Understanding the Non-Steady Electrochemical Mechanisms of the Stress Corrosion Cracking of X70 Pipeline Steel in a Marine Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Solutions
2.2. Electrochemical Test
2.3. SSRT Test
3. Results
3.1. Potentiodynamic Polarization Curve
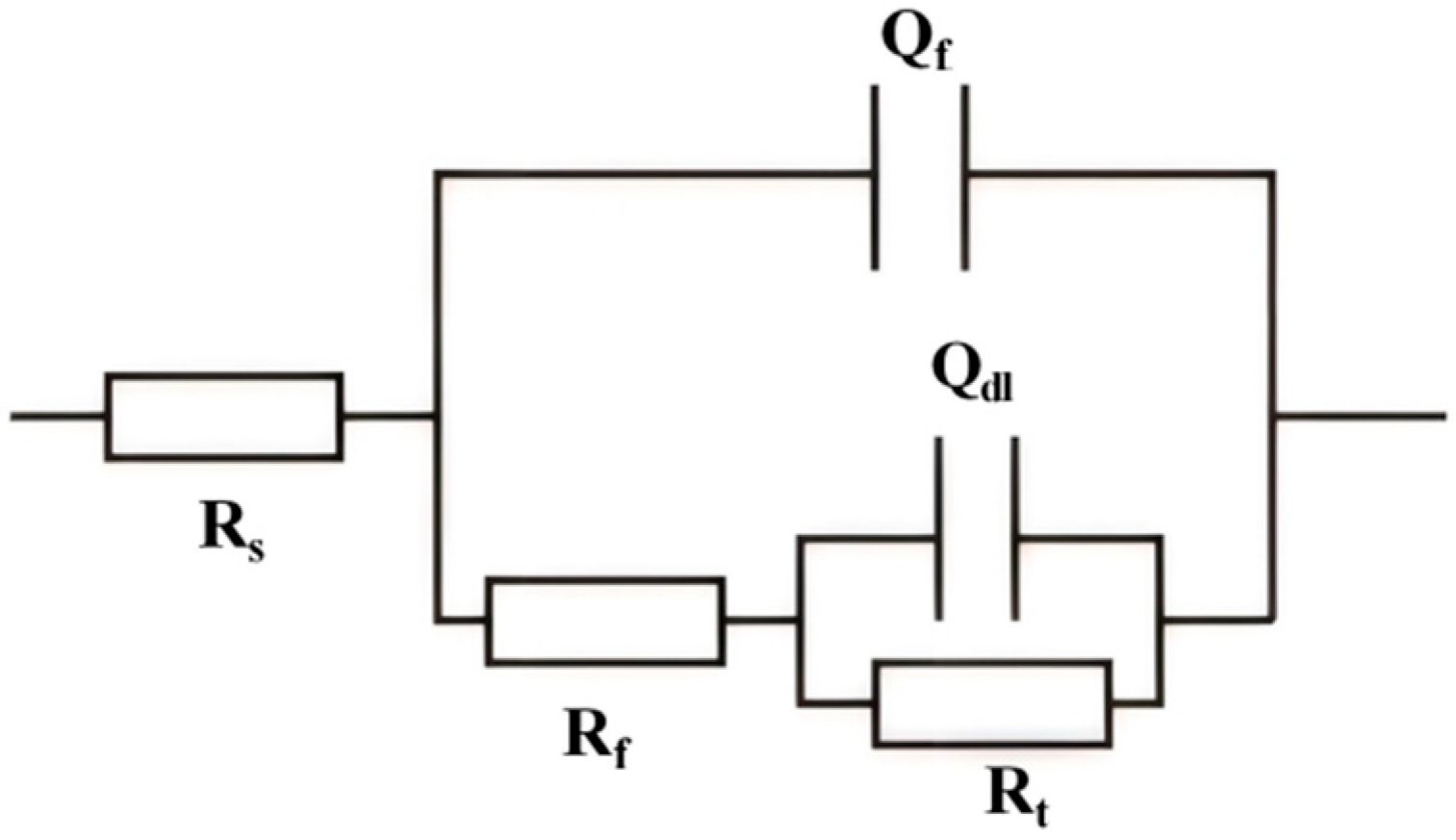
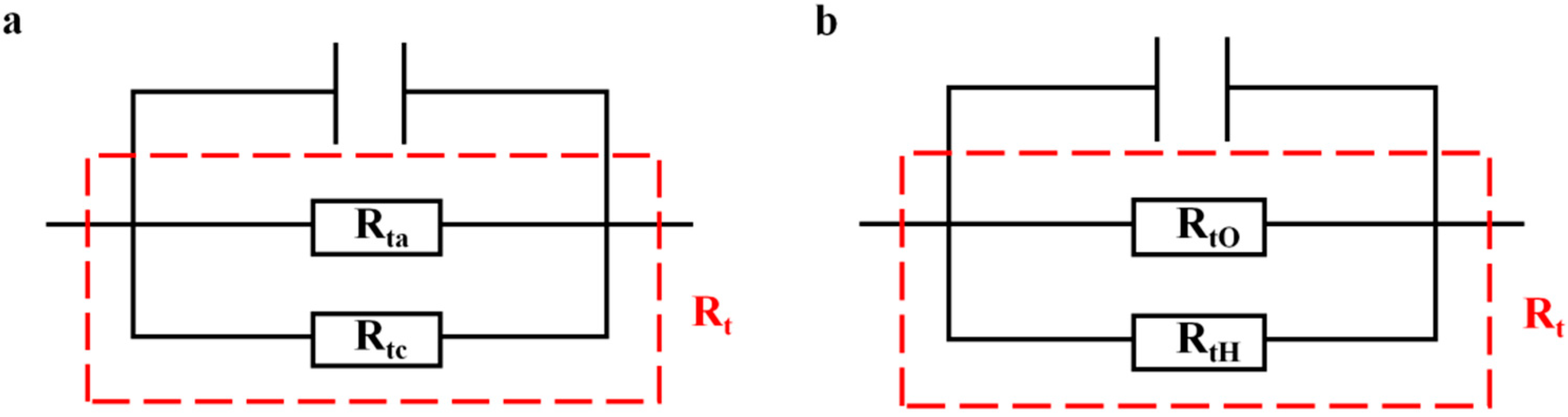
3.2. EIS Tests
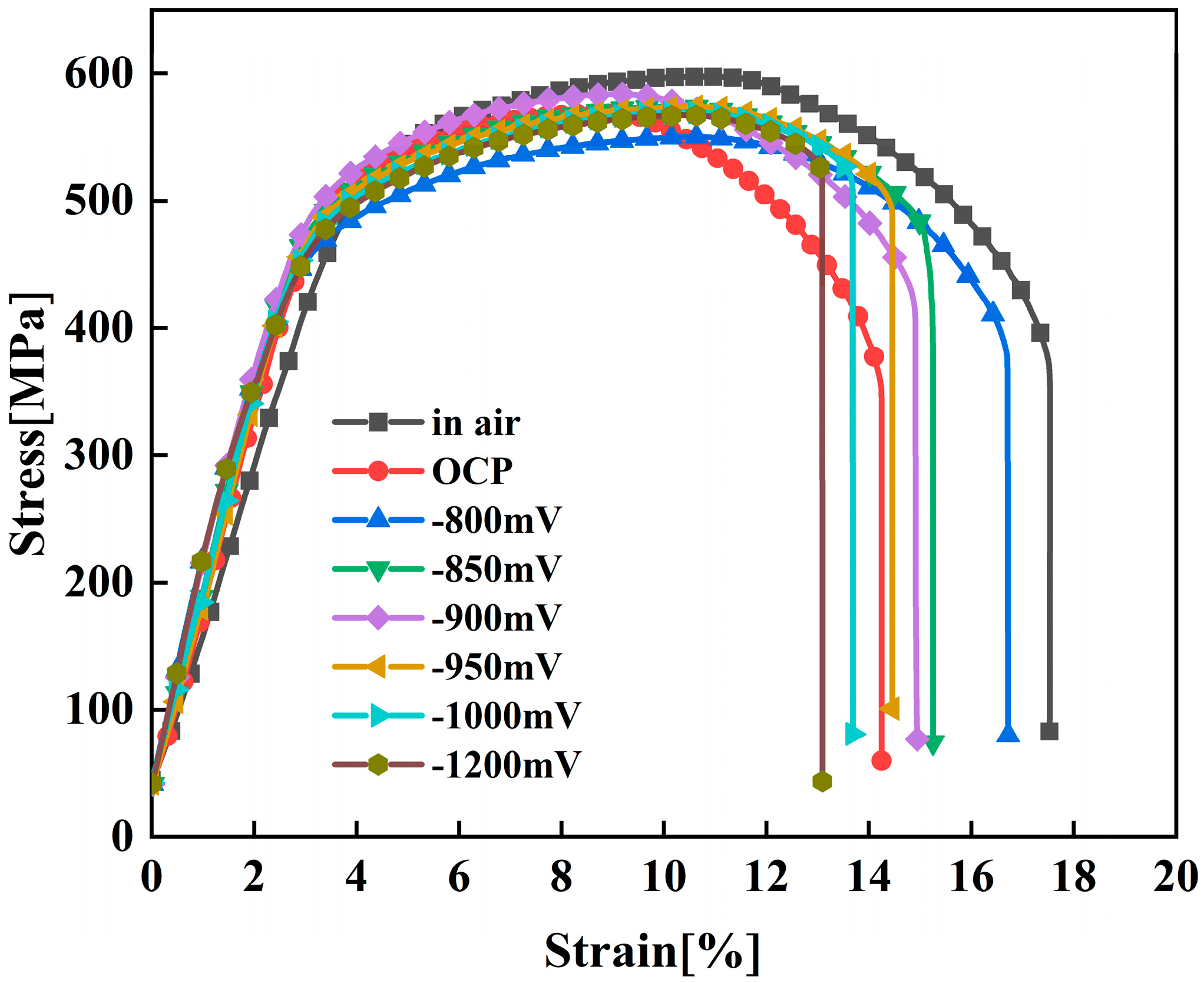
3.3. SSRT Tests
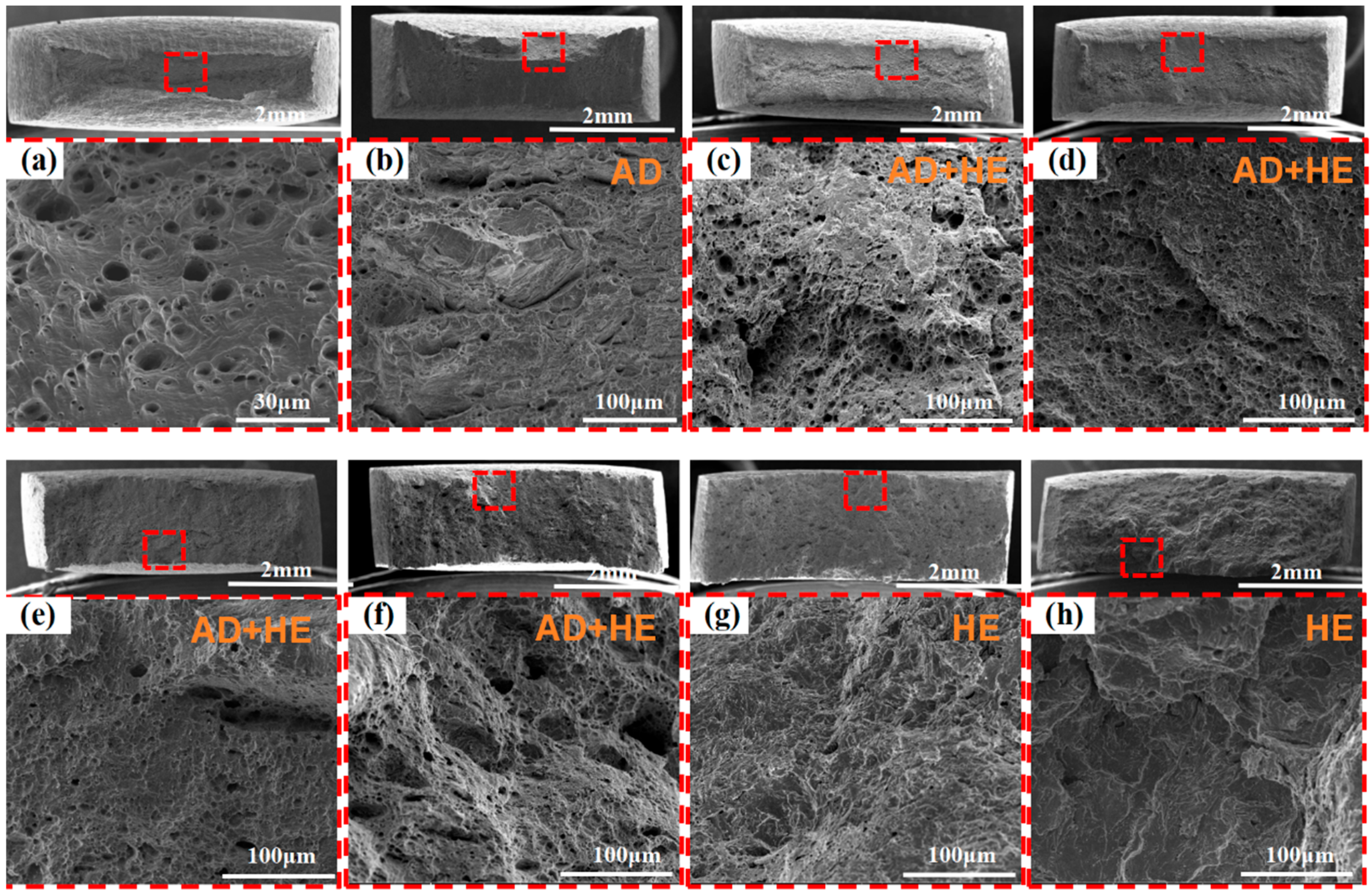
3.4. Fracture Morphology
4. Discussion
4.1. The Stress Corrosion Cracking Mechanism of X70 Pipeline Steel
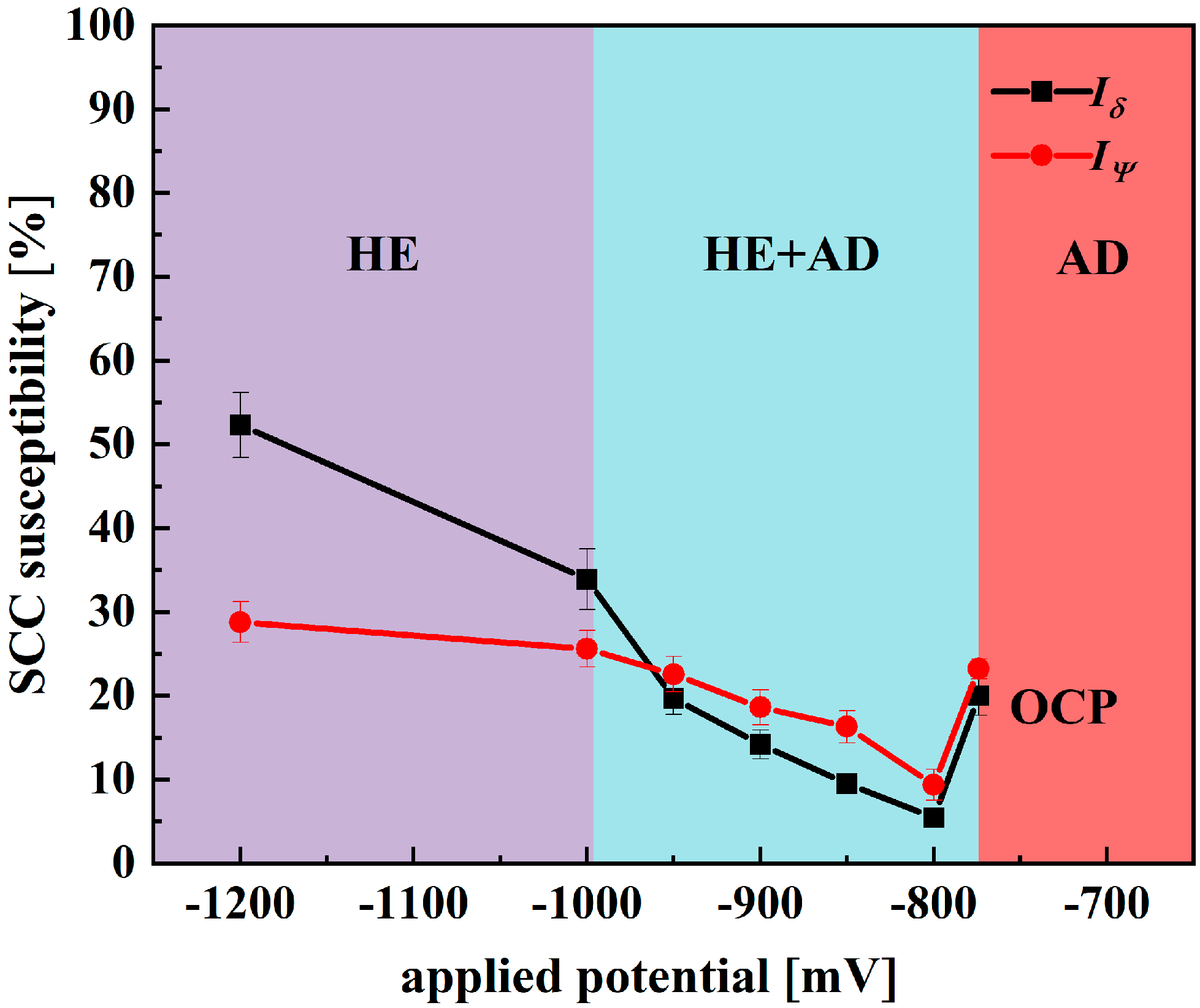
4.2. SCC Evaluation of X70 Pipeline Steel in Simulated Seawater Environment
5. Conclusions
- (1)
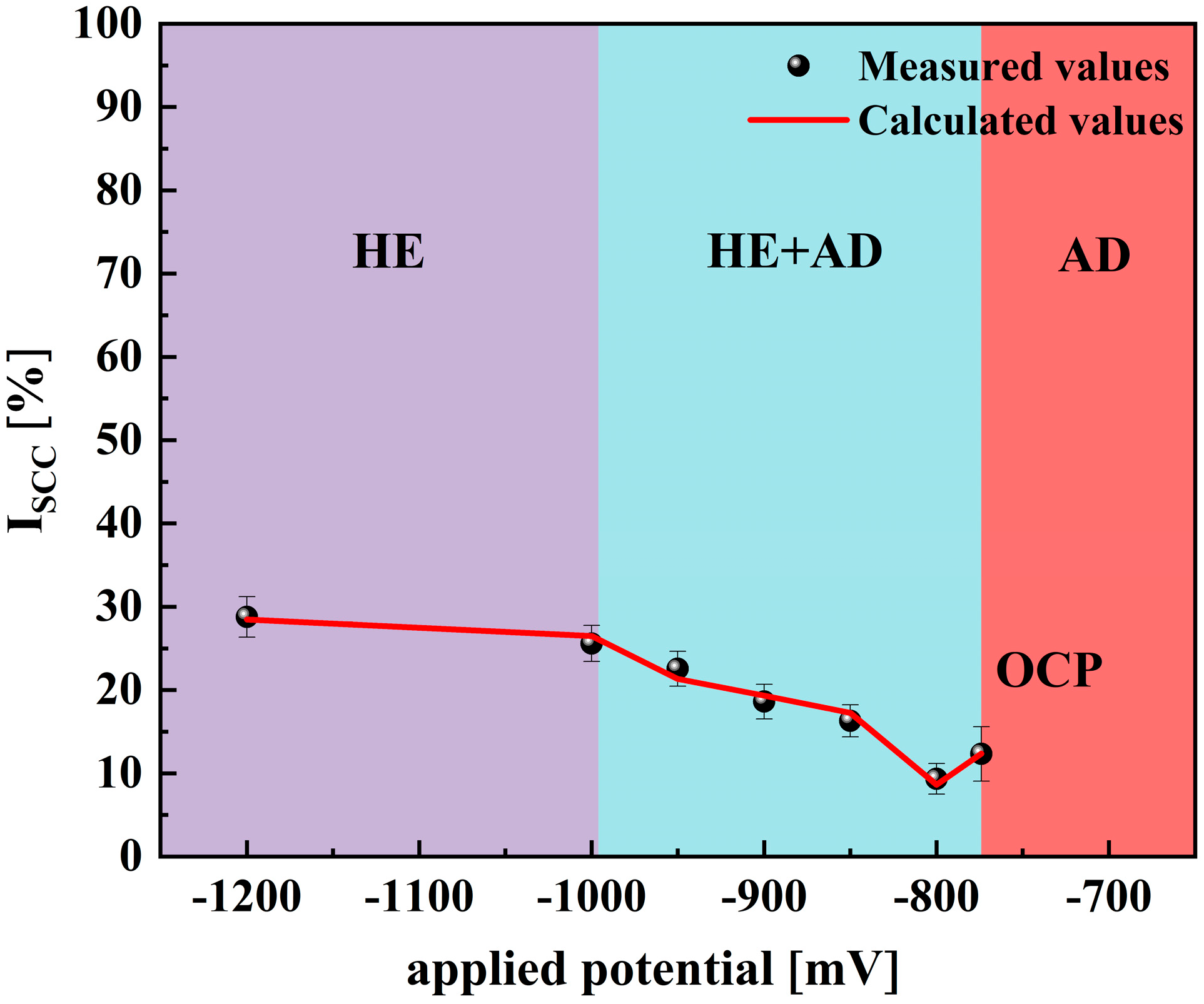
- In this study, it was found that the unsteady-state electrochemical model is also applicable to the rapid assessment of the stress corrosion of X70 pipeline steel under marine conditions. Relying only on electrochemical tests and a limited number of SSRT experiments to rapidly assess SCC susceptibility over the entire potential range, the model is easy to use and highly accurate.
- (2)
- X70—the mechanism of SCC non-stationary electrochemistry in simulated seawater environments depends on the applied potentials. Between −437 mV and −774 mV, SCC susceptibility is low and is controlled by AD. Between −774 mV and −996 mV, the increase in SCC susceptibility shifts to be controlled by both AD and HE, and when the potential falls below −996 mV, SCC susceptibility increases dramatically and is controlled by HE.
- (3)
- The model offers great potential for application in pipeline design, maintenance planning, and risk assessment frameworks, thus contributing to safer and more economically viable offshore infrastructure. Future research should focus on extending this approach to a wider range of pipeline steels such as X52, X65, and X100 to determine its general applicability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCC | Stress corrosion cracking |
| AD | Anodic dissolution |
| HE | Hydrogen embrittlement |
| CP | Cathodic protection |
| OCP | Open circuit potential |
| DFT | Density functional theory |
| EIS | Electrochemical impedance spectroscopy |
| SSRT | Slow strain rate testing |
| SEM | Scanning electron microscope |
| SE | Secondary electron |
| WD | Working distance |
| HER | Hydrogen evolution reaction |
| Rs | Solution resistance |
| Rf | Film resistance |
| Qf | Film constant phase element |
| Qdl | Double-layer constant phase element |
| Rt | Charge transfer resistance |
| Rta | Anodic charge transfer resistance |
| Rtc | Cathodic charge transfer resistance |
| RtO | Charge transfer resistance of oxygen reduction reaction |
| RtH | Charge transfer resistance of hydrogen evolution reaction |
| SCC susceptibility (the loss rate of elongation) | |
| SCC susceptibility (the reduction rate of area) | |
| The elongation measured in air | |
| The elongation measured in various potentials | |
| The reduction in the area measured in air | |
| The reduction in the area measured in various potentials | |
| Ka,Khe,Kad,Kc | A constant that varies with the current density, corrosion medium, and material |
| is | Current density obtained from the slow-sweep polarization curve |
| If | Current density obtained from the fast-sweep polarization curve |
| is,corr | Corrosion current density measured from the slow-sweep curve |
| I0 | The standard SCC index |
| Iac | A parameter that captures the synergistic effects of anodic dissolution and hydrogen embrittlement |
| Ic | The standard SCC index dominated by hydrogen embrittlement under identical experimental conditions |
| ISCC | The standard SCC index |
References
- Ohaeri, E.; Eduok, U.; Szpunar, J. Hydrogen related degradation in pipeline steel: A review. Int. J. Hydrogen Energy 2018, 43, 14584–14617. [Google Scholar] [CrossRef]
- Tekkaya, B.; Dölz, M.; Münstermann, S. Modeling of local hydrogen concentration on microscopic scale to characterize the influence of stress states and non-metallic inclusions in pipeline steels. Int. J. Hydrogen Energy 2024, 50, 1274–1287. [Google Scholar] [CrossRef]
- Tezdogan, T.; Demirel, Y.K. An Overview of Marine Corrosion Protection with a Focus on Cathodic Protection and Coatings. Brodogradnja 2014, 65, 49–59. [Google Scholar]
- Thodla, R.; Sridhar, N.; Amaya, H.; Fahimi, B.; Taylor, C. Hydrogen Embrittlement of Low Alloy Steels Under Cathodic Polarization. Corrosion 2020, 76, 299–311. [Google Scholar] [CrossRef]
- Ma, H.C.; Sun, L.Y.; Luo, H.; Li, X.G. Hydrogen embrittlement of high-strength marine steel as a weld joint in artificial seawater under cathodic polarization. Eng. Fail. Anal. 2022, 134, 105861. [Google Scholar] [CrossRef]
- Sun, B.Z.; Huang, X.K.; Pan, Y.; Yan, T.T.; Zhang, Y.X.; Sun, M.X.; Liu, Z.Y.; Fan, L.; Li, X.G. A comparative study on the passive film and SCC behavior of Ti-6Al-3Nb-2Zr-1Mo alloy at various test temperatures in simulated seawater. Corros. Sci. 2024, 233, 112046. [Google Scholar] [CrossRef]
- Wu, S.X.; Gao, Z.M.; Liu, Y.J.; Hu, W.B. Effect of cathodic protection potential on stress corrosion susceptibility of X80 steel. Corros. Sci. 2023, 218, 111174. [Google Scholar] [CrossRef]
- Li, S.L.; Zhang, L.G.; Wang, Y.; Hu, P.Y.; Jiang, N.; Guo, P.; Wang, X.D.; Feng, H. Effect of cathodic protection current density on corrosion rate of high-strength steel wires for stay cable in simulated dynamic marine atmospheric rainwater. Structures 2021, 29, 1655–1670. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, X.Z.; Du, C.W.; Li, J.K.; Li, X.G. Effect of hydrogen-induced plasticity on the stress corrosion cracking of X70 pipeline steel in simulated soil environments. Mater. Sci. Eng. A 2016, 658, 348–354. [Google Scholar] [CrossRef]
- Ramamurthy, S.; Atrens, A. Stress corrosion cracking of high-strength steels. Corros. Rev. 2013, 31, 1–31. [Google Scholar] [CrossRef]
- Sun, D.X.; Wu, M.; Xie, F. Effect of sulfate-reducing bacteria and cathodic potential on stress corrosion cracking of X70 steel in sea-mud simulated solution. Mater. Sci. Eng. A 2018, 721, 135–144. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Lu, L.; Huang, Y.Z.; Du, C.W.; Li, X.G. Mechanistic Aspect of Non-Steady Electrochemical Characteristic During Stress Corrosion Cracking of an X70 Pipeline Steel in Simulated Underground Water. Corrosion 2014, 70, 678–685. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B.B. External corrosion of oil and gas pipelines: A review of failure mechanisms and predictive preventions. J. Nat. Gas Sci. Eng. 2022, 100, 104467. [Google Scholar] [CrossRef]
- Sun, B.Z.; Wang, Q.Y.; Pan, Y.; Liu, Z.Y.; Du, C.W.; Li, X.G. Understanding the non-steady electrochemical mechanism on SCC of 304 SS under applied polarization potentials. Corros. Sci. 2024, 227, 111752. [Google Scholar] [CrossRef]
- Ohaeri, E.G. Enhancing the Corrosion Resistance of API 5L X70 Pipeline Steel Through Thermomechanically Controlled Processing. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2020. [Google Scholar]
- Catledge, K.E. Effect of Electrochemical Potential on Stress Corrosion Cracking of Age-Hardenable Al-Mg-Si Alloy 6111. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2023. [Google Scholar]
- Cui, C.J.; Ma, R.J.; Martinez-Paneda, E. A generalised, multi-phase-field theory for dissolution-driven stress corrosion cracking and hydrogen embrittlement. J. Mech. Phys. Solids 2022, 166, 104958. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Mechanistic aspect of near-neutral pH stress corrosion cracking of pipelines under cathodic polarization. Corros. Sci. 2012, 55, 54–60. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, B.Z.; Liu, Z.Y.; Wu, W.; Li, X.G. Hydrogen effects on passivation and SCC of 2205 DSS in acidified simulated seawater. Corros. Sci. 2022, 208, 110649. [Google Scholar] [CrossRef]
- Ma, H.C.; Liu, Z.Y.; Du, C.W.; Wang, H.R.; Li, C.Y.; Li, X.G. Effect of cathodic potentials on the SCC behavior of E690 steel in simulated seawater. Mater. Sci. Eng. A 2015, 642, 22–31. [Google Scholar] [CrossRef]
- Sun, F.L.; Ren, S.; Li, Z.; Liu, Z.Y.; Li, X.G.; Du, C.W. Comparative study on the stress corrosion cracking of X70 pipeline steel in simulated shallow and deep sea environments. Mater. Sci. Eng. A 2017, 685, 145–153. [Google Scholar] [CrossRef]
- Chen, X.; Xie, F.; Wang, D.; Sun, D.X.; Wu, M.; Li, Y.C. Hydrogen-induced cracking of X70 steel affected by sulfate-reducing bacteria and cathodic potential: Experiment and density functional theory study. Int. J. Hydrogen Energy 2024, 49, 798–810. [Google Scholar] [CrossRef]
- ASTM D1141-98; Standard Practice for Preparation of Substitute Ocean Water; (Reapproved 2021). ASTM International: West Conshohocken, PA, USA, 2021.
- GB/T 15970.10-2021; Corrosion of Metals and Alloys—Stress Corrosion Testing—Part 10: Reverse U-Bend Method. Standardization Administration of China: Beijing, China, 2021.
- Liu, Z.Y.; Li, Q.; Cui, Z.Y.; Wu, W.; Li, Z.; Du, C.W.; Li, X.G. Field experiment of stress corrosion cracking behavior of high strength pipeline steels in typical soil environments. Constr. Build. Mater. 2017, 148, 131–139. [Google Scholar] [CrossRef]
- Song, L.F.; Liu, Z.Y.; Hu, J.P.; Li, X.G.; Du, C.W.; Li, Y.; Pan, Y. Stress Corrosion Cracking of 2205 Duplex Stainless Steel with Simulated Welding Microstructures in Simulated Sea Environment at Different Depths. J. Mater. Eng. Perform. 2020, 29, 5476–5489. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, B.Z.; Chen, H.T.; Liu, Z.Y.; Dai, W.H.; Yang, X.J.; Yang, W.T.; Deng, Y.D.; Li, X.G. Stress corrosion cracking behavior and mechanism of 2205 duplex stainless steel under applied polarization potentials. Corros. Sci. 2024, 231, 111985. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Zhang, Y.; Du, C.W.; Zuo, G.L. Relationship between electrochemical characteristics and SCC of X70 pipeline steel in an acidic soil simulated solution. Acta Metall. Sin. 2009, 22, 58–64. [Google Scholar] [CrossRef]
- Javidi, M.; Horeh, S.B. Investigating the mechanism of stress corrosion cracking in near-neutral and high pH environments for API 5L X52 steel. Corros. Sci. 2014, 80, 213–220. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Du, C.; Huang, Y. Effect of applied potentials on stress corrosion cracking of X70 pipeline steel in alkali solution. Mater. Des. 2009, 30, 2259–2263. [Google Scholar] [CrossRef]
- Sun, B.Z.; Pan, Y.; Yang, J.K.; Guo, J.; Zhao, B.; Liu, X.; Liu, Z.Y.; Li, X.G. Microstructure evolution and SSCC behavior of strain-strengthened 304 SS pre-strained at room temperature and cryogenic temperature. Corros. Sci. 2023, 210, 110830. [Google Scholar] [CrossRef]
- Li, Z.; Sun, B.Z.; Liu, Q.; Yu, Y.F.; Liu, Z.Y. Fundamentally understanding the effect of Non-stable cathodic potential on stress corrosion cracking of pipeline steel in Near-neutral pH solution. Constr. Build. Mater. 2021, 288, 123104. [Google Scholar] [CrossRef]
- Ren, X.; Chu, W.; Su, Y.; Li, J.; Qiao, L.; Jiang, B.; Zhang, M.; Chen, G. The effects of atomic hydrogen and flake on mechanical properties of a tyre steel. Mater. Sci. Eng. A 2008, 491, 164–171. [Google Scholar] [CrossRef]
- Francis, R.; Byrne, G.; Warburton, G. Effects of cathodic protection on duplex stainless steels in seawater. Corrosion 1997, 53, 234–240. [Google Scholar] [CrossRef]
- Arafin, M.A.; Szpunar, J.A. Effect of bainitic microstructure on the susceptibility of pipeline steels to hydrogen induced cracking. Mater. Sci. Eng. A 2011, 528, 4927–4940. [Google Scholar] [CrossRef]
- Banerjee, K.; Chatterjee, U. Hydrogen permeation and hydrogen content under cathodic charging in HSLA 80 and HSLA 100 steels. Scr. Mater. 2001, 44, 213–216. [Google Scholar] [CrossRef]
- Behvar, A.; Haghshenas, M.; Djukic, M.B. Hydrogen embrittlement and hydrogen-induced crack initiation in additively manufactured metals: A critical review on mechanical and cyclic loading. Int. J. Hydrogen Energy 2024, 58, 1214–1239. [Google Scholar] [CrossRef]
- Needham, W.D. Stress corrosion cracking and hydrogen embrittlement of thick section high strength low alloy steel. Ph.D Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1986. [Google Scholar]
- Yu, H.Y.; Díaz, A.; Lu, X.; Sun, B.H.; Ding, Y.; Koyama, M.; He, J.Y.; Zhou, X.; Oudriss, A.; Feaugas, X.; et al. Hydrogen Embrittlement as a Conspicuous Material Challenge—Comprehensive Review and Future Directions. Chem. Rev. 2024, 124, 6271–6392. [Google Scholar] [CrossRef]
- Sun, H.J.; Xue, W.H.; Xu, J.X.; Chen, G.L.; Sun, J. Cathodic protection criteria of low alloy steel in simulated deep water environment. Anti-Corros. Methods Mater. 2020, 67, 427–434. [Google Scholar] [CrossRef]
- Li, C.J.; Du, M. Optimizing the cathodic protection system of pipeline under simulating thermocline environment in deep water by finite element method. Mater. Corros. 2017, 68, 868–875. [Google Scholar] [CrossRef]
- Gali, S.; Sharma, D.; Subramaniam, K.V.L. Influence of Steel Fibers on Fracture Energy and Shear Behavior of SCC. J. Mater. Civ. Eng. 2018, 30, 04018300. [Google Scholar] [CrossRef]
- Qu, H.Z.J.; Tao, F.; Gu, N.A.J.; Montoya, T.; Taylor, J.M.; Schaller, R.F.; Schindelholz, E.; Wharry, J.P. Crystallographic effects on transgranular chloride-induced stress corrosion crack propagation of arc welded austenitic stainless steel. Npj Mater. Degrad. 2022, 6, 9. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, B.; Wang, L.; Liu, Z.; Jiang, B.; Yang, W.; Deng, Y.; Li, X. Investigating the correlation of microstructure with stress corrosion cracking of the high-temperature heat treated 2205 duplex stainless steel. J. Mater. Res. Technol. 2025, 35, 6280–6295. [Google Scholar] [CrossRef]
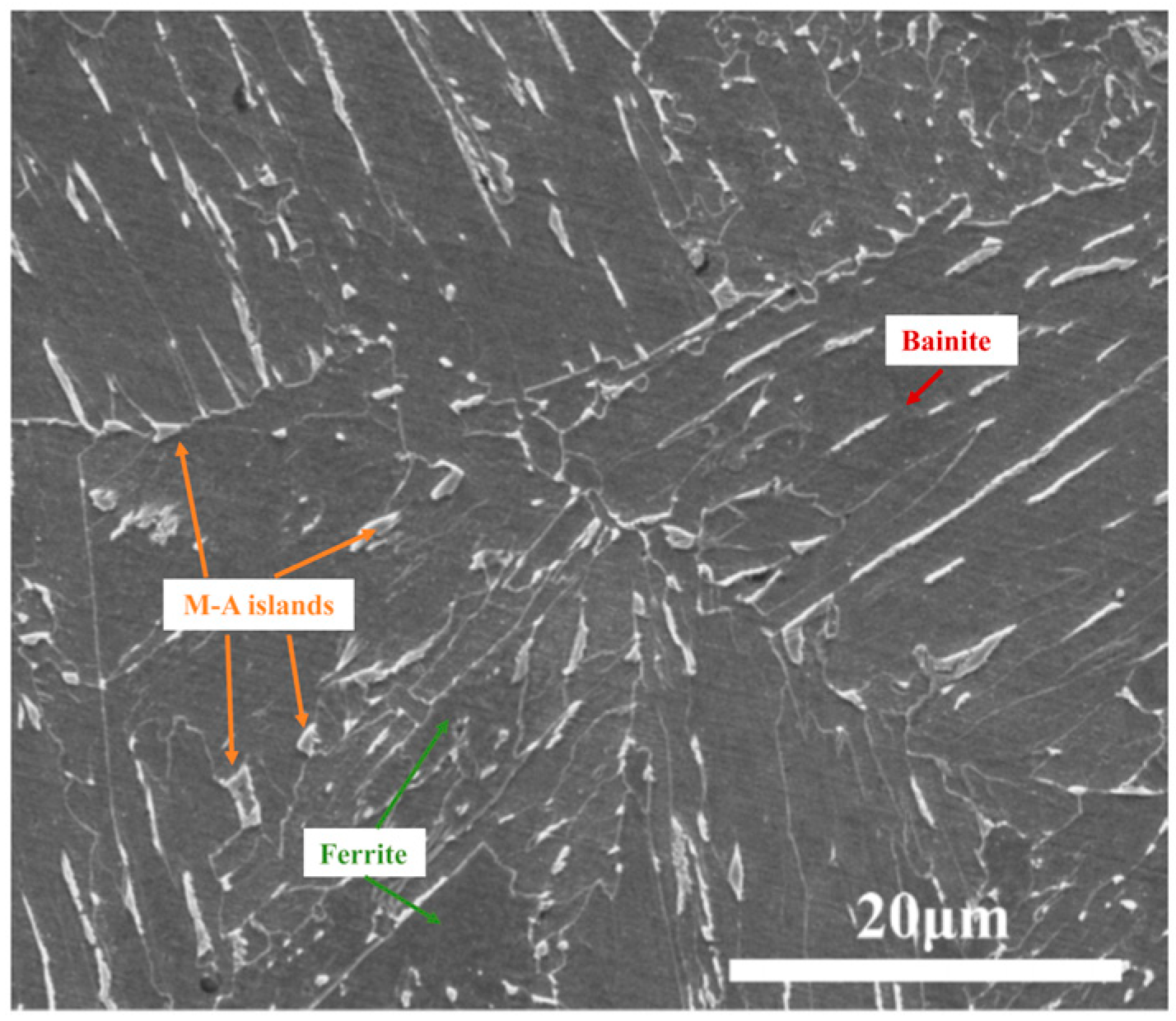

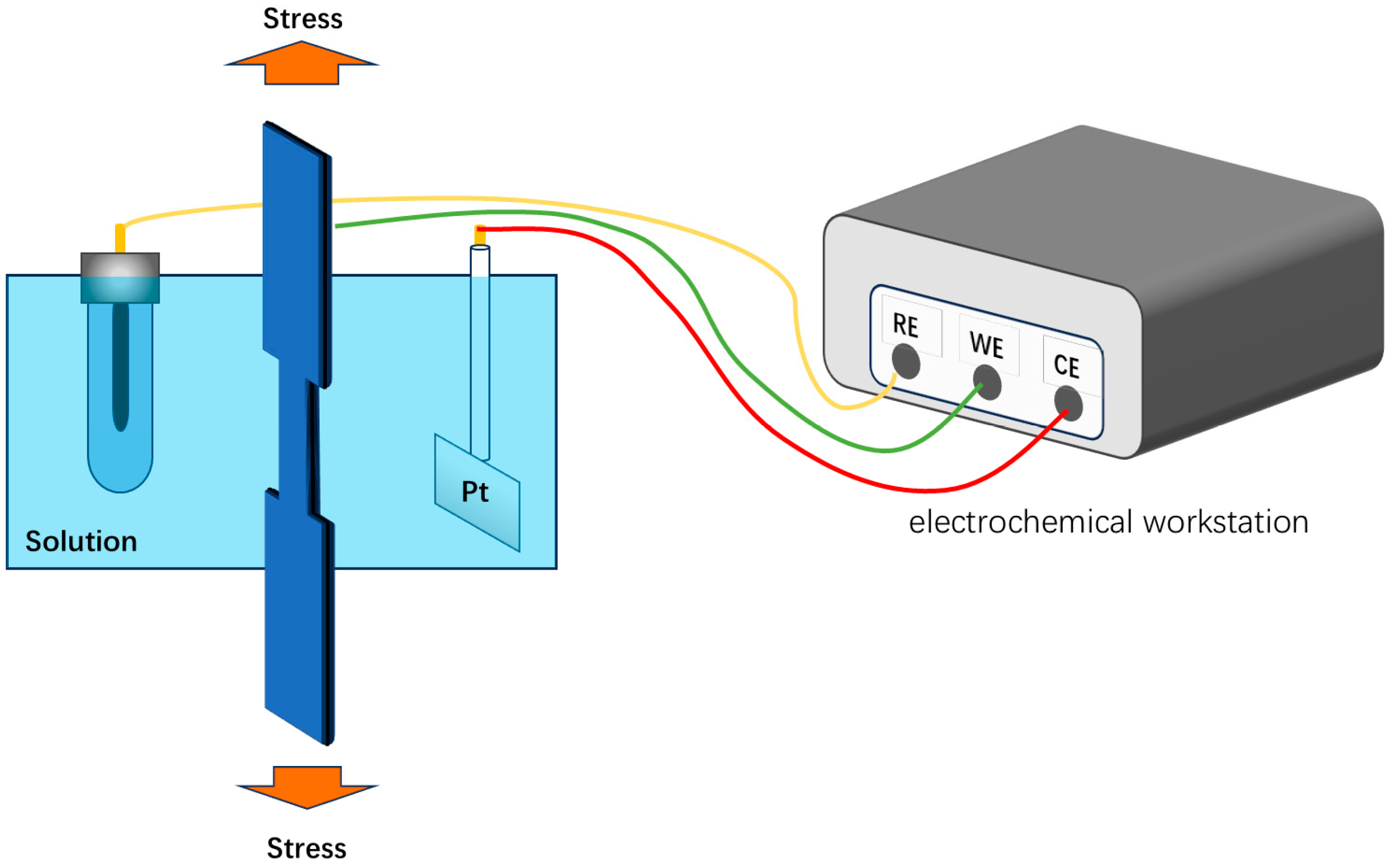

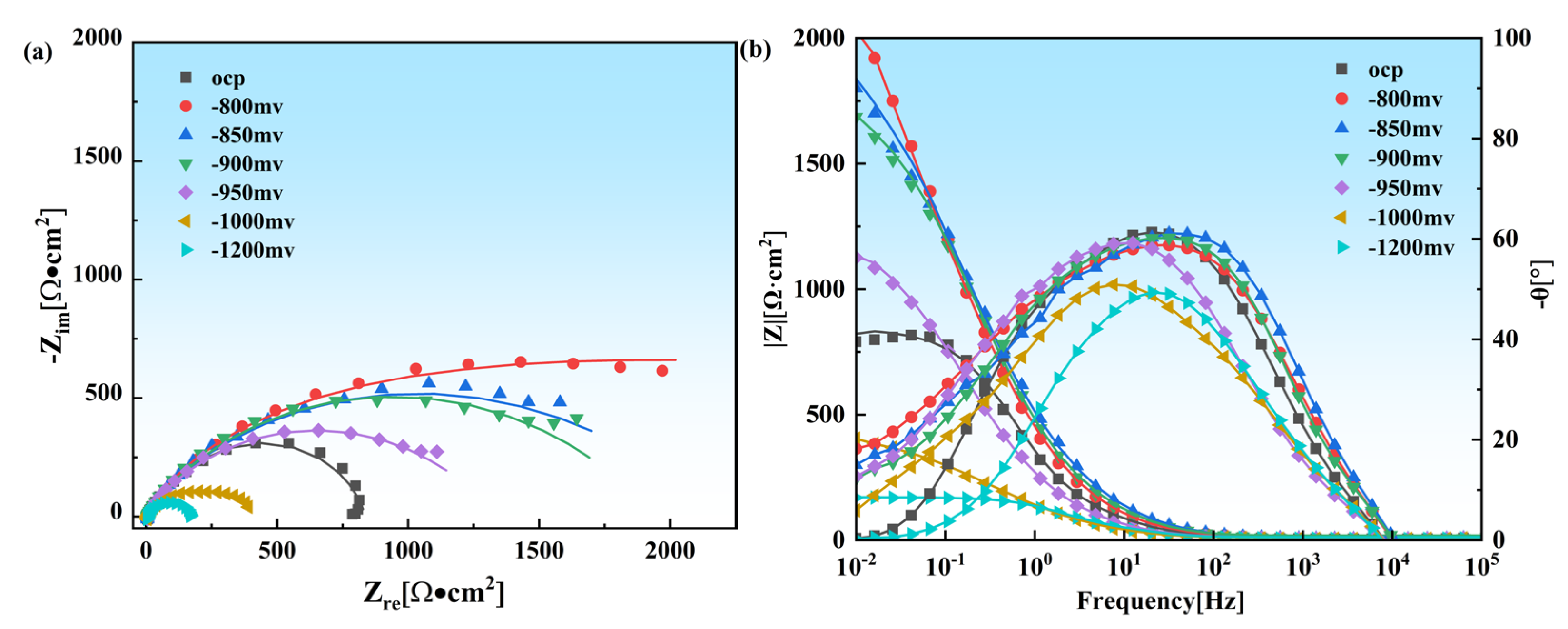


| C | Si | Mn | P | S | Cr | Ni | Mo | Nb | V | Ti | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.044 | 0.26 | 1.13 | 0.004 | 0.002 | 0.27 | 0.13 | 0.16 | 0.06 | 0.02 | 0.015 | 0.005 | Bal. |
| Elongation [%] | Yield Strength [MPa] | Tensile Strength [MPa] | Impact Energy [J] | Brinell Hardness [HB] |
|---|---|---|---|---|
| ≥16 | ≥485 | 570–760 | ≥120 | ≤255 |
| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | H2BO3 |
|---|---|---|---|---|---|---|---|
| 24.53 | 5.2 | 4.09 | 1.16 | 0.695 | 0.201 | 0.101 | 0.027 |
| Conditions | Specimen Number | Area Reduction [%] | [%] | Elongation [%] | [%] | Yield Strength [MPa] | Tensile Strength [MPa] |
|---|---|---|---|---|---|---|---|
| In air | 1# | 53.61 | 17.64 | 508.96 | 595.75 | ||
| 2# | 54.24 | 17.5 | 505.61 | 591.03 | |||
| 3# | 52.89 | 17.93 | 506.34 | 594.17 | |||
| At OCP | 4# | 43.9 | 19.99 ± 2.36 | 13.38 | 23.19 ± 1.21 | 473.27 | 563.84 |
| 5# | 43.3 | 13.47 | 476.95 | 567.52 | |||
| 6# | 41.47 | 13.79 | 469.76 | 558.91 | |||
| −800 mV | 7# | 51.03 | 5.47 ± 0.94 | 16.13 | 9.34 ± 1.84 | 486.07 | 546.57 |
| 8# | 50.68 | 15.61 | 487.64 | 550.79 | |||
| 9# | 50.31 | 16.23 | 489.40 | 552.88 | |||
| −850 mV | 10# | 49.12 | 9.51 ± 1.17 | 15.03 | 16.30 ± 1.93 | 492.94 | 560.58 |
| 11# | 48.53 | 14.38 | 493.87 | 562.35 | |||
| 12# | 47.88 | 14.88 | 490.76 | 558.29 | |||
| −900 mV | 13# | 46.94 | 14.16 ± 1.73 | 14.45 | 18.60 ± 2.08 | 495.07 | 573.60 |
| 14# | 46.04 | 13.95 | 493.79 | 572.15 | |||
| 15# | 45.07 | 14.67 | 492.33 | 570.94 | |||
| −950 mV | 16# | 44.17 | 19.70 ± 1.96 | 13.63 | 22.56 ± 2.10 | 492.45 | 569.23 |
| 17# | 42.90 | 13.30 | 491.18 | 567.08 | |||
| 18# | 42.08 | 14.04 | 493.93 | 569.83 | |||
| −1000 mV | 19# | 37.26 | 33.87 ± 3.61 | 13.17 | 25.59 ± 2.16 | 488.47 | 563.63 |
| 20# | 35.68 | 12.72 | 486.82 | 561.70 | |||
| 21# | 33.40 | 13.48 | 488.29 | 562.95 | |||
| −1200 mV | 22# | 27.49 | 52.32 ± 3.8 | 12.62 | 28.77 ± 2.43 | 479.36 | 560.14 |
| 23# | 25.83 | 12.11 | 482.05 | 562.96 | |||
| 24# | 23.36 | 12.97 | 481.87 | 561.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Sun, B.; Dai, Y.; Xie, F.; Huang, F.; Liu, Z.; Li, X. Understanding the Non-Steady Electrochemical Mechanisms of the Stress Corrosion Cracking of X70 Pipeline Steel in a Marine Environment. Materials 2025, 18, 2073. https://doi.org/10.3390/ma18092073
Xu B, Sun B, Dai Y, Xie F, Huang F, Liu Z, Li X. Understanding the Non-Steady Electrochemical Mechanisms of the Stress Corrosion Cracking of X70 Pipeline Steel in a Marine Environment. Materials. 2025; 18(9):2073. https://doi.org/10.3390/ma18092073
Chicago/Turabian StyleXu, Bo, Baozhuang Sun, Yang Dai, Fei Xie, Feng Huang, Zhiyong Liu, and Xiaogang Li. 2025. "Understanding the Non-Steady Electrochemical Mechanisms of the Stress Corrosion Cracking of X70 Pipeline Steel in a Marine Environment" Materials 18, no. 9: 2073. https://doi.org/10.3390/ma18092073
APA StyleXu, B., Sun, B., Dai, Y., Xie, F., Huang, F., Liu, Z., & Li, X. (2025). Understanding the Non-Steady Electrochemical Mechanisms of the Stress Corrosion Cracking of X70 Pipeline Steel in a Marine Environment. Materials, 18(9), 2073. https://doi.org/10.3390/ma18092073








