Evaluating the Chemical Resistance and Performance of Thermochromic Polymers for Food Packaging
Abstract
1. Introduction
- i.
- To assess thermochromic polymers’ optical stability and thermochromic activation before and after exposure to solutions representative of food-contact environments.
- ii.
- To determine the impact of repeated thermal cycling on thermochromic behavior post-chemical exposure.
- iii.
- To review mechanical integrity post-chemical exposure.
2. Materials and Methods
2.1. Materials
2.2. Blend Preparation
2.3. Injection Molding
2.4. Chemical Resistance Testing Solutions
2.5. Visual and Spectrophotometric Color Analysis
2.6. SEM Analysis
2.7. Moisture Content Analysis
2.8. Mass Stability
2.9. Thermal Cycling
2.10. Tensile Testing
2.11. Charpy Impact Testing
2.12. Statistical Analyses
2.13. Rationale for Testing Conditions
3. Results
3.1. Visual Appearance and Colorimetric Results
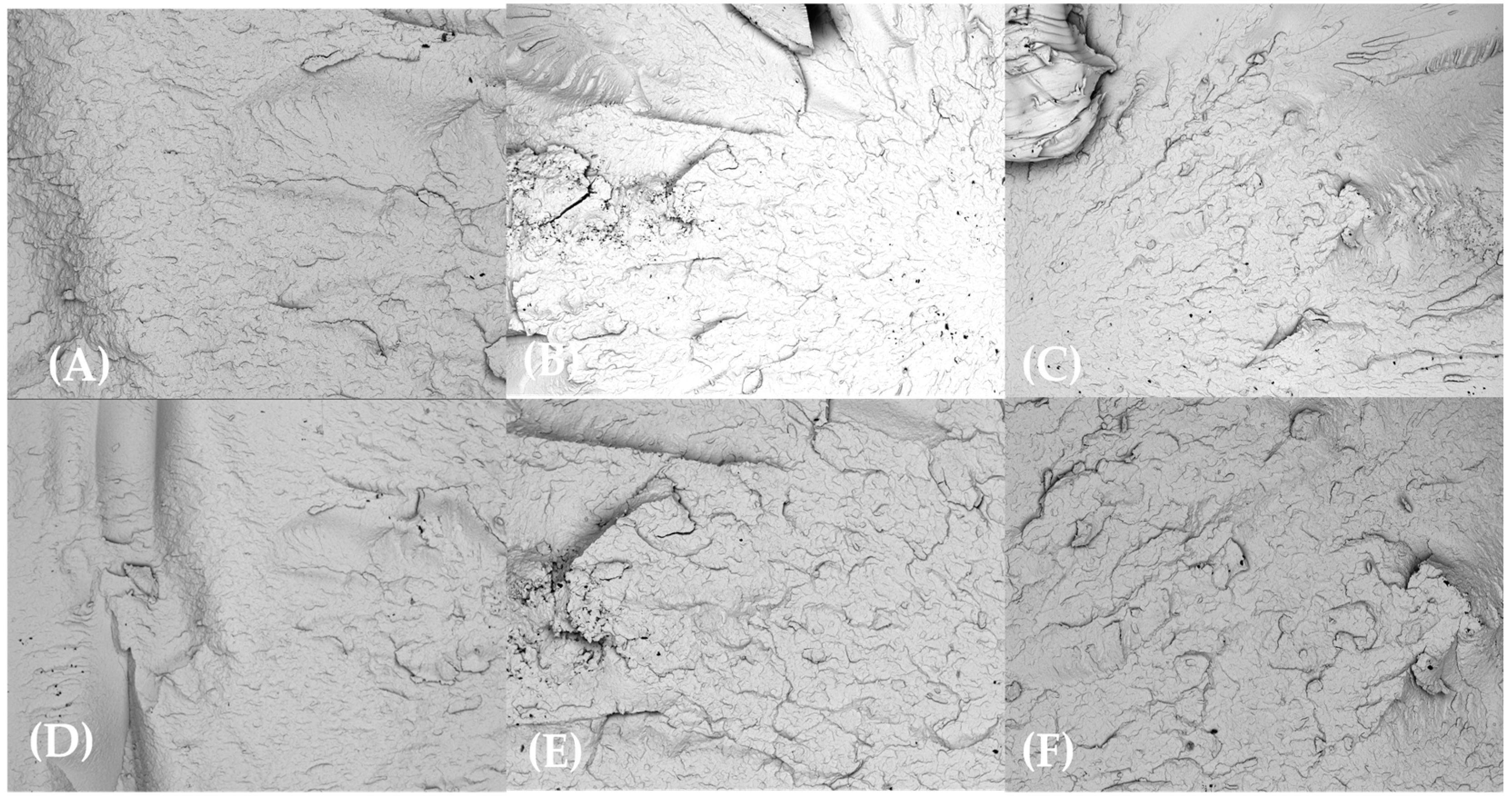
3.2. SEM Observations
3.3. Moisture Content Analysis Results
3.4. Mass Stability Results
3.5. Effect of Thermal Cycling
3.6. Tensile Properties
3.7. Charpy Impact Strength Results
3.8. Recommendations for Improving Chemical Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart monitoring of gas/temperature changes within food packaging based on natural colorants. Comp. Rev. Food Sci. Food Safe 2020, 19, 2885–2931. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Aparna, K. Food Packaging and Storage. In Research Trends in Home Science and Extension; AkiNik Publications: New Delhi, India, 2020; pp. 27–51. ISBN 978-93-90420-92-6. [Google Scholar]
- Yan, M.R.; Hsieh, S.; Ricacho, N. Innovative Food Packaging, Food Quality and Safety, and Consumer Perspectives. Processes 2022, 10, 747. [Google Scholar] [CrossRef]
- Jones, A.; Neal, B.; Reeve, B.; Ni Mhurchu, C.; Thow, A.M. Front-of-pack nutrition labelling to promote healthier diets: Current practice and opportunities to strengthen regulation worldwide. BMJ Glob. Health 2019, 4, e001882. [Google Scholar] [CrossRef]
- Verma, M.K.; Shakya, S.; Kumar, P.; Madhavi, J.; Murugaiyan, J.; Rao, M.V.R. Trends in packaging material for food products: Historical background, current scenario, and future prospects. J. Food Sci. Technol. 2021, 58, 4069–4082. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive Review of Biodegradable Polymer-Based Films and Coatings and Their Food Packaging Applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef]
- Mohamadi, M. Plastic Types and Applications. In Plastic Waste Treatment and Management; Hasanzadeh, R., Mojaver, P., Eds.; Engineering Materials; Springer Nature: Cham, Switzerland, 2023; pp. 1–19. ISBN 978-3-031-31159-8. [Google Scholar]
- AlMaadeed, M.A.A.; Ponnamma, D.; El-Samak, A.A. Polymers to improve the world and lifestyle: Physical, mechanical, and chemical needs. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–19. ISBN 978-0-12-816808-0. [Google Scholar]
- Wang, C.; Liu, Y.; Chen, W.; Zhu, B.; Qu, S.; Xu, M. Critical review of global plastics stock and flow data. J. Ind. Ecol. 2021, 25, 1300–1317. [Google Scholar] [CrossRef]
- The Busines Research Company. Plastics And Rubber Products Global Market Report 2025—By Type (Rubber Products, Plastic Products), By End-User Industry (Automotive & Transportation, Electrical & Electronics, Medical, Construction, Other End Users)—Market Size, Trends, And Global Forecast 2025–2034; Global Market Reports; The Business Research Company: London, UK, 2025; p. 400. [Google Scholar]
- White, A.; Lockyer, S. Removing plastic packaging from fresh produce—What’s the impact? Nutr. Bull. 2020, 45, 35–50. [Google Scholar] [CrossRef]
- Ugoeze, K.C.; Amogu, E.O.; Oluigbo, K.E.; Nwachukwu, N. Environmental and public health impacts of plastic wastes due to healthcare and food products packages: A Review. J. Environ. Sci. Public Health 2021, 5, 1–31. [Google Scholar] [CrossRef]
- Saalah, S.; Saallah, S.; Rajin, M.; Yaser, A.Z. Management of Biodegradable Plastic Waste: A Review. In Advances in Waste Processing Technology; Yaser, A.Z., Ed.; Springer: Singapore, 2020; pp. 127–143. ISBN 978-981-15-4820-8. [Google Scholar]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments. Appl. Sci. 2021, 11, 3532. [Google Scholar] [CrossRef]
- Sadeghi, K.; Yoon, J.Y.; Seo, J. Chromogenic Polymers and Their Packaging Applications: A Review. Polym. Rev. 2020, 60, 442–492. [Google Scholar] [CrossRef]
- Saber, D.; Abd El-Aziz, K. Advanced materials used in wearable health care devices and medical textiles in the battle against coronavirus (COVID-19): A review. J. Ind. Text. 2022, 51, 246S–271S. [Google Scholar] [CrossRef] [PubMed]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors—A Review. Front. Mater. 2020, 7, 82. [Google Scholar] [CrossRef]
- Udoh, I.I.; Shi, H.; Daniel, E.F.; Li, J.; Gu, S.; Liu, F.; Han, E.-H. Active anticorrosion and self-healing coatings: A review with focus on multi-action smart coating strategies. J. Mater. Sci. Technol. 2022, 116, 224–237. [Google Scholar] [CrossRef]
- Zaszczyńska, A.; Gradys, A.; Sajkiewicz, P. Progress in the Applications of Smart Piezoelectric Materials for Medical Devices. Polymers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Behera, A. Chromogenic Materials. In Advanced Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 157–191. ISBN 978-3-030-80358-2. [Google Scholar]
- Sadoh, A.; Hossain, S.; Ravindra, N.M. Thermochromic polymeric films for applications in active intelligent packaging—An overview. Micromachines 2021, 12, 1193. [Google Scholar] [CrossRef]
- Toan, V.N.; Tri, N.M.; Nguyen, X.H.; Nguyen, D.D.; Chung, W.; Chang, S.W.; La, D.D. Exploring the potential of organic thermochromic materials in textile applications. J. Mater. Sci. 2024, 59, 14924–14947. [Google Scholar] [CrossRef]
- Ramlow, H.; Andrade, K.L.; Immich, A.P.S. Smart textiles: An overview of recent progress on chromic textiles. J. Text. Inst. 2021, 112, 152–171. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, Y.; Li, J.; Liu, S.; Huang, W.; Zhao, Q. Stimuli-responsive photofunctional materials for green and security printing. InfoMat 2021, 3, 82–100. [Google Scholar] [CrossRef]
- Jeon, H.S.; Kim, J.H.; Jun, M.B.G.; Jeong, Y.H. Fabrication of Thermochromic Membrane and Its Characteristics for Fever Detection. Materials 2021, 14, 3460. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Akhila, K.; Kumar, P.; Deshmukh, R.K.; Gaikwad, K.K. Novel temperature-sensitive label based on thermochromic ink for hot food packaging and serving applications. J. Therm. Anal. Calorim. 2023, 148, 6061–6069. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Hanzer, S.J.; Kulčar, R.; Vukoje, M.; Maroševi’c, A.; Dolovski, M. Assessment of Thermochromic Packaging Prints’ Resistance to UV Radiation and Various Chemical Agents. Polymers 2023, 15, 1208. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Marques, M.A.; Alves, J.; Morais, M.; Figueira, J.; Pinto, J.V.; Moreira, F.T. Irreversible colorimetric bio-based curcumin bilayer membranes for smart food packaging temperature control applications. RSC Adv. 2024, 14, 8981–8989. [Google Scholar] [CrossRef]
- Crosby, P.H.N.; Netravali, A.N. Green Thermochromic Materials: A Brief Review. Adv. Sustain. Syst. 2022, 6, 2200208. [Google Scholar] [CrossRef]
- Gupta, R.K. Specialty Polymers: Fundamentals, Properties, Applications and Advances, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-003-27826-9. [Google Scholar]
- Vicoveanu, D.; Pîrvu, C. Polymer Composition with Improved Barrier Properties for Thermochromic Applications. WO2020079499A1, 23 April 2020. [Google Scholar]
- Abraham, J. Future of Food Packaging: Intelligent Packaging. In Nanotechnology in Intelligent Food Packaging; Annu, Bhattacharya, T., Ahmed, S., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 383–417. ISBN 978-1-119-81895-3. [Google Scholar]
- Chen, T.; Yang, Q.; Fang, C.; Deng, S.; Xu, B. Advanced Design for Stimuli-Reversible Chromic Wearables With Customizable Functionalities. Adv. Mater. 2025, 37, 2413665. [Google Scholar] [CrossRef]
- Kantola, R.; Lassila, L.V.J.; Tolvanen, M.; Valittu, P.K. Color stability of thermochromic pigment in maxillofacial silicone. J. Adv. Prosthodont. 2013, 5, 75. [Google Scholar] [CrossRef][Green Version]
- Shayan, M.; Koo, M.S.; Abouzeid, R.; She, Y.; Gwon, J.; Wu, Q. Functional electrospun polylactic acid core-shell thermochromic fibers with modified cellulose nanocrystals for enhanced temperature-responsive applications. Ind. Crops Prod. 2024, 222, 119594. [Google Scholar] [CrossRef]
- Kovačević, D.; Bota, J. Consumer Satisfaction With Packaging Materials: Kano Model Analysis Approach. Teh. Vjesn. 2021, 28, 1203–1210. [Google Scholar] [CrossRef]
- Sharma, G.K.; Dutt Semwal, A.; Kumar Yadav, D. Advances in Processing Technology; CRC Press: Boca Raton, FL, USA; New India Publishing Agency: Abingdon, UK, 2022; ISBN 978-1-000-50555-9. [Google Scholar]
- Breheny, C.; Donlon, K.; Harrington, A.; Colbert, D.M.; Nunes Bezerra, G.S.; Geever, L. Thermochromic Polymers in Food Packaging: A Comprehensive Systematic Review and Patent Landscape Analysis. Coatings 2024, 14, 1252. [Google Scholar] [CrossRef]
- Civan, L.; Kurama, S. A review: Preparation of functionalised materials/smart fabrics that exhibit thermochromic behaviour. J. Mater. Sci. Technol. 2021, 37, 1865–1877. [Google Scholar] [CrossRef]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Afr. 2023, 6, 117–144. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Env. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Hanzer, S.J.; Kulčar, R.; Vukoje, M.; Širol, P. Mechanical and chemical resistance of thermochromic packaging prints. In Proceedings of the Tenth International Symposium GRID 2020, Novi Sad, Serbia, 12–14 November 2020; Faculty of Technical Sciences, Department of Graphic Engineering and Design, University of Novi Sad: Novi Sad, Serbia, 2020; pp. 109–118. [Google Scholar]
- Kulčar, R.; Kračun, I.; Vukoje, M.; Ivanda, K.I.; Cigula, T. Investigation of the Impact of Chemical Agents and Substrates on the Stability of Thermochromic Printing Inks. In Proceedings of the 12th International Symposium GRID 2024, Novi Sad, Serbia, 14–16 November 2024; Faculty Of Technical Sciences Department of Graphic Engineering and Design, University of Novi Sad: Novi Sad, Serbia, 2024. [Google Scholar]
- Wang, J.; Zeng, S.; Liu, H.; Zheng, Y.; Ma, Y. Thermochromic behavior of pigment red 254 in nylon 6 polymer for high-chromaticity engineering plastics. Dye Pigment. 2025, 233, 112507. [Google Scholar] [CrossRef]
- ISO 527-2:2012; International Organization for Standardization Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. Polish Committee for Standardization: Warsaw, Poland, 2012.
- ISO 179-1:2023; International Organization for Standardization Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. Polish Committee for Standardization: Warsaw, Poland, 2023.
- Fu, H.; Xu, H.; Liu, Y.; Yang, Z.; Kormakov, S.; Wu, D.; Sun, J. Overview of Injection Molding Technology for Processing Polymers and Their Composites. ES Mater. Manuf. 2020, 8, 3–23. [Google Scholar] [CrossRef]
- ISO 294-1:2017; Plastics—Injection Moulding of Test Specimens of Thermoplastic Materials—Part 1: General Principles, and Moulding of Multipurpose and Bar Test Specimens. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Dadi, M.; Yasir, M. Spectroscopy and Spectrophotometry: Principles and Applications for Colorimetric and Related Other Analysis. In Colorimetry; Kumar Samanta, A., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-83962-940-2. [Google Scholar]
- Cinko, U.O.; Becerir, B. Computing characteristics of color difference formulas for regular coordinate changes in CIELAB color space. Text. Res. J. 2024, 00405175241278025. [Google Scholar] [CrossRef]
- Belasco, R.; Edwards, T.; Munoz, A.J.; Rayo, V.; Buono, M.J. The Effect of Hydration on Urine Color Objectively Evaluated in CIE L*a*b* Color Space. Front. Nutr. 2020, 7, 576974. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Zhang, S.; Li, J.; Feng, J.; Yang, Y.; Meng, H.; Zhang, Z. Correlation between Colour Traits and Intrinsic Quality of Dalbergiae Odoriferae Lignum. Molecules 2023, 28, 7635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.; Li, W.; Gan, X.; Wang, J. Reflectance model for filament yarn composed of different color monofilaments. J. Text. Inst. 2021, 112, 2039–2047. [Google Scholar] [CrossRef]
- ISO/CIE 11664-4:2019; Colorimetry—Part 4: CIE 1976 Lab Colour Space*. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- Davies, T.E.; Li, H.; Bessette, S.; Gauvin, R.; Patience, G.S.; Dummer, N.F. Experimental methods in chemical engineering: Scanning electron microscopy and X-ray ultra-microscopy—SEM and XuM. Can. J. Chem. Eng. 2022, 100, 3145–3159. [Google Scholar] [CrossRef]
- Lyu, Z.; Yao, L.; Chen, W.; Kalutantirige, F.C.; Chen, Q. Electron Microscopy Studies of Soft Nanomaterials. Chem. Rev. 2023, 123, 4051–4145. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Rotzler, S.; Schneider-Ramelow, M. Washability of E-Textiles: Failure Modes and Influences on Washing Reliability. Textiles 2021, 1, 37–54. [Google Scholar] [CrossRef]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A Review on Antimicrobial Packaging for Extending the Shelf Life of Food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Mirbach, B.; Boguslawski, M. How accurate can combined measurements be—Experiment, simulation, and theory. arXiv 2022, arXiv:2205.07879. [Google Scholar] [CrossRef]
- Szumska, A.A.; Maria, I.P.; Flagg, L.Q.; Savva, A.; Surgailis, J.; Paulsen, B.D.; Moia, D.; Chen, X.; Griggs, S.; Mefford, J.T.; et al. Reversible Electrochemical Charging of n-Type Conjugated Polymer Electrodes in Aqueous Electrolytes. J. Am. Chem. Soc. 2021, 143, 14795–14805. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Majka, T.M. Thermal Degradation of Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-823142-5. [Google Scholar]
- Bridson, J.H.; Abbel, R.; Smith, D.A.; Northcott, G.L.; Gaw, S. Release of additives and non-intentionally added substances from microplastics under environmentally relevant conditions. Environ. Adv. 2023, 12, 100359. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Zhang, D.; Tan, S.Y.; Chong, Y.T.; Hui, H.K.; Sng, A.; Wei, F.; Suwardi, A.; Png, Z.M.; Zhu, Q.; et al. Ultra-high Performance Thermochromic Polymers via a Solid-solid Phase Transition Mechanism and Their Applications. Adv. Mater. 2024, 36, e2405430. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Pi, A.; Zhao, Z.; Wang, S.; Wei, C.; Jie, Z.; Huang, F. Study on Intrinsic Influence Law of Specimen Size and Loading Speed on Charpy Impact Test. Materials 2022, 15, 3855. [Google Scholar] [CrossRef]
- Patterson, A.E.; Pereira, T.R.; Allison, J.T.; Messimer, S.L. IZOD impact properties of full-density fused deposition modeling polymer materials with respect to raster angle and print orientation. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 235, 1891–1908. [Google Scholar] [CrossRef]
- Vandever, C. Introduction to Research Statistical Analysis: An Overview of the Basics. HCA Healthc. J. Med. 2020, 1, 71–75. [Google Scholar] [CrossRef]
- Papachristou, E.; Korres, D.; Mamma, D.; Kekos, D.; Tarantili, P.A.; Polyzois, G. Titanium Dioxide/Polysiloxane Composites: Preparation, Characterization and Study of Their Color Stability Using Thermochromic Pigments. J. Compos. Sci. 2022, 6, 195. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.; Zhao, W. Effect of Thermochromic and Photochromic Microcapsules on the Surface Coating Properties for Metal Substrates. Coatings 2022, 12, 1642. [Google Scholar] [CrossRef]
- Wang, J.; Xie, M.; An, Y.; Tao, Y.; Sun, J.; Ji, C. All-season thermal regulation with thermochromic temperature-adaptive radiative cooling coatings. Sol. Energy Mater. Sol. Cells 2022, 246, 111883. [Google Scholar] [CrossRef]
- White, M.A.; Bourque, A. Colorant, Thermochromic. In Encyclopedia of Color Science and Technology; Shamey, R., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 571–582. ISBN 978-3-030-89861-8. [Google Scholar]
- Rossi, S.; Simeoni, M.; Quaranta, A. Behavior of chromogenic pigments and influence of binder in organic smart coatings. Dye Pigment. 2021, 184, 108879. [Google Scholar] [CrossRef]
- Hakami, A.; Srinivasan, S.S.; Biswas, P.K.; Krishnegowda, A.; Wallen, S.L.; Stefanakos, E.K. Review on thermochromic materials: Development, characterization, and applications. J. Coat. Technol. Res. 2022, 19, 377–402. [Google Scholar] [CrossRef]
- Nagare, S.M.; Hakami, A.; Biswas, P.K.; Stefanakos, E.K.; Srinivasan, S.S. A review of thermochromic materials for coating applications: Production, protection, and degradation of organic thermochromic materials. J. Coat. Technol. Res. 2025, 22, 91–115. [Google Scholar] [CrossRef]
- Dungani, R.; Sumardi, I.; Alamsyah, E.M.; Aditiawati, P.; Karliati, T.; Malik, J.; Sulistyono. A study on fracture toughness of nano-structured carbon black-filled epoxy composites. Polym. Bull. 2021, 78, 6867–6885. [Google Scholar] [CrossRef]
- Han, W.; Yin, M.; Zhang, W.; Liu, Z.; Wang, N.; Yong, K.T.; An, Q. Acid-Resistance and Self-Repairing Supramolecular Nanoparticle Membranes via Hydrogen-Bonding for Sustainable Molecules Separation. Adv. Sci. 2021, 8, 2102594. [Google Scholar] [CrossRef]
- Ramezani Dana, H.; Ebrahimi, F. Synthesis, properties, and applications of polylactic acid-based polymers. Polym. Eng. Sci. 2023, 63, 22–43. [Google Scholar] [CrossRef]
- Feng, W.; Li, M.; Hao, Z.; Zhang, J. Analytical Methods of Isolation and Identification. In Phytochemicals in Human Health; Rao, V., Mans, D., Rao, L., Eds.; IntechOpen: Switzerland, UK, 2020; ISBN 978-1-78985-587-6. [Google Scholar]
- Bîrleanu, E.; Mihăilă, I.; Topală, I.; Borcia, C.; Borcia, G. Adhesion Properties and Stability of Non-Polar Polymers Treated by Air Atmospheric-Pressure Plasma. Polymers 2023, 15, 2443. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, Q.; Li, M.; Jin, S.; Fan, J.; Zhao, L.; Yao, Z. Surface modification of activated carbon fiber by low-temperature oxygen plasma: Textural property, surface chemistry, and the effect of water vapor adsorption. Chem. Eng. J. 2021, 418, 129474. [Google Scholar] [CrossRef]
- Rakić, V.; Poklar Ulrih, N. Influence of pH on color variation and stability of cyanidin and cyanidin 3-O-β-glucopyranoside in aqueous solution. CyTA-J. Food 2021, 19, 174–182. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A Review on Degradation Mechanisms of Polylactic acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. Available online: https://4spepublications.onlinelibrary.wiley.com/doi/abs/10.1002/pen.25511 (accessed on 22 March 2025). [CrossRef]
- Jouenne, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; He, Y.; Song, H.; Chen, X.; Guo, J. The effect of thermo-oxidative ageing on crystallization, dynamic and static mechanical properties of long glass fibre-reinforced polyamide 10T composites. R. Soc. Open Sci. 2018, 5, 172029. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
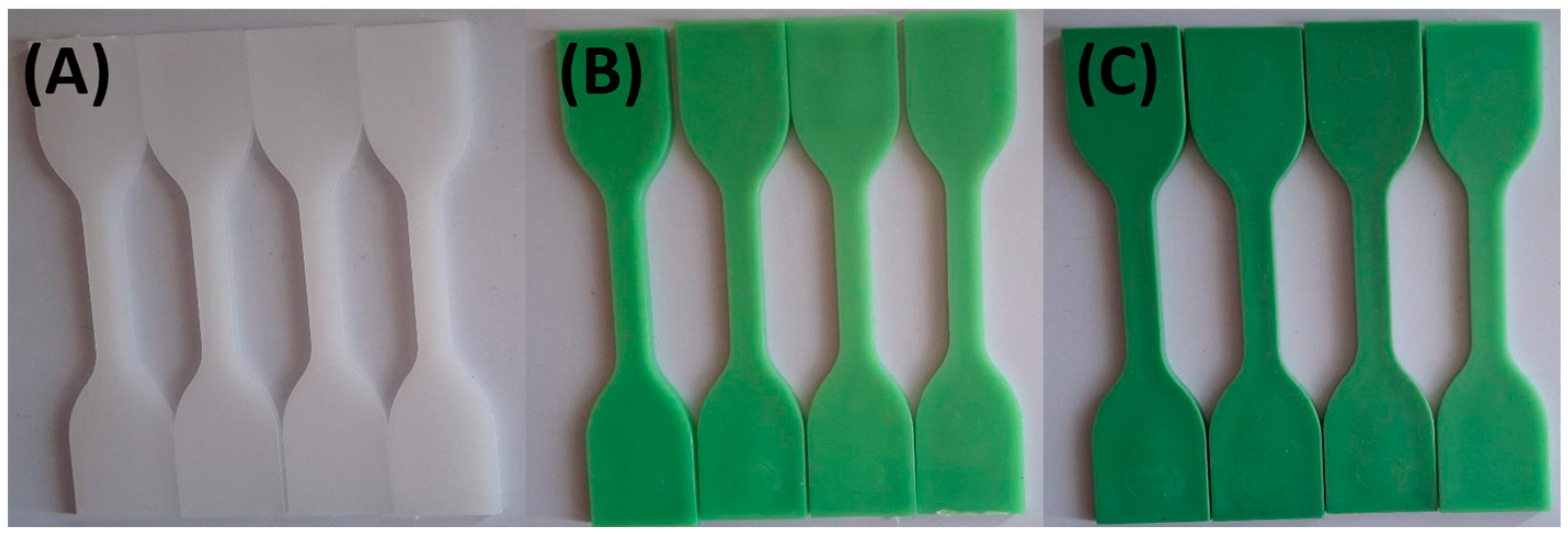
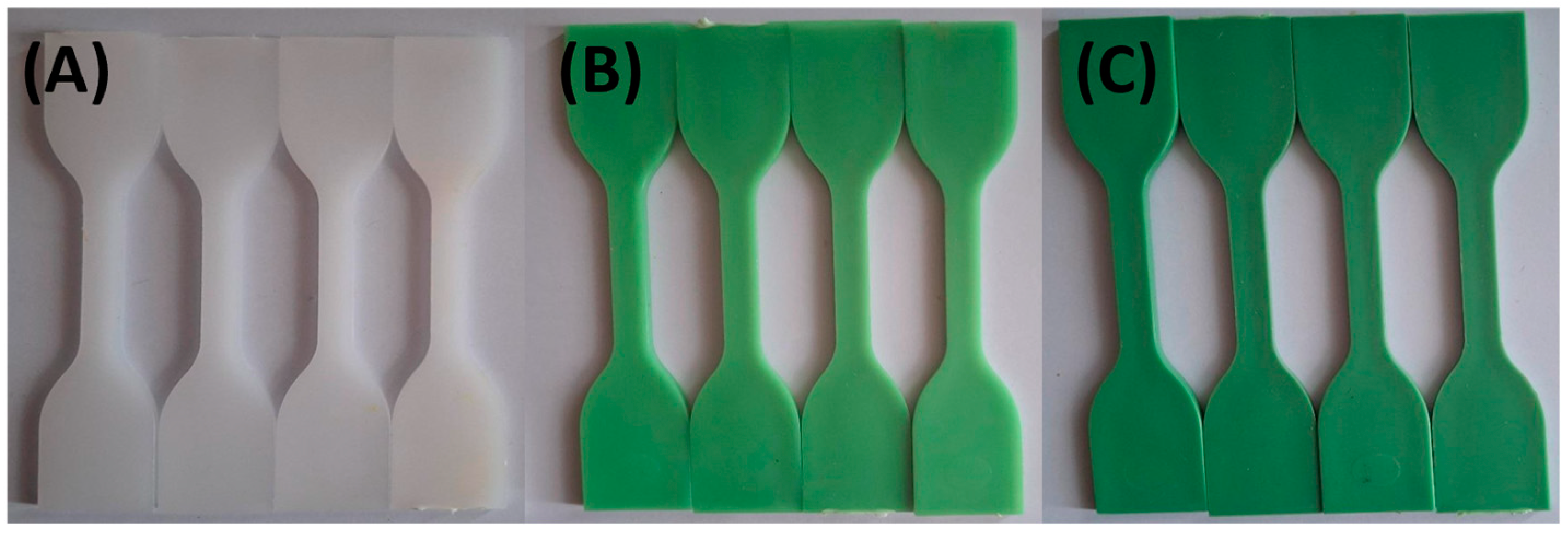

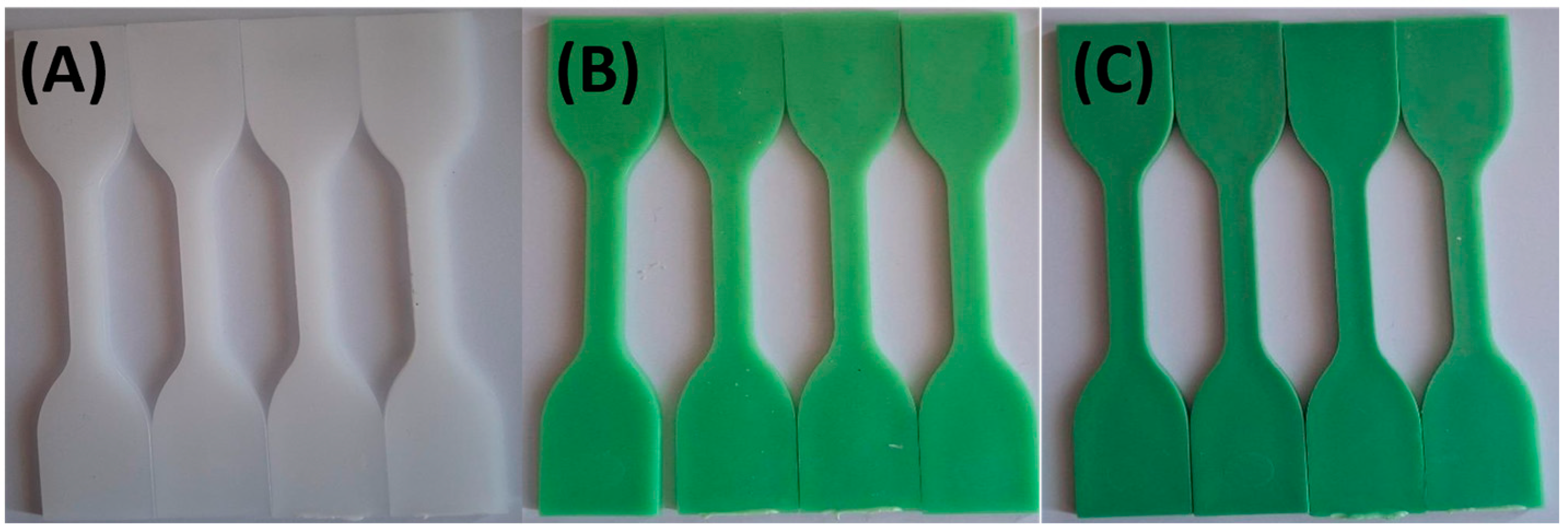
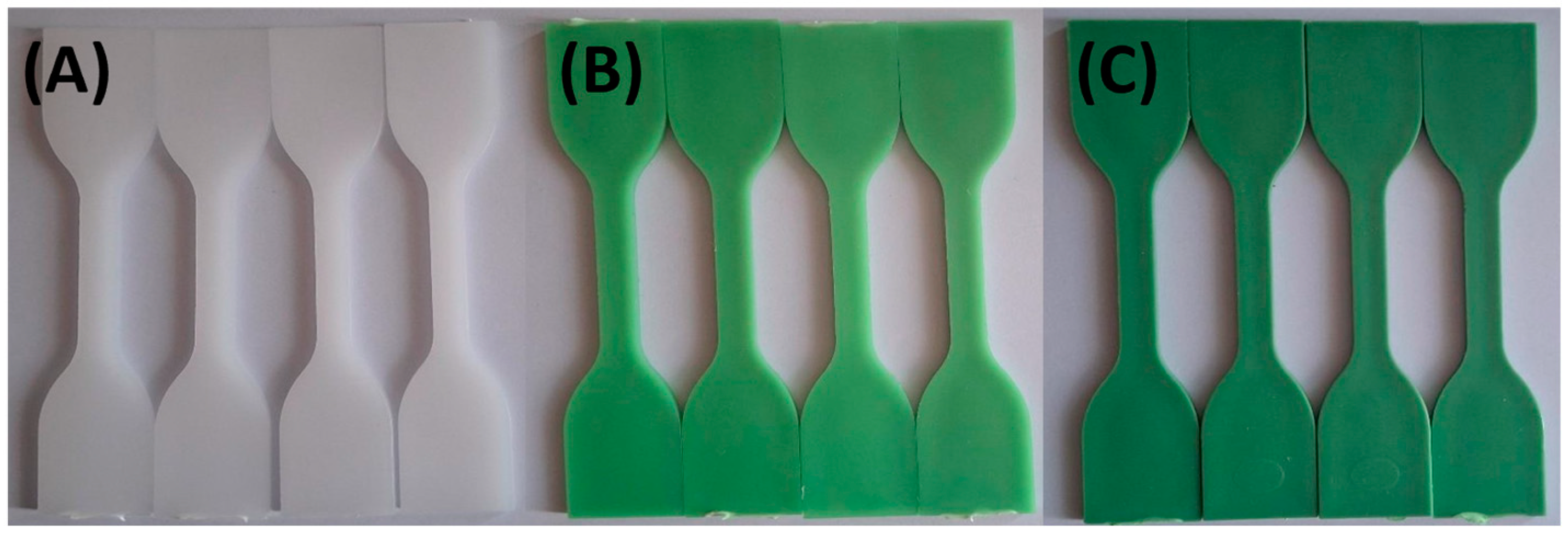
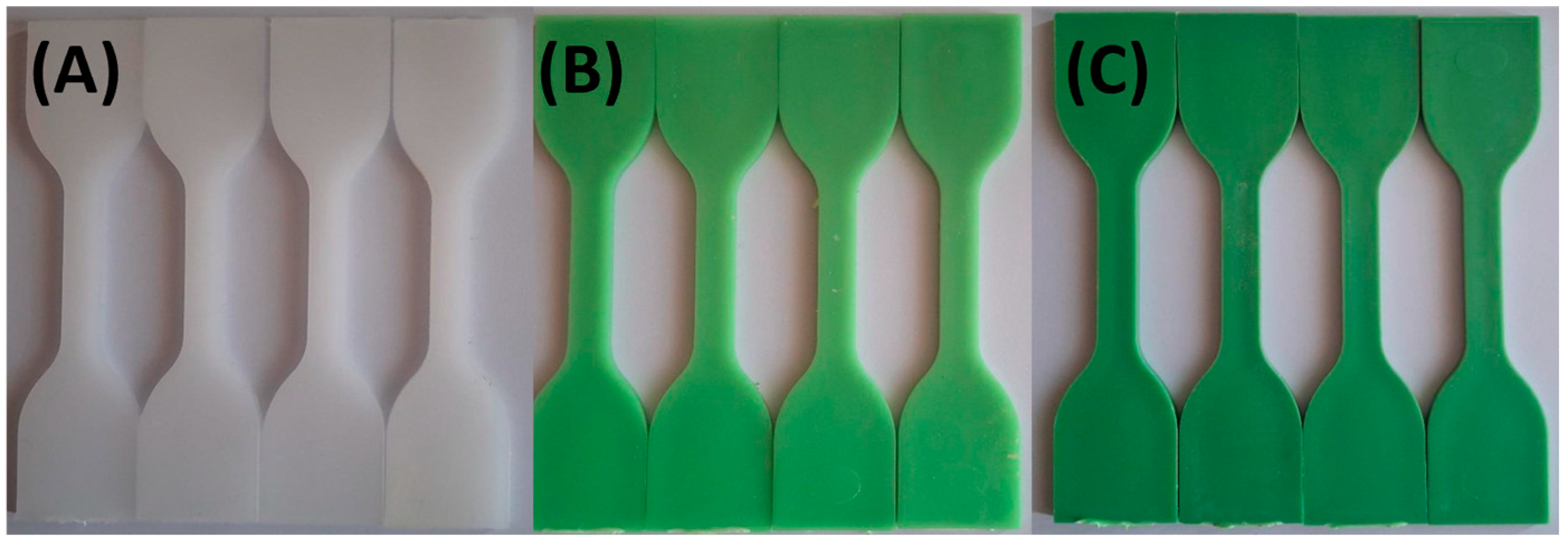


| Sample Name | HDPE (wt. %) | TP (wt. %) |
|---|---|---|
| HDPE100/TP0 | 100 | 0 |
| HDPE98/TP2 | 98 | 2 |
| HDPE92/TP8 | 92 | 8 |
| Solution Type | Test Substance | Concentration (%) | Measured pH | Exposure Time (h) |
|---|---|---|---|---|
| Acidic | Lemon juice | 100% | 2.3 | 0, 24, 48, 72 |
| Acidic/Aqueous | Orange juice | 100% | 3.8 | 0, 24, 48, 72 |
| Neutral Solution | Tap water | 100% | 7.0 | 0, 24, 48, 72 |
| Solvent-Based | Hydroalcoholic solution | 40% | 7.2 | 0, 24, 48, 72 |
| Oil-based Solution | Vegetable oil | 100% | N/A | 0, 24, 48, 72 |
| Alkaline Solution | Bleach solution | 5% | 12.5 | 0, 24, 48, 72 |
| Specimen ID | Solution Type | 24 h (Mean ± SD) | 48 h (Mean ± SD) | 72 h (Mean ± SD) |
|---|---|---|---|---|
| HDPE100/TP0 | Lemon juice | 0.42 (±0.03) | 1.61 (±0.09) | 1.73 (±0.04) |
| Orange juice | 0.48 (±0.02) | 1.64 (±0.07) | 1.89 (±0.09) | |
| Tap water | 0.11 (±0.01) | 0.19 (±0.01) | 0.23 (±0.02) | |
| Hydroalcoholic solution | 0.22 (±0.09) | 0.31 (±0.07) | 0.42 (±0.05) | |
| Vegetable oil | 0.27 (±0.03) | 0.61 (±0.09) | 0.73 (±0.04) | |
| Bleach solution | 0.40 (±0.11) | 1.51 (±0.06) | 1.66 (±0.03) | |
| HDPE98/TP2 | Lemon juice | 0.92 (±0.11) | 1.61 (±0.14) | 1.94 (±0.19) |
| Orange juice | 1.05 (±0.13) | 1.64 (±0.17) | 2.09 (±0.21) | |
| Tap water | 0.19 (±0.09) | 0.27 (±0.11) | 0.42 (±0.12) | |
| Hydroalcoholic solution | 0.38 (±0.12) | 0.50 (±0.18) | 0.62 (±0.22) | |
| Vegetable oil | 0.42 (±0.23) | 0.61 (±0.19) | 0.93 (±0.14) | |
| Bleach solution | 0.83 (±0.21) | 1.46 (±0.22) | 1.73 (±0.19) | |
| HDPE92/TP8 | Lemon juice | 1.21 (±0.11) | 2.52 (±0.22) | 3.54 (±0.32) |
| Orange juice | 1.37 (±0.12) | 2.76 (±0.17) | 3.75 (±0.29) | |
| Tap water | 0.31 (±0.11) | 0.45 (±0.11) | 0.51 (±0.12) | |
| Hydroalcoholic solution | 0.23 (±0.09) | 0.42 (±0.12) | 0.98 (±0.21) | |
| Vegetable oil | 0.69 (±0.11) | 0.87 (±0.19) | 0.95 (±0.14) | |
| Bleach solution | 1.11 (±0.13) | 2.34 (±0.21) | 3.30 (±0.19) |
| Specimen ID. | Test Substance | Moisture Content (%) (0 Exposure) (Mean ± SD) | Moisture Content (%) (After 24 h) (Mean ± SD) | Moisture Content (%) (After 48 h) (Mean ± SD) | Moisture Content (%) (After 72 h) (Mean ± SD) |
|---|---|---|---|---|---|
| HDPE100/TP0 | Control | 0.02 (±0.01) | 0.02 (±0.01) | 0.02 (±0.01) | 0.02 (±0.01) |
| Lemon juice | 0.02 (±0.02) | 0.05 (±0.02) | 0.09 (±0.02) | 0.12 (±0.02) | |
| Orange juice | 0.02 (±0.02) | 0.07 (±0.02) | 0.12 (±0.02) | 0.15 (±0.02) | |
| Tap water | 0.02 (±0.01) | 0.04 (±0.01) | 0.06 (±0.01) | 0.08 (±0.01) | |
| Hydroalcoholic solution | 0.02 (±0.01) | 0.02 (±0.01) | 0.06 (±0.01) | 0.08 (±0.01) | |
| Vegetable oil | 0.02 (±0.01) | 0.03 (±0.01) | 0.07 (±0.01) | 0.08 (±0.01) | |
| Bleach solution | 0.02 (±0.02) | 0.04 (±0.02) | 0.08 (±0.02) | 0.10 (±0.02) | |
| HDPE98/TP2 | Control | 0.03 (±0.01) | 0.03 (±0.01) | 0.03 (±0.01) | 0.03 (±0.01) |
| Lemon juice | 0.03 (±0.02) | 0.07 (±0.01) | 0.12 (±0.01) | 0.15 (±0.01) | |
| Orange juice | 0.03 (±0.02) | 0.10 (±0.01) | 0.16 (±0.01) | 0.19 (±0.01) | |
| Tap water | 0.03 (±0.01) | 0.06 (±0.01) | 0.08 (±0.01) | 0.10 (±0.01) | |
| Hydroalcoholic solution | 0.03 (±0.01) | 0.03 (±0.01) | 0.03 (±0.01) | 0.03 (±0.01) | |
| Vegetable oil | 0.03 (±0.01) | 0.03 (±0.01) | 0.03 (±0.01) | 0.03 (±0.01) | |
| Bleach solution | 0.03 (±0.02) | 0.06 (±0.02) | 0.10 (±0.03) | 0.12 (±0.03) | |
| HDPE92/TP8 | Control | 0.04 (±0.01) | 0.04 (±0.01) | 0.04 (±0.01) | 0.04 (±0.01) |
| Lemon juice | 0.04 (±0.02) | 0.09 (±0.02) | 0.15 (±0.02) | 0.18 (±0.02) | |
| Orange juice | 0.04 (±0.02) | 0.12 (±0.03) | 0.19 (±0.03) | 0.22 (±0.03) | |
| Tap water | 0.04 (±0.01) | 0.08 (±0.01) | 0.10 (±0.01) | 0.12 (±0.01) | |
| Hydroalcoholic solution | 0.04 (±0.01) | 0.07 (±0.01) | 0.11 (±0.01) | 0.12 (±0.04) | |
| Vegetable oil | 0.04 (±0.01) | 0.07 (±0.01) | 0.10 (±0.01) | 0.13(±0.03) | |
| Bleach solution | 0.04 (±0.02) | 0.08 (±0.02) | 0.14 (±0.03) | 0.16 (±0.04) |
| Specimen ID. | Test Substance | Mass Measurement (g) (0 Exposure) (Mean ± SD) | Mass Measurement (g) (After 24 h) (Mean ± SD) | Mass Measurement (g) (After 48 h) (Mean ± SD) | Mass Measurement (g) (After 72 h) (Mean ± SD) |
|---|---|---|---|---|---|
| HDPE100/TP0 | Control | 3.724 (±0.00) | 3.724 (±0.00) | 3.724 (±0.00) | 3.724 (±0.00) |
| Lemon juice | 3.724 (±0.02) | 3.726 (±0.02) | 3.736 (±0.03) | 3.738 (±0.04) | |
| Orange juice | 3.724 (±0.02) | 3.727 (±0.04) | 3.738 (±0.03) | 3.741 (±0.05) | |
| Tap water | 3.724 (±0.01) | 3.725 (±0.01) | 3.725 (±0.02) | 3.725 (±0.01) | |
| Hydroalcoholic solution | 3.724 (±0.01) | 3.725 (±0.02) | 3.726 (±0.01) | 3.726 (±0.02) | |
| Vegetable oil | 3.724 (±0.01) | 3.725 (±0.02) | 3.726 (±0.02) | 3.726 (±0.02) | |
| Bleach solution | 3.724 (±0.01) | 3.725 (±0.02) | 3.732 (±0.03) | 3.735 (±0.04) | |
| HDPE98/TP2 | Control | 3.724 (±0.00) | 3.724 (±0.00) | 3.724 (±0.00) | 3.724 (±0.00) |
| Lemon juice | 3.724 (±0.02) | 3.727 (±0.02) | 3.730 (±0.03) | 3.732 (±0.04) | |
| Bleach | 3.724 (±0.02) | 3.728 (±0.04) | 3.737 (±0.03) | 3.739 (±0.05) | |
| Tap water | 3.724 (±0.01) | 3.725 (±0.01) | 3.727 (±0.02) | 3.728 (±0.01) | |
| Hydroalcoholic solution | 3.724 (±0.01) | 3.724 (±0.02) | 3.724 (±0.01) | 3.725 (±0.02) | |
| Vegetable oil | 3.724 (±0.01) | 3.725 (±0.02) | 3.725 (±0.02) | 3.726 (±0.02) | |
| Bleach solution | 3.724 (±0.02) | 3.726 (±0.04) | 3.729 (±0.03) | 3.730 (±0.05) | |
| HDPE92/TP8 | Control | 3.724 (±0.00) | 3.724 (±0.00) | 3.724 (±0.00) | 3.724 (±0.00) |
| Lemon juice | 3.724 (±0.01) | 3.728 (±0.02) | 3.730 (±0.03) | 3.733 (±0.04) | |
| Orange juice | 3.724 (±0.02) | 3.729 (±0.02) | 3.732 (±0.03) | 3.733 (±0.04) | |
| Tap water | 3.724 (±0.01) | 3.726 (±0.01) | 3.727 (±0.02) | 3.728 (±0.01) | |
| Hydroalcoholic solution | 3.724 (±0.01) | 3.725 (±0.02) | 3.726 (±0.01) | 3.727 (±0.02) | |
| Vegetable oil | 3.724 (±0.01) | 3.725 (±0.02) | 3.726 (±0.02) | 3.726 (±0.02) | |
| Bleach solution | 3.724 (±0.02) | 3.727 (±0.04) | 3.729(±0.03) | 3.731 (±0.05) |
| Specimen ID. | Test Substance | Initial Activation Temp. (°C) | Final Activation Temp. (°C) |
|---|---|---|---|
| HDPE98/TP2 | Control | 38.1 | 38.1 |
| Lemon juice | 38.2 | 36.2 | |
| Orange juice | 38.2 | 35.5 | |
| Tap water | 38.3 | 37.9 | |
| Hydroalcoholic solution | 38.2 | 37.6 | |
| Vegetable oil | 38.0 | 37.5 | |
| Bleach solution | 38.2 | 36.4 | |
| HDPE92/TP8 | Control | 38.1 | 38.1 |
| Lemon juice | 38.1 | 35.5 | |
| Orange juice | 38.4 | 34.2 | |
| Tap water | 38.3 | 37.6 | |
| Hydroalcoholic solution | 38.1 | 37.2 | |
| Vegetable oil | 38.2 | 36.9 | |
| Bleach solution | 38.3 | 35.9 |
| Specimen ID. | Test Substance | Time (h) | Tensile Strain (Displacement at Break) (%) (Mean ± SD) | Maximum Tensile Stress (MPa) (Mean ± SD) | Youngs Modulus (MPa) (Mean ± SD) |
|---|---|---|---|---|---|
| HDPE100/TP0 | Lemon juice | 0 | 90.27 (±7.89) | 29.85 (±1.32) | 785.83 (±12.30) |
| 24 | 85.26 (±11.23) | 28.26 (±1.48) | 741.55 (±14.41) | ||
| 48 | 80.04 (±12.31) | 28.96 (±1.13) | 716.80 (±11.32) | ||
| 72 | 103.29 (±13.76) | 28.07 (±1.54) | 710.20 (±13.85) | ||
| Orange juice | 0 | 100.84 (±7.11) | 30.80 (±1.21) | 743.49 (±12.23) | |
| 24 | 110.05 (±12.97) | 31.25 (±1.41) | 708.60 (±12.11) | ||
| 48 | 85.27(±10.11) | 32.24 (±1.56) | 760.16 (±12.56) | ||
| 72 | 80.22 (±12.45) | 33.30 (±1.43) | 797.10 (±12.87) | ||
| Tap water | 0 | 80.24 (±7.76) | 28.48 (±1.43) | 741.57 (±13.21) | |
| 24 | 100.41 (±8.56) | 30.88 (±1.76) | 715.07 (±11.99) | ||
| 48 | 101.05 (±9.98) | 29.40 (±1.34) | 782.69 (±12.54) | ||
| 72 | 100.36 (±8.09) | 30.00 (±1.32) | 786.18 (±11.13) | ||
| Hydroalcoholic solution | 0 | 86.73 (±7.09) | 28.38 (±1.21) | 727.93 (±11.83) | |
| 24 | 100.84 (±8.11) | 30.97 (±1.98) | 808.31 (±11.54) | ||
| 48 | 95.61 (±10.12) | 29.17 (±1.56) | 704.57 (±12.12) | ||
| 72 | 90.73 (±11.59) | 33.72 (±1.45) | 797.33 (±12.89) | ||
| Vegetable oil | 0 | 100.38 (±8.12) | 28.69 (±1.65) | 749.32 (±13.32) | |
| 24 | 80.22 (±11.67) | 28.35 (±1.25) | 761.54 (±14.01) | ||
| 48 | 100.37 (±12.21) | 30.31 (±1.71) | 748.11 (±12.03) | ||
| 72 | 95.49 (±9.11) | 31.47 (±2.44) | 750.82 (±13.72) | ||
| Bleach solution | 0 | 100.38 (±9.12) | 28.69 (±1.87) | 749.32 (±11.58) | |
| 24 | 95.28 (±9.78) | 29.61 (±1.94) | 782.14 (±13.91) | ||
| 48 | 90.52(±8.99) | 29.12 (±1.43) | 742.75 (±14.32) | ||
| 72 | 85.56 (±11.45) | 27.60 (±1.02) | 694.36 (±13.98) |
| Specimen ID. | Test Substance | Time (h) | Tensile Strain (Displacement at Break) (%) (Mean ± SD) | Maximum Tensile Stress (MPa) (Mean ± SD) | Youngs Modulus (MPa) (Mean ± SD) |
|---|---|---|---|---|---|
| HDPE98/TP2 | Lemon juice | 0 | 95.75 (±7.89) | 28.89 (±1.12) | 647.29 (±13.11) |
| 24 | 100.55 (±7.31) | 29.60 (±1.54) | 755.85 (±14.23) | ||
| 48 | 96.47 (±7.64) | 28.92 (±1.56) | 766.31 (±14.51) | ||
| 72 | 90.45 (±7.19) | 28.55 (±1.93) | 756.33 (±13.25) | ||
| Orange juice | 0 | 100.32 (±8.49) | 33.62 (±1.89) | 816.49 (±14.23) | |
| 24 | 95.24 (±9.34) | 32.87 (±1.54) | 784.96 (±12.65) | ||
| 48 | 96.01 (±10.24) | 31.86 (±1.53) | 765.22 (±12.13) | ||
| 72 | 102.00 (±11.32) | 30.68 (±1.72) | 688.22 (±13.45) | ||
| Tap water | 0 | 95.34 (±7.99) | 28.01 (±1.89) | 662.96 (±14.54) | |
| 24 | 90.28 (±8.63) | 29.16 (±1.31) | 704.47 (±14.39) | ||
| 48 | 85.32 (±9.65) | 31.58 (±1.65) | 708.79 (±14.32) | ||
| 72 | 80.56 (±8.35) | 31.61 (±1.42) | 724.91 (±12.65) | ||
| Hydroalcoholic solution | 0 | 86.73 (±8.65) | 28.38 (±1.89) | 727.93 (±13.90) | |
| 24 | 80.26 (±8.43) | 31.42 (±1.68) | 759.33 (±14.94) | ||
| 48 | 100.90 (±8.12) | 28.03 (±1.75) | 655.97 (±13.10) | ||
| 72 | 95.52 (±8.99) | 30.24 (±1.24) | 662.82 (±14.82) | ||
| Vegetable oil | 0 | 86.73 (±9.47) | 28.38 (±1.48) | 727.93 (±15.12) | |
| 24 | 100.66 (±9.57) | 28.66 (±1.02) | 683.2 (±12.13) | ||
| 48 | 95.55 (±8.62) | 28.66 (±1.49) | 751.81 (±12.63) | ||
| 72 | 90.52 (±9.60) | 31.67 (±1.56) | 729.66 (±12.91) | ||
| Bleach solution | 0 | 80.48 (±10.49) | 30.60 (±1.46) | 707.51 (±13.12) | |
| 24 | 114.13 (±11.94) | 28.17 (±1.95) | 721.67 (±13.34) | ||
| 48 | 100.62 (±10.34) | 31.27 (±1.59) | 751.92 (±13.89) | ||
| 72 | 90.52 (±9.87) | 31.67 (±1.50) | 729.67 (±13.24) |
| Specimen ID. | Test Substance | Time (h) | Tensile Strain (Displacement at Break) (%) (Mean ± SD) | Maximum Tensile Stress (MPa) (Mean ± SD) | Youngs Modulus (MPa) (Mean ± SD) |
|---|---|---|---|---|---|
| PR92/TP8 | Lemon juice | 0 | 85.14 (±8.81) | 27.77 (±1.21) | 679.85 (±12.13) |
| 24 | 80.24 (±8.12) | 30.02 (±1.18) | 722.47 (±13.15) | ||
| 48 | 100.56 (±8.31) | 28.63 (±1.17) | 677.96 (±13.26) | ||
| 72 | 95.22 (±8.92) | 32.83 (±1.16) | 746.61 (±13.71) | ||
| Orange juice | 0 | 95.94 (±8.15) | 27.62 (±1.15) | 693.08 (±13.11) | |
| 24 | 90.44 (±8.12) | 28.96 (±1.72) | 680.49 (±12.81) | ||
| 48 | 85.29 (±8.65) | 30.96 (±1.64) | 716.28 (±13.33) | ||
| 72 | 83.73 (±7.38) | 28.64 (±1.27) | 655.66 (±13.82) | ||
| Tap water | 0 | 100.44 (±7.19) | 27.82 (±1.61) | 711.64 (±13.10) | |
| 24 | 96.15 (±8.89) | 28.64 (±1.77) | 715.85 (±13.27) | ||
| 48 | 100.35 (±7.12) | 28.34 (±1.35) | 769.71 (±13.93) | ||
| 72 | 95.64 (±7.38) | 29.03 (±1.82) | 768.21 (±13.41) | ||
| Hydroalcoholic solution | 0 | 101.00 (±7.89) | 28.63 (±1.34) | 729.54 (±13.11) | |
| 24 | 103.65 (±7.23) | 27.99 (±1.78) | 603.05 (±13.31) | ||
| 48 | 92.97 (±7.15) | 27.76 (±1.12) | 679.39 (±13.14) | ||
| 72 | 85.87 (±7.79) | 30.46 (±1.99) | 769.73 (±13.79) | ||
| Vegetable oil | 0 | 90.27 (±7.19) | 29.85 (±1.56) | 785.83 (±13.88) | |
| 24 | 85.25 (±7.68) | 27.58 (±1.38) | 715.51 (±14.76) | ||
| 48 | 80.45 (±7.57) | 27.64 (±1.28) | 744.91 (±14.45) | ||
| 72 | 102.98 (±7.29) | 27.72 (±1.91) | 741.50 (±14.13) | ||
| Bleach solution | 0 | 100.51 (±8.96) | 30.82 (±1.56) | 700.70 (±15.43) | |
| 24 | 95.50 (±8.24) | 28.75 (±1.82) | 705.38 (±15.89) | ||
| 48 | 90.35 (±8.97) | 27.90 (±1.93) | 699.69 (±15.72) | ||
| 72 | 85.30 (±9.19) | 29.64 (±2.26) | 680.35 (±15.11) |
| Specimen ID. | Test Substances | Time (h) | Impact Strength (kJ/m2) Unnotched (Mean ± SD) | Impact Strength (kJ/m2) Notched (Mean ± SD) |
|---|---|---|---|---|
| HDPE100/TP0 | Lemon juice | 0 | 99.63 (±1.82) | 23.48 (±1.56) |
| 24 | 99.48 (±1.56) | 23.35 (±1.34) | ||
| 48 | 99.38 (±1.55) | 23.28 (±2.00) | ||
| 72 | 99.28 (±1.98) | 23.15 (±2.56) | ||
| Orange juice | 0 | 99.43 (±1.46) | 23.43 (±1.56) | |
| 24 | 99.38 (±1.76) | 23.28 (±1.65) | ||
| 48 | 99.30 (±2.09) | 23.23 (±1.32) | ||
| 72 | 99.20 (±2.98) | 23.10 (±2.86) | ||
| Tap water | 0 | 99.58 (±1.47) | 23.44 (±1.45) | |
| 24 | 99.43 (±1.95) | 23.38 (±1.72) | ||
| 48 | 99.40 (±1.63) | 23.37 (±1.88) | ||
| 72 | 99.38 (±1.79) | 23.33 (±1.56) | ||
| Hydroalcoholic solution | 0 | 99.68 (±2.56) | 23.36 (±1.06) | |
| 24 | 99.63 (±2.99) | 23.32 (±1.34) | ||
| 48 | 99.63 (±2.66) | 23.28 (±1.32) | ||
| 72 | 99.60 (±2.56) | 23.24 (±1.46) | ||
| Vegetable oil | 0 | 99.88 (±1.57) | 23.33 (±1.21) | |
| 24 | 99.80 (±1.88) | 23.26 (±1.32) | ||
| 48 | 99.78 (±1.65) | 23.21 (±1.11) | ||
| 72 | 99.70 (±1.70) | 23.18 (±1.26) | ||
| Bleach solution | 0 | 99.75 (±1.57) | 23.48 (±1.26) | |
| 24 | 99.70 (±1.64) | 23.37 (±1.45) | ||
| 48 | 99.60 (±1.87) | 23.31 (±1.32) | ||
| 72 | 99.56 (±2.05) | 23.21 (±2.13) |
| Specimen ID. | Test Substance | Time (h) | Impact Strength (kJ/m2) Unnotched (Mean ± SD) | Impact Strength (kJ/m2) Notched (Mean ± SD) |
|---|---|---|---|---|
| HDPE98/TP2 | Lemon juice | 0 | 99.68 (±1.87) | 23.48 (±2.56) |
| 24 | 99.40 (±1.99) | 23.35 (±1.34) | ||
| 48 | 99.30 (±1.88) | 23.28 (±2.00) | ||
| 72 | 99.18 (±2.10) | 22.77 (±2.56) | ||
| Orange juice | 0 | 99.48 (±2.11) | 23.44 (±2.42) | |
| 24 | 99.18 (±2.54) | 23.21 (±2.16) | ||
| 48 | 99.20 (±2.96) | 22.79 (±2.57) | ||
| 72 | 99.13 (±2.56) | 22.12 (±3.06) | ||
| Tap water | 0 | 99.60 (±1.80) | 23.44 (±1.36) | |
| 24 | 99.55 (±2.01) | 23.38 (±1.75) | ||
| 48 | 99.48 (±2.29) | 23.25 (±1.43) | ||
| 72 | 99.38 (±1.35) | 23.13 (±1.20) | ||
| Hydroalcoholic solution | 0 | 99.48 (±2.56) | 23.43 (±1.16) | |
| 24 | 99.46 (±2.43) | 23.16 (±1.22) | ||
| 48 | 99.40 (±2.42) | 23.14 (±1.34) | ||
| 72 | 99.35 (±2.76) | 23.02 (±1.97) | ||
| Vegetable oil | 0 | 99.65 (±2.67) | 23.32 (±1.67) | |
| 24 | 99.55 (±2.45) | 23.22 (±1.98) | ||
| 48 | 99.52 (±2.90) | 23.19 (±2.06) | ||
| 72 | 99.45 (±2.56) | 23.14 (±2.33) | ||
| Bleach solution | 0 | 99.60 (±2.67) | 23.31 (±2.46) | |
| 24 | 99.50 (±2.84) | 23.26 (±1.45) | ||
| 48 | 99.40 (±3.57) | 23.12 (±2.64) | ||
| 72 | 99.25 (±2.89) | 22.98 (±2.86) |
| Specimen ID. | Test Substance | Time (h) | Impact Strength (kJ/m2) Unnotched (Mean ± SD) | Impact Strength (kJ/m2) Notched (Mean ± SD) |
|---|---|---|---|---|
| HDPE92/TP8 | Lemon juice | 0 | 99.38 (±2.03) | 23.21 (±3.16) |
| 24 | 99.20 (±2.45) | 22.71 (±3.29) | ||
| 48 | 99.11 (±2.54) | 22.31 (±3.11) | ||
| 72 | 99.02 (±2.76) | 22.14 (±3.86) | ||
| Orange juice | 0 | 99.08 (±2.54) | 22.58 (±3.44) | |
| 24 | 98.95 (±2.55) | 22.11 (±3.66) | ||
| 48 | 98.55 (±2.78) | 21.64 (±3.12) | ||
| 72 | 98.13 (±3.10) | 21.02 (±3.96) | ||
| Tap water | 0 | 99.63 (±2.12) | 23.34 (±2.35) | |
| 24 | 99.58 (±2.93) | 23.18 (±2.15) | ||
| 48 | 99.50 (±2.56) | 23.02 (±2.05) | ||
| 72 | 99.50 (±2.74) | 23.00 (±2.03) | ||
| Hydroalcoholic solution | 0 | 99.63 (±2.45) | 23.12 (±1.06) | |
| 24 | 99.58 (±2.63) | 23.06 (±1.22) | ||
| 48 | 99.50 (±2.91) | 23.04 (±1.34) | ||
| 72 | 99.48 (±2.44) | 23.02 (±1.97) | ||
| Vegetable oil | 0 | 99.85 (±2.56) | 23.45 (±1.97) | |
| 24 | 99.80 (±2.76) | 23.40 (±1.96) | ||
| 48 | 99.73 (±2.77) | 23.29 (±2.31) | ||
| 72 | 99.68 (±2.94) | 23.34 (±2.43) | ||
| Bleach solution | 0 | 99.85 (±3.13) | 23.31 (±2.56) | |
| 24 | 99.54 (±3.01) | 23.06 (±1.54) | ||
| 48 | 99.23 (±3.54) | 22.92 (±2.01) | ||
| 72 | 98.97 (±3.56) | 22.64 (±2.86) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breheny, C.; Colbert, D.M.; Bezerra, G.; Geever, J.; Geever, L.M. Evaluating the Chemical Resistance and Performance of Thermochromic Polymers for Food Packaging. Materials 2025, 18, 2085. https://doi.org/10.3390/ma18092085
Breheny C, Colbert DM, Bezerra G, Geever J, Geever LM. Evaluating the Chemical Resistance and Performance of Thermochromic Polymers for Food Packaging. Materials. 2025; 18(9):2085. https://doi.org/10.3390/ma18092085
Chicago/Turabian StyleBreheny, Colette, Declan Mary Colbert, Gilberto Bezerra, Joseph Geever, and Luke M. Geever. 2025. "Evaluating the Chemical Resistance and Performance of Thermochromic Polymers for Food Packaging" Materials 18, no. 9: 2085. https://doi.org/10.3390/ma18092085
APA StyleBreheny, C., Colbert, D. M., Bezerra, G., Geever, J., & Geever, L. M. (2025). Evaluating the Chemical Resistance and Performance of Thermochromic Polymers for Food Packaging. Materials, 18(9), 2085. https://doi.org/10.3390/ma18092085







