineering | Lacks antibacterial properties; needs modification | High (abundant natural sources, cheap bacterial production) | High (widely researched in tissue engineering) |
| Chitosan | Wound healing, drug delivery, tissue engineering, antimicrobial applications | Antibacterial, antifungal; hemostatic properties; promising for drug delivery | Variable antimicrobial effects depending on molecular weight; solubility limitations | Moderate (cost depends on source and degree of deacetylation) | High (extensive biomedical research, though some variability in results) |
| Collagen | Tissue scaffolds, joint disease treatments, skin regeneration, hemostatic agents | Excellent biocompatibility; widely used in regenerative medicine and surgery | Risk of immunogenicity if not purified; variable degradation rates | From moderate to high (costly extraction and purification) | Very high (extensive clinical use, FDA-approved products) |
| Cyclodextrins | Drug delivery systems, pharmaceutical formulations, enhancing drug bioavailability | Effective drug carriers; improve solubility and stability of active compounds | Limited mechanical applications; mainly chemical carriers | Moderate (depends on derivative used) | High (several cyclodextrin-based drugs approved) |
| Gelatin | Wound healing, drug delivery, tissue regeneration, hemostatic preparations | Good for wound dressings, hemostats, drug delivery; promotes cell adhesion | Poor mechanical properties; stability issues; toxicity risk if chemically crosslinked | High (inexpensive, especially bovine/porcine sources) | High (extensively studied and used clinically) |
| Hyaluronic Acid | Joint treatments, wound healing, cosmetics, tissue regeneration | Excellent tissue regeneration properties; used in dermatology and joint injections | Rapid degradation; biological effects vary with molecular weight | From moderate to high (depends on molecular weight and source) | Very high (extensively used in orthopedics, dermatology, dentistry) |
| Hydroxyapatite | Bone repair, dental applications, bone substitutes, drug carriers | Promotes bone regeneration; osteoconductive; widely used in bone implants | Brittle; lacks antimicrobial properties; requires composites for enhanced properties | Moderate (natural and synthetic sources available) | Very high (long history of clinical use in orthopedics and dentistry) |
| Silk | Drug delivery (cancer therapy), tissue scaffolds, sutures, biomedical engineering | Biocompatible scaffolds; slow degradation; promising for advanced drug delivery | No inherent antibacterial activity; suture biofilm formation | Moderate (depends on production method—traditional or recombinant) | High (growing clinical research; some FDA-cleared products like sutures) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczkowska-Walendowska, M.; Kulawik, M.; Kwiatek, J.; Bikiaris, D.; Cielecka-Piontek, J. Novel Applications of Natural Biomaterials in Dentistry—Properties, Uses, and Development Perspectives. Materials 2025, 18, 2124. https://doi.org/10.3390/ma18092124
Paczkowska-Walendowska M, Kulawik M, Kwiatek J, Bikiaris D, Cielecka-Piontek J. Novel Applications of Natural Biomaterials in Dentistry—Properties, Uses, and Development Perspectives. Materials. 2025; 18(9):2124. https://doi.org/10.3390/ma18092124
Chicago/Turabian StylePaczkowska-Walendowska, Magdalena, Maciej Kulawik, Jakub Kwiatek, Dimitrios Bikiaris, and Judyta Cielecka-Piontek. 2025. "Novel Applications of Natural Biomaterials in Dentistry—Properties, Uses, and Development Perspectives" Materials 18, no. 9: 2124. https://doi.org/10.3390/ma18092124
APA StylePaczkowska-Walendowska, M., Kulawik, M., Kwiatek, J., Bikiaris, D., & Cielecka-Piontek, J. (2025). Novel Applications of Natural Biomaterials in Dentistry—Properties, Uses, and Development Perspectives. Materials, 18(9), 2124. https://doi.org/10.3390/ma18092124











