Using XRD to Assess the Strength of Fly-Ash- and Metakaolin-Based Geopolymers
Abstract
1. Introduction
2. Experimental Details
- Eva was used to model and separate the background data (including amorphous humps), thus removing crystalline peaks, and exported to TOPAS.
- In TOPAS, the precursor amorphous hump was fitted with a custom peak using a Split Pseudo-Voigt (SPV) peak profile with only the position and full width at half maximum parameters set to refine, and this centroid value was recorded.
- The geopolymer XRD pattern was loaded into TOPAS, and two SPV peaks were fitted, one fixed at the previously recorded precursor peak position and the other refined for the geopolymer peak position.
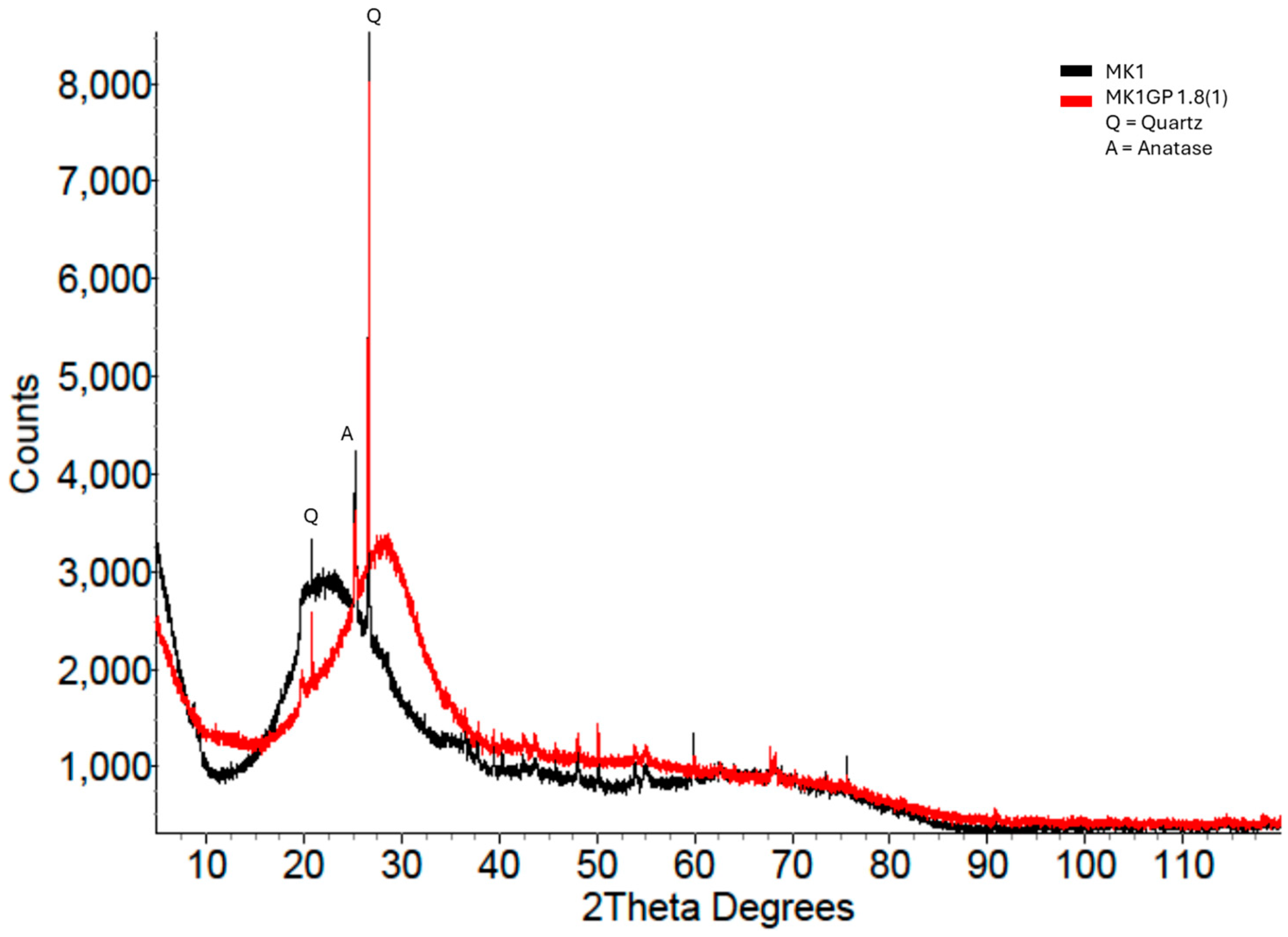
- The peak shift was calculated as the difference between the geopolymer peak position and the precursor peak position (Figure 1).
3. Results and Discussion
3.1. Precursors
3.1.1. Scanning Electron Microscopy
3.1.2. Particle Size Distribution
3.1.3. X-Ray Diffraction
3.2. Geopolymers
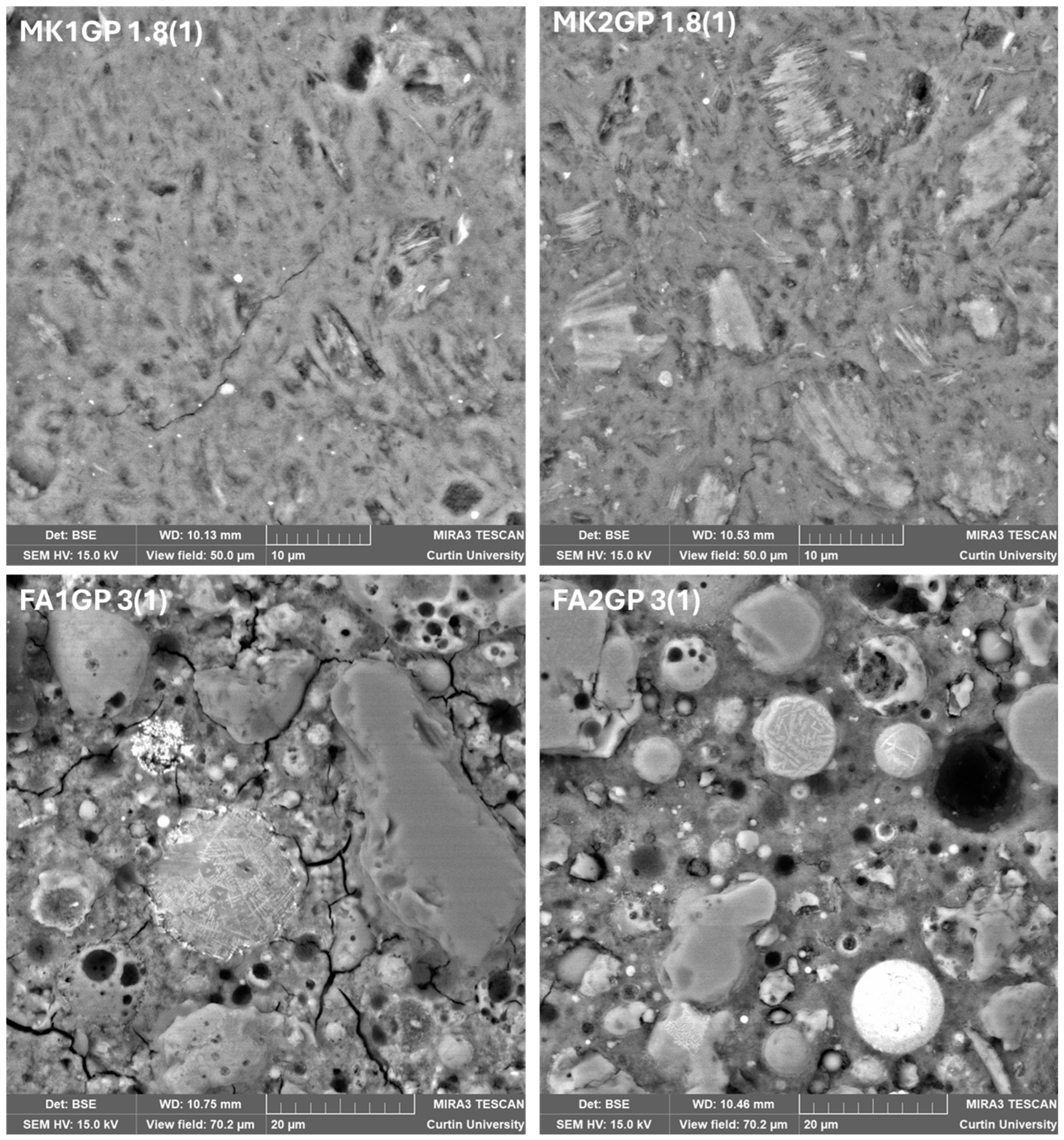
3.2.1. Scanning Electron Microscopy
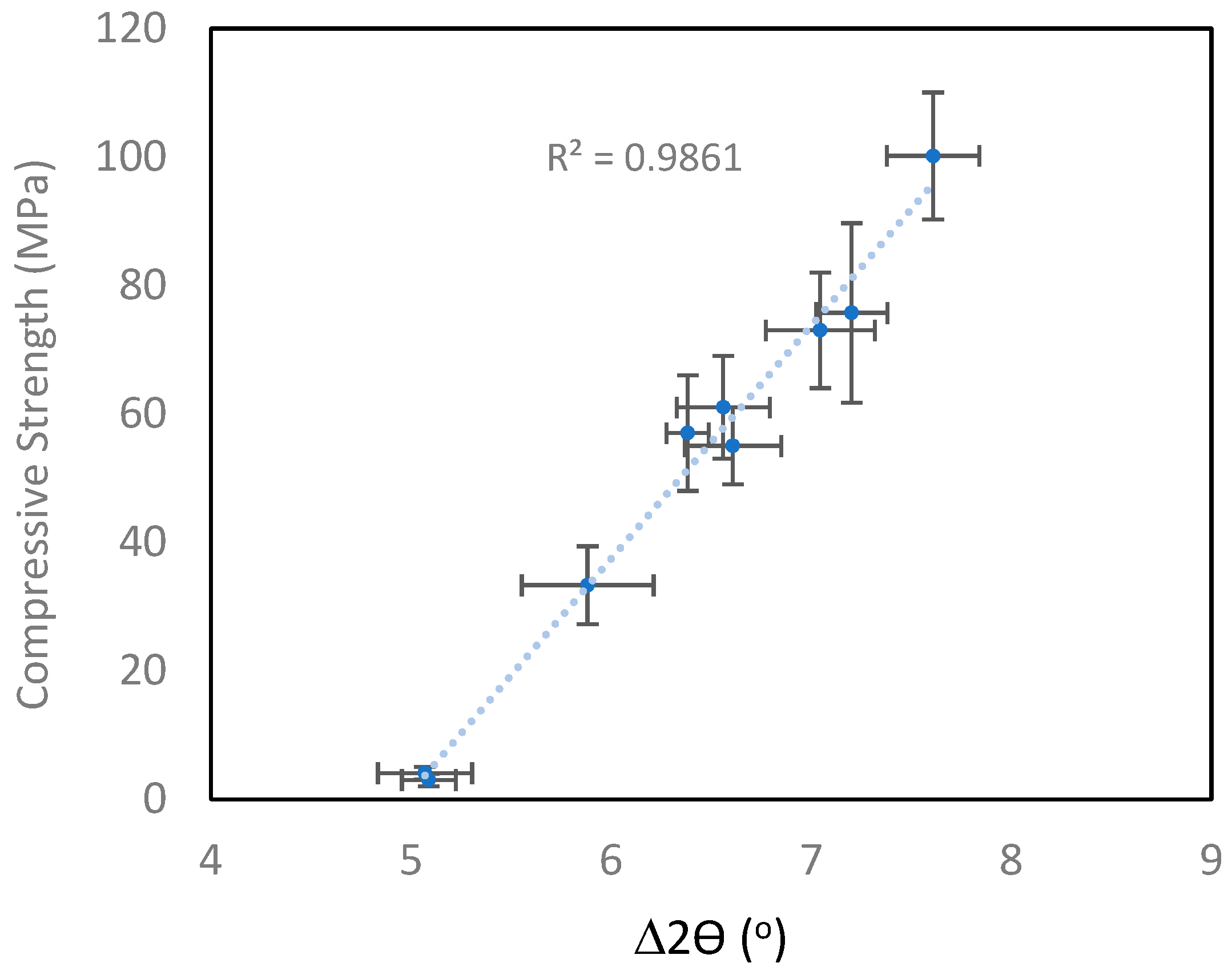
3.2.2. X-Ray Diffraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. Aust. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Jamieson, E.; McLellan, B.; van Riessen, A.; Nikraz, H. Comparison of embodied energies of Ordinary Portland Cement with Bayer-derived Geopolymer products. J. Clean. Prod. 2015, 99, 112–118. [Google Scholar] [CrossRef]
- Xiao, Q.; Tang, Z.; Xiang, Y.; Cai, Y.; Wang, J.; Xu, W.; Long, G. Early age reaction, rheological properties, and environment impact of NaOH-activated fly ash mixtures at ambient environment. J. Build. Eng. 2024, 88, 109145. [Google Scholar] [CrossRef]
- Cong, P.; Du, R.; Gao, H.; Chen, Z. Comparison and assessment of carbon dioxide emissions between alkali-activated materials and OPC cement concrete. J. Traffic Transp. Eng. 2024, 11, 918–938. [Google Scholar] [CrossRef]
- Temuujin, J.; Lee, M.; Chen-Tan, N.; van Riessen, A. Characterisation of class F fly ash geopolymer pastes immersed in acid and alkaline solutions. Cem. Concr. Compos. 2011, 33, 1086–1091. [Google Scholar] [CrossRef]
- van Riessen, A.; Chen-Tan, N. Beneficiation of Collie Fly Ash for Synthesis of Geopolymer—Part 2—Geopolymers. Fuel 2013, 111, 829–835. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Scherb, S.; Köberl, M.; Beuntner, N.; Thienel, K.-C.; Neubauer, J. Reactivity of Metakaolin in Alkaline Environment: Correlation of Results from Dissolution Experiments with XRD Quantifications. Materials 2020, 13, 2214. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S. Review on characteristics of metakaolin-based geopolymer and fast setting. J. Korean Ceram. Soc. 2020, 57, 368–377. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers based on natural and synthetic metakaolin—A critical review. In Proceedings of the 41st International Conference on Advanced Ceramics and Composites: Ceramic Engineering and Science Proceedings, Daytona Beach, FA, USA, 22–27 January 2017; Volume 38. [Google Scholar]
- Longhi, M.; Walkley, B.; Rodriguez, E.; Kirchheim, A.; Zhang, Z.; Wang, H. New selective dissolution process to quantify reaction extent and product stability in metakaolin-based geopolymers. Compos. Part B Eng. 2019, 176, 107172. [Google Scholar] [CrossRef]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Kunzel, C.; Vandeperre, L.; Donatello, S.; Boccaccini, A.; Cheeseman, C. Ambient temperature drying shrinkage and cracking in metakaolin-based geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, Z.; Tian, X.; Wu, J.; Wang, L. Review on the impact of metakaolin-based geopolymer’s reaction chemistry, nanostructure and factors on its properties. Constr. Build. Mater. 2024, 412, 134760. [Google Scholar] [CrossRef]
- van Riessen, A.; Chen-Tan, N. Beneficiation of Collie Fly Ash for Synthesis of Geopolymer Part 1—Beneficiation. Fuel 2013, 106, 569–575. [Google Scholar] [CrossRef]
- Oh, J.E.; Jun, Y.; Jeong, Y. Characterization of geopolymers from compositionally and physically different Class F fly ashes. Cem. Concr. Compos. 2014, 60, 16–26. [Google Scholar] [CrossRef]
- Hidayati, R.; Anindika, G.R.; Faradila, F.; Pamungkas, C.; Hidayati, I.; Prasetyoko, D.; Fansuri, H. Geopolymerization of fly-ashes from 6 Indonesian power plants using a standardised recipes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012017. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. Fly ash-based geopolymer concrete. Aust. J. Struct. Eng. 2005, 6, 2204–2261. [Google Scholar] [CrossRef]
- Kuenzel, C.; Ranjbar, N. Dissolution mechanism of fly ash to quantify the reactive aluminosilicates in geopolymerisation. Resour. Conserv. Recycl. 2019, 150, 104421. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Singh, N.B. Fly Ash-Based Geopolymer Binder: A Future Construction Material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- Murri, A.N.; Rickard, W.D.A.; Bignozzi, M.C.; van Riessen, A. High temperature behaviour of ambient cured alkali-activated materials based on ladle slag. Cem. Concr. Res. 2013, 43, 51–61. [Google Scholar] [CrossRef]
- Segura, P.I.; Luukkonen, T.; Yliniemi, J.; Sreenivasan, H.; Damø, A.J.; Jensen, L.S.; Canut, M.; Kantola, A.M.; Telkki, V.-V.; Jensen, P.A. Comparison of one-part and two-part alkali-activated metakaolin and blast furnace slag. J. Sustain. Metall. 2022, 8, 1816–1830. [Google Scholar] [CrossRef]
- Lau, C.; Rowles, M.; Parnham, G.; Htut, T.; Ng, T.S. Investigation of geopolymers containing fly ash and ground-granulated blast-furnace slag blended by amorphous ratios. Constr. Build. Mater. 2019, 222, 731–737. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 52, 32–39. [Google Scholar] [CrossRef]
- Gökçe, H.S. Durability of slag-based alkali-activated materials: A critical review. J. Aust. Ceram. Soc. 2024, 60, 885–903. [Google Scholar] [CrossRef]
- Furtos, G.; Prodan, D.; Sarosi, C.; Popa, D.; Moldovan, M.; Korniejenko, K. The Precursors Used for Developing Geopolymer Composites for Circular Economy—A Review. Materials 2024, 17, 1696. [Google Scholar] [CrossRef]
- Ferrara, P.L.; La Noce, M.; Sciuto, G. Sustainability of Green Building Materials: A Scientometric Review of Geopolymers from a Circular Economy Perspective. Sustainability 2023, 15, 16047. [Google Scholar] [CrossRef]
- Williams, R.; Hart, R.; van Riessen, A. Quantification of the extent of reaction of metakaolin based geopolymers using XRD, SEM and EDS. J. Am. Ceram. Soc. 2011, 94, 2663–2670. [Google Scholar] [CrossRef]
- Heidrich, C.; Ward, C.; Chalmers, D.; Heeley, P.; Ness, J.; Williams, R. Production and Handling of Coal Combustion Products—Chapter 1. In Coal Combustion Handbook, 2nd ed.; Ward, C., Heidrich, C., Yeatman, O., Eds.; Ash Development Association of Australia: Wollongong, Australia, 2014. [Google Scholar]
- van Riessen, A.; Rickard, W.D.A.; Williams, R.P.; van Riessen, G.A. Methods for Geopolymer Formulation Development and Microstructural Analysis. J. Ceram. Sci. Technol. 2017, 8, 421–431. [Google Scholar]
- Davidovits, J. Geopolymers—Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer—Chemistry and Applications; Institut Géopolymère: Saint-Quentin, France, 2008; pp. 61–63. [Google Scholar]
- Subaer. Influence of Aggregate on the Microstructure of Geopolymer. Ph.D. Thesis, Curtin University, Perth, Australia, 2004. [Google Scholar]
- Chen-Tan, N. Geopolymer from a Western Australian Fly Ash. Ph.D. Thesis, Curtin University, Perth, Australia, 2010. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodriguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- Weise, K.; Ukrainczyk, N.; Koenders, E. Pozzolanic reactions of metakaolin with calcium hydroxide: Review on hydrate phase formations and effect of alkali hydroxides, carbonates and sulfates. Mater. Des. 2023, 231, 112062. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Chen, Y.; Dolado, J.S.; Li, Z.; Yin, S.; Yu, Q.; Kostiuchenko, A.; Ye, G. A molecular dynamics study of N–A–S–H gel with various Si/Al ratios. J. Am. Ceram. Soc. 2022, 105, 6462–6474. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Pławecka, K.; Bazan, P.; Lin, W.-T.; Korniejenko, K.; Sitarz, M.; Nykiel, M. Development of Geopolymers Based on Fly Ashes from Different Combustion Processes. Polymers 2022, 14, 1954. [Google Scholar] [CrossRef]
- Bundy, W.M.; Ishley, J.N. Kaolin in paper filling and coating. Appl. Clay Sci. 1991, 5, 397–420. [Google Scholar] [CrossRef]
- Lee, S.; Moon, H.-S. Phase Transformation Sequence from Kaolinite to Mullite Investigated by an Energy-Filtering TEM. J. Am. Ceram. Soc. 1999, 82, 2841–2848. [Google Scholar] [CrossRef]
- Skane, R.; Schneider, P.; Rickard, W.D.A.; Jones, F.; van Riessen, A.; Jamieson, E.; Xiao, S. Predicting the Stability of Geopolymer Activator Solutions for Optimised Synthesis through Thermodynamic Modelling. arXiv 2025, arXiv:2503.12942. [Google Scholar] [CrossRef]
- ASTM C39/C39M—21; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Silverstrim, T.; Rostrami, H.; Larradale, J.; Samadi, A. Fly Ash Cementitious Material and Method of Making a Product. U.S. 5,601,643A, 11 February 1997. [Google Scholar]

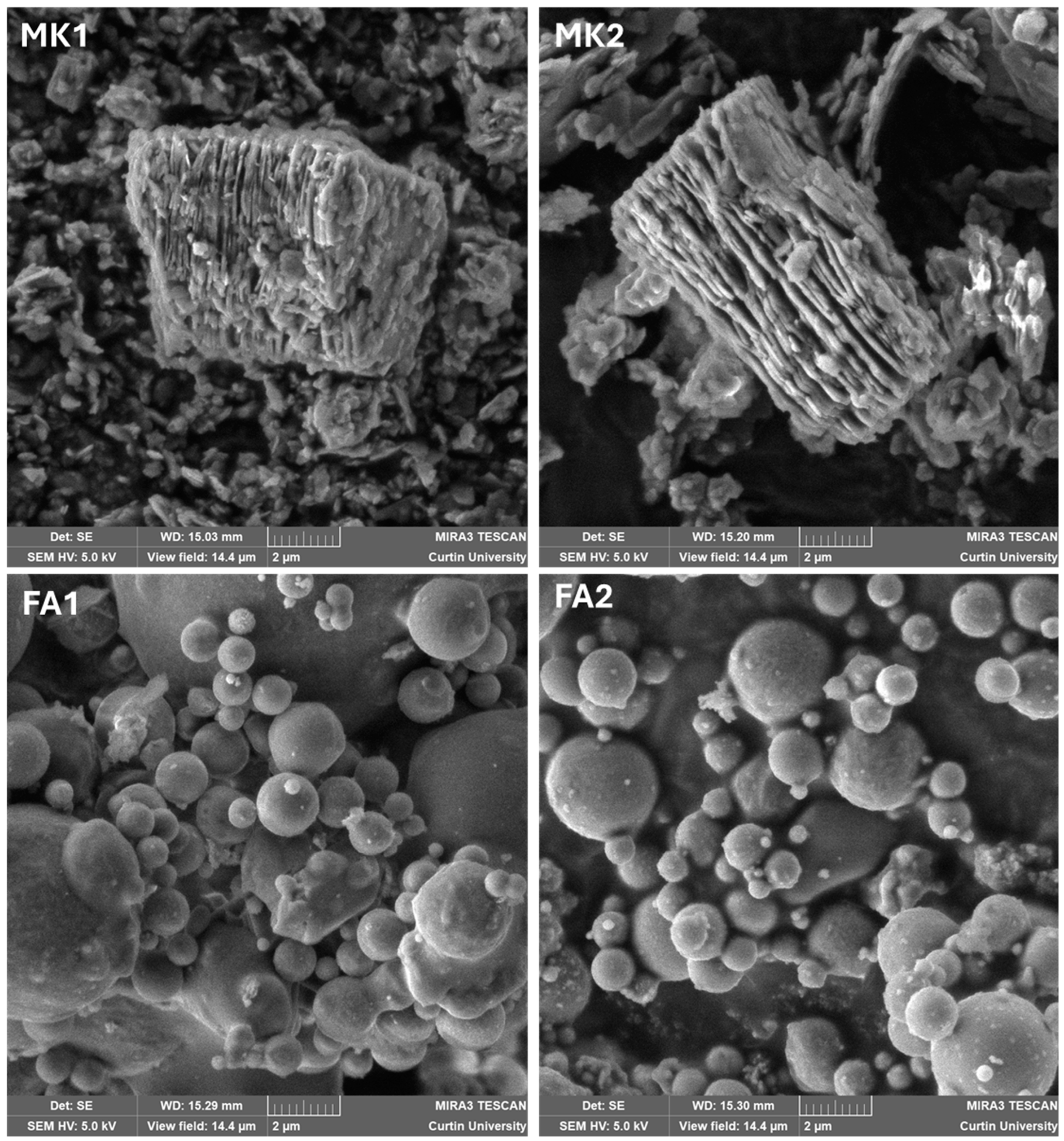

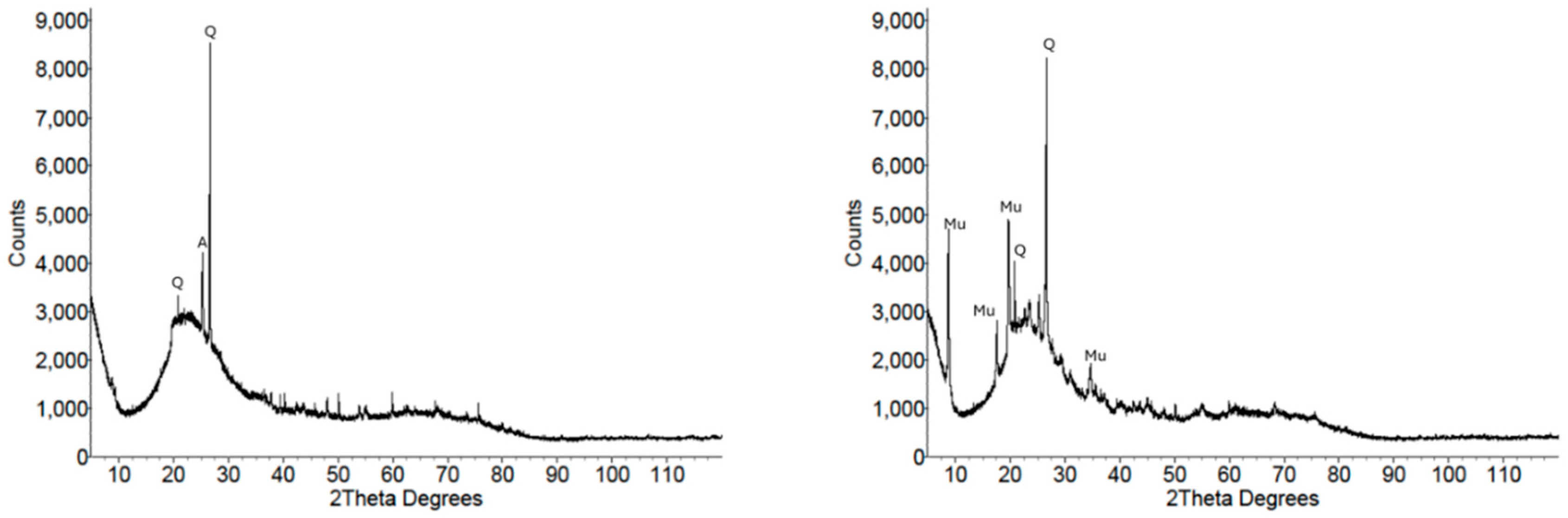
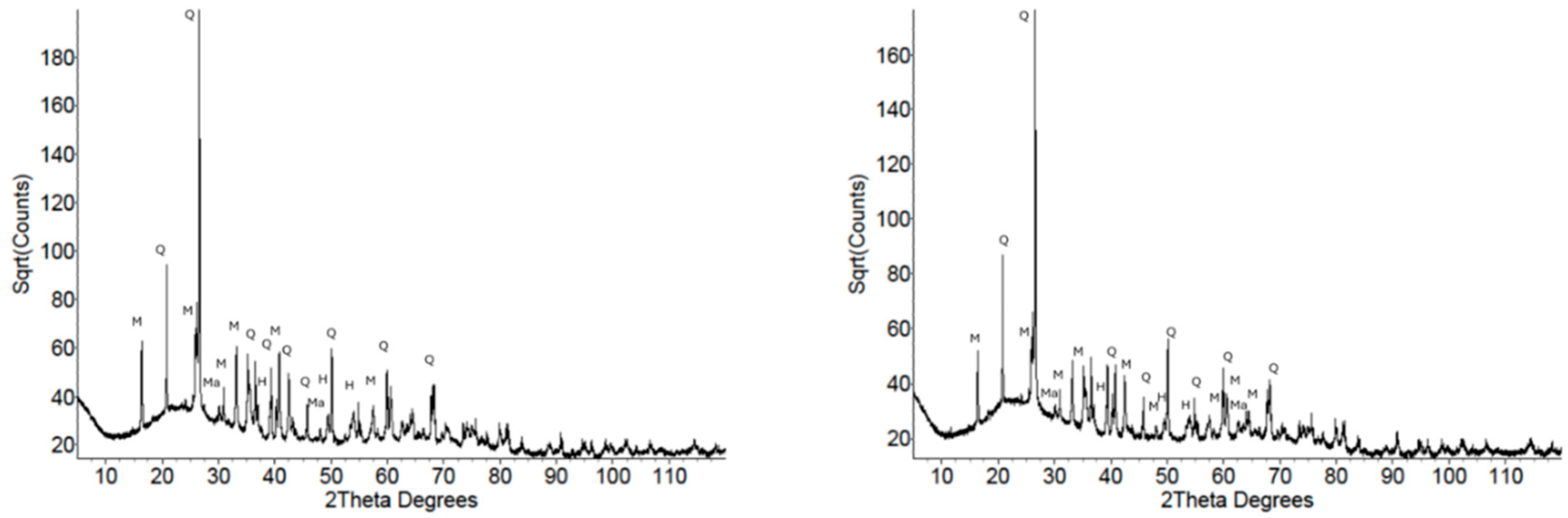




| Analyte | MK1 | MK2 | FA1 | FA2 |
|---|---|---|---|---|
| Al2O3 | 42.32 | 42.58 | 29.72 | 25.74 |
| BaO | 0.02 | 0.02 | 0.30 | 0.20 |
| CaO | 0.10 | 0.01 | 1.00 | 0.67 |
| Cr2O3 | X | X | 0.02 | 0.02 |
| Fe2O3 | 0.96 | 0.46 | 8.87 | 8.06 |
| K2O | 0.26 | 1.60 | 0.50 | 0.46 |
| MgO | 0.19 | 0.26 | 0.66 | 0.80 |
| MnO | X | X | 0.05 | 0.05 |
| Na2O | 0.07 | 0.05 | 0.22 | 0.24 |
| P2O5 | 0.01 | 0.01 | 1.23 | 0.38 |
| SO3 | 0.03 | X | 0.26 | 0.18 |
| SiO2 | 53.30 | 53.35 | 53.94 | 60.42 |
| TiO2 | 1.15 | 0.52 | 1.83 | 1.85 |
| LOI (1000 °C) | 1.49 | 0.88 | 1.17 | 0.74 |
| Total | 99.93 | 99.74 | 99.94 | 99.86 |
| Si/Al (wt.%) | 1.26 | 1.25 | 1.81 | 2.35 |
| Molar Si/Al | 1.36 | 1.06 | 1.54 | 1.99 |
| Sample ID | Amorphous Content | Amorphous Molar Si/Al | Quartz1 (SiO2) | Quartz2 (SiO2) | Mullite (Al4.7Si1.3O9.7) | Hematite (Fe2O3) | Magnetite (Fe3O4) | Spinel (MgAl2O4) | Muscovite (KAl3Si3O10) | Anatase (TiO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| MK1 | 95.96 | 1.00 | 3.28 | - | - | - | - | - | - | 0.76 |
| MK2 | 96.57 | 1.03 | 1.46 | - | - | - | - | - | 1.96 | - |
| FA1 | 52.00 | 2.39 | 13 | 6 | 25 | 1 | 2 | 1 | - | - |
| FA2 | 52.00 | 2.58 | 20 | 5 | 21 | 2 | 1 | 0 | - | - |
| Sample ID | Al2O3 | SiO2 | CaO | Fe2O3 | K2O | Na2O | TiO2 | SiO2/Al2O3 | Si/Al |
|---|---|---|---|---|---|---|---|---|---|
| MK1 | 42.32 | 50.02 | 0.10 | 0.96 | 0.26 | 0.07 | 0.39 | 1.18 | 1.04 |
| MK2 | 41.76 | 50.92 | 0.01 | 0.46 | 1.35 | 0.05 | 0.52 | 1.22 | 1.08 |
| FA1 | 10.20 | 28.81 | 1.00 | 6.95 | 0.50 | 0.22 | 1.83 | 2.83 | 2.49 |
| FA2 | 9.94 | 30.27 | 0.67 | 5.60 | 0.46 | 0.24 | 1.85 | 3.04 | 2.69 |
| Precursor | Si/Al | Na/Al | H/Si |
|---|---|---|---|
| MK1 | 1.8 | 1.0 | 5.02 |
| MK1 | 1.6 | 0.8 | 5.34 |
| MK1 | 1.2 | 0.6 | 6.22 |
| MK1 | 1.7 | 0.6 | 5.15 |
| MK2 | 2.0 | 1.0 | 5.62 |
| MK2 | 1.8 | 0.9 | 5.50 |
| MK2 | 1.6 | 0.8 | 5.50 |
| MK2 | 1.4 | 0.7 | 5.50 |
| MK2 | 1.2 | 0.6 | 6.50 |
| FA1 | 3.0 | 1.0 | 4.68 |
| FA1 | 2.4 | 1.0 | 5.48 |
| FA1 | 1.2 | 1.0 | 6.34 |
| FA2 | 3.0 | 1.0 | 3.69 |
| FA2 | 2.4 | 1.0 | 4.27 |
| FA2 | 1.6 | 1.0 | 4.65 |
| Sample ID | MPa | Peak Shift | Zeolite |
|---|---|---|---|
| FA 1 | |||
| 1.2 (1) | 43 (5) | 7.2 (0.3) | ✓ |
| 2.4 (1) | 7 (2) | 6.1 (0.2) | |
| 3 (1) | 33 (6) | 6.9 (0.5) | |
| FA 2 | |||
| 1.6 (1) | 40 (3) | 7.4 (0.3) | ✓ |
| 2.4 (1) | 18 (4) | 6.3 (0.2) | ✓ |
| 3 (1) | 70 (9) | 8.2 (0.6) | |
| MK 1 | |||
| 1.8 (1) | 100 (10) | 7.6 (0.2) | |
| 1.6 (0.8) | 76 (14) | 7.2 (0.2) | |
| 1.2 (0.6) | 33 (6) | 5.9 (0.3) | ✓ |
| 1.7 (0.6) | 4 (1) | 5.1 (0.2) | |
| MK 2 | |||
| 1.2 (0.6) | 3 (1) | 5.1 (0.1) | |
| 1.4 (0.7) | 57 (9) | 6.4 (0.1) | |
| 1.6 (0.8) | 61 (8) | 6.6 (0.2) | |
| 1.8 (0.9) | 55 (6) | 6.6 (0.2) | |
| 2.0 (1) | 73 (9) | 7.0 (0.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Riessen, A.; Jamieson, E.; Gildenhuys, H.; Skane, R.; Allery, J. Using XRD to Assess the Strength of Fly-Ash- and Metakaolin-Based Geopolymers. Materials 2025, 18, 2093. https://doi.org/10.3390/ma18092093
van Riessen A, Jamieson E, Gildenhuys H, Skane R, Allery J. Using XRD to Assess the Strength of Fly-Ash- and Metakaolin-Based Geopolymers. Materials. 2025; 18(9):2093. https://doi.org/10.3390/ma18092093
Chicago/Turabian Stylevan Riessen, Arie, Evan Jamieson, Hendrik Gildenhuys, Ramon Skane, and Jarrad Allery. 2025. "Using XRD to Assess the Strength of Fly-Ash- and Metakaolin-Based Geopolymers" Materials 18, no. 9: 2093. https://doi.org/10.3390/ma18092093
APA Stylevan Riessen, A., Jamieson, E., Gildenhuys, H., Skane, R., & Allery, J. (2025). Using XRD to Assess the Strength of Fly-Ash- and Metakaolin-Based Geopolymers. Materials, 18(9), 2093. https://doi.org/10.3390/ma18092093







