Effect of Annealing Temperature on the Photoluminescence Blue Shift of Nitrogen-Doped Graphene Quantum Dots
Abstract
1. Introduction
2. Experimental
2.1. Materials
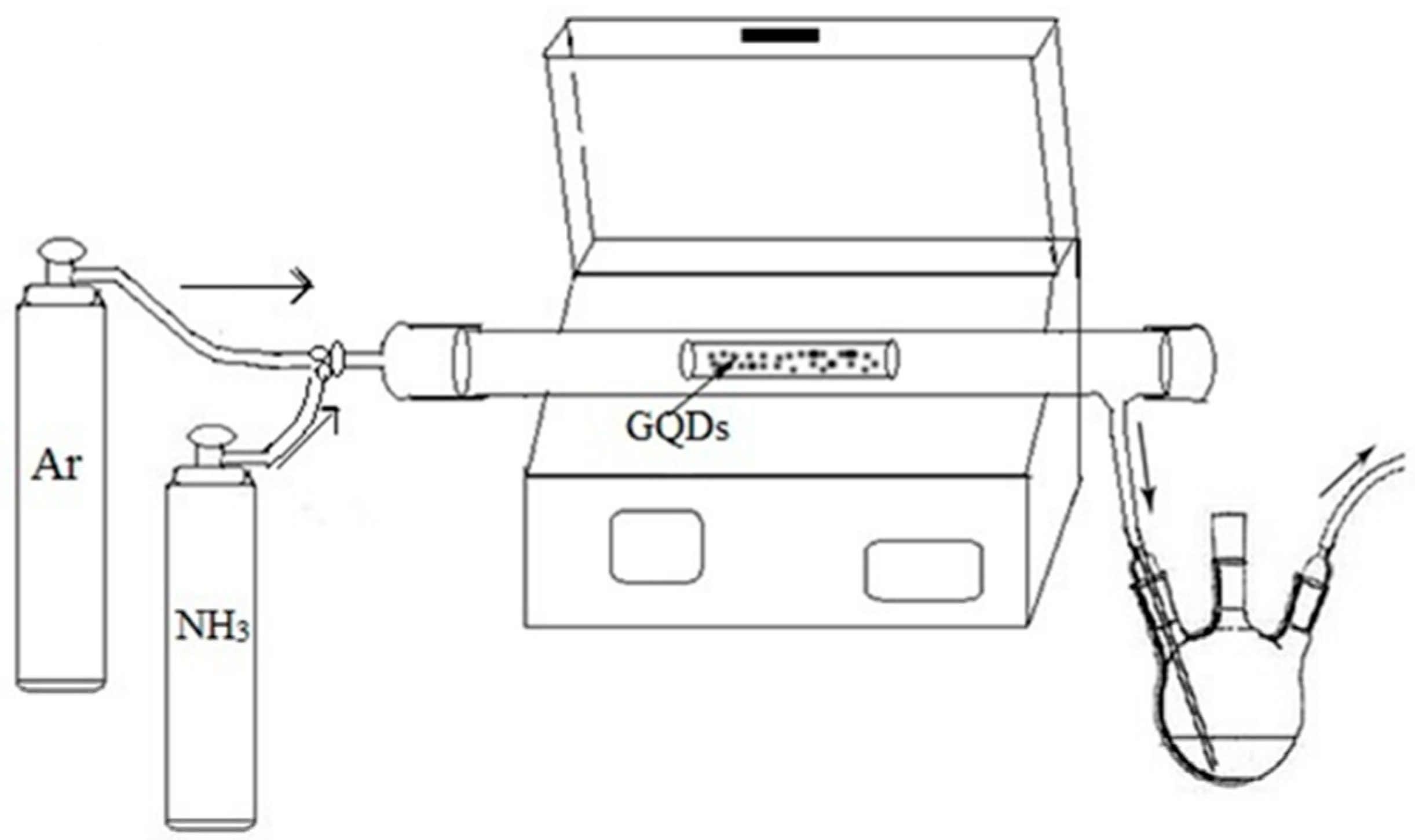
2.2. Production of NGQDs
2.3. Characterizations
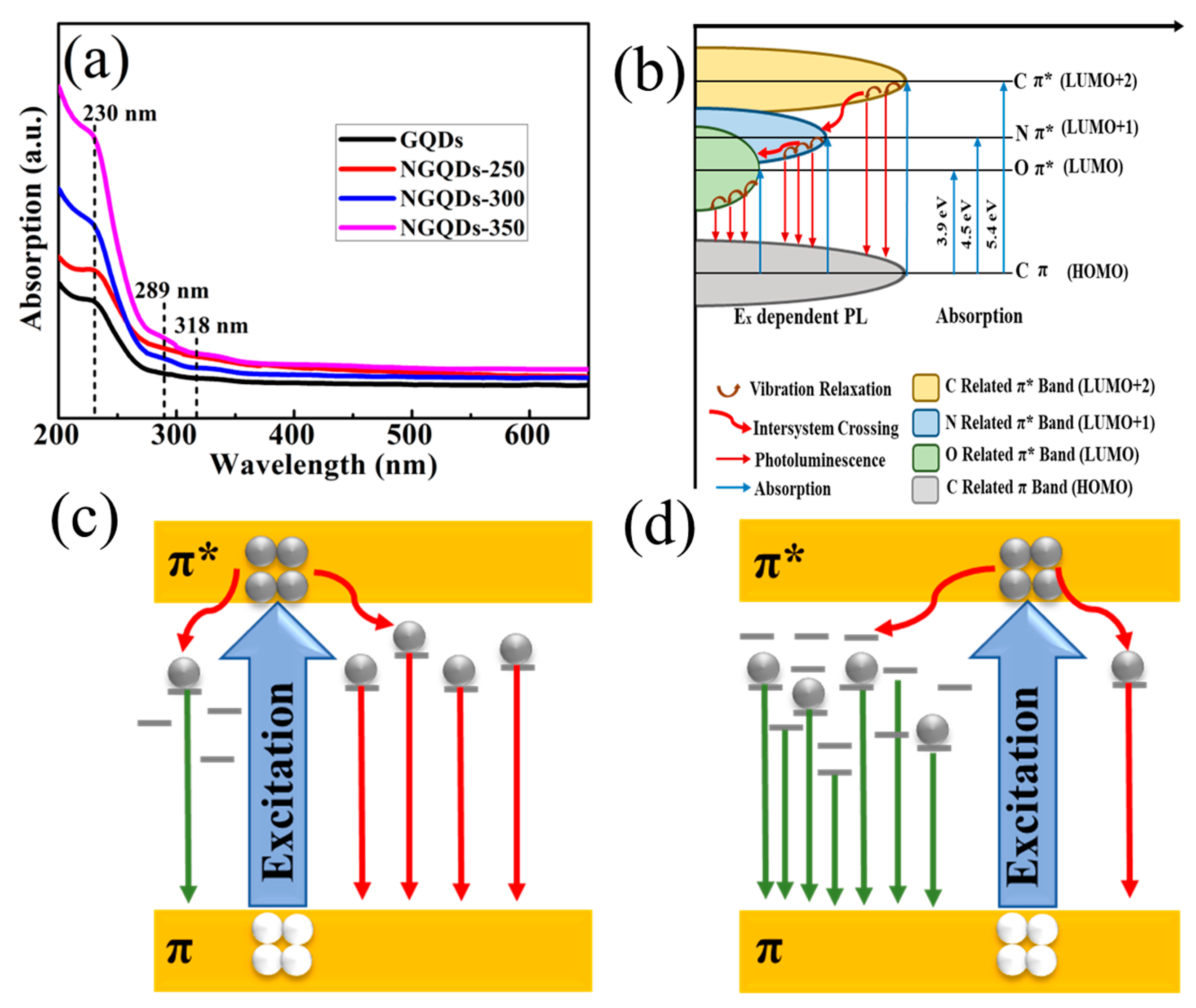
3. Results and Discussion
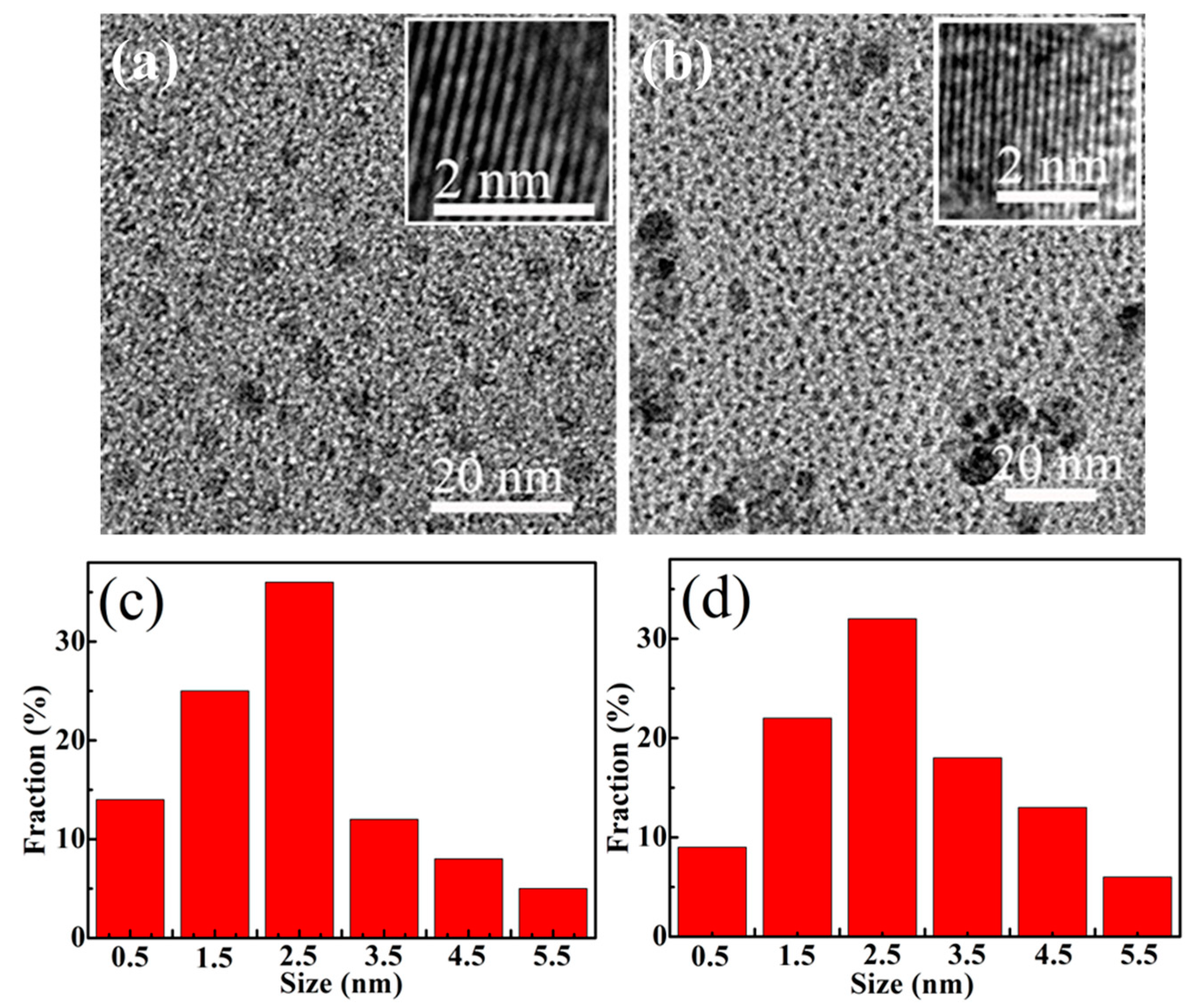
3.1. Microstructure Investigations
3.2. FTIR and Raman Investigations
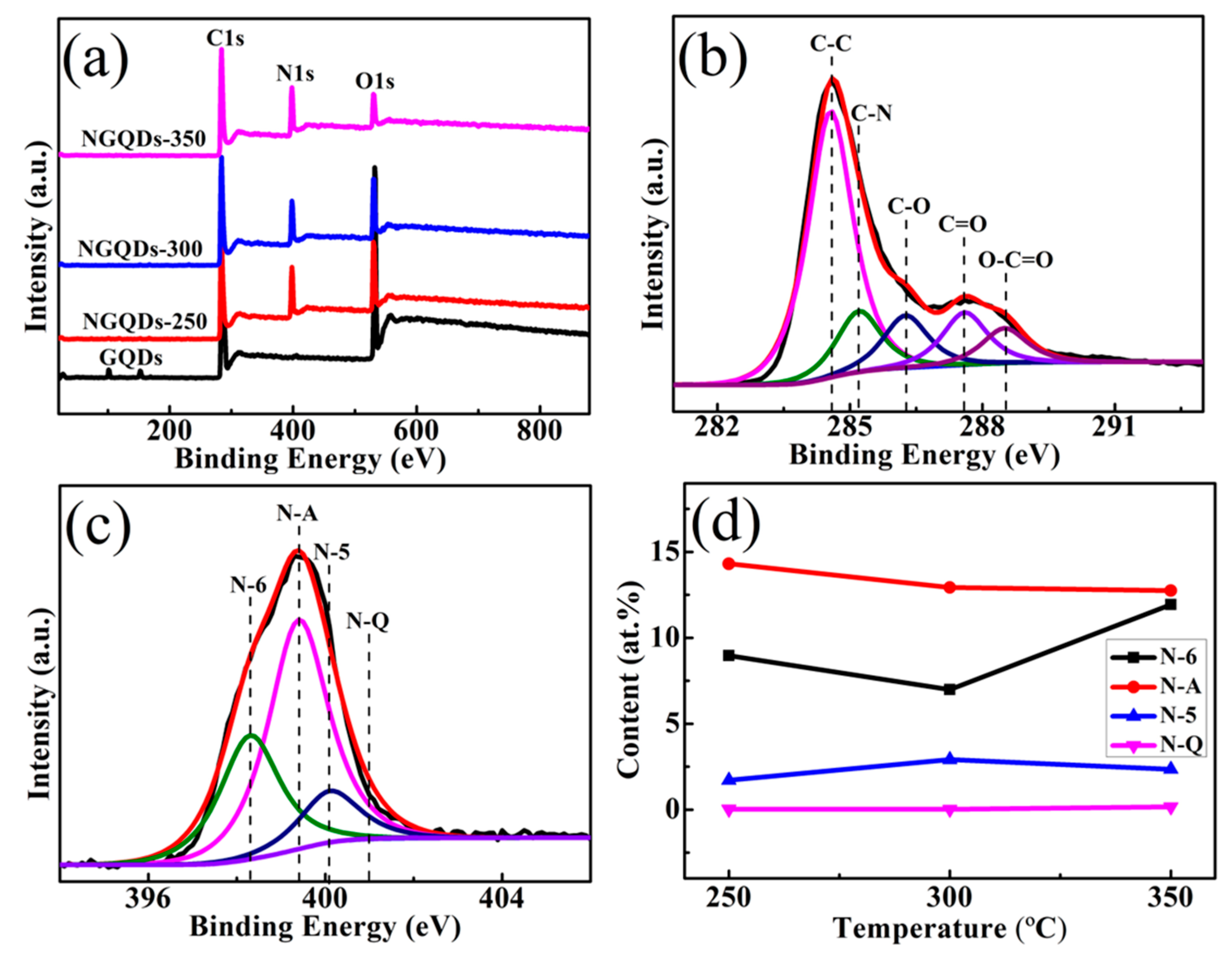
3.3. XPS Investigations
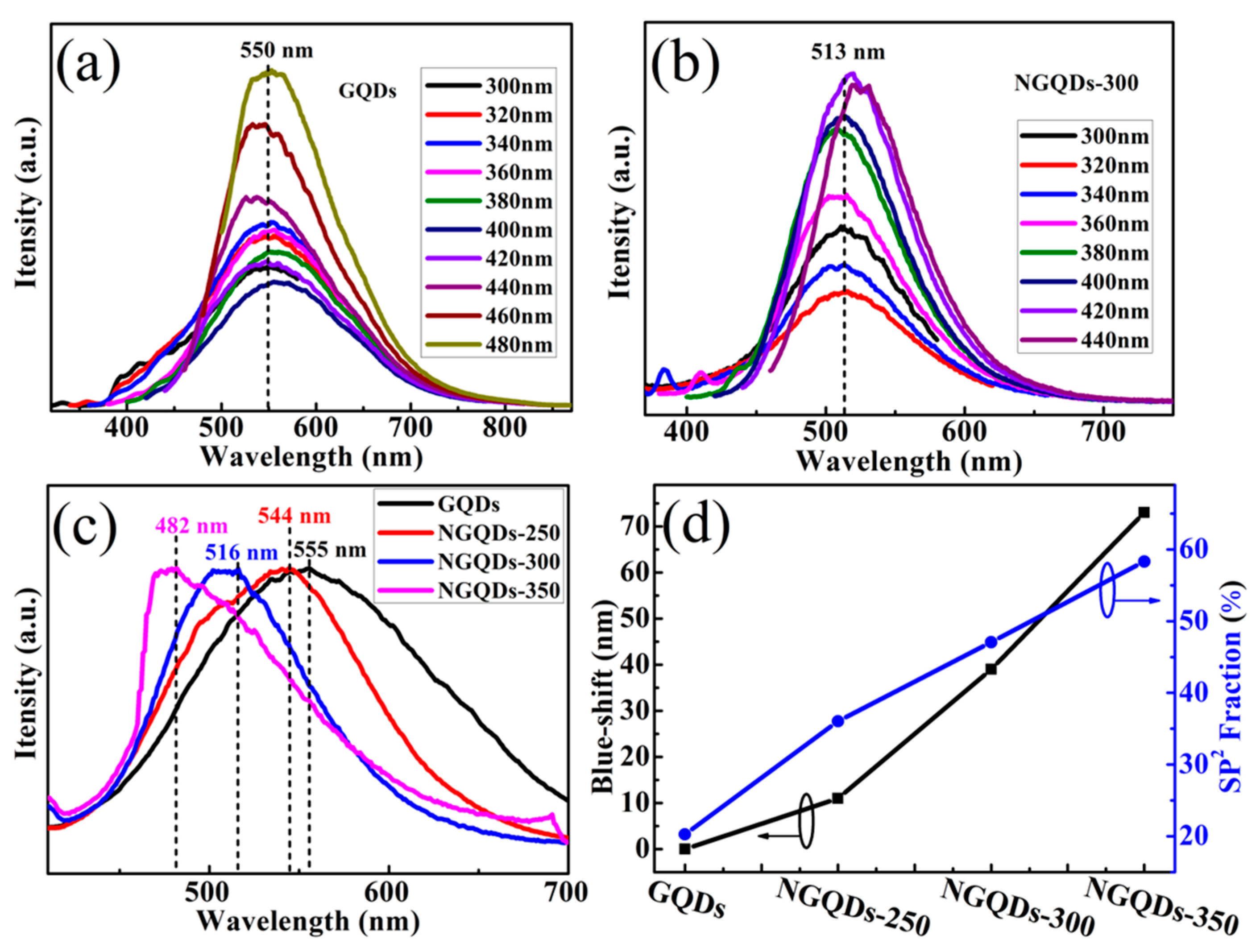
3.4. Optical and Photoelectrochemical Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, J.H.; Zhu, Y.H.; Yang, X.L.; Li, C.Z. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, S.W.; Kim, M.K.; Shin, D.Y.; Shin, D.H.; Kim, C.O.; Yang, S.B.; Park, J.H.; Hwang, E.; Choi, S.H.; et al. Anomalous Behaviors of Visible Luminescence from Graphene Quantum Dots: Interplay between Size and Shape. ACS Nano 2012, 6, 8203–8208. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, C.; Liu, Y.; Yang, D.; Zhang, Y.; Ren, Z.; Li, Q.; Hao, J.; Hu, W.; Wu, Y.; et al. Constructing Heterogeneous Photocatalysts Based on Carbon Nitride Nanosheets and Graphene Quantum Dots for Highly Efficient Photocatalytic Hydrogen Generation. Materials 2022, 15, 5390. [Google Scholar] [CrossRef]
- Thivyah, B.; Lun, A.W.; Ebrahim, M. Enhancing photoluminescence stability of blue emissive nitrogen doped graphene quantum dots by encapsulating inside zeolitic imidazole framework-8. J. Solid State Chem. 2023, 328, 124343. [Google Scholar]
- Shalini, R.B.; Sruthi, R.; Sunaja, D.K.R.; Dephan, P.; Resnik, J.U.J. N-doped graphene quantum dots incorporated cobalt ferrite/graphitic carbon nitride ternary composite for electrochemical overall water splitting. Int. J. Hydrogen Energy 2023, 48, 2906–2919. [Google Scholar]
- Singh, R.; Yadav, R.K.; Shukla, R.K.; Singh, S.; Singh, A.P.; Dwivedi, D.K.; Umar, A.; Gupta, N.K. Highly Selective Nitrogen-Doped Graphene Quantum Dots/Eriochrome Cyanine Composite Photocatalyst for NADH Regeneration and Coupling of Benzylamine in Aerobic Condition under Solar Light. Catalysts 2023, 13, 199. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, W.; Zhang, M.; Qiu, G.; Zhang, Y.; Luo, T.; Kong, C. Facile synthesis of nitrogen-doped graphene quantum dots as nanocarbon emitters for sensitive detection of catechol. RSC Adv. 2022, 12, 25778. [Google Scholar] [CrossRef] [PubMed]
- Minchul, A.; Jaekwang, S.; Hee, H.B. Facile Synthesis of N-Doped Graphene Quantum Dots as Novel Transfection Agents for mRNA and pDNA. Nanomaterials 2021, 11, 2816. [Google Scholar] [CrossRef]
- Chen, T.; Zhong, L.; Yang, Z.; Mou, Z.; Liu, L.; Wang, Y.; Sun, J.; Lei, W. Enhanced Visible-light Photocatalytic Activity of g-C3N4 /Nitrogen-doped Graphene Quantum Dots/TiO2 Ternary Heterojunctions for Ciprofloxacin Degradation with Narrow Band Gap and High Charge Carrier Mobility. Chem. Res. Chin. Univ. 2020, 36, 1083–1090. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Dai, L.; Li, L.S. Nitrogen-Doped Colloidal Graphene Quantum Dots and Their Size-Dependent Electrocatalytic Activity for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 18932–18935. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; Ma, Y.; Qin, H.; Liu, J.; Lv, F.; Zhu, C.; Wang, L.; Long, L.; Liu, F.; et al. Effects of pyridine-like and pyrrolic-like nitrogen on the photoluminescence blue-shift of nitrogen-doped graphene oxide quantum dots. J. Lumin. 2021, 235, 117983. [Google Scholar] [CrossRef]
- Hu, C.F.; Liu, Y.L.; Yang, Y.H.; Cui, J.H.; Huang, Z.R.; Wang, Y.L.; Yang, L.F.; Wang, H.B.; Xiao, Y.; Rong, J.H. One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J. Mater. Chem. B 2013, 1, 39–42. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Xia, T.; Ma, Q. A novel electrochemiluminescence sensor for the detection of nitroaniline based on the nitrogen-doped graphene quantum dots. Biosens. Bioelectron. 2016, 85, 903–908. [Google Scholar] [CrossRef]
- Achadu, O.J.; Nyokong, T. Interaction of Graphene Quantum Dots with 4-Acetamido-2,2,6,6-Tetramethylpiperidine-Oxyl Free Radicals: A Spectroscopic and Fluorimetric Study. J. Fluoresc. 2016, 26, 283–295. [Google Scholar] [CrossRef]
- Wang, W.; He, D.; Duan, J.; Fu, M.; Zhang, X.; Wu, H.; Hu, Y.; Wang, Y. Modulated photoluminescence of graphene quantum dots in the vicinity of an individual silver nano-octahedron. Phys. Chem. Chem. Phys. 2014, 16, 4504–4509. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Y.; Zheng, Y.; Tang, N.; Li, M.; Zhong, W.; Du, Y. Gram-scale synthesis of high-purity graphene quantum dots with multicolor photoluminescence. RSC Adv. 2015, 5, 103428–103432. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Lu, W.; Li, Y.; Li, R.; Shuang, S.; Dong, C.; Cai, Z. Facile Synthesis of N-Doped Carbon Dots as a New Matrix for Detection of Hydroxy-Polycyclic Aromatic Hydrocarbons by Negative-Ion Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. ACS Appl. Mater. Interfaces 2016, 8, 12976–12984. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.Y.; Zhang, Z.Y. Synthesis and surface photochemistry of graphitized carbon quantum dots. J. Colloid. Interface Sci. 2011, 356, 416–421. [Google Scholar] [CrossRef]
- Halder, A.; Zhang, M.; Chi, Q. Electroactive and biocompatible functionalization of graphene for the development of biosensing platforms. Biosens. Bioelectron. 2017, 87, 764–771. [Google Scholar] [CrossRef]
- Gupta, A.; Saha, S.K. Emerging photoluminescence in azo-pyridine intercalated graphene oxide layers. Nanoscale 2012, 4, 6562–6567. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, C.Y.; Chiu, P.W. Controllable graphene N-doping with ammonia plasma. Appl. Phys. Lett. 2010, 96, 133110. [Google Scholar] [CrossRef]
- Inbanathan, F.P.N.; Cimatu, K.L.A.; Ingram, D.C.; Erasquin, U.J.; Dasari, K.; Sultan, M.S.; Sajjad, M.; Makarov, V.; Weiner, B.R.; Morell, G.; et al. Paramagnetism in Microwave-Synthesized Metal-Free Nitrogen-Doped Graphene Quantum Dots. Materials 2023, 16, 3410. [Google Scholar] [CrossRef]
- Liu, D.; Kim, S.; Choi, W.M. Facile Synthesis of Nitrogen-Doped Graphene Quantum Dots/MnCO3/ZnMn2O4 on Ni Foam Composites for High-Performance Supercapacitor Electrodes. Materials 2024, 17, 884. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Afeworki, M.; Gorbaty, M.L.; Kwiatek, P.J.; Solum, M.S.; Hu, J.Z.; Pugmire, R.J. XPS and 15N NMR NMR study of nitrogen forms in carbonaceous solids. Energy Fuels 2002, 16, 1507–1515. [Google Scholar] [CrossRef]
- Wang, X.B.; Liu, L.Q.; Zhu, D.B.; Zhang, L.; Ma, H.Z.; Yao, N.; Zhang, B.L. Controllable growth, structure, and low field emission of well-aligned CNx nanotubes. J. Phys. Chem. B 2002, 106, 2186–2190. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Tetsuka, H.; Asahi, R.; Nagoya, A.; Okamoto, K.; Tajima, I.; Ohta, R.; Okamoto, A. Optically Tunable Amino-Functionalized Graphene Quantum Dots. Adv. Mater. 2012, 24, 5333–5338. [Google Scholar] [CrossRef]
- Jin, S.H.; Kim, D.H.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the Photoluminescence of Graphene Quantum Dots through the Charge Transfer Effect of Functional Groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef]
- Ritter, K.A.; Lyding, J.W. The influence of edge structure on the electronic properties of graphene quantum dots and nanoribbons. Nat. Mater. 2009, 8, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yeo, P.S.E.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C60 molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, G.; Liu, J.; Sun, X. Evaluation Criteria for Reduced Graphene Oxide. J. Phys. Chem. C 2011, 115, 11327–11335. [Google Scholar] [CrossRef]
- Liu, F.; Tang, T.; Feng, Q.; Li, M.; Liu, Y.; Tang, N.; Zhong, W.; Du, Y. Tuning photoluminescence of reduced graphene oxide quantum dots from blue to purple. J. Appl. Phys. 2014, 115, 164307. [Google Scholar] [CrossRef]
- Usachov, D.; Vilkov, O.; Gruneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.K.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; et al. Nitrogen-doped graphene: Efficient growth, structure, and electronic properties. Nano Lett. 2011, 11, 5401–5407. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, X.; Bourgeois, L.; Guan, H.; Chen, S.; Zhong, Y.; Tang, D.M.; Li, H.; Zhai, T.; Li, L.; et al. N-Doped Graphene-SnO2 Sandwich Paper for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2012, 22, 2682–2690. [Google Scholar] [CrossRef]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Energy-level structure of nitrogen-doped graphene quantum dots. J. Mater. Chem. C 2013, 1, 4908–4915. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, D.; Feng, Y.; Yang, D.; Wu, S.; Jiang, H.; Wang, D.; Bi, S. Study on the Synthesis and Electrochemical Properties of Nitrogen-Doped Graphene Quantum Dots. Materials 2024, 17, 6163. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, H.; Ou, M.; Sun, D.; Yang, C. Luminescence and magnetic properties of bifunctional nanoparticles composited by nitrogen-doped graphene quantum dots and gadolinium. J. Rare Earths 2024, 42, 716–723. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Liu, L.; Bao, R.; Yi, J.H.; Guo, S.D. Attaining synergetic equilibrium of electrical conductivity and tensile strength in GQDs@GN/Cu composites through multi-scale intragranular and intergranular reinforcements. Rare Met. 2024, 43, 366–379. [Google Scholar] [CrossRef]
- Huang, X.; He, Z.-L.; Chen, Y.; Li, L.; Yang, Z.; Zhai, C.; Zhu, M. Novel P-doping-tuned Pd nanoflowers/S,N-GQDs photo-electrocatalyst for high-efficient ethylene glycol oxidation. Chin. Chem. Lett. 2024, 35, 567–571. [Google Scholar] [CrossRef]
- Yan, J.A.; Xian, L.; Chou, M.Y. Structural and Electronic Properties of Oxidized Graphene. Phys. Rev. Lett. 2009, 103, 086802. [Google Scholar] [CrossRef]
- Eda, G.; Lin, Y.Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.A.; Chen, I.S.; Chen, C.W.; Chhowalla, M. Blue Photoluminescence from Chemically Derived Graphene Oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.T.; Li, S.S.; Lai, W.J.; Yeh, Y.C.; Chen, H.A.; Chen, I.S.; Chen, L.C.; Chen, K.H.; Nemoto, T.; Isoda, S.; et al. Tunable photoluminescence from graphene oxide. Angew. Chem. Int. Ed. 2012, 51, 6662–6666. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Guo, J.; Han, L.; Fan, H.; Tong, F. Effect of Annealing Temperature on the Photoluminescence Blue Shift of Nitrogen-Doped Graphene Quantum Dots. Materials 2025, 18, 2094. https://doi.org/10.3390/ma18092094
Xu X, Guo J, Han L, Fan H, Tong F. Effect of Annealing Temperature on the Photoluminescence Blue Shift of Nitrogen-Doped Graphene Quantum Dots. Materials. 2025; 18(9):2094. https://doi.org/10.3390/ma18092094
Chicago/Turabian StyleXu, Xiaofen, Jun Guo, Lu Han, Huimin Fan, and Fei Tong. 2025. "Effect of Annealing Temperature on the Photoluminescence Blue Shift of Nitrogen-Doped Graphene Quantum Dots" Materials 18, no. 9: 2094. https://doi.org/10.3390/ma18092094
APA StyleXu, X., Guo, J., Han, L., Fan, H., & Tong, F. (2025). Effect of Annealing Temperature on the Photoluminescence Blue Shift of Nitrogen-Doped Graphene Quantum Dots. Materials, 18(9), 2094. https://doi.org/10.3390/ma18092094




